Abstract
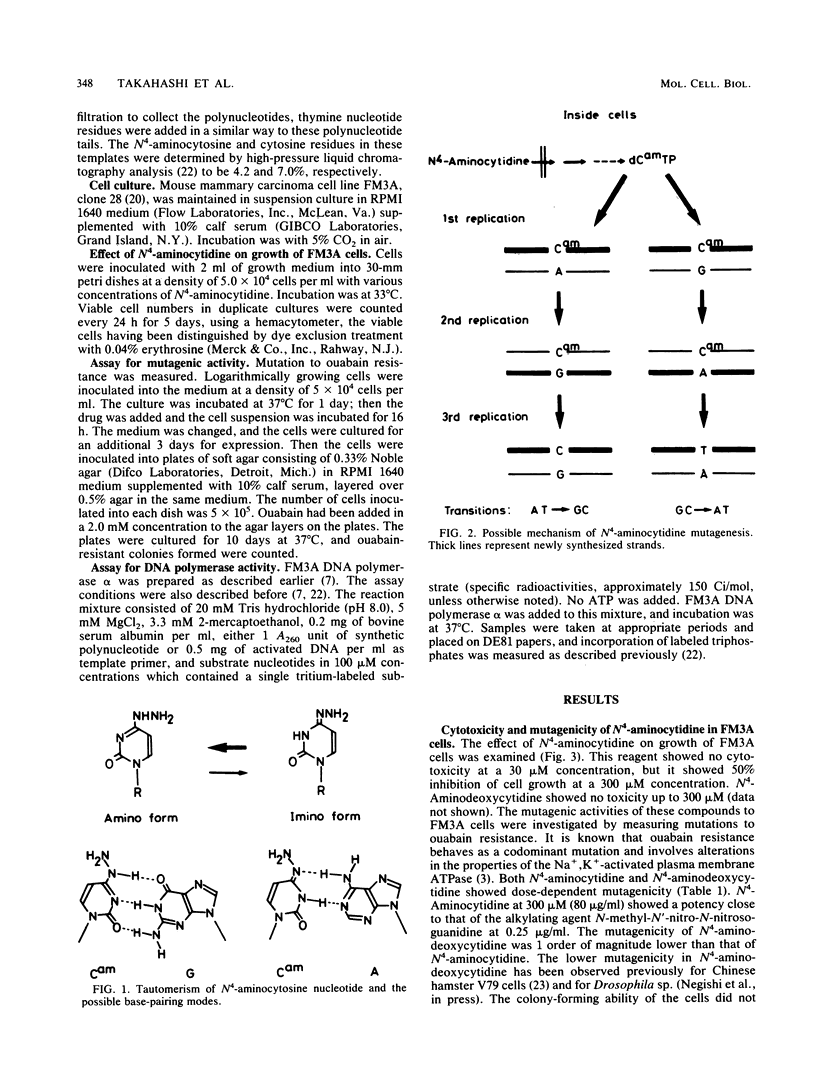
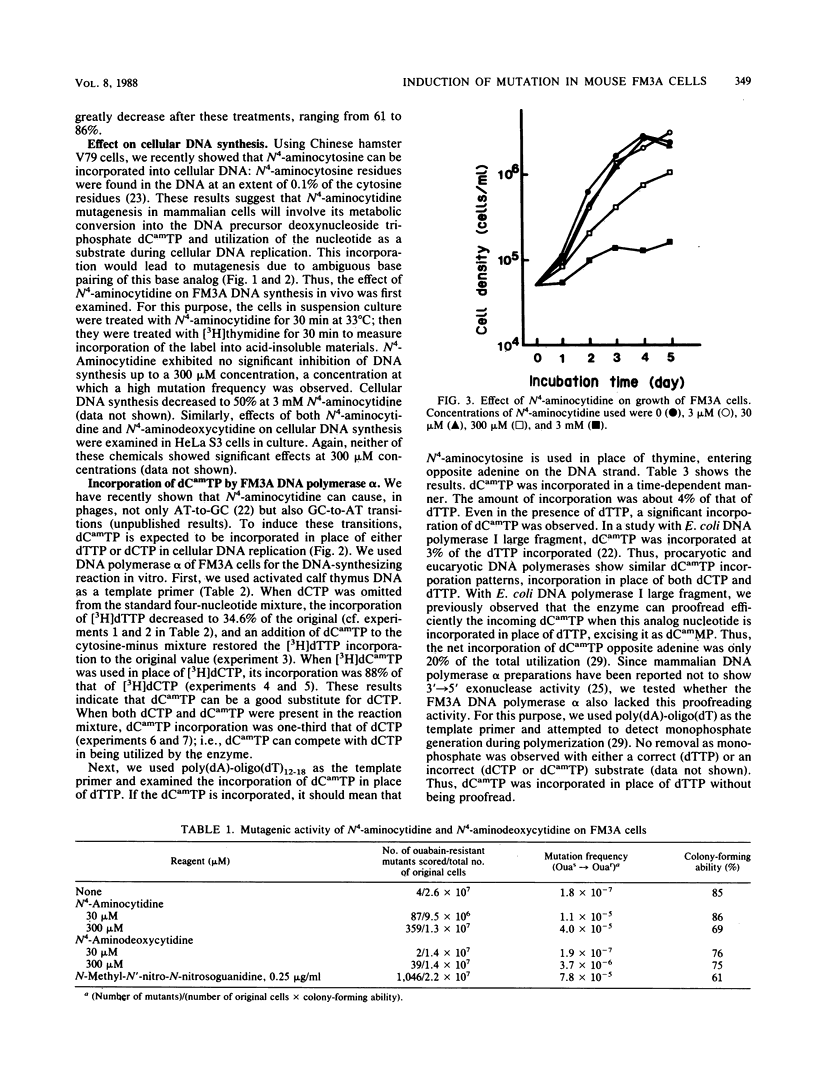
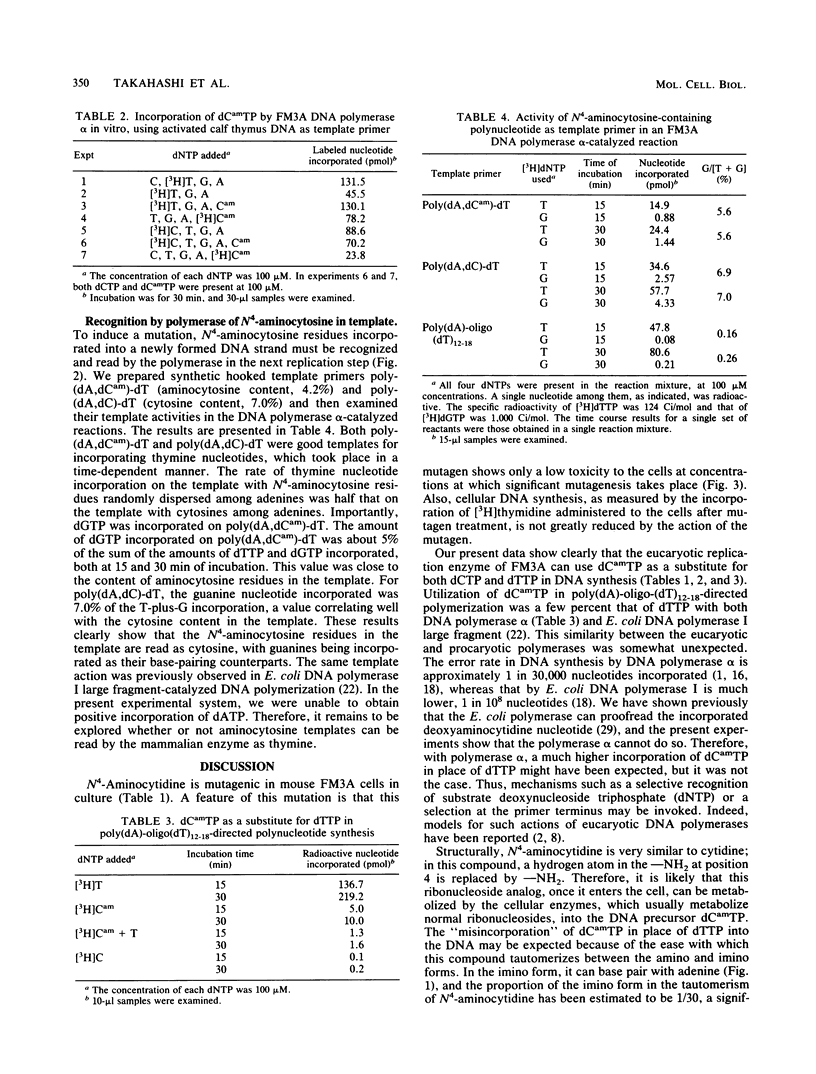
To explore the potential use of a nucleoside analog, N4-aminocytidine, in studies of cellular biology, the mechanism of mutation induced by this compound in mouse FM3A cells in culture was studied. On treatment of cells in suspension with N4-aminocytidine, the mutation to ouabain resistance was induced. The major DNA-replicating enzyme in mammalian cells, DNA polymerase alpha, was used to investigate whether the possible cellular metabolite of N4-aminocytidine, N4-aminodeoxycytidine 5'-triphosphate (dCamTP), can be incorporated into the DNA during replication. Using [3H]dCamTP in an in vitro DNA-synthesizing system, we were able to show that this nucleotide analog can be incorporated into newly formed DNA and that it can serve as a substitute for either dCTP or dTTP. dCamTP in the absence of dCTP maintained the activated calf thymus DNA-directed polymerization of deoxynucleoside triphosphates as efficiently as in its presence. Even in the presence of dCTP, dCamTP was incorporated into the polynucleotide. When dCamTP was used as a single substrate in the poly(dA)-oligo(dT)-directed polymerase reaction, it was incorporated into the polynucleotide fraction. The extent of incorporation was 4% of that of dTTP incorporation when dTTP was used as a single substrate. Even in the presence of dTTP, dCamTP incorporation was observed. A copolymer containing N4-aminocytosine residues was shown to incorporate guanine residues opposite the N4-aminocytosines. However, we were unable to observe adenine incorporation opposite N4-aminocytosine in templates. These cell-free experiments show that an AT-to-GC transition can take place in the presence of dCamTP during DNA synthesis, strongly suggesting that the mutation induced in the FM3A cells by N4-aminocytidine is due to replicational errors.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbotts J., Loeb L. A. DNA polymerase alpha and models for proofreading. Nucleic Acids Res. 1985 Jan 11;13(1):261–274. doi: 10.1093/nar/13.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbotts J., Loeb L. A. On the fidelity of DNA replication: use of synthetic oligonucleotide-initiated reactions. Biochim Biophys Acta. 1985 Jan 29;824(1):58–65. doi: 10.1016/0167-4781(85)90029-6. [DOI] [PubMed] [Google Scholar]
- Barrett J. C. Induction of gene mutation in and cell transformation of mammalian cells by modified purines: 2-aminopurine and 6-N-hydroxylaminopurine. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5685–5689. doi: 10.1073/pnas.78.9.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M., Hewlins M. J., Schell P. The tautomeric state of N(4)-hydroxy- and of N(4)-amino-cytosine derivatives. J Chem Soc Perkin 1. 1968;15:1925–1929. doi: 10.1039/j39680001925. [DOI] [PubMed] [Google Scholar]
- Crute J. J., Wahl A. F., Bambara R. A. Purification and characterization of two new high molecular weight forms of DNA polymerase delta. Biochemistry. 1986 Jan 14;25(1):26–36. doi: 10.1021/bi00349a005. [DOI] [PubMed] [Google Scholar]
- Enomoto T., Suzuki M., Takahashi M., Kawasaki K., Watanabe Y., Nagata K., Hanaoka F., Yamada M. Purification and characterization of two forms of DNA polymerase alpha from mouse FM3A cells: a DNA polymerase alpha-primase complex and a free DNA polymerase alpha. Cell Struct Funct. 1985 Jun;10(2):161–171. doi: 10.1247/csf.10.161. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Korn D. Ordered sequential mechanism of substrate recognition and binding by KB cell DNA polymerase alpha. Biochemistry. 1981 Aug 4;20(16):4560–4569. doi: 10.1021/bi00519a008. [DOI] [PubMed] [Google Scholar]
- Grossberger D., Clough W. Incorporation into DNA of the base analog 2-aminopurine by the Epstein-Barr virus-induced DNA polymerase in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7271–7275. doi: 10.1073/pnas.78.12.7271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. A., Saffhill R. The incorporation of O6-methyldeoxyguanosine and O4-methyldeoxythymidine monophosphates into DNA by DNA polymerases I and alpha. Nucleic Acids Res. 1983 Jun 25;11(12):4185–4193. doi: 10.1093/nar/11.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillebrand G. G., Beattie K. L. Influence of template primary and secondary structure on the rate and fidelity of DNA synthesis. J Biol Chem. 1985 Mar 10;260(5):3116–3125. [PubMed] [Google Scholar]
- Huberman E., Heidelberger C. The mutagenicity to mammalian cells of pyrimidine nucleoside analogs. Mutat Res. 1972 Jan;14(1):130–132. doi: 10.1016/0027-5107(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Kaufman E. R. Reversion analysis of mutations induced by 5-bromodeoxyuridine mutagenesis in mammalian cells. Mol Cell Biol. 1985 Nov;5(11):3092–3096. doi: 10.1128/mcb.5.11.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Alexander P. S. The base substitution fidelity of eucaryotic DNA polymerases. Mispairing frequencies, site preferences, insertion preferences, and base substitution by dislocation. J Biol Chem. 1986 Jan 5;261(1):160–166. [PubMed] [Google Scholar]
- Lasken R. S., Goodman M. F. The biochemical basis of 5-bromouracil-induced mutagenesis. Heteroduplex base mispairs involving bromouracil in G x C----A x T and A x T----G x C mutational pathways. J Biol Chem. 1984 Sep 25;259(18):11491–11495. [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Loquet C., Andrae U. Mutagenicity of 5-diazouracil in V79 Chinese hamster cells. Mutat Res. 1982 Aug;105(1-2):129–131. doi: 10.1016/0165-7992(82)90219-6. [DOI] [PubMed] [Google Scholar]
- Nakano N. Establishment of cell lines in vitro from a mammary ascites tumor of mouse and biological properties of the established lines in a serum containing medium. Tohoku J Exp Med. 1966 Jan 25;88(1):69–84. doi: 10.1620/tjem.88.69. [DOI] [PubMed] [Google Scholar]
- Negishi K., Harada C., Ohara Y., Oohara K., Nitta N., Hayatsu H. N4-aminocytidine, a nucleoside analog that has an exceptionally high mutagenic activity. Nucleic Acids Res. 1983 Aug 11;11(15):5223–5233. doi: 10.1093/nar/11.15.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K., Takahashi M., Yamashita Y., Nishizawa M., Hayatsu H. Mutagenesis by N4-aminocytidine: induction of AT to GC transition and its molecular mechanism. Biochemistry. 1985 Dec 3;24(25):7273–7278. doi: 10.1021/bi00346a038. [DOI] [PubMed] [Google Scholar]
- Nomura A., Negishi K., Hayatsu H., Kuroda Y. Mutagenicity of N4-aminocytidine and its derivatives in Chinese hamster lung V79 cells. Incorporation of N4-aminocytosine into cellular DNA. Mutat Res. 1987 Apr;177(2):283–287. doi: 10.1016/0027-5107(87)90012-1. [DOI] [PubMed] [Google Scholar]
- Prelich G., Kostura M., Marshak D. R., Mathews M. B., Stillman B. The cell-cycle regulated proliferating cell nuclear antigen is required for SV40 DNA replication in vitro. Nature. 1987 Apr 2;326(6112):471–475. doi: 10.1038/326471a0. [DOI] [PubMed] [Google Scholar]
- Sedwick W. D., Shu-Fong Wang T., Korn D. "Cytoplasmic" deoxyribonucleic acid polymerase. Structure and properties of the highly purified enzyme from human KB cells. J Biol Chem. 1975 Sep 10;250(17):7045–7056. [PubMed] [Google Scholar]
- Singer B. C4-methyldeoxythymidine replacing deoxythymidine in poly[d(A-T)] renders the polymer resistant to the 3'----5' exonuclease activity of the Klenow and T4 DNA polymerases. Nucleic Acids Res. 1986 Aug 26;14(16):6735–6743. doi: 10.1093/nar/14.16.6735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Negishi K., Hayatsu H. Induction of mutation in vitro in phage phi X174 am3 by N4-aminodeoxycytidine triphosphate. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1277–1283. doi: 10.1016/0006-291x(85)90229-3. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Negishi K., Hayatsu H. Proofreading of a mutagenic nucleotide, N4-aminodeoxycytidylic acid, by Escherichia coli DNA polymerase I. Biochem Biophys Res Commun. 1987 Feb 27;143(1):104–109. doi: 10.1016/0006-291x(87)90636-x. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Castillo C., So A. G., Downey K. M. An auxiliary protein for DNA polymerase-delta from fetal calf thymus. J Biol Chem. 1986 Sep 15;261(26):12310–12316. [PubMed] [Google Scholar]
- Topal M. D., Hutchison C. A., 3rd, Baker M. S. DNA precursors in chemical mutagenesis: a novel application of DNA sequencing. Nature. 1982 Aug 26;298(5877):863–865. doi: 10.1038/298863a0. [DOI] [PubMed] [Google Scholar]
- Wahl A. F., Crute J. J., Sabatino R. D., Bodner J. B., Marraccino R. L., Harwell L. W., Lord E. M., Bambara R. A. Properties of two forms of DNA polymerase delta from calf thymus. Biochemistry. 1986 Dec 2;25(24):7821–7827. doi: 10.1021/bi00372a006. [DOI] [PubMed] [Google Scholar]
- Watanabe S. M., Goodman M. F. On the molecular basis of transition mutations: frequencies of forming 2-aminopurine.cytosine and adenine.cytosine base mispairs in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2864–2868. doi: 10.1073/pnas.78.5.2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]



