Abstract
Stem cell therapy is a promising strategy in promoting cardiac repair in the setting of ischemic heart disease. Clinical and preclinical studies have shown that cell therapy improves cardiac function. Whether autologous or allogeneic cells should be used, and the need for immunosuppression in non-autologous settings, is a matter of debate. Cyclosporin A (CsA) is frequently used in preclinical trials to reduce cell rejection after non-autologous cell therapy. The direct effect of CsA on the function and survival of stem cells is unclear. Furthermore, the appropriate daily dosage of CsA in animal models has not been established. In this review, we discuss the pros and cons of the use of CsA on an array of stem cells both in vitro and in vivo. Furthermore, we present a small collection of data put forth by our group supporting the efficacy and safety of a specific daily CsA dosage in a pig model.
Keywords: Immunosuppression, Allogeneic cell therapy, Autologous cell therapy, Cardiac regeneration, Cyclosporine
Cardiac Regenerative Therapy
Stem Cell Therapy for Cardiac Repair
Stem cell therapy (or progenitor- or precursor cell therapy) has emerged as a promising therapy for cardiac repair. Despite the presence of endogenous cardiac stem cells [1, 2], the heart’s ability to self-renew is inadequate for compensating the extensive ischemic injury [3]. In the acute setting, delivery of stem cells may modulate the post-inflammatory response, while regeneration and prevention of further cardiac remodelling may be achieved in a more chronic phase.
Apart from differentiation of stem cells into cardiomyocytes, a more likely mechanism of action is through paracrine signalling [2–6]. Paracrine signalling may reduce the inflammatory response, promote vasculogenesis, and stimulate endogenous (cardiac) stem cells [7].
Stem cell therapy has successfully been investigated for the recovery of cardiac function in ischemic heart disease in clinical and preclinical setting [8–10]. Although these results are promising, low delivery efficiency and engraftment rates (≤10 %) should be emphasized [5, 11–14].
Mechanical washout and/or loss, cell death [15], and redistribution to other organs [12] play a role. Additionally, in non-autologous therapy, cell rejection may cause even lower engraftment, due to decreased survival of transplanted cells in the hostile environment.
Allogeneic Versus Autologous Stem Cells
Allogeneic cell therapy enables prior preparation of the right cell type and immediate “off-the-shelf” therapy but may require immune suppression to avoid cell rejection. Autologous cell therapy lacks immunologic concerns but is associated with low cost-effectiveness, logistic concerns and lifelong exposure of cells to ageing, comorbidity, and risk factors [3, 4, 16]. A meta-analysis of preclinical trials showed no difference in effect size between autologous and allogeneic cell therapy for cardiac repair, irrespective of immunosuppressive therapy [17]. This underscores the potential paracrine working mechanism of cell therapy and might even imply that immunosuppression is not necessary.
The use of mesenchymal stem cells (MSCs) for allogeneic cell therapy may obviate the need for immune suppression due to the MSC’s proposed immunomodulatory effect and apparent immune-privileged state [18–20]. The immunosuppressive capability of MSCs can even be enhanced by pharmacological agents like cyclosporine (CsA) [21, 22]. Conflicting studies, however, have shown that MSCs are indeed immunogenic and provoke an immune response [23, 24]. Thus, the potential role of immunosuppressive drugs cannot be ignored for MSCs as well.
The need of immunosuppression in clinical application of allogeneic cells for cardiac regeneration is unknown, as is the role of CsA in this setting. An overview of preclinical data might be elucidating and guiding for future clinical studies.
Alloreactivity
Alloreactivity depends on foreign peptide presentation by major histocompatibility complex (MHC) on antigen presenting cells and detection by T cells [25]. Immunomodulation for prevention of alloreactivity should therefore act on T cell suppression. T cell suppressors include calcineurin inhibitors, corticosteroids, antimetabolites, and target-of-rapamycin inhibitors. As CsA, a calcineurin inhibitor, is most often used in preclinical trials of allogeneic cell therapy, it will be the focus of this review. Little information exists on the pharmacokinetics and subsequent correct dosage of CsA in large animals.
Cyclosporin
Mechanism of Action of CsA
CsA suppresses T cell activity by forming a complex with the intracellular receptor cyclophilin. This CsA-cyclophilin complex subsequently binds to calcineurin A, inhibiting its phosphatase activity [26–30]. Inhibition of calcineurin A blocks activity of nuclear factor of activated T cells (NFAT). The inhibition of the calcineurin/NFAT pathway reduces IL-2, IL-4, and interferon-γ production [31–35], leading to cell cycle arrest, DNA and RNA inhibition, and inhibition of protein synthesis and growth factor production of T cells [36].
In clinical practice, CsA is used as an immunosuppressor in organ transplantation, bone marrow transplantation, and inflammatory diseases like rheumatoid arthritis and psoriasis. CsA is known for its interaction with several other pharmacological agents and its serious adverse effects. The most often described adverse effect is nephrotoxicity [37].
CsA in Clinical Practice
In clinical application of CsA, dosages differ based on indication [38]. Defining the correct daily CsA dosage and therapeutic serum levels in organ transplantation proves to be challenging as a fine balance must be found between organ rejection (underdose) and toxicity (overdose) [39–41]. Large intra- and interindividual differences in absorption, distribution, metabolism, and elimination are observed, and no true definitions of cell rejection and toxicity exist [42]. Nevertheless, serum concentrations exceeding 300 ng/mL are associated with toxicity, nephrotoxicity in particular, while lower levels were linked to organ rejection [39].
In renal transplant patients, an initial serum level of 250–400 ng/mL and a maintenance level of 100–250 ng/mL are achieved with dosages of 4–6 and 2–6 mg/kg/day, respectively [43, 44].
In a survey carried out among 87 “European Group for Blood and Marrow Transplantation” centers, the median daily oral CsA maintenance dose was 10 mg/kg/day, with a range of 2–12.5 mg/kg/d [45]. Most target serum concentration ranges were 100–500 ng/mL.
CsA in Cardiology
In the field of cardiology, CsA has been investigated for reduction of infarct size after myocardial ischemia. In a meta-analysis of animal models of myocardial ischemia/reperfusion, cyclosporine was associated with a smaller infarct size compared to control [46]. The proposed mechanism is prevention of opening of the mitochondrial permeability transition pores, which is triggered by the oxygen-derived free radicals coupled with Ca2+ during reperfusion. However, in a third of the included studies, there is no effect of CsA, and there was evidence for publication bias, based on funnel plotting. The direct effect of CsA on myocardial infarct size, on top of the effect of CsA on stem cells, must be emphasized, in a setting of stem cells therapy for acute myocardial ischemia.
In order to identify studies of the effect of CsA on stem cells in vitro and in vivo in the setting of myocardial infarction, we conducted a search in the PubMed database on August 1, 2013, using the search terms ((((((((ciclosporin[Title/Abstract]) OR cyclosporin[Title/Abstract]) OR ciclosporine [Title/Abstract]) OR cyclosporine [Title/Abstract]) OR Neoral[Title/Abstract]) OR Sandimmune [Title/Abstract]) OR CsA[Title/Abstract])) AND ((((((“stem cells”[Title/Abstract]) OR “stem cell”[Title/Abstract]) OR progenitor*[Title/Abstract]) OR “bone marrow cells”[Title/Abstract]) OR “bone marrow derived cells”[Title/Abstract]) OR pluripotent [Title/Abstract]).
In Vitro Experiments
Aside from both a (nephro) toxic and immunosuppressive effect, CsA may have a direct effect on stem cells. In several experiments, stem cells were incubated with varying concentrations of CsA and proliferation [47–57], survival [49], apoptosis [58, 59], differentiation [53, 55, 57], migration [53], and angiogenesis [59] were analysed. Table 1 summarizes the studies that revealed a positive effect (enhanced differentiation, proliferation, survival, or decreased apoptosis) or negative impact (reduced differentiation, proliferation, survival, or increased apoptosis) of CsA on different cell types, compared to control.
Table 1.
In vitro effects of CsA on different cell types
| Reference | Cell type | CsA concentration | Conclusion |
|---|---|---|---|
| Positive effect | |||
| Chen at al. [58] | Rat MSC | 0.5–5 µM (0.6–6 µg/mL) | Reduced apoptosis after hypoxia/reoxygenation |
| Fujiwara et al. [47] | Mouse/Human iPSC | 1–3 µg/mL | Enhanced cardiac differentiation |
| Hunt et al. [49] | Mouse NPC | 0.1–0.5 µg/mL | Enhanced proliferation, enhanced survival |
| Perry et al. [50] | Mouse HSPC/BMC | 0.1–100 µg/mL | Enhanced proliferation in low dose, inhibition in high dose |
| Sachinidis et al. [51] | Mouse ESCs | 1 µM (1.2 µg/mL) | Enhanced cardiac differentiation |
| Yan et al. [56] | Mouse ESC | 1–3 µg/mL | Enhanced cardiac differentiation |
| Negative effect | |||
| Byun et al. [52] | Rat MSCs | 50–500 nM (0.06–0.6 µg/mL) | No effect on proliferation |
| Davies et al. [53] | Porcine MNCs | 0.001–0.2 µg/mL | Decreased proliferation, differentiation, migration of smooth muscle and endothelial outgrowth cells |
| Guo et al. [57] | Rat NSC | 0.5–5 µg/mL | Decreased proliferation |
| Poncelet et al. [54] | Swine MSC | 0.2 µg/mL | Reduced proliferation |
| Song et al. [55] | Mouse MSC | 1–10 µM (1.2–12 µg/mL) | Reduced proliferation |
| Yang et al. [59] | Human EPC | 0.01–10 µg/mL | Reduced proliferation, increased apoptosis, reduced angiogenesis |
iPSC induced pluripotent stem cell, HSPC hematopoietic stem/progenitor cell, BMC Bone marrow cells, EB embryonic bodies, ESC embryonic stem cell, NPC neural precursor cell, MSC mesenchymal stem cell, MNC mononuclear cell, EPC endothelial progenitor cell, NSC neural stem cell
As can be seen in Table 1, numerous studies have shown a pro-proliferative effect of CsA on stem cells. A calcineurin/NFAT pathway-independent effect has been proposed [47, 49, 56], because of the absence of a significant effect when using other calcineurin/NFAT inhibitors such as tacrolimus and 11R-VIVIT [56].
Apoptosis
Reduced apoptosis and increased survival, as seen in Chen et al. and Hunt et al, are explained by prevention of mitochondrial dysfunction [49, 58] and promotion of bcl-2, an antiapoptotic factor [58]. However, Hunt et al. showed decreased cell viability and decreased cell proliferation at higher concentrations (≥5 µg/mL) [49]. Yang et al. reported increased apoptosis of endothelial progenitor cells by all concentrations tested (0.1–10 µg/ml) and attributed this to decreased NO generation, which promotes EPC apoptosis [59].
A dose dependent effect is endorsed by Perry et al. showing enhanced proliferation in dosages until 1 µg/mL and decreased proliferation in higher dosages.
Proliferation
Different studies showed both anti- and pro-proliferative effects of CsA. The mechanism behind the anti-proliferative effect remains unclear. Disruption of the calcineurin pathway may influence the fate of stem cells [57]. Other studies showed or stated the reduced proliferation to be caused through alteration in the NOS/NO pathway by CsA, where a decrease in NO production causes decrease in proliferation and differentiation [55, 59, 60].
The pro-proliferative effect is ascribed to inhibition of IFN-γ [50], decreased cell-adhesion by decreased cyclophilin, and subsequently decreased matrix-metalloproteinase production [49].
Cell Types
Incubation of CsA with MSCs seems to negatively influence stem cell growth. Two studies showed reduced proliferation [54, 55], one study showed no effect on proliferation [52], and only one study showed a positive effect in the form of reduced MSC apoptosis [58]. Two studies used embryonic stem cells (ESCs) [51, 56], and both revealed a positive pro-proliferative effect of CsA, suggesting ESCs may benefit from the presence of CsA.
In Vivo Experiments
We identified only four studies investigating the role of CsA in cell therapy in an animal model of ischemic heart disease. Stem cells were transplanted into the ischemic myocardium following ligation of the left anterior descending artery, with or without CsA [61–64]. Immune cell infiltration, cell survival, contractile performance, and/or ejection fraction (EF) were monitored. Table 2 summarizes the positive and negative effects (positive: enhanced survival or engraftment, reduced immune reaction, improved cardiac function; negative: decreased survival or engraftment, immune reaction, less improved cardiac function) of CsA on non-autologous stem cells in vivo.
Table 2.
In vivo effects of CsA on different non-autologous cell types, compared to control
| Reference | Cell type | Animal model | N | CsA dosage (mg/kg/day) |
Administration | Conclusion |
|---|---|---|---|---|---|---|
| Positive effect | ||||||
| Guo et al. [61] | Human SkM | Rat | 208 | 10 | Intraperitoneal | Reduced immunocyte infiltration, enhanced cell survival, improved EF |
| Westrich et al. [62] | Rat MSC | Rat | 12 | 10 | NA | Enhanced cell survival |
| Negative or neutral effect | ||||||
| Chiavegato et al. [63] | Human AFS | Rat | 42 | 5 | Intramuscular | Unsuccessful inhibition of cell rejection |
| Zeng et al. [64] | Swine pMultistem | Swine | 30 | 15 | Oral | No effect on engraftment and cardiac function |
SkM skeletal myoblasts, MSC mesenchymal stem cells, AFS amniotic fluid-derived stem cells, MNC mononuclear cell, pMultistem bone marrow-derived adherent stem cells, EF ejection fraction
Guo et al. investigated immune cell kinetics, myoblast survival, and cardiac function after myocardial injection of human myoblasts, human myoblast + CsA, rat myoblasts and rat myoblasts + CsA, in rats 1 week after MI. CsA caused less infiltration of immune cells, more myoblast survival, and improved cardiac function, compared to cells alone. The observed improvement in cardiac function was largely attributed to the reduced infiltration of macrophages and CD4+/CD8+ cells, causing prolonged myoblast survival [61]. However, 10 min after injection, stated as baseline, two-thirds of the injected cells were already lost. After 1 day, only 10 % of the numbers of cells at baseline were still present in all groups.
Westrich et al. compared intramyocardial syngeneic and allogeneic MSCs injections in rats, both with and without CsA, 5 days after MI. Cell survival of syngeneic cells was higher than allogeneic, regardless of use of CsA. However, cell survival per cell source was higher in CsA-treated groups compared to cells only, explained by the anti-inflammatory and anti-apoptotic effect of CsA [62].
In contrast, Chiavegato et al. showed the activity of macrophages and dendritic cells remains unaffected by CsA and graft cell rejection still occurs [63]. In this study, human amniotic fluid-derived stem cells (AFS) were injected in the myocardium of three groups of rats: healthy animals; 20 min after MI; and athymic animals. The authors were surprised by the results because of their previous experience with engraftment and differentiation of human amniotic MSCs in immune competent rats after MI and engraftment of human “amnion cells” in neonatal swine and rats. Because of the rejection in athymic rats and rats treated with CsA, a T cell independent mechanism is proposed, where natural antibodies, complement factors, NK cells, or macrophages may play a role [63].
Zeng et al. demonstrated that injection of allogeneic pMultistem cells (porcine derived multipotent adult progenitor cells) in pigs immediately after MI, led to improvement in cardiac function, irrespective of CsA treatment. Engraftment rate was also not affected by CsA, and only 0.35%of the cells could be detected after 4 weeks. Differentiation of present cells into cardiomyocytes was limited, and infarct size was not affected. Therefore, paracrine signalling was proposed as the main mechanism of effect [64].
Endogenous Recruitment
Hunt et al., showing increased proliferation and survival in vitro (Table 1), also investigated the effects of CsA in vivo in healthy mice [49]. Authors observed an increased number of neurospheres from endogenous neural progenitors in CsA-treated animals compared to control animals. Furthermore, an increased number of neural stem cells was observed. This recruitment of endogenous progenitor cells is confirmed by Wang et al. [65] In this study, authors show endothelial progenitor cell mobilization by CsA in a hind limb ischemia mouse model. Proposed mechanism is by decreased activity of CD26, which causes an increase in the level of chemo attractants for progenitor cells (like stroma-derived factor-1α). In contrast to this data, Davies et al. showed in a porcine cardiac transplant model that endothelial and smooth muscle outgrowth colony numbers decreased after treatment with CsA in sham-operated as well as transplanted animals [53]. In the sham-operated + CsA-treated animals, the number of colonies increased to above baseline levels at 4 weeks. In sham alone, without CsA, the endothelial colony numbers remained stable. This detrimental effect in vivo is in accordance with the effects shown by Davies et al. in vitro (Table 1).
Target CsA Levels in Large Animals
The therapeutic range and the subsequent recommended daily dosage of CsA in large laboratory animals are largely unknown. The pig serves as an excellent model for cardiac and pharmacological studies due to its similar physiology and anatomy compared to men [66]. Nevertheless, Frey et al. demonstrated that pigs require two times higher intravenous doses and up to four to six times higher oral doses of CsA in order to reach the same serum levels as in humans [67]. In accordance with Frey et al., Cibulskyte et al. demonstrated that an oral dosage of 30 kg/mg/day in pigs is needed to reach blood concentration levels of 475 ng/ml. However, at such high dosages acute nephrotoxicity was observed within 4 weeks. A dosage of 15 mg/kg/day was stated to be safe and caused a trough concentration of 338 ng/ml [68].
Dosing and Safety in a Pig Model of Myocardial Infarction
Rationale
In order to address the problem of the inconsistency in recommended CsA dosages in the setting of cardiac repair, we performed a small study investigating serum levels and animal safety after administration of CsA in a myocardial ischemia/reperfusion pig model.
Methods
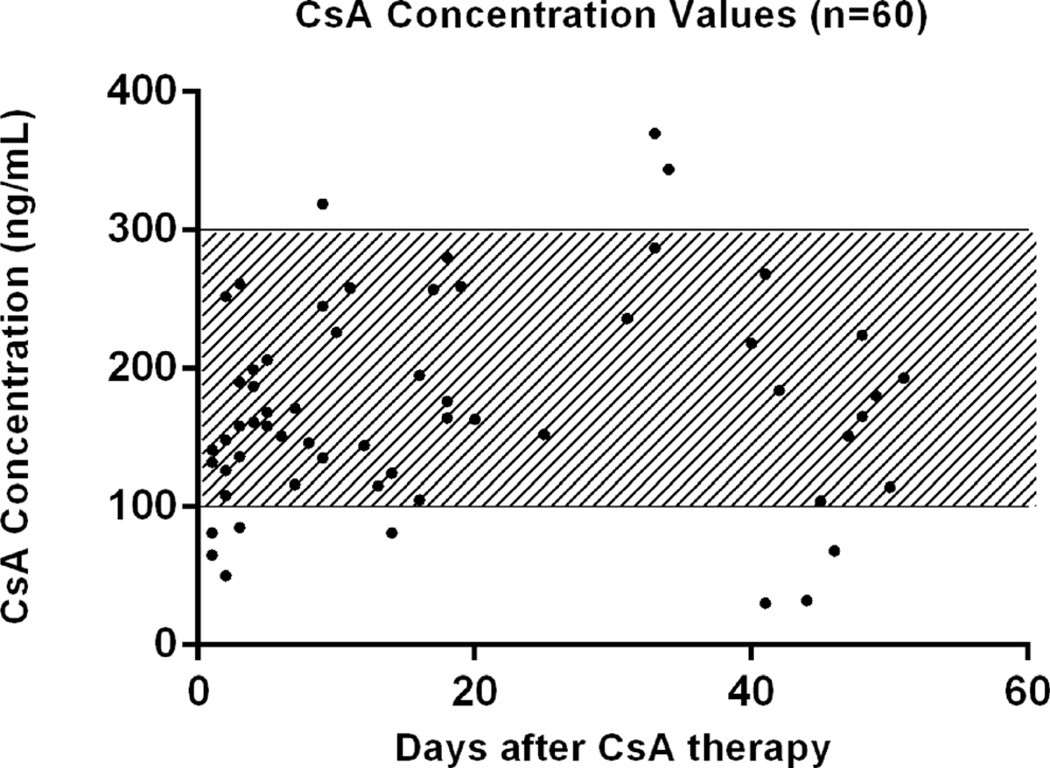
Open chest LAD occlusion was performed for 90 min in pigs (n=5, mean weight 32.5±2.1 kg at start of study). CsA was orally administered twice daily (15 mg/kg/day), and venous blood was collected at different time points through a peripherally inserted central line, tunneled to the back. The mean follow-up was 44.6 days. We determined the target range to be between 100–300 ng/mL, based on levels for safety and efficacy suggested by others [39, 43, 53].
Results
CsA serum levels were within the therapeutic range in 81.7 % of all cases (49/60) (Fig. 1). The mean CsA levels (and the upper and lower limit) per pig were as follows: pig 1, 182 ng/mL (280–85); pig 2, 177 ng/mL (344–108); pig 3, 182 ng/mL (261–81), pig 4, 167 ng/mL (287–50); pig 5, 153 ng/mL (319–30).
Fig. 1.
CsA concentration values of all five pigs (n=60). Therapeutic range determined at 100–300 ng/mL
To investigate the safety of the given CsA dosage and the significance of the determined therapeutic range, serum values were analyzed and compared to existing reference values (Table 3).
Table 3.
Mean serum value per pig throughout the follow-up period
| Reference | Pig 1 | Pig 2 | Pig 3 | Pig 4 | Pig 5 | Mean | ||
|---|---|---|---|---|---|---|---|---|
| Creatinine | 1.2–2.0 | mg/dL | 1.7 | 1.4 | 1.1 | 1.5 | 1.6 | 1.5±0.23 |
| AST | 14–56 | IU/L | 26.1 | 46.2 | 52.6 | 43.2 | 44.0 | 42.4±9.8 |
| ALT | 5–78 | IU/L | 37.6 | 59.8 | 51.9 | 50.2 | 51.2 | 50.1±8.0 |
| Hb | 9.0–16.2 | g/dL | 9.0 | 11.1 | 10.2 | 10.4 | 11.2 | 10.4±0.9 |
| WBC | 6.3–21.1 | 103/mL | 14.2 | 15.4 | 19.6 | 13.9 | 18.5 | 16.3±2.6 |
| BUN | 8–24 | mg/dL | 11.1 | 10.1 | 7.6 | 10.0 | 9.4 | 9.6±1.3 |
| Sodium | 133–153 | meq/dL | NA | 141.3 | 135.4 | 139.3 | 143.6 | 139.9±3.5 |
| Potassium | 3.1–6.2 | meq/dL | NA | 4.3 | 4.4 | 4.4 | 4.4 | 4.4±0.1 |
Number of measurements per pig: pig 1 n=19; pig 2 n=10; pig 3 n=9; pig 4 n=8; pig 5 n=5. The items in bold represent the mean values of the 5 pigs together
AST aspartate transaminase, ALT alanine aminotransferase, Hb hemoglobin, WBC white blood cells, BUN blood urea nitrogen
None of the serum levels exceeded the reference values. In conclusion, our data suggest that the administration of 15 mg/kg/day twice a day in a pig model of myocardial infarction is feasible and represents a safe dosage that ensures adequate CsA serum concentration values with 82 % of the values within the determined therapeutic range.
Conclusion
This essay summarizes current knowledge on the role of CsA in non-autologous stem cell therapy. CsA has been reported to exert inconsistent effects on stem cells both in vitro and in vivo. In vitro, a positive effect was observed through enhanced proliferation, enhanced survival, or reduced cell apoptosis. At the same time, different studies using a similar methodology reported negative effects, namely reduced proliferation, angiogenesis, differentiation and migration, and increased apoptosis. In both the positive and negative studies, approximately the same range of CsA concentration was used.
Interestingly, enhanced proliferation was reported, while cell viability was drastically reduced at higher concentrations [48–50], suggesting CsA may only have a beneficial effect up to a certain concentration.
In vivo, similar conflicting results were observed. In both the positive and negative studies, CsA dosage was fairly equal. Studies focused on both cell engraftment and cardiac function, but little is known on the link between engraftment rate and cardiac function.
The conflicting results in both in vivo and in vitro data, maybe explained by heterogeneity in included studies. Methodology of included studies is quite similar, but cell type and cell source are different. From these data, the exact role of CsA in animal models cannot be fully elucidated.
Non-autologous cells will eventually be rejected, with or without immune suppression [4]. A large proportion of the observed beneficial effect of stem cell therapy is thought to be through indirect pathways. Cell rejection may not be a matter of concern as long as enough time has passed for these paracrine effects to occur [4]. At the same time, avoiding cell rejection and hence prolonging graft survival may contribute to overall improvement in cardiac function.
We have also presented data showing that oral administration of CsA twice a day in a pig model of myocardial infarction is feasible and safe and blood concentrations remain within the therapeutic range. As others have suggested, the daily dose in our pig study is higher than in humans.
In conclusion, there is no evidence to clearly demonstrate whether CsA, or immune suppression in general, is essential and beneficial in non-autologous cell therapy. Regardless of this limited knowledge, the issue of immune suppression is one of great importance and thus should be further elucidated.
Limitations
First, the effect of CsA on endogenous stem cell properties was not discussed. Since one of the suggested mechanisms of effect of cell therapy is paracrine signaling, this might be of importance. Second, we focused on animal models of myocardial infarction. The effect of, and need for CsA, in other disease models might be different. Finally, no reliable meta-analysis could be performed, because of the large variability of cell type, CsA dosages, and measures of effect size.
Future Directions
Although CsA is commonly used as an immunosuppressant in organ transplantation, its contribution to cell therapy for cardiac regeneration remains unclear. If immune suppression is deemed redundant, future studies should focus on the impact of cell rejection (and low engraftment) on cardiac function following cell transplantation.
On the other hand, if immune suppression is a requirement for cell engraftment in non-autologous stem cell therapy, future experiments should focus on the effect of CsA on cardiac function after cell therapy in a myocardial ischemia large animal model. Furthermore, a dose-response experiment could shed light on the fine balance between successful cell engraftment and animal safety.
Acknowledgment
Part of this work is supported by the Project P1.04 SMARTCARE of the research program of the BioMedical Materials institute, co-funded by the Dutch Ministry of Economic Affairs and by a Fellowship Grant of the Interuniversity Cardiology Institute of the Netherlands (ICIN).
Contributor Information
S. J. Jansen of Lorkeers, Department of Cardiology, University Medical Center Utrecht, Utrecht, The Netherlands
E. Hart, Department of Cardiology, University Medical Center Utrecht, Utrecht, The Netherlands
X. L. Tang, Institute of Molecular Cardiology, University of Louisville, Louisville, KY, USA
M. E. D. Chamuleau, Department of Hematology, VU Medical Center, Amsterdam, The Netherlands
P. A. Doevendans, Department of Cardiology, University Medical Center Utrecht, Utrecht, The Netherlands
R. Bolli, Institute of Molecular Cardiology, University of Louisville, Louisville, KY, USA
S. A. J. Chamuleau, Email: s.a.j.chamuleau@umcutrecht.nl, Department of Cardiology, University Medical Center Utrecht, Utrecht, The Netherlands.
References
- 1.Beltrami AP, Urbanek K, Kajstura J, Shao-Min Y. Evidence that human cardiac myocytes divide after myocardial infarcation. The New England Journal of Medicine. 2001;344(23) doi: 10.1056/NEJM200106073442303. [DOI] [PubMed] [Google Scholar]
- 2.Tang YL, Wang YJ, Chen LJ, Pan YH, Zhang L, Weintraub NL. Cardiac-derived stem cell-based therapy for heart failure: progress and clinical applications. Experimental Biology and Medicine (Maywood, N.J.) 2013;238(3):294–300. doi: 10.1177/1535370213477982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segers VFM, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451(7181):937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 4.Malliaras K, Marbán E. Cardiac cell therapy: where we’ve been, where we are, and where we should be headed. British Medical Bulletin. 2011;98:161–185. doi: 10.1093/bmb/ldr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Jameel MN, Li Q, Mansoor A, Qiang X, Swingen C, et al. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120(11 Suppl):S238–S246. doi: 10.1161/CIRCULATIONAHA.109.885236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(2):208–216. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation Research. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Spoel TIG, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyöngyösi M, Sluijter JPG, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovascular Research. 2011;91(4):649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 10.Clifford D, Fisher S, Brunskill S, Doree C, Mathur A, Watt S. Stem cell treatment for acute myocardial infarction (Review) The Cochrane Collaboration. 2012;(2) doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Toma C. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 12.Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. Journal of the American College of Cardiology. 2009;54(17):1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, et al. Aquantitative, randomized study evaluating threemethods of mesenchymal stem cell delivery following myocardial infarction. European Heart Journal. 2006;27(9):1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 14.Van der Spoel TIG, Vrijsen KR, Koudstaal S, Sluijter JPG, Nijsen JFW, de Jong HW, et al. Transendocardial cell injection is not superior to intracoronary infusion in a porcine model of ischaemic cardiomyopathy: a study on delivery efficiency. Journal of Cellular and Molecular Medicine. 2012;16(11):2768–2776. doi: 10.1111/j.1582-4934.2012.01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malliaras K, Kreke M, Marbán E. The stuttering progress of cell therapy for heart disease. Clinical Pharmacology and Therapeutics. 2011;90(4):532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 16.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circulation Research. 2008;102(11):1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen of Lorkeers S, Eding JEC, Spoel TIG, van der Vesterinen HM, Sena ES, Doevendans PA, et al. Similar effect of autologous and allogeneic cell therapy for ischaemic heart disease: results from a meta-analysis of large animal studies. Journal of American College of Cardiology. 2014;63(12):A1762. [Google Scholar]
- 18.Halkos ME, Zhao Z-Q, Kerendi F, Wang N-P, Jiang R, Schmarkey LS, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Research in Cardiology. 2008;103(6):525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 19.Tse WTT, Pendleton JDD, Beyer WMM, Egalka MCC, Guinan ECC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75(3):389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 20.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Experimental Hematology. 2002;30(1):42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 21.Maccario R, Moretta A, Cometa A, Montagna DD, Comoli P, Locatelli F, et al. Human mesenchymal stem cells and cyclosporin a exert a synergistic suppressive effect on in vitro activation of alloantigen-specific cytotoxic lymphocytes. Biology of Blood and Marrow Transplantation. 2005;1032(12):1031–1032. doi: 10.1016/j.bbmt.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 22.Buron F, Perrin H, Malcus C, Héquet O, Thaunat O, Kholopp-Sarda M-N, et al. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: an in vitro study using human cells. Transplantation Proceedings. 2009;41(8):3347–3352. doi: 10.1016/j.transproceed.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Nauta AJJ, Westerhuis G, Kruisselbrink ABB, Lurvink EGAGA, Willemze R, Fibbe WEE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108(6):2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poncelet AJ, Vercruysse J, Saliez A, Gianello P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation. 2007;83(6):783–790. doi: 10.1097/01.tp.0000258649.23081.a3. [DOI] [PubMed] [Google Scholar]
- 25.Felix NJ, Allen PM. Specificity of T-cell alloreactivity. Nature Reviews. Immunology. 2007;7(12):942–953. doi: 10.1038/nri2200. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber S, Crabtree GR. The mechanism of action of cyclosporin A and FK506. Clinical Immunology and Immunopathology. 1996;80(3 Pt 2):S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- 27.Kiani A, Rao A, Aramburu JIMK, Carus G. Manipulating immune responses with immunosuppressive agents that target NFAT. Immunity. 2000;12:359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 28.Kaye RE, Fruman DA, Bierer BE, Albers MW, Zydowsky LD, Ho SI, et al. Effects of cyclosporin A and FK506 on Fc epsilon receptor type I-initiated increases in cytokine mRNA in mouse bone marrow-derived progenitor mast cells: resistance to FK506 is associated with a deficiency in FK506-binding protein FKBP12. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(18):8542–8546. doi: 10.1073/pnas.89.18.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Farmer JDD, Lane WSS, Friedman J, Weissman I, Schreiber SLL. Calcineurin Is a common target of cyclophili-cyclosporin A and FKBP-FK506 Complexes. Cell. 1991;66 doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 30.Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47(2–3):119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 31.Fric J, Zelante T, Wong AYW, Mertes A, Yu H-B, Ricciardi-Castagnoli P. NFAT control of innate immunity. Blood. 2012;120(7):1380–1389. doi: 10.1182/blood-2012-02-404475. [DOI] [PubMed] [Google Scholar]
- 32.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 33.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annual Review of Immunology. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 34.Krönke M, Leonard WJJ, Depper JMM, Arya SKK, Wong-Staal F, Gallo RCC, et al. Cyclosporin A inhibits T-cell growth factor gene expression at the level of mRNA transcription. Proceedings of the National Academy of Sciences of the United States of America. 1984;81(16):5214–5218. doi: 10.1073/pnas.81.16.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herold KC, Lanckl DW, Moldwin RL, Fitch FW. Immonusuppressive effects of cyclosporin a on cloned cells. The Journal of Immunology. 1986;136(4):1315–1321. [PubMed] [Google Scholar]
- 36.Hogan WJJ, Storb R. Use of cyclosporine in hematopoietic cell transplantation. Transplantation Proceedings. 2004;36(2 Suppl):367S–371S. doi: 10.1016/j.transproceed.2004.01.043. [DOI] [PubMed] [Google Scholar]
- 37.Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM. Chronic cyclosporine nephropathy: the Achilles’ heel of immunosuppressive therapy. Kidney International. 1996;50(4):1089–1100. doi: 10.1038/ki.1996.415. [DOI] [PubMed] [Google Scholar]
- 38.Wong SH. Therapeutic drug monitoring for immunosuppressants. Clinica Chimica Acta. 2001;313(1–2):241–253. doi: 10.1016/s0009-8981(01)00678-7. [DOI] [PubMed] [Google Scholar]
- 39.Bowers LD. Therapeutic monitoring for cyclosporine: difficulties in establishing a therapeutic window. Clinical Biochemistry. 1991;24(1):81–87. doi: 10.1016/0009-9120(91)90315-6. [DOI] [PubMed] [Google Scholar]
- 40.Irschik E, Tilg H, Niederwieser D, Gastl G. Cyclosporin blood levels do correlate with clinical complications. Lancet. 1984;2:692–693. doi: 10.1016/s0140-6736(84)91244-3. [DOI] [PubMed] [Google Scholar]
- 41.Kahan BD, Welsh M, Rutzky LP. Challenges in cyclosporine therapy: the role of therapeutic monitoring by area under the curve monitorin. Therapeutic Drug Monitoring. 1995;17:621–624. doi: 10.1097/00007691-199512000-00013. [DOI] [PubMed] [Google Scholar]
- 42.Kahan BD. Therapeutic drug monitoring of cyclosporine: 20 years of progress. Transplantation Proceedings. 2004;36(2 Suppl):378S–391S. doi: 10.1016/j.transproceed.2004.01.091. [DOI] [PubMed] [Google Scholar]
- 43.Gaston RS. Maintenance immunosuppression in the renal transplant recipient: an overview. American Journal of Kidney Diseases. 2001;38(6):S25–S35. doi: 10.1053/ajkd.2001.28923. [DOI] [PubMed] [Google Scholar]
- 44.Belitsky P, Dunn S, Johnston A, Levy G. Impact of absorption profiling on efficacy and safety of cyclosporin therapy in transplant recipients. Clinical Pharmacokinetics. 2000;39(2):117–125. doi: 10.2165/00003088-200039020-00003. [DOI] [PubMed] [Google Scholar]
- 45.Ruutu T, Niederwieser D, Gratwohl A. A survey of the prophylaxis and treatment of acute GVHD in Europe : a report of the European Group for Blood and Marrow Transplantation (EBMT) Bone Marrow Transplantation. 1997;19:759–764. doi: 10.1038/sj.bmt.1700745. [DOI] [PubMed] [Google Scholar]
- 46.Lim WY, Messow CM, Berry C. Cyclosporin variably and inconsistently reduces infarct size in experimental models of reperfused myocardial infarction: a systematic review and meta-analysis. British Journal of Pharmacology. 2012;165(7):2034–2043. doi: 10.1111/j.1476-5381.2011.01691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fujiwara M, Yan P, Otsuji TG, Narazaki G, Uosaki H, Fukushima H, et al. Induction and enhancement of cardiac cell differentiation from mouse and human induced pluripotent stem cells with cyclosporin-A. PloS One. 2011;6(2):e16734. doi: 10.1371/journal.pone.0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hostettler KE, Roth M, Burgess JK, Johnson PRA, Glanville AR, Tamm M, et al. Cyclosporine A mediates fibroproliferation through epithelial cells. Transplantation. 2004;77(12):1886–1893. doi: 10.1097/01.tp.0000131149.78168.dd. [DOI] [PubMed] [Google Scholar]
- 49.Hunt J, Cheng A, Hoyles A, Jervis E, Morshead CM. Cyclosporin A has direct effects on adult neural precursor cells. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30(8):2888–2896. doi: 10.1523/JNEUROSCI.5991-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Perry, Spangrude Direct effects of cyclosporin A on proliferation of hematopoietic stem and progenitor cells. Cell Transplantation. 1999;8:339–344. doi: 10.1177/096368979900800401. [DOI] [PubMed] [Google Scholar]
- 51.Sachinidis A, Schwengberg S, Hippler-altenburg R, Mariappan D, Kamisetti N, Seelig B, et al. Identification of small signalling molecules promoting cardiac-specific differentiation of mouse embryonic stem cells. Cellular Physiology and Biochemisty. 2006;18:303–314. doi: 10.1159/000097608. [DOI] [PubMed] [Google Scholar]
- 52.Byun Y, Kim K, Kim S, Kim Y, Koo K, Kim T, et al. Cyclosporin A, on the osteogenic differentiation of rat mesenchymal stem cells. Journal of Periodontal and Implant Science. 2012;42:73–80. doi: 10.5051/jpis.2012.42.3.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies WR, Wang S, Oi K, Bailey KR, Tazelaar HD, Caplice NM, et al. Cyclosporine decreases vascular progenitor cell numbers after cardiac transplantation and attenuates progenitor cell growth in vitro. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation. 2005;24(11):1868–1877. doi: 10.1016/j.healun.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Poncelet AJ, Nizet Y, Vercruysse J, Hiel AL, Saliez A, Gianello P. Inhibition of humoral response to allogeneic porcine mesenchymal stem cell with 12 days of tacrolimus. Transplantation. 2008;86(11):1586–1595. doi: 10.1097/TP.0b013e31818bd96f. [DOI] [PubMed] [Google Scholar]
- 55.Song LH, Pan W, Yu YH, Quarles LD, Zhou HH, Xiao ZS. Resveratrol prevents CsA inhibition of proliferation and osteoblastic differentiation of mouse bone marrow-derived mesenchymal stem cells through an ER/NO/cGMP pathway. Toxicology In Vitro: An International Journal Published in Association with BIBRA. 2006;20(6):915–922. doi: 10.1016/j.tiv.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 56.Yan P, Nagasawa A, Uosaki H, Sugimoto A, Yamamizu K, Teranishi M, et al. Cyclosporin-A potently induces highly cardiogenic progenitors from embryonic stem cells. Biochemical and Biophysical Research Communications. 2009;379(1):115–120. doi: 10.1016/j.bbrc.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 57.Guo J, Zeng Y, Liang Y, Wang L, Huanxing S, Wutain W. Cyclosporine affects the proliferation and differentiation of neural stem cells in culture. Regeneration and Transplantation. 2007;18(9):63–68. doi: 10.1097/WNR.0b013e32811d6d36. [DOI] [PubMed] [Google Scholar]
- 58.Chen TL, Wang JA, Shi H, Gui C, Luo RH, Xie XJ, et al. Cyclosporin A pre-incubation attenuates hypoxia/reoxygenation-induced apoptosis in mesenchymal stem cells. Scandinavian Journal of Clinical and Laboratory Investigation. 2008;68(7):585–593. doi: 10.1080/00365510801918761. [DOI] [PubMed] [Google Scholar]
- 59.Yang L, Yang X-C, Yang J-K, Guo Y-H, Yi F-F, Fan Q, et al. Cyclosporin A suppresses proliferation of endothelial progenitor cells: involvement of nitric oxide synthase inhibition. Internal Medicine. 2008;47(16):1457–1464. doi: 10.2169/internalmedicine.47.1042. [DOI] [PubMed] [Google Scholar]
- 60.Esposito C, Fornoni A, Cornacchia F, Bellotti N, Fasoli G, Foschi A, et al. Cyclosporine induces different responses in human epithelial, endothelial and fibroblast cell cultures. Kidney International. 2000;58(1):123–130. doi: 10.1046/j.1523-1755.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- 61.Guo C, Haider HK, Shim WSN, Tan R-S, Ye L, Jiang S, et al. Myoblast-based cardiac repair: xenomyoblast versus allomyoblast transplantation. The Journal of Thoracic and Cardiovascular Surgery. 2007;134(5):1332–1339. doi: 10.1016/j.jtcvs.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 62.Westrich J, Yaeger P, He C, Stewart J, Chen R, Seleznik G, et al. Factors affecting residence time of mesenchymal stromal cells (MSC) injected into the myocardium. Cell Transplantation. 2010;19(8):937–948. doi: 10.3727/096368910X494911. [DOI] [PubMed] [Google Scholar]
- 63.Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, et al. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. Journal of Molecular and Cellular Cardiology. 2007;42(4):746–759. doi: 10.1016/j.yjmcc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 64.Zeng L, Hu Q, Wang X, Mansoor A, Lee J, Feygin J, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115(14):1866–1875. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 65.Wang C-H, Cherng W-J, Yang N-I, Hsu C-M, Yeh C-H, Lan Y-J. Cyclosporine increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;294(3):R811–R818. doi: 10.1152/ajpregu.00543.2007. [DOI] [PubMed] [Google Scholar]
- 66.Suenderhauf C, Parrott N. A physiologically based pharmacokinetic model of the minipig: data compilation and model implementation. Pharmaceutical Research. 2013;30(1):1–15. doi: 10.1007/s11095-012-0911-5. [DOI] [PubMed] [Google Scholar]
- 67.Frey BM, Sieber M, Mettler H, Ganger H. Marked interspecies differences between humans and pigs in cyclosporine and prednisolone disposition. Drug Metabolism and Disposition. 1985;16(2):285–289. [PubMed] [Google Scholar]
- 68.Cibulskyte D, Pedersen M, Hjelm-Poulsen J, Hansen HE, Madsen M, Mortensen J. The pharmacokinetics and acute renal effects of oral microemulsion ciclosporin A in normal pigs. International Immunopharmacology. 2006;6(4):627–634. doi: 10.1016/j.intimp.2005.09.013. [DOI] [PubMed] [Google Scholar]