Abstract
Purpose
Interstitial cystitis (IC) is a highly prevalent pain condition, estimated to affect 3-6% of women in the United States. Emerging data suggests there are central neurobiological components to the etiology of this disease. Here we report the first brain structural imaging findings from the Multidisciplinary Approach to Pelvic Pain (MAPP) network, with data on over 300 participants.
Materials and Methods
We used Voxel-Based Morphometry (VBM) to determine whether human patients with chronic IC display changes in brain morphology as compared to healthy controls (HCs). 33 female IC patients without comorbidities and 33 age- and sex-matched controls, taken from the larger sample, underwent structural magnetic resonance imaging at 5 different MAPP sites across the United States.
Results
When compared to controls, females with IC displayed significant increased gray matter (GM) volume in several regions of the brain including the right primary somatosensory cortex (S1), the superior parietal lobule bilaterally, and the right supplementary motor area. GM volume in the right S1 was associated with greater pain, mood (anxiety), and urological symptoms. We explored these correlations in a linear regression model and found independent effects of these three measures on S1 GM volume: clinical pain (McGill pain sensory total), a measure of “urgency,” and anxiety (HADS).
Conclusions
These data support the notion that changes in somatosensory GM may play an important role in pain sensitivity as well as affective and sensory aspects of IC. Further studies are needed to confirm the generalizability of these findings to other pain conditions.
Keywords: interstitial cystitis, voxel based morphometry, pain, primary somatosensory cortex, MAPP network
Introduction
Interstitial Cystitis (IC), also known as painful bladder syndrome, is a prevalent pain condition, estimated to affect 3-6% of women in the USA1. Symptoms include urinary frequency, urgency, and pelvic pain, with no pathognomonic clinical findings in these patients2. Originally considered a disease of the bladder3, it is now recognized that some patients have a systemic condition, involving in pain outside the bladder and comorbid symptoms2. With no general agreement about the pathophysiology, a wide variety of management options includes dietary, behavioral, pharmacologic and surgical therapies4.
Chronic pain disorders are considered to be partially mediated by a “centralized” pathology (i.e. pain is augmented/maintained by alterations in CNS processing). Neuroimaging is increasingly used to assess alterations in brain shape and function in chronic pain conditions5-8. Voxel-Based Morphometry (VBM), an imaging technique used to assess changes in brain anatomy, has been employed previously in pelvic pain conditions9-12. Compared to healthy controls (HCs), chronic pelvic pain patients were found to have increased and decreased regional GM volumes in specific brain areas involved in nociceptive processing. These changes were associated with self-report measures of clinical symptoms10, 11. Analogous structural neuroimaging studies have not been published for IC/BPS.
We present data from a, multi-site neuroimaging study of female IC patients, originating from the Multidisciplinary Approach to Pelvic Pain (MAPP) network (http://www.mappnetwork.org). We examined the relationship between GM volume and clinical symptoms in women with non-comorbid IC compared to HCs. We hypothesized IC patients would show GM alterations in brain regions previously found to be involved in chronic pain conditions, and that these changes would be associated with clinical measures.
Methods
Anatomical Data Acquisition
Anatomical MRI data were acquired across five MAPP discovery sites. Each site utilized a whole-body scanner with an 8-channel phased-array head coil, previously described13. High resolution T1 structural images were acquired for each subject using the acquisition protocol: 2200 millisecond repetition time, 3.26 millisecond echo time, 1 millimeter slice thickness, 256 x 256 voxel matrices, and 1 millimeter3 voxel size.
Trans-MAPP neuroimaging data was collected, quality controlled and archived according to multi-site imaging procedures developed by the MAPP Research Network, UCLA PAIN repository and UCLA Laboratory of Neuroimaging. Scanner compatible acquisition parameters were developed and all sites passed a qualification test. Initial scans were reviewed for quality control by the UCLA site. Recommendations and adjustments were made as necessary prior to patient enrollment.
Subjects
Subjects were 33 female non-comorbid IC patients (mean age ± SD; 39.5 ± 12 years; mean symptom duration ± SD; 9.1 ± 9 years) and 33 age- and sex-matched HCs (mean age ± SD; 39 ± 11.6 years). Subjects were selected from 225 women in the MAPP network data as of July 2012 (see Table 1 for inclusion/exclusion criteria). Because our primary research focus was brain GM in non-comorbid IC patients, we selected “pure” IC patients without fibromyalgia, irritable bowel syndrome, or chronic fatigue syndrome. Subjects were from five discovery sites: NWU (2 IC patients, 5 HCs), UCLA (6 IC, 9 HCs), UM (10 IC, 7 HCs), UAB (6 IC, 7 HCs), and SU (9 IC, 5 HCs). This study was approved by the institutional review boards (IRB) of each site.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Patients | • At least 18 years of age • English speaking • Right handed • Rating of at least “1” on SYM-Q, question #1 • Unpleasant pain localised to pelvis • IC symptoms present for the majority of the time during any 3 months in the past 6 months • IC symptoms present for the majority of the time in the most recent 3 months |
• On-going neurological disease or disorder affecting the bladder or bowel fistula • A history of cystitis caused by tuberculosis, radiation therapy or Cytoxan/cyclophosphamide therapy • Augmentation cystoplasty or cystectomy • Active autoimmune or infectious disorder (e.g. Crohn's Disease or Lupus) • A history of cancer (with the exception of skin cancer) • Current major psychiatric disorder or other psychiatric or medical issues that would interfere with study participation • Severe cardiac, pulmonary, renal, or hepatic disease • CNS disease, including structural brain abnormalities (e.g. neoplasms, subarachnoid cysts) and cerebrovascular disease • Ongoing infectious disease (e.g. abcess • History of other neurological disease including stroke or seizure disorders • Claustrophobia • Vision/hearing impairments • Any metal implants |
| Healthy Controls | • At least 18 years of age • English speaking • Right handed • Rating of “0” on SYM-Q, question #1 • No chronic pain in pelvic or bladder region, and chronic pain in no more than one o than body region • No urological symptoms that have been evaluated, but are still present |
Assessment of IC Symptoms (Collected Day of Scan)
Subjects’ MRI sessions were conducted within 14 days of a baseline visit. Symptom measure data analyzed in this study was restricted to those obtained on the day of the scan, which included: the Symptom and Health Care Utilization Questionnaire (SYM-Q), Concomitant Medications (CMED), the Female Genitourinary Pain Index (FGUPI), PROMIS (Patient-reported Outcomes Information System) sleep disturbance scale (www.nihpromis.org), Short Form McGill Pain Questionnaire (MPQ)14, Hospital Anxiety and Depression Scale (HADS)15, Positive and Negative Affect Scale (PANAS)16, and the Gracely Box Scales (GBS) to measure pain and unpleasantness during the scan.
Data Processing
Voxel Based Morphometry
T1-weighted structural images were segmented into WM, GM and CSF using the “new segment” function in SPM8, under MATLAB 7.6. (2008a, The MathWorks, Natick, Massachusetts, 2012). Resulting GM segments were then processed using the diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL) toolbox17. DARTEL increases accuracy of inter-subject alignment by modeling the shape of each participant's brain using millions of parameters (three parameters per voxel). DARTEL works by aligning GM among the images, while simultaneously aligning WM. Thus, an increasingly high resolution average template was created, to which the data were then aligned17. Data were normalized to a standard brain in Montreal Neurological Institute space. Spatial normalization expands and contracts some brain regions, thus the images were modulated involving scaling by the amount of contraction, so that the total amount of GM in the modulated image remains the same as it would be in the original images. Normalized, modulated images were then smoothed with a Gaussian kernel of 8 full-width, half-maximum.
Statistical Analyses
Smoothed GM images were entered into a full factorial (ANCOVA) analysis using the general linear model within SPM8. Three covariates of no interest were included in the model: age, total brain volume (the sum of total GM and WM), and discovery site ID. An absolute threshold mask of 0.1 (i.e. voxels with GM values <0.1 were excluded from analysis) was applied to avoid possible edge effects around the border between GM and WM, and to include only relatively homogeneous voxels. We performed three statistical analyses for significant group difference results using: 1) a whole brain cluster-level corrected family wise error (FWE) threshold of p<0.05, 2) a small volume correction (SVC) using a cluster-level corrected FWE threshold of p<0.05 for a priori regions involved in pain processing. SVC was performed using a 5mm sphere centered around literature coordinates (S1: x=19, y=-37, z=57; L superior parietal lobule/precuneus: x=0, y=-68, z=60)18, 19 3) we performed an exploratory analysis with a voxel-level uncorrected threshold of p<0.001 for clusters with >100 contiguous voxels. Brain regions found to be significantly different between IC patients and HCs were extracted from the normalized smoothed data, corrected for age using standardized residuals, and analyzed using SPSS v19. Raw GM values from each group were extracted and entered into a one-way analysis of variance (ANOVA) to test for differences in regional VBM findings across sites. To determine if there were differences in total intracranial volume (TIV; the sum of the raw whole-brain GM, WM, and CSF values) between groups or discovery sites, global GM, WM and CSF were calculated and entered into a two-sample t-test and ANOVA respectively.
Regions with significant GM volume differences were entered into regression models. GM volume values were extracted from the normalized, smoothed images and correlated with measures of pain, urological symptoms, and anxiety, acquired by self-report on the day of scan, using standard bivariate correlations. No correction was made for multiple comparisons. A linear regression model was used to dissociate the differential independent effects of pain, anxiety, and urological symptoms on GM changes in these regions. A p-value threshold of 0.05 was used for significance.
Results
VBM Analysis
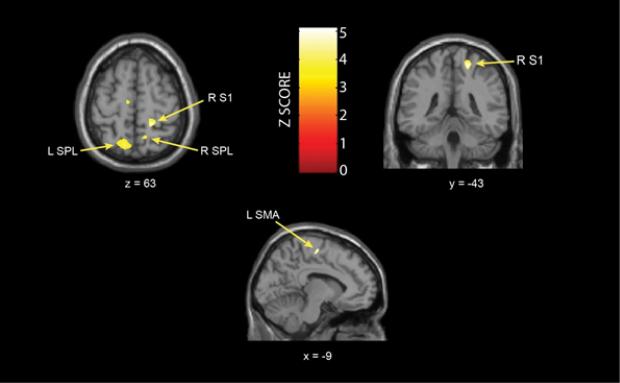
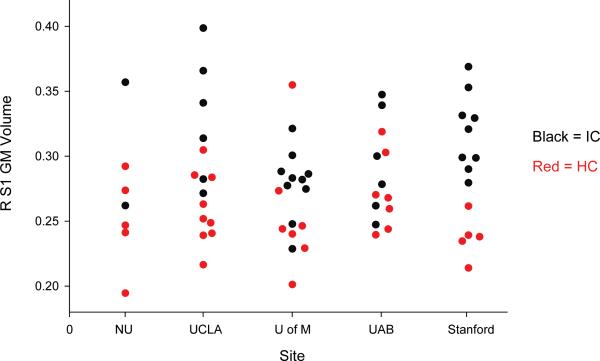
No significant whole brain cluster-level FWE differences between IC and HCs were found. Compared to HCs, females with IC displayed significantly more GM volume in several regions including the: right S1 (p<0.05, FWE SVC), superior parietal lobule/precuneus bilaterally (Left: p<0.05, FWE SVC; Right: p<0.001, uncorrected), and left supplementary motor area (p<0.001, uncorrected) (Table 2, Figure 1). No decreases in GM were found, and no effect of site on right S1 GM volume (p=0.556) (Figure 2) was found. Patients had significantly greater TIV than controls (p=0.039), however there were no differences in TIV across sites (F=0.755, p=0.559). To further assess the contribution of site as a factor to the GM VBM differences, we ran a general linear model predicting GM volume differences between IC patients and HCs for the S1 region. There was a significant main effect by group (p < 0.001), but no significant group*site interaction (p = 0.114).
Table 2.
Summary of results from a whole brain VBM analysis indicating greater GM volume in several regions of the brain in IC patients when compared to healthy controls.
| Region | Brodmann Area | Cluster size (# of voxels) | Z-score (peak value) | Coordinates (MNI) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Grey Matter Volume: Female IC > Female HCs | ||||||
| R Postcentral gyrus (S1)* | 1,2,3 | 796 | 5.04 | 19 | −43 | 61 |
| L Precuneus/superior parietal lobule* | 7 | 1379 | 4.95 | −16 | −64 | 64 |
| R Precuneus/superior parietal lobule† | 7 | 108 | 4.32 | 12 | −57 | 59 |
| L Supplementary motor area/medial frontal gyrus† | 6 | 126 | 4.29 | −9 | −15 | 61 |
L=left, R=right, MNI=Montreal Neurological Institute
significant when correcting for multiple comparisons (p<.05, small-volume correction for multiple comparisons) at a voxel-level threshold of p<0.001
resultant exploratory regions found at a voxel-level threshold of p<.001 (uncorrected)
Figure 1. Brain Regions Showing Increased GM Volume in IC patients Relative to Controls.
Results from general linear model overlaid on standard t1spgr brain template in SPM8. Images are in neurological orientation. L=left; R=right; SPL: superior parietal lobule; S1: primary somatosensory cortex; SMA: supplementary motor area
Figure 2. Comparison of GM Volume in Right Primary Somatosensory Cortex Between Groups, and Across Sites.
NWU=Northwestern University; UCLA=University of California, Los Angeles; U of M=University of Michigan; UAB=University of Alabama at Birmingham; Stanford=Stanford University, R=right, S1=primary somatosensory cortex, GM=gray matter, IC=interstitial cystitis, HC=healthy control
Relationship between Gray Matter Regions of Interest and Clinical Measures
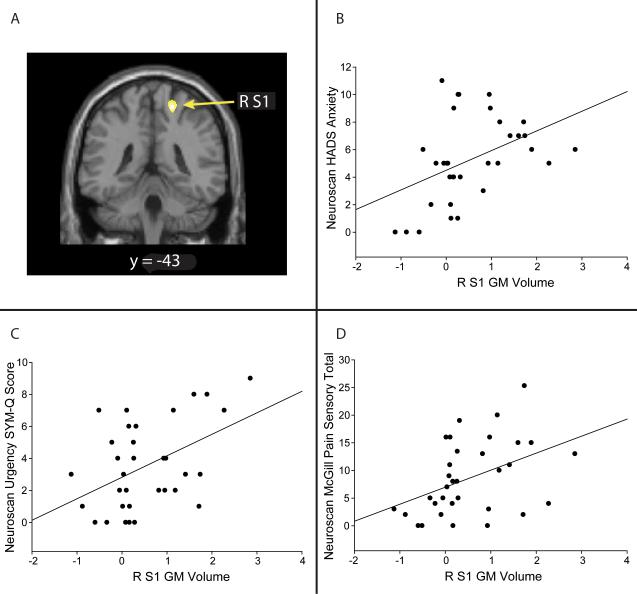
Correlations between the ROIs obtained in our whole-brain analysis and clinical measures obtained on the day of scan showed positive correlations between the right S1 GM values and measures of pain, anxiety, and urological symptoms (Figure 3). No other regions displayed significant correlations with our clinical outcomes. We further explored these correlations with the S1 in a linear regression model and found independent effects of: clinical pain (McGill pain sensory total; r=.396, p=.025), anxiety (HADS; r=.447, p=.01), and a measure of “urgency” (r=.449, p=.01) on S1 GM volume (Table 3).
Figure 3. Clinical Correlates of Brain Gray Matter Volume in IC Patients.
A. VBM data overlaid on standard SPM template illustrating where the GM increase was found in the S1. B: Correlation between HADS anxiety scores and S1 GM volume. Greater GM in the S1 positively correlated with anxiety scores taken the day of the scan (r = 0.447, p = 0.01); C. Correlation between SYM-Q urgency score and S1 GM volume. Greater GM in the S1 positively correlated with SYM-Q urgency scores taken day of scan (r = 0.449, p = 0.01). D: Correlation between McGill pain sensory scores and S1 GM volume (r = 0.396, p = 0.025).
Table 3.
Somatosensory gray matter is independently related to pain, anxiety, and urgency SYM-Q scores.
| Dependent Variable | Predictors | Standardized Beta | S.E. Unstandardized | p-Value | R Square Full Model |
|---|---|---|---|---|---|
| Right S1 GM Volume | 0.53 | ||||
| Age | −0.15 | 0.001 | 0.225 | ||
| Pain (SF-MPQ sensory) | 0.30 | 0.001 | 0.01 | ||
| Anxiety (HADS) | 0.50 | 0.002 | 0.008 | ||
| Urgency | 0.44 | 0.002 | 0.004 |
A linear regression model was constructed with the gray matter ROI in the right primary somatosensory cortex as the dependent variable and age, pain sensory, anxiety, and urgency as predictors. The total model explains approximately 53% of the variance in GM values. S1=primary somatosensory cortex, GM=gray matter
Discussion
This is the first study to detect differences in regional brain morphology between female IC patients and HCs. Our results indicate that women with IC have regional GM volume increases within brain regions involved in pain processing including: the S1, superior parietal lobule, and the supplementary motor area, when compared to HCs. Based on correlations with clinical outcome measures, as well as previous research20, 21, these changes may play an important role in pain sensitivity as well as affective and attentional aspects of IC.
Although the exact biological mechanism remains unclear, GM variability can be due to a number of different factors such as differences in cell volume, synaptic densities, or blood flow and interstitial fluid22. Generally, decreases in GM volume are more commonly reported in chronic pain populations, including chronic pelvic pain, when using VBM10, 11. However an emerging theme in structural MRI pain studies now shows there to be increases as well as decreases in GM values12, 23. This was the case in a study of patients with cyclic menstrual pain in which they found increased and decreased GM volumes in pain processing regions9. In HCs, there is evidence of increased GM volume associated with pain perception, specifically in the S121. In this study, the authors have found that GM volume increased following repetitive thermal stimulation. Similarly, a recent study 20 utilized 80 healthy controls (40 females) that underwent a thermal pain paradigm while inside the fMRI scanner. These researchers found that participants showed thickening in the S1 as an effect of thermal pain stimulation. Although this study examined cortical thickness and not GM volumetric differences, these two studies are in line with our positive correlation between clinical pain and GM volume in the S1, indicating that increased GM volume in the S1 may be related to increased pain in these patients. Not all pelvic pain conditions have found similar GM results as ours10, 12. No studies report regional GM increases in the S1 or SMA; however, there are reports of regional GM volume increases in the precuneus in patients with dysmenorrhea9. We speculate that our cohort may be somewhat different based on the lack of other comorbid conditions that were not controlled for in these other studies.
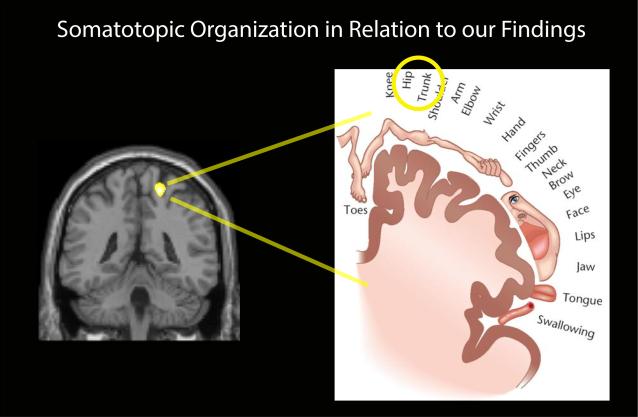
A comprehensive review of altered brain morphology in chronic pain by Borsook and colleagues suggests that these volumetric changes could be driven by the interaction between adaptive and maladaptive processes24. The review notes that cortical thickening of the somatosensory cortex is observed in migraine patients and is explained as an effect of recurrent nociceptive input. The S1 region identified in our study with increased GM corresponds to the trunk/hip area which projects to the pelvis (Figure 4). Interestingly, other studies have found this same region to be activated during stimulation of the clitoris and vaginal region in healthy females25. One possible hypothesis for the S1 GM increase is that this region of the S1 represents an enlarged receptive field due to increased/prolonged nociceptive and/or proprioceptive input, thereby commanding a larger sensory representation in the somatosensory cortex. It is possible that the increase in S1 GM volume in our IC patients could result from repeated exposure to pain in the bladder and pelvic regions derived from common symptoms including urinary urgency and frequency, bladder filling or emptying, or even during menstruation and vaginal intercourse. Of note, a recent resting state fMRI study using data from some of the same IC patients as studied here uncovered alterations in resting state low frequency power in the sensory-motor brain region neighboring our S1 region13. It is possible that changes in brain connectivity, which these authors suggest may be related to pelvic floor contraction during bladder voiding, could in turn influence brain GM volume or vice versa. Further studies investigating the evocation of these symptoms in patients with IC could help shed light on whether these augmented symptoms are directly related to GM increases in female IC patients.
Figure 4. Somatotopic Organization of the Primary Somatosensory Cortex.
The S1 GM increase is shown to be somatotopically mapped to the hip/trunk, or pelvic region. S1=primary somatosensory cortex, GM=gray matter.
Many chronic pain patients have been found to experience comorbidities such as anxiety and depression, which has been shown in IC patients26. Consistent with these findings, positive relationships between S1 GM and both anxiety and urgency were observed in the present study. In a sample of patients with IC, urgency was found to be more related to fear of pain (65%), than fear of incontinence27. It is important to draw the distinction between the urgency experienced by patients with IC compared to that experienced by patients with overactive bladder (OAB).
Finally, we also found evidence of increased GM in the supplementary motor area (SMA) and the superior parietal lobule/precuneus. The precuneus is a region previously shown to be part of the default mode network (DMN), an assemblage of brain regions activated when one engages in internally focused tasks such as autobiographical memory retrieval or other forms of self-referential thinking28. It was shown that patients with fibromyalgia have enhanced intrinsic connectivity between commonly activated pain regions and regions of the DMN, and the degree of this connectivity was related to spontaneous clinical pain29. Although distinct from fibromyalgia, the common thread with IC patients is the chronic nature of their pain. We speculate that an increase in GM in this region may be associated with alterations in connectivity between the pain regions we have found in the present study, and the DMN. Future analyses will address this question.
Although this is the first comprehensive examination of GM differences in IC patients, there are some limitations to our study that should be taken into account. We did find a significant difference in TIV between groups. However, the significance came from differences in GM and WM and we controlled for these factors by entering them in our general linear model as nuisance variables. A second concern is the data was collected at different discovery sites, and thus on different MRI scanners. This could potentially be problematic, however all data were rigorously inspected for quality and integrity, and we controlled for this factor by homogenizing scanning sequences across scanners (see www.painrepository.org for detailed information).
Conclusions
These data are the first to show increased GM in a population of women with IC. Specifically IC is associated with increases in regional GM volume in the S1 as well as the superior parietal lobule and SMA, when compared to HCs. Future research studies will aim to further explore the relationship between S1 GM volume and pain sensitivity. More research is needed to determine whether comorbid symptoms lead to differences in GM volumes of patients in patterns distinct from those with “pure” IC. Finally, variations among chronic pelvic pain conditions due to gender and clinical presentation need to be examined.
Supplementary Material
Acknowledgements
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (DK82370, DK82342, DK82315, DK82344, DK82325, DK82345, DK82333, and DK82316). This study was also supported by an additional NIH grant (K24 DA029262) and the Redlich Pain Research Endowment.
Footnotes
The authors declare no competing financial interests.
References
- 1.Berry SH, Elliott MN, Suttorp M, et al. Prevalence of symptoms of bladder pain syndrome/interstitial cystitis among adult females in the United States. J Urol. 2011;186:540. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanno P, Nordling J, Fall M. Bladder pain syndrome. Med Clin North Am. 2011;95:55. doi: 10.1016/j.mcna.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Janicki TI. Chronic pelvic pain as a form of complex regional pain syndrome. Clin Obstet Gynecol. 2003;46:797. doi: 10.1097/00003081-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Giannantoni A, Bini V, Dmochowski R, et al. Contemporary management of the painful bladder: a systematic review. Eur Urol. 2012;61:29. doi: 10.1016/j.eururo.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 5.Gracely RH, Petzke F, Wolf JM, et al. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 6.Harris RE, Clauw DJ, Scott DJ, et al. Decreased central mu-opioid receptor availability in fibromyalgia. Journal of Neuroscience. 2007;27:10000. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt-Wilcke T. Variations in brain volume and regional morphology associated with chronic pain. Curr Rheumatol Rep. 2008;10:467. doi: 10.1007/s11926-008-0077-7. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia - A voxel-based morphometry study. Pain. 2007;132:S109. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Tu CH, Niddam DM, Chao HT, et al. Brain morphological changes associated with cyclic menstrual pain. Pain. 2010;150:462. doi: 10.1016/j.pain.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 10.As-Sanie S, Harris RE, Napadow V, et al. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain. 2012;153:1006. doi: 10.1016/j.pain.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer MA, Chanda ML, Parks EL, et al. Brain Functional and Anatomical Changes in Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Journal of Urology. 2011;186:117. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweinhardt P, Kuchinad A, Pukall CF, et al. Increased gray matter density in young women with chronic vulvar pain. Pain. 2008;140:411. doi: 10.1016/j.pain.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in Resting State Oscillations and Connectivity in Sensory and Motor Networks in Women with Interstitial Cystitis/Painful Bladder Syndrome. J Urol. 2014 doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 16.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Michels L, Mehnert U, Boy S, et al. The somatosensory representation of the human clitoris: an fMRI study. Neuroimage. 2010;49:177. doi: 10.1016/j.neuroimage.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Hampson JP, Reed BD, Clauw DJ, et al. Augmented central pain processing in vulvodynia. J Pain. 2013;14:579. doi: 10.1016/j.jpain.2013.01.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erpelding N, Moayedi M, Davis KD. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153:1602. doi: 10.1016/j.pain.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Teutsch S, Herken W, Bingel U, et al. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845. doi: 10.1016/j.neuroimage.2008.05.044. [DOI] [PubMed] [Google Scholar]
- 22.Gage FH. Neurogenesis in the adult brain. J Neurosci. 2002;22:612. doi: 10.1523/JNEUROSCI.22-03-00612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol. 2013;8:518. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- 24.Borsook D, Erpelding N, Becerra L. Losses and gains: chronic pain and altered brain morphology. Expert Rev Neurother. 2013;13:1221. doi: 10.1586/14737175.2013.846218. [DOI] [PubMed] [Google Scholar]
- 25.Michels L, Mehnert U, Boy S, et al. The somatosensory representation of the human clitoris: An fMRI study. Neuroimage. 2010;49:177. doi: 10.1016/j.neuroimage.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Wu EQ, Birnbaum H, Mareva M, et al. Interstitial Cystitis: Cost, treatment and comorbidities in an employed population. Pharmacoeconomics. 2006;24:55. doi: 10.2165/00019053-200624010-00005. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg P, Brown J, Yates T, et al. Voiding urges perceived by patients with interstitial cystitis/painful bladder syndrome. Neurourol Urodyn. 2008;27:287. doi: 10.1002/nau.20516. [DOI] [PubMed] [Google Scholar]
- 28.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 29.Napadow V, LaCount L, Park K, et al. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.