Abstract
Adipose tissue plays an essential role in regulating energy balance through its metabolic, cellular and endocrine functions. Adipose tissue has been historically classified into anabolic white adipose tissue and catabolic brown adipose tissue. An explosion of new data, however, points to the remarkable heterogeneity among the cells types that can become adipocytes, as well as the inherent metabolic plasticity of mature cells. These data indicate that targeting cellular and metabolic plasticity of adipose tissue might provide new avenues for treatment of obesity-related diseases. This review will discuss the developmental origins of adipose tissue, the cellular complexity of adipose tissues, and the identification of progenitors that contribute to adipogenesis throughout development. We will touch upon the pathological remodelling of adipose tissue and discuss how our understanding of adipose tissue remodeling can uncover new therapeutic targets.
1. Introduction
Adipose tissue (AT) is an organ that typically functions as the body’s energy reservoir by storing energy in the form of triglyceride (TG) in times of surfeit, and mobilizing energy in the form of fatty acids in times of need. Because fatty acids and their metabolic products can be toxic, homeostatic mechanisms exist to finely balance lipid storage and mobilization to prevent accumulation of potentially toxic lipids in peripheral organs [1]. The ability of the adipose organ to buffer variations in energy supply and demand is achieved by integrated endocrine and metabolic responses, as well as through dynamic changes in cellular composition [2]. The buffering capacity of adipose tissue can be exceeded during chronic overnutrition, resulting in the spillover of lipids from fat tissue and their pathological accumulation within other major metabolic organs. Metabolites derived from this ectopic lipid accumulation impair insulin action in peripheral tissues and insulin production by the pancreas [1, 3] in a process termed lipotoxicity [1]. Thus, one might anticipate that promoting the ability of AT to store or oxidize excess lipid energy would have beneficial effects on whole body metabolism.
White adipose tissue (WAT) can expand its energy-buffering capacity by fat cell hypertrophy and/or by hyperplasia from committed progenitors. Although AT mass is a rough predictor of diabetes risk, the amount of fat tissue matters less than the ability of the tissue to act as an energy buffer [3]. For example, patients with mutation of phosphatase and tensin homolog [4] are obese yet insulin sensitive, whereas patients with familial partial lipodystrophy exhibit ectopic lipid accumulation and are severely insulin resistant and diabetic [5]. Similarly, limiting AT expansion during overnutrition in rodents promotes ectopic lipid accumulation and accelerates diabetes [6], whereas expanding storage capability reduces ectopic triglyceride accumulation and improves insulin sensitivity [7].
In contrast to WAT, brown adipose tissue (BAT) is a highly oxidative tissue containing multilocular fat cells with abundant mitochondria that oxidize fatty acids and generate heat via uncoupling protein 1 (UCP1). Classic BAT is clearly the dominant site of nonshivering thermogenesis in rodents [8], and there is little doubt that variations in thermoregulatory thermogenesis can contribute greatly to disturbances in energy balance [9]. Whether classic BAT is important for energy balance during nutritional challenges continues to be a matter of debate [10–12]. However, the recent identification of BAT in humans [13] raises the possibility that pharmacological activation of BAT might combat obesity and improve insulin action [14], as it clearly does in rodents [15].
WAT has been historically defined by anatomical location and the presence of parenchymal cells containing a single large lipid droplet and sparse mitochondria lacking UCP1. However, WAT can be remodeled under various physiological and pharmacological conditions to a more oxidative phenotype resembling that of BAT, including the presence of multilocular cells that are rich in mitochondria containing UCP1. This catabolic remodeling is prominent in rodents and can be brought about by activating adipocyte receptors such as β3-adrenergic receptors (β3-AR) and peroxisome proliferator-activated receptor γ (PPARγ). Importantly, activation of BAT, and possibly brown adipocytes (BA) in WAT [16], can have anti-obesity effects and improve glucose homeostasis in rodent models of type 2 diabetes [15]. Recent observations indicating that BA in human supraclavicular BAT resemble those that can be recruited in rodent WAT raises the possibility of expanding these cells for therapeutic benefit in man [17, 18].
Work in experimental models has demonstrated the remarkable plasticity of the adipose organ [19], and it is clear that beneficial effects can be achieved by promoting either anabolic or catabolic phenotypes. If modulation of adipocyte phenotypes is to be realized as a therapeutic approach, then it becomes essential to develop a better understanding of the cell types and extrinsic signals that contribute to AT development and plasticity. In this review, we discuss the origin of AT, including a historic description of AT ontogeny and the current understanding of adipocyte progenitors. We examine depot-specific heterogeneity in the origin and transcriptional requirements of adipogenesis, and introduce the concept of an adipogenic tissue niche that involves cellular and matrix components. Finally, we discuss AT plasticity and how targeting adipocyte receptors can be used to improve AT function.
2. Development of adipose tissue
Adipogenesis is a highly ordered process that is initiated during development and continues throughout life [20]. Seminal histological experiments conducted in the 1960s defined the ontogeny of AT and provided a foundation for delineating adipocyte progenitors using modern genetic tracing techniques [21, 22]. In brief, AT organogenesis can be divided into distinct stages, which are characterized by cellular composition and differentiation status. Presumptive AT first appears as loose connective tissues which contains extracellular matrix with sparse, mostly mesenchymal, cells [21]. These unvascularized “prestructural territories” appear to provide a framework for further recruitment of progenitors and subsequent adipogenesis [22]. Over time, mesenchymal cells aggregate to form “mesenchymal lobules,” which have a reticular structure [21]. Major events of this stage include vascularization and expansion of mesenchymal cells [23–25]. Proliferation kinetic studies in rodents have shown that mitoses are mostly found in poorly differentiated mesenchymal cells that subsequently differentiate into adipocytes [26, 27]. Differentiating progenitors accumulate micro-lipid droplets to form “primitive fat lobules” [22, 28]. Adipogenesis appears to be guided in a central to peripheral gradient by the developing capillary networks that bud from the central vasculature [22]. Eventually, the primitive AT matures to form “definitive fat lobules” that are enclosed by connective tissue septae [23, 29]. Finally, terminal differentiation and enlargement of individual adipocytes occur, which are the major modes of postnatal AT growth [30].
The developmental timing of adipogenesis differs among species, and varies by anatomical location. AT development is initiated during the second trimester in humans [23, 24, 29], and during the perinatal period in rodents [27, 31]. Subcutaneous AT generally develops before abdominal adipose tissue in rodents [26, 27] and humans [32]. Overall, differentiation progresses in an anterior to posterior, rostral to caudal, and dorsal to ventral direction in various species, including man [25, 28]. BAT develops before WAT, and can easily be identified at birth in most mammals [33]. BAT histogenesis resembles that of WAT, but has slightly denser and more compact reticular structures during primitive fat lobule formation [22].
3. Adipocyte progenitors
Application of the stem cell concept to AT biology launched an explosion of research into the molecular characteristics and differentiation potential of progenitor cells that reside in AT. Although the precise identity of adipocyte progenitors in humans remains unknown, modern approaches, including in vivo lineage tracing and FACS-based prospective analyses, have enabled extensive characterization of adipocyte progenitors in mouse models. As discussed below, growing evidence suggests that several cell types can give rise to adipocytes in vivo, depending on developmental stage, anatomic location, and physiological conditions.
3.1. Adipocyte progenitors during development
Based on earlier histogenetic studies, a mesodermal origin of AT has been widely accepted. However, the fact that AT depots appear at different times and possess distinct molecular characteristics suggests that there may be regional heterogeneity among AT depots. In addition, genetic lineage tracing using Cre-LoxP recombination has shown diverse developmental origins of AT (Table 1). For example, lineage tracing with a marker of neural crest (Sox10) demonstrated that adipocytes in the head and neck are generated from the neuroectoderm, rather than the mesoderm [34]. Interestingly, the bones and muscles of the skull and face also appear to be of neural crest origin [35], suggesting that local mesenchymal lineages have a common origin in a given anatomic location. Similarly, lineage tracing showed that SM22-Cre, which is active in vascular smooth muscle cells (VSMC) and surrounding mesenchyme, marks perivascular adipose tissue (PVAT), indicating a close lineage relationship between PVAT and VSMC [36, 37].
Table 1.
Genetic tracing of adipocyte progenitors during development and in adult adipose tissue
| Lineage markers | Developmental origin | Lineage-derived adipose depots | Reference |
|---|---|---|---|
| Myf5 | Paraxial mesoderm | BAT (interscapular, perirenal) | [39] |
| Myogenic | WAT | [41] | |
|
| |||
| Pax7 | Somite (dermomyotome) | BAT (interscapular) | [43] |
| Myogenic | |||
|
| |||
| PDGFRβ | Mesenchyme | WAT (retroperitoneal, inguinal) | [52] |
| MSC | |||
|
| |||
| PPARγ | Adipogenic | WAT (retroperitoneal, inguinal) | [52] |
|
| |||
| SM22 | Vascular smooth muscle cells (VSMC) | Perivascular adipose tissue | [36] |
| [37] | |||
|
| |||
| Sox10 | Neural crest | Facial WAT (salivary gland and ear) | [34] |
|
| |||
| VE-Cadherin | Endothelial cells | WAT and BAT | [60] |
| [64] | |||
|
| |||
| LysM | Myeloid cells | BM-derived adipocytes | [68] |
|
| |||
| PDGFRα | Paraxial mesoderm | WA/ Inducible BA | [58] |
| MSC | Inducible BA | ||
Notably, in vivo fate-mapping and lineage-tracing studies have established that cells expressing the early myogenic factor Myf5 can give rise to both skeletal myocytes and BA in the interscapular and perirenal BAT depots [38, 39]. Moreover, the BA that emerge in WAT in response to β3-AR stimulation or cold stress are not tagged by Myf5-Cre, indicating that the origins of classic BA (which reside in BAT) and inducible BA in WAT are distinct [39]. Because the Myf5 promoter is transiently activated in undifferentiated paraxial mesoderm, cells with a history of Myf5 expression give rise to multiple types of cells, including cartilage and bone [40]. In this regard, recent findings demonstrate that adipocytes derived from Myf5-expressing progenitors are heterogeneously expressed within a given WAT depot [41]. As mentioned earlier, AT depots develop over time in a rostral to caudal direction [20, 42]. Conditional deletion of PTEN in Myf5+ expressing cells specifically increases adiposity in the upper body [41], implying earlier developmental timing of Myf5+ cell-derived adipocytes. It is not clear, however, whether Myf5 is an important determinant of adipogenesis or whether tagging by Myf5-Cre reflects transient expression within undifferentiated paraxial mesoderm. Although constitutive Cre-LoxP systems have provided definitive evidence of the contribution of specific lineages to AT, use of inducible reporter systems is required to define the timing of lineage specification. In this regard, lineage tracing with an inducible Pax7- reporter demonstrated that the developmental divergence between BAT and muscle occurs around E10.5 to E12.5. [43].
Gene knockout studies in mice indicate that the transcriptional program controlling adipogenesis varies among fat depots. For example, genetic ablation of CEBPα impairs development of subcutaneous and gonadal WAT, whereas mammary WAT and interscapular BAT are unaffected [44]. On the other hand, CEBPβ/CEBPδ deletion causes defects in interscapular BAT formation, whereas WAT is relatively unaffected [45]. Conversely, fat-specific inactivation of canonical hedgehog signaling cause loss of WAT, but not BAT [46]. In humans, inherited partial lipodystrophies often do not appear until adolescence, and then affect certain fat depots (e.g., subcutaneous WAT) and not others [47–49]. Together, these observations support a theory of inter-depot heterogeneity in developmental origin and transcriptional control of adipogenesis.
Despite variations in molecular signatures of adipocyte progenitors among individual depots [50, 51] , it is widely agreed that PPARγ is the requisite master regulator for differentiation of committed progenitors into adipocytes in all AT depots. Utilizing inducible PPARγ reporter strains, Tang et al. identified a population of committed progenitors that give rise to adipocytes during perinatal expansion of inguinal and retroperitoneal WAT [52]. Commitment, as deduced from reporter gene expression, occurs prenatally and the progenitors undergo dramatic postnatal expansion. Surprisingly, the PPARγ gene was active, as indicated by reporter gene expression, yet known PPARγ targets (e.g., Cebpα, fatty acid binding protein 4, leptin) were not expressed. This may reflect the sensitivity of the reporter system to very low levels of PPARγ that are found in pre-adipocytes, or the absence of mediators that initiate PPARγ activity of quiescent preadipocytes. Interestingly, many of these committed preadipocytes in this study had a perivascular location and expressed pericyte markers.
3.2. Adipocyte progenitors in adult adipose tissue
Mesenchymal stem cells
Mesenchymal stem cells (MSC) are postnatal progenitors with multiple lineage potential and can be found in adipose tissue, bone marrow, and other connective tissues [53]. MSC that reside in adipose tissue are generally believed to be a principal source for adipocytes during postnatal growth and maintenance of adipose tissue [54]. Numerous studies have isolated subpopulations of SVC and identified reliable cell surface markers that can be used to enrich highly adipogenic subpopulations (see review [54]). Among those studies, Rodeheffer et al. identified Lin-CD29+CD34+Sca-1+CD24+ cells (CD24+ cells) as cells that can reconstitute a functional epididymal fat pad when transplanted into lipodystropic mice [55]. CD24+ cells are relatively rare (0.08% of SVC), yet have a remarkable capacity for self-renewal and can form a functional organ with a limited number of cells. In subsequent studies, these investigators confirmed that all adipocyte progenitors express platelet-derived growth factor receptor alpha (PDGFRα) [56], and these cells can be further subdivided into CD24+ and CD24− fractions. CD24 expression in PDGFRα cells appears to reflect the state of adipogenic commitment, since CD24− PDGFRα cells are unable to form functional WAT in the adipogenic environment of a lipodystrophic mouse, yet do give rise adipocytes at an ectopic site after transplantation. The PDGFRα+CD24− cells observed by the Rodeheffer [57] and Granneman labs [56] appear to be a highly similar, if not identical, population of lineage-committed progenitors. This indicates that CD24+ cells may be early-stage stem cells (i.e., long-term reconstituting stem cells), and can give rise to lineage restricted adipogenic progenitors (i.e., short-term reconstituting stem cells). In line with this observation, subpopulations of MSC in adult adipose tissue have been reported to express the multipotency markers OCT4 and telomerase [58]. Additional studies, such as global transcriptome analysis of the CD24+ and CD24− populations might identify additional markers of adipose stem cells. Long term reconstituting stem cells in other tissues often occupy a specialized niche that is important for ensuring stemness. However, it is presently unknown whether such a specialized stem cell niche exists within AT.
Although immunotyping studies have identified potential adipocyte progenitor markers, genetic or chemical tracing is required to demonstrate lineage potential in vivo [59]. Taking advantage of inducible Cre-LoxP technology, we have identified cells in WAT that are capable of becoming BA or WA, depending on inductive signals [56]. These cells express the surface markers CD34+Sca1+ PDGFRα+ cells (PDGFRα+ cells) and have a unique dendritic morphology. Although these cells are often found in the perivascular region, they are negative for pericyte markers and thus are distinct from developmental adipocyte progenitors The morphology, localization, and immunophenotypes of PDGFRα+ cells are strikingly similar to adipogenic CD34+ cells in human adipose, which also do not express pericyte markers [60]. Interestingly, PDGFRα+ cells isolated from various depots exhibit similar adipogenic behavior in vitro, yet PDGFRα+ cells in gonadal WAT are much more prone to be activated by β3-AR stimulation and high fat diet (HFD). These observations suggest that extrinsic cues from the AT microenvironments might be important in depot-specific progenitor recruitment in vivo.
Endothelial origin
The close temporal-spatial association between angiogenesis and adipogenesis suggests that adipocytes may have endothelial origins; however, whether adipocytes arise from the endothelium or its underlying mesenchyme has long been a matter of debate. Recent lineage tracing of VE-cadherin expressing cells [61] suggests a contribution of endothelial lineages to WA and BA. In addition, the endothelial cells expressed a preadipocyte determination marker, ZFP432 [62], although ZFP432 is expressed in numerous cell types. Adipocytes derived from VE-cadherin+ progenitors may represent a subpopulation, as they do not appear to be uniformly distributed throughout WAT pads. In this regard, committed endothelial cells (CD31+CD34+) from dissociated AT are not adipogenic in vitro [63, 64], and the original characterization of VE-cadherin Cre mouse model showed little, if any, contribution of these progenitors to WAT [65]. This discrepancy may be due to the technical difficulty of discriminating adipocytes from surrounding endothelial cells, particularly when the lacZ reaction product is used as a reporter. To address controversies, a recent study used a membrane- targeted fluorescent reporter line, and demonstrated that endothelial cells, marked by either Cdh5 or Tie2 promoter activation, do not contribute to adipogenesis during normal development and HFD feeding [57]. Alternatively, MSC in adipose tissue could be common progenitors for endothelial cells and adipocytes, as SVC can differentiate into endothelial cells in vitro [66, 67] and in vivo [68].
BM-derived hematopoietic progenitors
Several studies indicate that circulating hematopoietic progenitors derived from bone marrow (BM) can participate in adipogenesis [69–71]. Genetic lineage tracing with LysM-Cre indicates that adipogenic progenitors are derived from the myeloid lineage [69] and BM-derived progenitors contribute to adipogenesis in a sex- and depot-specific fashion [72]. However, transplantation experiments [73] and genetic tracing with Tie2 and Vav1-reporter lines failed to confirm a hematopoietic contribution to adipogenesis during normal development and high fat feeding [57]. Nonetheless, compared to conventional adipocytes, BM-derived adipocytes have decreased expression of genes involved in mitochondriogenesis and lipid metabolism, and increased expression of pro-inflammatory markers [69]. Surprisingly, expression of adiponectin and leptin are nearly absent in these cells. This unique molecular phenotype suggests that BM-derived adipocytes may be deleterious to metabolic health, and certainly warrants further investigation.
3.3. Potential adipocyte progenitor niches
Stem cells typically reside in specialized anatomical locations, known as stem cell niches, where neighboring cells and cues from the microenvironment maintain quiescence or trigger activity [74–76]. The stem cell niche may be compartmentalized into distinct domains that enhance dormancy, or provide cues for proliferation and differentiation [75, 77]. As mentioned above, preadipocytes have been localized to a perivascular niche in developing [52, 78, 79] and mature AT [58, 60, 80]. Nonetheless, the cell types and extrinsic signals that balance quiescence and recruitment of progenitors are currently unknown. It is important to note that the vast majority of cells in AT are not mature fat cells[81, 82], but rather are a dynamic mixture of cellular and non-cellular elements, including progenitors, resident/recruited immune cells, fibroblasts, blood vessels, lymphatic vessels, peripheral nerves, and the extracellular matrix (ECM). While growing evidence suggests roles for these elements in progenitor recruitment and adipocyte function, the mechanistic details are scant. Therefore, in this section, we will provide a brief overview of the cell types and niche factors that may be involved in adipocyte progenitor recruitment. We also summarize the potential contribution of pathologic remodeling of AT to disease states.
3.3.1. Adipose tissue macrophages
Adipose tissue macrophages have been intensively investigated as a crucial cell type that affects the metabolic and endocrine functions of AT [83, 84]. Macrophages have been classified into subpopulations based on functional phenotypes [85]. Classically-activated M1 macrophages are associated with immune defense and pro-inflammatory responses, whereas alternatively-activated M2 macrophages are involved in tissue repair and restoration [86]. The M1/M2 classification has been largely established by the in vitro characterization of mouse macrophages, and it is important to emphasize that the in vivo polarization status of AT macrophages exists on a dynamic continuum [86, 87].
Interestingly, macrophages are believed to have trophic effects on AT development [88, 89]. Depletion of macrophages [89] or ablation of macrophage colony stimulating factor impairs adipogenesis [90]. Macrophage-conditioned medium can inhibit preadipocyte apoptosis and promote survival in a PDGF-dependent manner [91]. In addition, macrophages are a major source of angiogenic factors and can indirectly mediate AT expansion [92]. However, the specific effects of macrophages on in vivo adipocyte progenitor activation have not been investigated.
Pro-inflammatory M1 macrophages accumulate in AT during obesity [93] and thereby promote insulin resistance [94]. In contrast, polarization of macrophages toward alternative activation preserves insulin sensitivity via a PPARγ-dependent mechanism [95, 96]. Additionally, a recent study found that AT M2 macrophages secrete catecholamines, which increase catabolism and sustain thermoregulatory functions during cold exposure [97]. Alternatively-activated macrophages may also prevent the adverse effects of excessive free fatty acid efflux during weight loss by suppressing lipolysis [98].
3.3.2. Vasculature
Mounting data suggest that angiogenesis and adipogenesis are tightly coupled during development [25, 89, 99] and postnatal fat expansion [92, 100–102]. In vitro studies suggest that signals derived from surrounding vascular units directly influence preadipocyte proliferation and differentiation. For example, Hutley et al. isolated macrovascular endothelial cells and preadipocytes from human AT, and found that conditioned media from endothelial cell cultures promote proliferation of preadipocytes [103], whereas ECM obtained from rat AT-derived endothelial cell cultures enhances differentiation of preadipocytes [104]. However, the vascular-derived signals involved remain poorly characterized.
Antiangiogenic reagents have been investigated as potential anti-obesity therapies. Pharmacological inhibition of angiogenesis reduces fat mass in obese mouse models [101, 105]. However, the consequences of modulating angiogenic activity appear to vary based on context. Gain of function studies show that overexpression of VEGFA improves metabolic profiles and prevents tissue dysfunction during the early phase of diet-induced obesity [102]. In contrast, reducing vascularization in established obesity may improve metabolic outcomes by inducing apoptosis in dysfunctional adipocytes [102].
3.3.3. Innervation
The sympathetic nervous system plays an important role in regulating fatty acid mobilization and blood flow in AT (reviewed in [106]). In addition, adipocyte cellularity appears to be under sympathetic neuronal control. Chemical sympathectomy or surgical denervation increases proliferation and differentiation of WA progenitors [107–110], whereas sensory denervation has no effect [107] . Consistent with in vivo findings, physiological concentrations of norepinephrine inhibit WA preadipocyte proliferation in vitro [111]. In contrast, α2 adrenergic receptor activation induces proliferation of cultured preadipocytes [112], and upregulation of α2 adrenergic receptors may be involved in denervation-induced adipocyte hypertrophy [110].
It is well known that adrenergic stimulation triggers the proliferation and differentiation of BA in classical BA depots [113], and in vitro studies suggest this effect is mediated by β1-AR [114]. Interestingly, selective agonists of β3-AR have no effect on progenitor recruitment in classic BA [56]. In contrast, β3-AR agonists can increase proliferation and differentiation of BA progenitors in gonadal WAT and, to a lesser extent, in subcutaneous WAT [56]. It seems likely that these effects are indirectly mediated by effects on mature fat cells, since β3-AR are not expressed by preadipocytes. Recent study has demonstrated that deficiency of BAT created by genetic or surgical manipulation increased sympathetic tone in WAT and contributed to the recruitment of BA in WAT [115]. One of intriguing question would be whether de novo BA generation from progenitor proliferation is involved in this compensatory browning of WAT driven by augmented sympathetic innervation.
3.3.4. Extracellular matrix (ECM)
The extracellular matrix (ECM) is an obligatory component of the adipose tissue stroma that provides structural support and biochemical signals to maintain proper AT function. Importantly, physical and biochemical elements of ECM can direct MSC lineage specification, proliferation, and differentiation (reviewed in [116]). Adipocyte progenitors play an important role in ECM remodeling during development. Numerous studies have documented changes in the abundance and composition of ECM produced by preadipocytes during differentiation (reviewed in [117, 118]). For example, differentiating preadipocytes rapidly upregulate expression of collagen IV and various laminin complexes, which remain high throughout the differentiation [119]. On the other hand, fibronectin expression decreases during adipocyte differentiation in vivo [120] and in vitro [121]. Together, these findings suggest that ECM configuration undergoes a transition from a fibrillar to a laminar structure to accommodate adipocyte growth and fat storage [118].
ECM degrading enzymes also play a crucial role in modifying ECM structure and creating a permissive environment for tissue growth/remodeling. The proteolytic system includes fibrinolytic enzymes and numerous matrix metalloproteinases (MMP) that affect AT development (reviewed in [122]). For example, genetic inactivation of MMP3 enhances hyperplastic and hypertrophic expansion of AT during HFD feeding [123, 124]. Knockout of MMP3 also accelerates development of mammary WAT [125]. Inhibition of MMP2 and MMP9 by pharmacologic reagents or neutralizing antibodies reduces preadipocyte differentiation in vitro [126, 127]. General pharmacological inhibition of MMP activity decreases the AT mass of mice fed a HFD [128], although interpretation of pharmacological inhibition studies is complicated by the fact that broad-spectrum inhibitors can affect multiple MMP subtypes and other proteolytic enzymes.
While the aforementioned in vivo and in vitro studies support involvement of multiple MMPs in adipogenesis under various conditions, MT1-MMP seems to play an especially important role. WAT development is not adversely affected by knockout of MMP2, MMP9, or MMP3 [129]; however, ablation of MT1-MMP produces a lipodystrophic phenotype [129]. MT1-MMP is a membrane-tethered collagenase that degrades pericellular type 1 collagen, which is abundantly expressed in both undifferentiated and mature AT [129]. Interestingly, MT1-MMP activity is crucial for adipogenesis in a 3D-specific fashion. Preadipocytes from MT1-MMP null mice readily differentiate in 2D-culture condition upon adipogenic induction, but not when restricted by a 3D-collagen gel scaffold. Similarly, transplanted preadipocytes lacking MT1-MMP fail to become adipocytes in wild type recipient mice.
Dysregulation of ECM synthesis and turnover can lead to fibrosis and dysfunction of AT. Fibrosis is a common pathophysiological response to chronic injury in which excess accumulation of fibrotic ECM distorts tissue architecture and impairs the function of parenchymal cells [130]. During obesity, metabolically-challenged AT increases fibrous collagen production and upregulates related genes [84, 131, 132]. Consequently, increased ECM stiffness restricts adipocyte hypertrophy, and may cause ectopic fat accumulation [131]. Deletion of collagen VI in ob/ob mice enhances hypertrophic expansion and improves metabolic profile [131]. Adipose tissue macrophages, which are recruited in obese states, contribute to fibrosis by stimulating fibroblast ECM synthesis [133, 134]. In addition, local hypoxia may contribute to fibrosis via HIF1α dependent mechanisms [132]. Although ECM-producing fibroblasts are a key mediator of pathogenesis of fibrosis, the cellular origin of adipose tissue fibroblasts is not known. However, since PDGFRα+ cells have been identified as adipo-fibrogenic progenitors in muscle [135–138], it is likely that these cells can become fibrogenic under pathological conditions.
4. Adipose tissue plasticity
Adipocytes are replenished throughout an organism’s life, and are dynamically regulated in terms of their numbers, size, and metabolic characteristics. Adipocyte progenitors are activated during homeostatic turnover and hyperplastic expansion, and can also participate in catabolic remodeling, or “browning” of AT. Certain differentiated adipocytes can also contribute to AT plasticity by undergoing a phenotypic switch between anabolic and catabolic states. Here, we review recent work that highlights the range of cellular and metabolic phenotypes that exist in typical “white” adipose tissue depots, and points to possible avenues for engineering beneficial phenotypes in vivo.
4.1. Control of adipocyte cellularity
Adipocyte cellularity is determined early in life [139–142], yet there is clear propensity for cell renewal throughout the lifetime. Metabolic labeling studies with deuterium indicate that the half-life of adipocytes in humans is about 6 months, representing a turnover rate of 60% per year [143]. On the other hand, 14C decay analysis by Spalding et al. found that turnover is much less, with adipocytes replaced on average every 8.3 years. Interestingly, turnover is independent of body mass index, further suggesting that adipocyte number is determined at an early age and is relatively static in adulthood [141]. Despite these discrepancies in estimates of adipocyte lifetime, the cells within AT clearly turnover in the adult, and this phenomenon might be exploited for therapeutic purposes. Prior to the 1960s, it was thought that adipocytes were static in the rodent, but recent estimates indicate that mouse adipocytes are turned over every six months, with 1–5% of adipocytes being replaced every day [144].
Whether AT expansion occurs by adipocyte hypertrophy or hyperplasia depends upon anatomic location in humans and rodents [51, 145, 146]. The in vitro proliferation potential of progenitors correlates with depot-specific adipocyte hyperplasia in response to a high fat diet [51, 145]. These data agree with human studies in which overfeeding increased lower-body fat depots by hyperplasia, whereas abdominal fat increased by hypertrophy [146]. Adipogenesis is thought to occur once hypertrophied fat cells reach a critical size [147]. This process involves adipocyte death [141] and the proliferation of progenitors, followed by the appearance of smaller adipocytes [148]. Inherent differences in the ability of depots to expand by proliferation might be important in determining metabolic outcomes, considering the poor metabolic profile of visceral fat in humans [149].
4.2. Plasticity of adipocyte phenotype
Adipocytes within WAT depots have a remarkable capacity to alter their metabolic phenotype from typical WA to those resembling BA [150–152]. This phenomenon has been referred to as “browning” and is readily induced by cold exposure and β-adrenergic stimulation [151]. Browning has been extensively demonstrated only in small mammals, and the extent to which this occurs in humans is an open question. Typically, browning involves the appearance of multilocular adipocyte clusters that are dispersed among unilocular WA [153, 154]. The browning phenomenon varies among WAT depots and is strongly influence by numerous genetic factors [155, 156]. These multilocular, UCP1+ adipocytes were initially called “convertible,” since the phenotype can be induced from existing unilocular UCP1- cells without cell proliferation [157–159]. Although recent groups have named these cells “brown in white” (BRITE) [160] or beige [39], it is not clear whether these various designations refer to the same cell type [56, 150, 160, 161]. Indeed, limited genetic tracing experiments suggest that there may be heterogeneity among cells types that are capable of browning [56, 162].
Analyses of adipocyte ultrastructure suggests that the WA/BA transition can be bidirectional [159, 163]. Interestingly, those depots that are most susceptible to browning, such as retroperitoneal and inguinal fat pads, are densely populated with multilocular UCP1+ adipocytes early in development, and these cells likely revert to a WA-like phenotype in adulthood [161, 164, 165]. BA in WAT can also be derived from the recruitment of progenitors, which appears to be the major mode of browning of the gonadal fat depot [56]. Early work from our group established that BA in abdominal WAT can arise from new cellular proliferation [150]. More recently, our group utilized lineage tracing studies to demonstrate that greater than 90% of BA in abdominal WAT arise from a subpopulation of CD34+ cells that express PDGFRα [56]. The appearance of these inducible BA is dependent upon the stimulus, as a dose of β3-AR agonist that promotes fatty acid-induced inflammation in WAT reduces their appearance [166–168]. In addition, prospective analyses have identified progenitor cells that respond to activation of the cyclooxygenase 2 /prostaglandin pathway [16] and give rise to BA both in vitro and in vivo [160, 162].
While some BA-like adipocytes express UCP1 [153, 163], UCP1 is not essential for the chronic thermogenic actions of β3-AR agonists, or for the appearance of multilocular fat cells in white fat depots [163, 169]. These BA-like cells, which are found mostly in abdominal (gonadal) fat pads, are multilocular and have abundant mitochondria, yet clearly lack UCP1 [163]. UCP1- multilocular adipocytes exhibit elevated metabolic rate [170] and PPARα-dependent upregulation of fatty acid oxidation [166].
5. Pathological remodeling of adipose tissue
Substantial evidence suggests that adverse consequences of obesity have their origins in the pathological remodeling of AT [84]. Numerous studies demonstrate that obesity in mice and man is associated with elevated expression of inflammatory cytokines, adipocyte death, and recruitment of inflammatory macrophages [171–174]. Importantly, genetic and/or pharmacological approaches that reduce AT inflammation restore anabolic functions and improve systemic insulin action [171, 175]. Here we briefly summarize mechanisms of inflammation in obesity; recent reviews have covered this topic in greater depth [176, 177].
Chronic overnutrition increases the occurrence of hypertrophic fat cells, which upon reaching a critical size become distressed and undergo necrosis/apoptosis. Adipocyte cell death recruits macrophages, which surround and ultimately remove the dead fat cells [174]. These adipocyte clearance structures are often called “crown-like structures,” (CLS) which describes their appearance in histological sections. The formation of these CLS further triggers a local inflammatory response that impairs the functioning of nearby adipocytes [178, 179]. Inflammation disrupts the balance between fatty acid storage and mobilization and contributes to the production of lipid mediators that impair insulin signalling [180]. In addition, AT fibrosis promotes inflammatory responses and exacerbates insulin resistance in fat tissue [132]. Notably, reducing the constraints of the ECM increases fat cell expansion and improves whole body metabolism [131]. In humans with obesity, the exhaustion [181] and impaired commitment of adipocyte progenitors [182, 183] may also limit beneficial AT expansion.
Aging is associated with deleterious alterations in AT. Preadipocytes from aged rats [184] and older individuals [185] have reduced ability to proliferate and differentiate. The diminished ability of aged progenitors to replicate might be due to cellular senescence, which can be observed in vitro [186] and in vivo [187, 188]. Importantly, genetic approaches that reduce senescence improve insulin sensitivity [187], and removal of senescent cells improves age-related loss of AT [188]. Aging in mice is also associated with reduced adrenergic tone and loss of brown adipocytes in subcutaneous WAT [189].
6. Therapeutic remodeling of adipose tissue
As mentioned in the introduction, one means of addressing the adverse metabolic consequences of obesity is to increase the capacity of AT to buffer excess energy. PPARγ is the master regulator of adipogenesis, and its activation with thiazolidinediones (TZDs) induces metabolic and cellular remodeling that improves the ability of AT to sequester fatty acids. On the other hand, β3-AR agonists improve metabolism in rodent models of type 2 diabetes by expanding the ability of brown and white adipose tissues to oxidize fatty acids in situ, and thereby prevent systemic lipotoxicity. Although β3-AR agonists have not been successful in humans [190], their experimental use in rodent models has provided new information on the mechanisms of AT plasticity. This new appreciation of AT plasticity has sparked an explosion of work that is likely to lead to new means of expanding beneficial phenotypes in vivo.
6.1. Peroxisome proliferator-activated receptor γ
PPARγ is a nuclear receptor that is highly expressed in AT [191] and is a master regulator of adipogenesis [192] (reviewed in [193]). PPARγ is selectively activated by a class of anti-diabetic agents, the TZDs [193]. In humans, TZDs ameliorate insulin resistance, increase peripheral glucose utilization and improve serum lipid levels [194]. The therapeutic actions of TZDs in rodents are mediated by several molecular targets in multiple tissues [195–197]; nonetheless, activation of the AT PPARγ appears to play a central role [198, 199]. TZDs improve the ability of AT to sequester excess energy by upregulating the expression of genes involved in de novo lipogenesis and fatty acid uptake [200]. TZDs promote fat storage preferentially in subcutaneous WAT without increasing whole body energy expenditure [201]. TZDs also improve insulin sensitivity by improving adipokine profiles [202]. Adiponectin levels are reduced in obesity [203] and are correlated with insulin sensitivity [202]. TZD treatment rapidly increases adiponectin levels [204], and in mice, these effects involve activation of AMP kinase by adiponectin [205]. TZDs also activate PPARγ in certain lipid-sensing regulatory T-cells that regulate the inflammatory state of AT [195]. Similarly, TZDs suppress the expression of pro-inflammatory genes [206, 207] and promote the recruitment of anti-inflammatory tissue-remodeling macrophages [208]. Consistent with an adipocentric view, transgenic overexpression of PPARγ in AT mimics the systemic delivery of TZDs by reducing inflammation and improving adipokine profiles [199]. In addition, new work demonstrates that the crosstalk between PPARγ and the growth factors FGF1 and FGF21 is critical for the actions of TZDs in maintaining healthy AT [209, 210].
TZDs might impart therapeutic effects by increasing catabolic functions of WAT (Fig. 1). TZDs promote fatty acid re-esterification and futile cycling [211], which are energetically-demanding processes that increase mitochondrial biogenesis, energy expenditure, and fatty acid oxidation [212]. The clinical responsiveness of diabetic patients to TZDs is correlated with improvements in mitochondrial gene expression and reductions in inflammation [213]. In vitro, PPARγ agonism promotes the appearance of UCP1+ brown adipocytes in a population of EWAT-derived progenitors [160]. The mechanisms by which PPARγ agonism facilitates the appearance of brown adipocytes include stabilization of PRDM16 [214], suppression of WA-specific genes [215], and SirT1-dependent deacetylation of PPARγ [216]. Interestingly, the binding of PPARγ to genes that determine brown or white adipocyte expression patterns is depot-dependent, suggesting that the ability of TZDs to promote a brown or white phenotype is epigenetically pre-programmed [217]. Whether TZDs promote recruitment and activation of brown adipocytes in humans is not known; however, PPARγ agonism promotes characteristics of brown adipocytes in a human pre-adipocyte cell line [218].
Fig. 1.
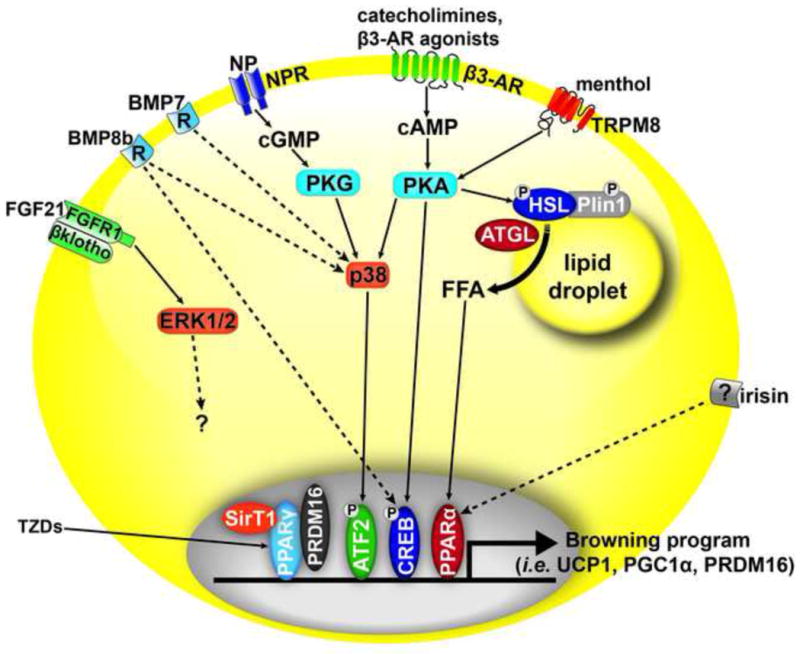
Adipocyte receptors that have been proposed to promote the browning of white fat or maintenance of a brown adipocyte (BA) phenotype. β3-adrenergic receptor (β3-AR) which signals through protein kinase A(PKA) to phosphorylate (P) and activate transcription factors such as cAMP response element binding protein(CREB). β3-ARs also activate p38 which phosphorylates ATF2 to promote the transcription of genes such as PGC1α and UCP1. In parallel, β3-ARs stimulate lipolysis by adipose triglyceride lipase (ATGL) and hormone sensitive lipase (HSL). Free fatty acids (FFA) mobilized by these lipases activate peroxisome proliferator-activated receptor (PPARα) and PPARδ (not shown) to promote the expression of BA genes. Natrietic peptides (NP) bind natrietic peptide receptors (NPR) and activate protein kinase G (PKG). In addition, bone morphogenetic protein (BMP8b) and BMP7 signal through p38 by unknown mechanisms (dashed line). Fibroblast growth factor 21 (FGF21) produced by the liver and in brown adipose tissue, acts through the receptor FGF1 and scaffold βKlotho to activate ERK1/2 and promote a BA phenotype through unknown pathways. Transient receptor potential cation channel subfamily M member 8 (TRPM8), which is activated by menthol, promotes thermogenesis in BAT in a PKA-dependent manner. Thiazolidinediones (TZDs) activate PPARγ, which through PRDM16 and the deacetylase sirtuin1 (SirT1), promote the browning of white fat. Finally, the hormone irisin, secreted from muscle, binds a yet unknown receptor to promote the appearance of BA by signaling through a PPARα dependent pathway.
6.2. β3-Adrenergic Receptors
In rodents, physiological or pharmacological stimulation of β3-ARs rapidly activates nonshivering thermogenesis in brown fat [219]. Although the thermogenic actions of β3-AR agonists have been attributed to activation of brown fat, activation of both BAT and WAT is required for a full thermogenic response [154]. The anti-diabetic effects of β3-AR agonists require chronic treatment [219], and are correlated with dramatic remodeling of WAT [150, 220]. Interestingly, improvement in insulin sensitivity can be seen prior to changes in body weight [221].
The mechanisms by which β-ARs enhance the catabolic character of brown fat and the appearance of BA in WAT are not completely understood, but are complex and involve synergy among various signalling pathways [156, 222] (Fig. 1). Many of the factors involved in promoting the browning of white fat, such as activation of p38 [223] and cAMP response element-binding protein [224], likely lie downstream of the cAMP/protein kinase A signalling pathway [156]. One critical factor that is upregulated by PKA is PGC1α, transcriptional co-activator of nuclear receptors that is necessary for the maximal induction of mitochondrial biogenesis and UCP1 expression [225–227].
In addition, PPARα and type 2 diodinase have been identified as genes associated with maximal induction of UCP1 expression in WAT depots [228]. In addition to the direct activation of transcription factors, PKA also triggers lipolysis, which provides ligands for the nuclear receptors PPARα and PPARδ. Dynamic imaging shows that ligands for PPARα and PPARδ are produced within minutes of activating lipolysis and function to match the oxidative capacity with the supply of fatty acids [229]. Moreover, knockout of hormone sensitive lipase or PPARα delays induction of genes involved in mitochondrial biogenesis and fatty acid oxidation during β3-AR agonist treatment [220, 230]. Similarly, overexpression of adipose triglyceride lipase (ATGL) enhances the catabolic phenotype of white fat, while AT-specific deletion of ATGL promotes a white fat phenotype in BAT, with reduced expression of PPARα target genes [231]. While these data indicate that intracellular lipolysis supports a catabolic phenotype, excessive mobilization of fatty acids can trigger inflammation in WAT [98, 150, 167, 220, 230].
6.3. Other factors that promote a BA phenotype
The role of β-ARs in browning of WAT has been known for some time, but recent evidence demonstrates that the appearance of BA and maintenance of a BA program can be induced by other hormones, cytokines, and growth factors (Fig. 1). Bostrom et al. demonstrated that a muscle-derived cytokine, irisin, is increased by exercise and induces the browning of subcutaneous AT [232]. Interestingly, the induction of BA genes by irisin involves activation of PPARα; however, whether irisin has a similar role of improving metabolic outcomes in humans is a matter of debate [233, 234]. BMP7, a morphogen important for development, can promote the differentiation of a population of preadipocytes towards BA and is necessary for the formation of BAT, while overexpression increases energy expenditure [162]. BMP7 also increases mitochondrial activity in mature brown adipocytes [235]. Additionally, BMP8b acts directly on brown fat and via the central nervous system to increase thermogenic activity [236]. Fibroblast growth factor 21 (FGF21) has been suggested to drive BAT-dependent thermogenesis after birth [237] and contribute to browning of white fat in adults [238, 239]. The metabolic effects of FGF21 require signalling through FGFR1 and the scaffold βKlotho [240–242]. Interestingly, cold-induced elevation of circulating FGF21 levels is associated with thermogenesis in humans [243]. In addition, transient receptor potential cation channel subfamily M member 8 (TRPM8), the cold-sensing receptor that is activated by menthol, is expressed in BAT and promotes UCP1-dependent thermogenesis [244]. The browning of WAT can also be induced in transgenic animals that express transcription factors such as PRDM16 [245], RIP140 [246], pRB and p107 [247], and FoxC2 [248]. Finally, cardiac-derived natrietic peptides (NP) [249], which are potent activators of lipolysis in human fat cells, can promote the browning of white fat and thermogenesis in mice, and drive increased respiration in human fat cell cultures [250]. Importantly, NP treatment stimulates lipolysis and increases lipid oxidation and energy expenditure in humans [249, 251]. An important question to address is whether natrietic peptides enhance energy expenditure by acting on brown fat, skeletal muscle, or both [252].
7. Concluding Remarks
The mechanistic study of cellular and metabolic plasticity of AT will raise new opportunities in treating obesity and its related metabolic disorders. Understanding the signals that determine progenitor fate may lead to new therapeutic approaches. For example, PDGFα progenitors in WAT appear to be capable of differentiating into WA, BA, or fibroblasts. Clearly, understanding the cues that drive the fate of this population could be key to preserving metabolically healthy WAT. Interestingly, recent evidence suggests that TZD-enhanced insulin sensitization is associated with the appearance of new adipocytes in muscle [213]. Thus, an understanding of how TZDs target specific progenitor populations is also important.
The metabolic improvements associated with activation of BAT in rodents have led to the suggestion that human BAT (hBAT) might be developed as a therapeutic target. Agonists of β3-ARs have potent anti-obesity and anti-diabetes effects in rodent models [219, 253]. However, β3-AR expression is low in human AT, and selective β3-AR agonists have not proven effective in man [190]. Thus far, indirect (ephedrine) and direct (isoproterenol) sympathomimetics have also failed to activate hBAT at doses that raise the metabolic rate [254–256]. Clearly, additional means other than cold stress will be needed for BAT activation to be a practical target in humans. Since fatty acids are necessary and sufficient to drive brown fat thermogenesis [257], promoting lipolysis [229, 231, 258] by mechanisms that are parallel or distal to adrenergic receptors may be effective. Whether the reported amounts of BAT in humans are sufficient to have an effect on whole energy expenditure is a matter of debate. Direct measures of blood flow and O2 metabolism in hBAT during cold stress indicate that the amount of activity of human BAT would need to increase by approximately 50 times to impact energy balance [259]. Increasing the amount of BAT or BA in WAT could be accomplished by manipulating transcription factors such as PRDM16 or Rb/p107; however, the critical roles of these factors in organogenesis and transformation will need to be considered [260, 261]. Targeting microRNA (miRNA) that modulate brown fat differentiation [262, 263] or browning of white fat [264] are additional possibilities. Others have suggested fat transplants [265], and inducible pluripotent stem cells [266, 267] could be used to expand brown AT in vivo. However, BAT is not simply a single cell type, but a complex organ that requires a vasculature to supply oxygen and energy to be burned and a means of thermogenic activation. Future studies are required to address these hurdles; however, given recent advances in knowledge, targeting adipose tissue plasticity seems to be an increasingly feasible avenue for therapeutic intervention.
Acknowledgments
This work was supported by National Institutes of Health grants RO1DK076629 and RO1DK62292. E.P. Mottillo was supported by Canadian Institutes of Health Research Doctoral Research Award MDR-214349. We thank Rachel Granneman for editorial assistance.
References
- 1.Unger RH. LIPOTOXIC DISEASES. Annual Review of Medicine. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 2.Cinti S. The adipose organ: morphological perspectives of adipose tissues. Proc Nutr Soc. 2001;60:319–328. doi: 10.1079/pns200192. [DOI] [PubMed] [Google Scholar]
- 3.Unger R, Scherer P. Gluttony, sloth and the metabolic syndrome: a roadmap to lipotoxicity. Trends in endocrinology and metabolism: TEM. 2010;21:345–352. doi: 10.1016/j.tem.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, Cooper NS, Linden H, Levy JC, Wakelam MJ, Walker L, Karpe F, Gloyn AL. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med. 2012;367:1002–1011. doi: 10.1056/NEJMoa1113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hegele RA, Joy TR, Al-Attar SA, Rutt BK. Thematic review series: Adipocyte Biology. Lipodystrophies: windows on adipose biology and metabolism. J Lipid Res. 2007;48:1433–1444. doi: 10.1194/jlr.R700004-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Wang MY, Grayburn P, Chen S, Ravazzola M, Orci L, Unger RH. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc Natl Acad Sci U S A. 2008;105:6139–6144. doi: 10.1073/pnas.0801981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster DO, Frydman ML. Nonshivering thermogenesis in the rat. II. Measurements of blood flow with microspheres point to brown adipose tissue as the dominant site of the calorigenesis induced by noradrenaline. Can J Physiol Pharmacol. 1978;56:110–122. doi: 10.1139/y78-015. [DOI] [PubMed] [Google Scholar]
- 9.Thurlby PL, Trayhurn P. The role of thermoregulatory thermogenesis in the development of obesity in genetically-obese (ob/ob) mice pair-fed with lean siblings. British journal of nutrition. 1979;42:377–385. doi: 10.1079/bjn19790127. [DOI] [PubMed] [Google Scholar]
- 10.Ma SW, Foster DO, Nadeau BE, Triandafillou J. Absence of increased oxygen consumption in brown adipose tissue of rats exhibiting “cafeteria” diet-induced thermogenesis. Canadian journal of physiology and pharmacology. 1988;66:1347–1354. doi: 10.1139/y88-221. [DOI] [PubMed] [Google Scholar]
- 11.Kozak LP. Brown Fat and the Myth of Diet-Induced Thermogenesis. Cell Metabolism. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedergaard J, Cannon B. The Changed Metabolic World with Human Brown Adipose Tissue: Therapeutic Visions. Cell Metabolism. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Ravussin E, Galgani J. The implication of brown adipose tissue for humans. Annual review of nutrition. 2011;31:33–47. doi: 10.1146/annurev-nutr-072610-145209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arch JRS. β3-Adrenoceptor agonists: potential, pitfalls and progress. Eur J of Pharmacol. 2002;440:99–107. doi: 10.1016/s0014-2999(02)01421-8. [DOI] [PubMed] [Google Scholar]
- 16.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Diaz MB, Rozman J, Hrabe de Angelis M, Nüsing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 Controls Energy Homeostasis in Mice by de Novo Recruitment of Brown Adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 17.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT Possesses Molecular Signatures That Resemble Beige/Brite Cells. PLoS ONE. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cinti S. The adipose organ, Prostaglandins. Leukotrienes and Essential Fatty Acids. 2005;73:9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Wassermann P. The development of Adipose Tissue. American Physiological Society; Washington, DC: 1965. [Google Scholar]
- 22.Simon G. Histogenesis, handbook of physiology. American Physiological Society; Washington, DC: 1965. [Google Scholar]
- 23.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Human Development. 1984;10:1–11. doi: 10.1016/0378-3782(84)90106-3. [DOI] [PubMed] [Google Scholar]
- 24.Poissonnet CM, Burdi AR, Bookstein FL. Growth and development of human adipose tissue during early gestation. Early Human Development. 1983;8:1–11. doi: 10.1016/0378-3782(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 25.Crandall DL, Hausman GJ, Kral JG. A Review of the Microcirculation of Adipose Tissue: Anatomic, Metabolic, and Angiogenic Perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 26.Ochi M, Sawada T, Hattori T. Tritiated thymidine autoradiographic study on postnatal development of epididymal adipose tissue in the normal mouse. Anat Embryol (Berl) 1987;177:139–145. doi: 10.1007/BF00572538. [DOI] [PubMed] [Google Scholar]
- 27.Pilgrim C. DNA synthesis and differentiation in developing white adipose tissue. Developmental Biology. 1971;26:69–76. doi: 10.1016/0012-1606(71)90108-4. [DOI] [PubMed] [Google Scholar]
- 28.Poulos SP, Hausman DB, Hausman GJ. The development and endocrine functions of adipose tissue. Molecular and Cellular Endocrinology. 2010;323:20–34. doi: 10.1016/j.mce.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Poissonnet CM. Growth and development of adipose tissue. The Journal of pediatrics. 1988;113:1–9. doi: 10.1016/s0022-3476(88)80520-1. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood MRC, Hirsch J. Postnatal development of adipocyte cellularity in the normal rat. Journal of Lipid Research. 1974;15:474–483. [PubMed] [Google Scholar]
- 31.Kirtland J. Changes in adipose tissue of the rat due early undernutrition followed by rehabilitation. 3. Changes in cell replication studied with tritiated thymidine. British journal of nutrition. 1980;43:33–43. doi: 10.1079/bjn19800062. [DOI] [PubMed] [Google Scholar]
- 32.Harrington TAM. Distribution of adipose tissue in the newborn. Pediatric research. 2004;55:437–441. doi: 10.1203/01.PDR.0000111202.29433.2D. [DOI] [PubMed] [Google Scholar]
- 33.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 34.Billon N, Iannarelli P, Monteiro MC, Glavieux-Pardanaud C, Richardson WD, Kessaris N, Dani C, Dupin E. The generation of adipocytes by the neural crest. Development. 2007;134:2283–2292. doi: 10.1242/dev.002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bronner-Fraser M. Neural crest cell formation and migration in the developing embryo. The FASEB Journal. 1994;8:699–706. doi: 10.1096/fasebj.8.10.8050668. [DOI] [PubMed] [Google Scholar]
- 36.Olson Lorin E, Soriano P. PDGFRβ Signaling Regulates Mural Cell Plasticity and Inhibits Fat Development. Developmental Cell. 2011;20:815–826. doi: 10.1016/j.devcel.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of Perivascular Adipose Tissue on Peroxisome Proliferator Activated Receptor-γ Deletion in Smooth Muscle Cells Impairs Intravascular Thermoregulation and Enhances Atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmons JA, Wennmalm K, Larsson O, Walden TB, Lassmann T, Petrovic N, Hamilton DL, Gimeno RE, Wahlestedt C, Baar K, Nedergaard J, Cannon B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development. 2008;135:1597–1604. doi: 10.1242/dev.019331. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez-Gurmaches J, Hung C-M, Sparks Cynthia A, Tang Y, Li H, Guertin David A. PTEN Loss in the Myf5 Lineage Redistributes Body Fat and Reveals Subsets of White Adipocytes that Arise from Myf5 Precursors. Cell Metabolism. 2012;16:348–362. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Billon ND. Christian, Developmental origins of the adipocyte lineage: new insights from genetics and genomics studies. Stem cell reviews. 2012;8:55–66. doi: 10.1007/s12015-011-9242-x. [DOI] [PubMed] [Google Scholar]
- 43.Lepper C, Fan C-M. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Linhart HG, Ishimura-Oka K, DeMayo F, Kibe T, Repka D, Poindexter B, Bick RJ, Darlington GJ. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proceedings of the National Academy of Sciences. 2001;98:12532–12537. doi: 10.1073/pnas.211416898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka T, Yoshida N, Kishimoto T, Akira S. Defective adipocyte differentiation in mice lacking the C/EBP[beta] and/or C/EBP[delta] gene. EMBO J. 1997;16:7432–7443. doi: 10.1093/emboj/16.24.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pospisilik JA, Schramek D, Schnidar H, Cronin SJF, Nehme NT, Zhang X, Knauf C, Cani PD, Aumayr K, Todoric J, Bayer M, Haschemi A, Puviindran V, Tar K, Orthofer M, Neely GG, Dietzl G, Manoukian A, Funovics M, Prager G, Wagner O, Ferrandon D, Aberger F, Hui C-c, Esterbauer H, Penninger JM. Drosophila Genome-wide Obesity Screen Reveals Hedgehog as a Determinant of Brown versus White Adipose Cell Fate. Cell. 2010;140:148–160. doi: 10.1016/j.cell.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 47.Garg A. Lipodystrophies: Genetic and Acquired Body Fat Disorders. Journal of Clinical Endocrinology & Metabolism. 2011;96:3313–3325. doi: 10.1210/jc.2011-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jeninga EH, Kalkhoven E. Central players in inherited lipodystrophies. Trends in Endocrinology & Metabolism. 2010;21:581–588. doi: 10.1016/j.tem.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 49.Hegele RA, Joy TR, Al-Attar SA, Rutt BK. Thematic review series: Adipocyte Biology. Lipodystrophies: windows on adipose biology and metabolism. Journal of Lipid Research. 2007;48:1433–1444. doi: 10.1194/jlr.R700004-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Gesta S, Blüher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proceedings of the National Academy of Sciences. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macotela Y, Emanuelli B, Mori MA, Gesta S, Schulz TJ, Tseng Y-H, Kahn CR. Intrinsic Differences in Adipocyte Precursor Cells From Different White Fat Depots. Diabetes. 2012;61:1691–1699. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White Fat Progenitor Cells Reside in the Adipose Vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bianco P, Robey PG, Simmons PJ. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell. 2008;2:313–319. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodeheffer MS, Birsoy K, Friedman JM. Identification of White Adipocyte Progenitor Cells In Vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 56.Lee Y-H, Petkova Anelia P, Mottillo Emilio P, Granneman James G. In Vivo Identification of Bipotential Adipocyte Progenitors Recruited by β3-Adrenoceptor Activation and High-Fat Feeding. Cell Metab. 2012;15:480–491. doi: 10.1016/j.cmet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Berry R, Rodeheffer MS. Characterization of the adipocyte cellular lineage in vivo. Nat Cell Biol. 2013;15:302–308. doi: 10.1038/ncb2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Keymeulen A, Rocha AS, Ousset M, Beck B, Bouvencourt G, Rock J, Sharma N, Dekoninck S, Blanpain C. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 60.Bouloumie A, Bourin P, Casteilla L, Dangelo R, Fournier-Wirth C, Maumus M, Peyrafitte JA, Sengenes C. Native human adipose stromal cells: localization, morphology and phenotype. International Journal of Obesity. 2011;35:1141. doi: 10.1038/ijo.2010.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tran K-V, Gealekman O, Frontini A, Zingaretti Maria C, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, Cinti S. The Vascular Endothelium of the Adipose Tissue Gives Rise to Both White and Brown Fat Cells. Cell Metabolism. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta Rana K, Mepani Rina J, Kleiner S, Lo James C, Khandekar Melin J, Cohen P, Frontini A, Bhowmick Diti C, Ye L, Cinti S, Spiegelman Bruce M. Zfp423 Expression Identifies Committed Preadipocytes and Localizes to Adipose Endothelial and Perivascular Cells. Cell Metabolism. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gimble JM, Katz AJ, Bunnell BA. Adipose-Derived Stem Cells for Regenerative Medicine. Circulation Research. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sengenès C, Lolmède K, Zakaroff-Girard A, Busse R, Bouloumié A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. Journal of Cellular Physiology. 2005;205:114–122. doi: 10.1002/jcp.20381. [DOI] [PubMed] [Google Scholar]
- 65.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. VE-cadherin-CreERT2 transgenic mouse: A model for inducible recombination in the endothelium. Developmental Dynamics. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 66.Fraser JK, Schreiber R, Strem B, Zhu M, Alfonso Z, Wulur I, Hedrick MH. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med. 2006 doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 67.Planat-Benard V, Silvestre J-S, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L. Plasticity of Human Adipose Lineage Cells Toward Endothelial Cells. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 68.Ferraro GA, De Francesco F, Nicoletti G, Paino F, Desiderio V, Tirino V, D'Andrea F. Human adipose CD34+CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. Journal of Cellular Biochemistry. 2012 doi: 10.1002/jcb.24443. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 69.Majka SM, Fox KE, Psilas JC, Helm KM, Childs CR, Acosta AS, Janssen RC, Friedman JE, Woessner BT, Shade TR, Varella-Garcia M, Klemm DJ. De novo generation of white adipocytes from the myeloid lineage via mesenchymal intermediates is age, adipose depot, and gender specific. Proceedings of the National Academy of Sciences. 2010;107:14781–14786. doi: 10.1073/pnas.1003512107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crossno JT, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sera Y, LaRue AC, Moussa O, Mehrotra M, Duncan JD, Williams CR, Nishimoto E, Schulte BA, Watson PM, Watson DK, Ogawa M. Hematopoietic stem cell origin of adipocytes. Experimental hematology. 2009;37:1108–1120.e1104. doi: 10.1016/j.exphem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majka SM, Miller HL, Sullivan T, Erickson PF, Kong R, Weiser-Evans M, Nemenoff R, Moldovan R, Morandi SA, Davis JA, Klemm DJ. Adipose lineage specification of bone marrow-derived myeloid cells. Adipocyte. 2012;1:215–229. doi: 10.4161/adip.21496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koh YJ, Kang S, Lee HJ, Choi T-S, Lee HS, Cho C-H, Koh GY. Bone marrow derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 75.Greco V, Guo S. Compartmentalized organization: a common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moore KA, Lemischka IR. Stem Cells and Their Niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 77.Li L, Clevers H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iyama K. Electron microscopical studies on the genesis of white adipocytes: differentiation of immature pericytes into adipocytes in transplanted preadipose tissue, Virchows Archiv. B. Cell pathology. 1979;31:143–155. doi: 10.1007/BF02889932. [DOI] [PubMed] [Google Scholar]
- 79.Cinti S. A morphological study of the adipocyte precursor. Journal of submicroscopic cytology. 1984;16:243–251. [PubMed] [Google Scholar]
- 80.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A Population of Multipotent CD34-Positive Adipose Stromal Cells Share Pericyte and Mesenchymal Surface Markers, Reside in a Periendothelial Location and Stabilize Endothelial Networks. Circulation Research. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 81.Eto H, Suga H, Matsumoto D, Inoue K, Aoi N, Kato H, Araki J, Yoshimura K. Characterization of Structure and Cellular Components of Aspirated and Excised Adipose Tissue. Plastic and Reconstructive Surgery. 2009;124:1087–1097. doi: 10.1097/PRS.0b013e3181b5a3f1. doi:1010.1097/PRS.1080b1013e3181b1085a1083f1081. [DOI] [PubMed] [Google Scholar]
- 82.Granneman JG, Li P, Lu Y, Tilak J. Seeing the trees in the forest: selective electroporation of adipocytes within adipose tissue. American Journal of Physiology - Endocrinology And Metabolism. 2004;287:E574–E582. doi: 10.1152/ajpendo.00567.2003. [DOI] [PubMed] [Google Scholar]
- 83.Biswas Subhra K, Mantovani A. Orchestration of Metabolism by Macrophages. Cell Metabolism. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 84.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 86.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas.(Science in medicine)(Report) Journal of Clinical Investigation. 2012;122:787–789. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Levine JA, Jensen MD, Eberhardt NL, O'Brien T. Adipocyte macrophage colony-stimulating factor is a mediator of adipose tissue growth. The Journal of Clinical Investigation. 1998;101:1557–1564. doi: 10.1172/JCI2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han J, Lee J-E, Jin J, Lim JS, Oh N, Kim K, Chang S-I, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 90.Wiktor-Jedrzejczak W. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Experimental hematology. 1991;19:1049–1054. [PubMed] [Google Scholar]
- 91.Molgat AS, Gagnon A, Sorisky A. Preadipocyte apoptosis is prevented by macrophage-conditioned medium in a PDGF-dependent manner. American Journal of Physiology - Cell Physiology. 2009;296:C757–C765. doi: 10.1152/ajpcell.00617.2008. [DOI] [PubMed] [Google Scholar]
- 92.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. American Journal of Physiology - Endocrinology And Metabolism. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. The Journal of Clinical Investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Odegaard JI, Chawla A. Alternative Macrophage Activation and Metabolism. Annual Review of Pathology: Mechanisms of Disease. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPAR[g] controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nguyen KD, Qiu Y, Cui X, Goh YPS, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of Clinical Investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cao Y. Angiogenesis modulates adipogenesis and obesity. Journal of Clinical Investigation. 2007;117:2362–2368. doi: 10.1172/JCI32239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in Obesity Requires Close Interplay Between Differentiating Adipocytes, Stromal Cells, and Blood Vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 101.Rupnick MA, Panigrahy D, Zhang C-Y, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proceedings of the National Academy of Sciences. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun K, Asterholm IW, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proceedings of the National Academy of Sciences. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hutley LJ, Herington AC, Shurety W, Cheung C, Vesey DA, Cameron DP, Prins JB. Human adipose tissue endothelial cells promote preadipocyte proliferation. American Journal of Physiology - Endocrinology And Metabolism. 2001;281:E1037–E1044. doi: 10.1152/ajpendo.2001.281.5.E1037. [DOI] [PubMed] [Google Scholar]
- 104.Varzaneh FE, Shillabeer G, Wong KL, Lau DCW. Extracellular matrix components secreted by microvascular endothelial cells stimulate preadipocyte differentiation in vitro. Metabolism. 1994;43:906–912. doi: 10.1016/0026-0495(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 105.Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. Angiogenesis Inhibitor, TNP-470 Prevents Diet-Induced and Genetic Obesity in Mice. Circulation Research. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 106.Bartness TJ, Song CK. Thematic review series: Adipocyte Biology. Sympathetic and sensory innervation of white adipose tissue. Journal of Lipid Research. 2007;48:1655–1672. doi: 10.1194/jlr.R700006-JLR200. [DOI] [PubMed] [Google Scholar]
- 107.Foster MT, Bartness TJ. Sympathetic but not sensory denervation stimulates white adipocyte proliferation, American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2006;291:R1630–R1637. doi: 10.1152/ajpregu.00197.2006. [DOI] [PubMed] [Google Scholar]
- 108.Shi H, Song CK, Giordano A, Cinti S, Bartness TJ. Sensory or sympathetic white adipose tissue denervation differentially affects depot growth and cellularity, American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 2005;288:R1028–R1037. doi: 10.1152/ajpregu.00648.2004. [DOI] [PubMed] [Google Scholar]
- 109.Hausman DB, DiGirolamo M, Bartness TJ, Hausman GJ, Martin RJ. The biology of white adipocyte proliferation. Obesity Reviews. 2001;2:239–254. doi: 10.1046/j.1467-789x.2001.00042.x. [DOI] [PubMed] [Google Scholar]
- 110.Cousin B, Casteilla L, Lafontan M, Ambid L, Langin D, Berthault MF, Pénicaud L. Local sympathetic denervation of white adipose tissue in rats induces preadipocyte proliferation without noticeable changes in metabolism. Endocrinology. 1993;133:2255–2262. doi: 10.1210/endo.133.5.8404678. [DOI] [PubMed] [Google Scholar]
- 111.Jones D, Ramsay T, Hausman G, Martin R. Norepinephrine inhibits rat pre-adipocyte proliferation. International journal of obesity and related metabolic disorders. 1992;16:349–354. [PubMed] [Google Scholar]
- 112.Bouloumié A, Planat V, Devedjian JC, Valet P, Saulnier-Blache JS, Record M, Lafontan M. Alpha 2-adrenergic stimulation promotes preadipocyte proliferation. Involvement of mitogen-activated protein kinases. Journal of Biological Chemistry. 1994;269:30254–30259. [PubMed] [Google Scholar]
- 113.Geloen A, Collet AJ, Bukowiecki LJ. Role of sympathetic innervation in brown adipocyte proliferation, American Journal of Physiology - Regulatory. Integrative and Comparative Physiology. 1992;263:R1176–R1181. doi: 10.1152/ajpregu.1992.263.6.R1176. [DOI] [PubMed] [Google Scholar]
- 114.Bronnikov G, Bengtsson T, Kramarova L, Golozoubova V, Cannon B, Nedergaard J. {beta}1 to {beta}3 Switch in Control of Cyclic Adenosine Monophosphate during Brown Adipocyte Development Explains Distinct {beta}-Adrenoceptor Subtype Mediation of Proliferation and Differentiation. Endocrinology. 1999;140:4185–4197. doi: 10.1210/endo.140.9.6972. [DOI] [PubMed] [Google Scholar]
- 115.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E, Tseng Y-H. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. doi: 10.1038/nature11943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Divoux A, Clément K. Architecture and the extracellular matrix: the still unappreciated components of the adipose tissue. Obesity Reviews. 2011;12:e494–e503. doi: 10.1111/j.1467-789X.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 118.Mariman EM, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nakajima I, Muroya S, Tanabe R-i, Chikuni K. Extracellular matrix development during differentiation into adipocytes with a unique increase in type V and VI collagen. Biology of the Cell. 2002;94:197–203. doi: 10.1016/s0248-4900(02)01189-9. [DOI] [PubMed] [Google Scholar]
- 120.Pierleoni C, Verdenelli F, Castellucci MCS. Fibronectins and basal lamina molecules expression in human subcutaneous white adipose tissue. European journal of histochemistry. 1998;42:183–188. [PubMed] [Google Scholar]
- 121.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3t3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 122.Christiaens V, Scroyen I, Lijnen HR. Role of proteolysis in development of murine adipose tissue. Thrombosis and haemostasis. 2008;99:290–294. doi: 10.1160/TH07-10-0589. [DOI] [PubMed] [Google Scholar]
- 123.Maquoi E. Enhanced nutritionally induced adipose tissue development in mice with stromelysin-1 gene inactivation. Thrombosis and haemostasis. 2003;89:696–704. [PubMed] [Google Scholar]
- 124.Lijnen HR. Adipocyte hypertrophy in stromelysin-3 deficient mice with nutritionally induced obesity. Thrombosis and haemostasis. 2002;87:530–535. [PubMed] [Google Scholar]
- 125.Alexander CM, Selvarajan S, Mudgett J, Werb Z. Stromelysin-1 Regulates Adipogenesis during Mammary Gland Involution. The Journal of Cell Biology. 2001;152:693–703. doi: 10.1083/jcb.152.4.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Croissandeau G, Chrétien M, Mbikay M. Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem J. 2002;364:739–746. doi: 10.1042/BJ20011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte Produces Matrix Metalloproteinases 2 and 9: Involvement in Adipose Differentiation. Diabetes. 2001;50:2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 128.Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. Matrix Metalloproteinase Inhibition Impairs Adipose Tissue Development in Mice, Arteriosclerosis, Thrombosis. and Vascular Biology. 2002;22:374–379. doi: 10.1161/hq0302.104522. [DOI] [PubMed] [Google Scholar]
- 129.Chun T-H, Hotary KB, Sabeh F, Saltiel AR, Allen ED, Weiss SJ. A Pericellular Collagenase Directs the 3-Dimensional Development of White Adipose Tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 130.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic Dysregulation and Adipose Tissue Fibrosis: Role of Collagen VI. Molecular and Cellular Biology. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia- Inducible Factor 1α Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Molecular and Cellular Biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spencer M, Yao-Borengasser A, Unal R, Rasouli N, Gurley CM, Zhu B, Peterson CA, Kern PA. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. American Journal of Physiology - Endocrinology And Metabolism. 2010;299:E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bourlier V, Sengenès C, Zakaroff-Girard A, Decaunes P, Wdziekonski B, Galitzky J, Villageois P, Esteve D, Chiotasso P, Dani C, Bouloumié A. TGFbeta Family Members Are Key Mediators in the Induction of Myofibroblast Phenotype of Human Adipose Tissue Progenitor Cells by Macrophages. PLoS ONE. 2012;7:e31274. doi: 10.1371/journal.pone.0031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uezumi A, Fukada S-i, Yamamoto N, Takeda Si, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 136.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda Si, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S-i. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–3664. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- 137.Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12+ perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- 139.Hollenberg CH, Vost A. Regulation of DNA synthesis in fat cells and stromal elements from rat adipose tissue. J Clin Invest. 1969;47:2485–2498. doi: 10.1172/JCI105930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63:239–246. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Spalding KL, Arner E, Westermark PO, Bernard S, Buchholz BA, Bergmann O, Blomqvist L, Hoffstedt J, Naslund E, Britton T, Concha H, Hassan M, Ryden M, Frisen J, Arner P. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 142.Knittle JL, Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968;47:2091–2098. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Strawford A, Antelo F, Christiansen M, Hellerstein MK. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. American Journal of Physiology - Endocrinology And Metabolism. 2004;286:E577–E588. doi: 10.1152/ajpendo.00093.2003. [DOI] [PubMed] [Google Scholar]
- 144.Rigamonti A, Brennand K, Lau F, Cowan CA. Rapid Cellular Turnover in Adipose Tissue. PLoS ONE. 2011;6:e17637. doi: 10.1371/journal.pone.0017637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Joe AWB, Yi L, Even Y, Vogl AW, Rossi FMV. Depot-Specific Differences in Adipogenic Progenitor Abundance and Proliferative Response to High-Fat Diet. STEM CELLS. 2009;27:2563–2570. doi: 10.1002/stem.190. [DOI] [PubMed] [Google Scholar]
- 146.Tchoukalova Y, Votruba S, Tchkonia T, Giorgadze N, Kirkland J, Jensen M. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hirsch J, Batchelor B. Adipose tissue cellularity in human obesity. Clinics in Endocrinology and Metabolism. 1976;5:299–311. doi: 10.1016/s0300-595x(76)80023-0. [DOI] [PubMed] [Google Scholar]
- 148.Maumus M, Sengenès C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, Galitzky J, Bouloumié A. Evidence of in Situ Proliferation of Adult Adipose Tissue-Derived Progenitor Cells: Influence of Fat Mass Microenvironment and Growth. Journal of Clinical Endocrinology & Metabolism. 2008;93:4098–4106. doi: 10.1210/jc.2008-0044. [DOI] [PubMed] [Google Scholar]
- 149.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19:81–87. doi: 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Granneman JG, Li P, Zhu Z, Lu Y. Metabolic and cellular plasticity in white adipose tissue I: effects of beta3-adrenergic receptor activation. Am J Physiol Endocrinol Metab. 2005;289:E608–616. doi: 10.1152/ajpendo.00009.2005. [DOI] [PubMed] [Google Scholar]
- 151.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am J Physiol. 1994;266:R1371–1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 152.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 153.Ghorbani M, Himms-Hagen J. Appearance of brown adipocytes in white adipose tissue during CL 316,243-induced reversal of obesity and diabetes in Zucker fa/fa rats. Int J Obes Relat Metab Disord. 1997;21:465–475. doi: 10.1038/sj.ijo.0800432. [DOI] [PubMed] [Google Scholar]
- 154.Grujic D, Susulic VS, Harper ME, Himms-Hagen J, Cunningham BA, Corkey BE, Lowell BB. Beta3-adrenergic receptors on white and brown adipocytes mediate beta3-selective agonist-induced effects on energy expenditure, insulin secretion, and food intake. A study using transgenic and gene knockout mice. J Biol Chem. 1997;272:17686–17693. doi: 10.1074/jbc.272.28.17686. [DOI] [PubMed] [Google Scholar]
- 155.Xue B, Rim J-S, Hogan J, Coulter A, Koza R, Kozak L. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 156.Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne) 2011;2:64. doi: 10.3389/fendo.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. II. Mitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101:199–209. doi: 10.1016/0889-1605(88)90010-9. [DOI] [PubMed] [Google Scholar]
- 158.Loncar D, Afzelius BA, Cannon B. Epididymal white adipose tissue after cold stress in rats. I. Nonmitochondrial changes. J Ultrastruct Mol Struct Res. 1988;101:109–122. doi: 10.1016/0889-1605(88)90001-8. [DOI] [PubMed] [Google Scholar]
- 159.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L, Casteilla L. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 160.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic Peroxisome Proliferator-activated Receptor γ (PPARγ ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. Journal of Biological Chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xue B, Rim J-S, Hogan J, Coulter A, Koza R, Kozak L. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. Journal of Lipid Research. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 162.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng Y-H. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Himms-Hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. 2000;279:C670–681. doi: 10.1152/ajpcell.2000.279.3.C670. [DOI] [PubMed] [Google Scholar]
- 164.Gouon-Evans V, Pollard J. Unexpected deposition of brown fat in mammary gland during postnatal development. Molecular endocrinology (Baltimore, Md) 2002;16:2618–2627. doi: 10.1210/me.2001-0337. [DOI] [PubMed] [Google Scholar]
- 165.Loncar D. Development of thermogenic adipose tissue. Int J Dev Biol. 1991;35:321–333. [PubMed] [Google Scholar]
- 166.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-α. Am J of Physiol Endocrinol Metab. 2005;289:E617–E626. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 167.Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta. 2010;1801:1048–1055. doi: 10.1016/j.bbalip.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab. 2007;293:E1188–1197. doi: 10.1152/ajpendo.00051.2007. [DOI] [PubMed] [Google Scholar]
- 169.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 170.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 171.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 175.Goldfine AB, Fonseca V, Shoelson SE. Therapeutic approaches to target inflammation in type 2 diabetes. Clin Chem. 2011;57:162–167. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Gregor M, Hotamisligil G. Inflammatory mechanisms in obesity. Annual review of immunology. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 177.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J Clin Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O'Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Glass C, Olefsky J. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metabolism. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50:151–157. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]
- 182.Roldan M, Macias-Gonzalez M, Garcia R, Tinahones FJ, Martin M. Obesity short-circuits stemness gene network in human adipose multipotent stem cells. The FASEB Journal. 2011;25:4111–4126. doi: 10.1096/fj.10-171439. [DOI] [PubMed] [Google Scholar]
- 183.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58:1550–1557. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Karagiannides I, Tchkonia T, Dobson DE, Steppan CM, Cummins P, Chan G, Salvatori K, Hadzopoulou-Cladaras M, Kirkland JL. Altered expression of C/EBP family members results in decreased adipogenesis with aging. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1772–1780. doi: 10.1152/ajpregu.2001.280.6.R1772. [DOI] [PubMed] [Google Scholar]
- 185.Caso G, McNurlan MA, Mileva I, Zemlyak A, Mynarcik DC, Gelato MC. Peripheral fat loss and decline in adipogenesis in older humans. Metabolism. 2012 doi: 10.1016/j.metabol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tchkonia T, Morbeck D, Von Zglinicki T, Van Deursen J, Lustgarten J, Scrable H, Khosla S, Jensen M, Kirkland J. Fat tissue, aging, and cellular senescence. Aging cell. 2010;9:667–684. doi: 10.1111/j.1474-9726.2010.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med. 2009;15:1082–1087. doi: 10.1038/nm.2014. [DOI] [PubMed] [Google Scholar]
- 188.Baker D, Wijshake T, Tchkonia T, LeBrasseur N, Childs B, van de Sluis B, Kirkland J, van Deursen J. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rogers N, Landa A, Park S, Smith R. Aging leads to a programmed loss of brown adipocytes in murine subcutaneous white adipose tissue. Aging cell. 2012;11:1074–1083. doi: 10.1111/acel.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Arch J. The discovery of drugs for obesity, the metabolic effects of leptin and variable receptor pharmacology: perspectives from β3-adrenoceptor agonists. Naunyn-Schmiedeberg's Archives of Pharmacology. 2008;378:225–240. doi: 10.1007/s00210-008-0271-1. [DOI] [PubMed] [Google Scholar]
- 191.Chawla A, Schwarz EJ, Dimaculangan DD, Lazar MA. Peroxisome proliferator-activated receptor (PPAR) gamma: adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 192.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2 a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 193.Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- 194.Seufert J, Lubben G, Dietrich K, Bates PC. A comparison of the effects of thiazolidinediones and metformin on metabolic control in patients with type 2 diabetes mellitus. Clin Ther. 2004;26:805–818. doi: 10.1016/s0149-2918(04)90125-7. [DOI] [PubMed] [Google Scholar]
- 195.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson S, Benoist C, Mathis D. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hevener AL, He W, Barak Y, Le J, Bandyopadhyay G, Olson P, Wilkes J, Evans RM, Olefsky J. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 198.He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sugii S, Olson P, Sears DD, Saberi M, Atkins AR, Barish GD, Hong SH, Castro GL, Yin YQ, Nelson MC, Hsiao G, Greaves DR, Downes M, Yu RT, Olefsky JM, Evans RM. PPARgamma activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci U S A. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Laplante M, Festuccia WT, Soucy G, Gelinas Y, Lalonde J, Deshaies Y. Involvement of adipose tissues in the early hypolipidemic action of PPAR{gamma} agonism in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1408–1417. doi: 10.1152/ajpregu.00761.2006. [DOI] [PubMed] [Google Scholar]
- 201.Smith SR, De Jonge L, Volaufova J, Li Y, Xie H, Bray GA. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metabolism. 2005;54:24–32. doi: 10.1016/j.metabol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 202.Shadid S, Stehouwer CD, Jensen MD. Diet/Exercise versus pioglitazone: effects of insulin sensitization with decreasing or increasing fat mass on adipokines and inflammatory markers. J Clin Endocrinol Metab. 2006;91:3418–3425. doi: 10.1210/jc.2006-0015. [DOI] [PubMed] [Google Scholar]
- 203.Fruebis J, Tsao T-S, Javorschi S, Ebbets-Reed D, Erickson MRS, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 205.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 206.Miles PD, Romeo OM, Higo K, Cohen A, Rafaat K, Olefsky JM. TNF-alpha-induced insulin resistance in vivo and its prevention by troglitazone. Diabetes. 1997;46:1678–1683. doi: 10.2337/diab.46.11.1678. [DOI] [PubMed] [Google Scholar]
- 207.Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- 208.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. J Biol Chem. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 209.Jonker J, Suh J, Atkins A, Ahmadian M, Li P, Whyte J, He M, Juguilon H, Yin Y-Q, Phillips C, Yu R, Olefsky J, Henry R, Downes M, Evans R. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Dutchak P, Katafuchi T, Bookout A, Choi J, Yu R, Mangelsdorf D, Kliewer S. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Guan HP, Li Y, Jensen MV, Newgard CB, Steppan CM, Lazar MA. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat Med. 2002;8:1122–1128. doi: 10.1038/nm780. [DOI] [PubMed] [Google Scholar]
- 212.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione-mediated insulin sensitization. Proc Natl Acad Sci U S A. 2009;106:18745–18750. doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Vernochet C, Peres SB, Davis KE, McDonald ME, Qiang L, Wang H, Scherer PE, Farmer SR. C/EBPalpha and the corepressors CtBP1 and CtBP2 regulate repression of select visceral white adipose genes during induction of the brown phenotype in white adipocytes by peroxisome proliferator-activated receptor gamma agonists. Mol Cell Biol. 2009;29:4714–4728. doi: 10.1128/MCB.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Siersbaek MS, Loft A, Aagaard MM, Nielsen R, Schmidt SF, Petrovic N, Nedergaard J, Mandrup S. Genome-wide profiling of peroxisome proliferator-activated receptor gamma in primary epididymal, inguinal and brown adipocytes reveals depot-selective binding correlated with gene expression. Mol Cell Biol. 2012;32:3452–3463. doi: 10.1128/MCB.00526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Elabd C, Chiellini C, Carmona M, Galitzky J, Cochet O, Petersen R, Penicaud L, Kristiansen K, Bouloumie A, Casteilla L, Dani C, Ailhaud G, Amri EZ. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. STEM CELLS. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 219.Yoshida T, Sakane N, Wakabayashi Y, Umekawa T, Kondo M. Anti-obesity and anti-diabetic effects of CL 316,243, a highly specific beta 3-adrenoceptor agonist, in yellow KK mice. Life Sci. 1994;54:491–498. doi: 10.1016/0024-3205(94)00408-0. [DOI] [PubMed] [Google Scholar]
- 220.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue II: role of peroxisome proliferator-activated receptor-alpha. Am J Physiol Endocrinol Metab. 2005;289:E617–626. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 221.Cawthorne MA, Sennitt MV, Arch JR, Smith SA. BRL 35135, a potent and selective atypical beta-adrenoceptor agonist. Am J Clin Nutr. 1992;55:252S–257S. doi: 10.1093/ajcn/55.1.252s. [DOI] [PubMed] [Google Scholar]
- 222.Collins S, Yehuda-Shnaidman E, Wang H. Positive and negative control of Ucp1 gene transcription and the role of beta-adrenergic signaling networks. Int J Obes (Lond) 2010;34(Suppl 1):S28–33. doi: 10.1038/ijo.2010.180. [DOI] [PubMed] [Google Scholar]
- 223.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Rim JS, Kozak LP. Regulatory motifs for CREB-binding protein and Nfe2l2 transcription factors in the upstream enhancer of the mitochondrial uncoupling protein 1 gene. J Biol Chem. 2002;277:34589–34600. doi: 10.1074/jbc.M108866200. [DOI] [PubMed] [Google Scholar]
- 225.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 226.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 227.Chang JS, Fernand V, Zhang Y, Shin J, Jun HJ, Joshi Y, Gettys TW. NT-PGC-1alpha protein is sufficient to link beta3-adrenergic receptor activation to transcriptional and physiological components of adaptive thermogenesis. J Biol Chem. 2012;287:9100–9111. doi: 10.1074/jbc.M111.320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Xue B, Coulter A, Rim JS, Koza RA, Kozak LP. Transcriptional synergy and the regulation of Ucp1 during brown adipocyte induction in white fat depots. Mol Cell Biol. 2005;25:8311–8322. doi: 10.1128/MCB.25.18.8311-8322.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Mottillo EP, Bloch AE, Leff T, Granneman JG. Lipolytic Products Activate Peroxisome Proliferator-activated Receptor (PPAR) α and δ in Brown Adipocytes to Match Fatty Acid Oxidation with Supply. Journal of Biological Chemistry. 2012;287:25038–25048. doi: 10.1074/jbc.M112.374041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mottillo EP, Shen XJ, Granneman JG. Role of hormone-sensitive lipase in beta-adrenergic remodeling of white adipose tissue. Am J Physiol Endocrinol Metab. 2007;293:E1188–1197. doi: 10.1152/ajpendo.00051.2007. [DOI] [PubMed] [Google Scholar]
- 231.Ahmadian M, Abbott Marcia J, Tang T, Hudak Carolyn SS, Kim Y, Bruss M, Hellerstein Marc K, Lee H-Y, Samuel Varman T, Shulman Gerald I, Wang Y, Duncan Robin E, Kang C, Sul Hei S. Desnutrin/ATGL Is Regulated by AMPK and Is Required for a Brown Adipose Phenotype. Cell Metab. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Timmons JA, Baar K, Davidsen PK, Atherton PJ. Is irisin a human exercise gene? Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]
- 234.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased Mitochondrial Activity in BMP7-Treated Brown Adipocytes, Due to Increased CPT1- and CD36-Mediated Fatty Acid Uptake. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2012.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Whittle A, Carobbio S, Martins L, Slawik M, Hondares E, Vázquez M, Morgan D, Csikasz R, Gallego R, Rodriguez-Cuenca S, Dale M, Virtue S, Villarroya F, Cannon B, Rahmouni K, López M, Vidal-Puig A. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell. 2012;149:871–885. doi: 10.1016/j.cell.2012.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hondares E, Rosell M, Gonzalez F, Giralt M, Iglesias R, Villarroya F. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metabolism. 2010;11:206–212. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fisher F, Kleiner S, Douris N, Fox E, Mepani R, Verdeguer F, Wu J, Kharitonenkov A, Flier J, Maratos-Flier E, Spiegelman B. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes & Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chartoumpekis D, Habeos I, Ziros P, Psyrogiannis A, Kyriazopoulou V, Papavassiliou A. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Molecular medicine (Cambridge, Mass) 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 Requires betaklotho to Act In Vivo. PLoS ONE. 2012;7:e49977. doi: 10.1371/journal.pone.0049977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ding X, Boney-Montoya J, Owen BM, Bookout AL, Coate KC, Mangelsdorf DJ, Kliewer SA. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metab. 2012;16:387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N, Gupte J, Komorowski R, Sekirov L, Hager T, Arora T, Ge H, Baribault H, Wang F, Sheng J, Karow M, Wang M, Luo Y, McKeehan W, Wang Z, Veniant MM, Li Y. Treating Diabetes and Obesity with an FGF21-Mimetic Antibody Activating the betaKlotho/FGFR1c Receptor Complex. Sci Transl Med. 2012;4:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- 243.Lee P, Brychta RJ, Linderman J, Smith S, Chen KY, Celi FS. Mild Cold Exposure Modulates Fibroblast Growth Factor 21 (FGF21) Diurnal Rhythm in Humans: Relationship between FGF21 Levels, Lipolysis, and Cold-Induced Thermogenesis. J Clin Endocrinol Metab. 2012 doi: 10.1210/jc.2012-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, Jin R, Ma L, Wang P, Zhu Z, Li L, Zhong J, Liu D, Nilius B, Zhu Z. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol. 2012;4:88–96. doi: 10.1093/jmcb/mjs001. [DOI] [PubMed] [Google Scholar]
- 245.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Christian M, Kiskinis E, Debevec D, Leonardsson G, White R, Parker M. RIP140-targeted repression of gene expression in adipocytes. Molecular and cellular biology. 2005;25:9383–9391. doi: 10.1128/MCB.25.21.9383-9391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Scime A, Grenier G, Huh MS, Gillespie MA, Bevilacqua L, Harper ME, Rudnicki MA. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1alpha. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 248.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 249.Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19:130–137. doi: 10.1016/j.tem.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 250.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, Berlan M, Marques MA, Sweep FC, Luft FC, Lafontan M, Jordan J. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes. 2008;57:3199–3204. doi: 10.2337/db08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, Thalamas C, Montastier E, Larrouy D, Harant I, de Glisezinski I, Lieske S, Reinke J, Beckmann B, Langin D, Jordan J, Moro C. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–4679. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Arch JR, Ainsworth AT, Cawthorne MA, Piercy V, Sennitt MV, Thody VE, Wilson C, Wilson S. Atypical beta-adrenoceptor on brown adipocytes as target for anti-obesity drugs. Nature. 1984;309:163–165. doi: 10.1038/309163a0. [DOI] [PubMed] [Google Scholar]
- 254.Cypess A, Chen Y-C, Sze C, Wang K, English J, Chan O, Holman A, Tal I, Palmer M, Kolodny G, Kahn C. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Carey A, Formosa M, Van Every B, Bertovic D, Eikelis N, Lambert G, Kalff V, Duffy S, Cherk M, Kingwell B. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2012 doi: 10.1007/s00125-012-2748-1. [DOI] [PubMed] [Google Scholar]
- 256.Vosselman MJ, van der Lans AA, Brans B, Wierts R, van Baak MA, Schrauwen P, van Marken Lichtenbelt WD. Systemic beta-Adrenergic Stimulation of Thermogenesis Is Not Accompanied by Brown Adipose Tissue Activity in Humans. Diabetes. 2012;61:3106–3113. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Nicholls DG, Rial E. A History of the First Uncoupling Protein, UCP1. Journal of Bioenergetics and Biomembranes. 1999;31:399–406. doi: 10.1023/a:1005436121005. [DOI] [PubMed] [Google Scholar]
- 258.Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, Schreiber R, Eichmann T, Kolb D, Kotzbeck P, Schweiger M, Kumari M, Eder S, Schoiswohl G, Wongsiriroj N, Pollak NM, Radner FP, Preiss-Landl K, Kolbe T, Rulicke T, Pieske B, Trauner M, Lass A, Zimmermann R, Hoefler G, Cinti S, Kershaw EE, Schrauwen P, Madeo F, Mayer B, Zechner R. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne) 2012;3:15. doi: 10.3389/fendo.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shing DC, Trubia M, Marchesi F, Radaelli E, Belloni E, Tapinassi C, Scanziani E, Mecucci C, Crescenzi B, Lahortiga I, Odero MD, Zardo G, Gruszka A, Minucci S, Di Fiore PP, Pelicci PG. Overexpression of sPRDM16 coupled with loss of p53 induces myeloid leukemias in mice. J Clin Invest. 2007;117:3696–3707. doi: 10.1172/JCI32390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Viatour P, Sage J. Newly identified aspects of tumor suppression by RB. Dis Model Mech. 2011;4:581–585. doi: 10.1242/dmm.008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol. 2011;13:958–965. doi: 10.1038/ncb2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol. 2012;14:1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- 264.Mori M, Nakagami H, Rodriguez-Araujo G, Nimura K, Kaneda Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS biology. 2012;10 doi: 10.1371/journal.pbio.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nishio M, Yoneshiro T, Nakahara M, Suzuki S, Saeki K, Hasegawa M, Kawai Y, Akutsu H, Umezawa A, Yasuda K, Tobe K, Yuo A, Kubota K, Saito M, Saeki K. Production of Functional Classical Brown Adipocytes from Human Pluripotent Stem Cells using Specific Hemopoietin Cocktail without Gene Transfer. Cell Metabolism. 2012;16:394–406. doi: 10.1016/j.cmet.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 267.Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP, Clish CB, Deo RC, Shen T, Lau FH, Cowley A, Mowrer G, Al-Siddiqi H, Nahrendorf M, Musunuru K, Gerszten RE, Rinn JL, Cowan CA. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 14(2012):209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]


