Summary
Background
Oxaliplatin-based adjuvant therapy is the standard of care for stage III colon cancer. Adjuvant capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin has not been directly compared; therefore, we aimed to analyse the efficacy and safety of these treatments using individual patient data pooled from four randomised controlled trials. We also assessed post-relapse survival, which has been postulated to be worse in patients receiving adjuvant oxaliplatin.
Methods
Patients with resected stage III colon cancer who were 18 years of age or older, with an Eastern Cooperative Oncology Group performance status of 0 or 1, from four randomised controlled trials (NSABP C-08, XELOXA, X-ACT, and AVANT; 8734 patients in total) were pooled and analysed. The treatment regimens included in our analyses were: XELOX (oxaliplatin and capecitabine); leucovorin and fluorouracil; capecitabine; FOLFOX-4 (leucovorin, fluorouracil, and oxaliplatin); and modified FOLFOX-6 (mFOLFOX-6). Disease-free survival was the primary endpoint for all trials that supplied patients for this analysis. Here, we compared disease-free, relapse-free, and overall survival between the patient groups who received capecitabine with or without oxaliplatin and those who received leucovorin and fluorouracil with or without oxaliplatin. Post-relapse survival was compared between the combined XELOX and FOLFOX groups, and the leucovorin and fluorouracil groups. Post-relapse survival was also compared between the capecitabine with or without oxaliplatin and leucovorin and fluorouracil with or without oxaliplatin groups.
Findings
Disease-free survival did not differ significantly between patients who received leucovorin and fluorouracil versus those who received capecitabine in adjusted analyses (hazard ratio [HR] 1·02 [0·93–1·11; p=0·72]) or in unadjusted analyses (HR 1·01 [95% CI 0·92–1·10; p=0·86]). Relapse-free survival was similar (adjusted HR 1·02 [0·93–1·12; p=0·72] and unadjusted HR 1·01 [95% CI 0·92–1·11; p=0·86]), as was overall survival (adjusted HR 1·04 [95% CI 0·93–1·15; p=0·50] and unadjusted HR 1·02 [0·92–1·14]; p=0·65). For overall survival, a significant interaction between oxaliplatin and fluoropyrimidine was recorded in the multiple Cox regression analysis (p=0·014). Post-relapse survival was similar in adjusted (p=0·23) and unadjusted analyses (p=0·33) for the comparison of XELOX or FOLFOX versus leucovorin and fluorouracil, and was also similar for capecitabine-based regimens versus leucovorin and fluorouracil-based regimens (unadjusted p=0·26).
Interpretation
Combination therapy with oxaliplatin provided consistently improved outcomes without adversely affecting post-relapse survival in the adjuvant treatment of stage III colon cancer, irrespective of whether the fluoropyrimidine backbone was capecitabine or leucovorin and fluorouracil. These data add to the existing evidence that oxaliplatin plus capecitabine or leucovorin and fluorouracil is the standard of care for the adjuvant treatment of stage III colon cancer, and offers physicians flexibility to treat patients according to the patients' overall physical performance and preference.
Funding
Genentech Inc.
Introduction
Adjuvant treatment with a fluoropyrimidine plus oxaliplatin is the standard of care for resected stage III colon cancer,1,2 as supported by the results of three randomised controlled trials.3,4,5,6,7 Oxaliplatin plus infusional leucovorin and fluorouracil (FOLFOX) significantly increased disease-free survival and overall survival compared with leucovorin and fluorouracil alone in the MOSAIC trial.3,4 Oxaliplatin plus bolus leucovorin and fluorouracil (FLOX) significantly increased disease-free survival compared with leucovorin and fluorouracil alone in the NSABP C-07 trial, albeit with no significant overall survival difference at 8 years' follow-up.5,6 Oxaliplatin plus oral capecitabine (Xeloda, F Hoffmann-La Roche, Basel, Switzerland) (XELOX) significantly increased disease-free survival and overall survival compared with bolus leucovorin and fluorouracil in the XELOXA trial.7 Single-agent leucovorin and fluorouracil or capecitabine is also recommended in patients for whom oxaliplatin is unsuitable;1 in the non-inferiority X-ACT trial, capecitabine was shown to be as efficacious as leucovorin and fluorouracil.8,9 Despite these results, data from the NSABP C-07 and MOSAIC trials—which both included patients with stage II or III disease—suggested that adjuvant oxaliplatin might reduce post-relapse survival.4,10
No direct comparisons of capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin have been done in the adjuvant setting, and such a study is unlikely because treatment patterns are now well established. However, use of XELOX and FOLFOX in both the first-line and second-line treatment of metastatic colorectal cancer have led to similar survival outcomes.11,12,13 In the absence of randomised controlled trials addressing the comparative efficacy of adjuvant oxaliplatin combined with capecitabine or leucovorin and fluorouracil, and to further assess the effect of oxaliplatin on post-relapse survival, we compared the efficacy and safety of adjuvant capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin for resected stage III colon cancer by pooling individual patient data from four large, randomised controlled phase 3 trials: NSABP C-08, XELOXA, X-ACT, and AVANT. These trials were selected on the basis of access to the complete clinical datasets, which enabled the use of individual patient data to address relevant scientific questions.
We postulated that efficacy outcomes (disease-free survival, relapse-free survival, and overall survival) would be the same with capecitabine-based and leucovorin and fluorouracil-based regimens, but that post-relapse survival would be reduced in patients receiving adjuvant oxaliplatin.
Methods
Study design and participants
The designs and patient eligibility criteria of these four studies have been reported previously.7,8,14,15 Further details are provided in appendix pp 1–2. Patients with stage II colon cancer were excluded from this comparison of the standards of care for stage III disease. Tumor stage was recorded variably across the studies; therefore, for this analysis, stage was derived from existing tumor–node–metastasis (TNM) staging information, based on the American Joint Committee on Cancer (7th edition) definition of stage III colon cancer as any stage T, and nodal stage N1 or N2.16 Patients receiving bevacizumab in AVANT and NSABP C-08 were excluded from the primary analysis because its efficacy has not been demonstrated in this setting.14,15 However, selected post-hoc sensitivity analyses were done on the complete dataset of patients with stage III disease, and these included patients who received bevacizumab.
Disease-free survival, relapse-free survival, and overall survival were compared according to the fluoropyrimidine received—ie, capecitabine or XELOX (capecitabine with or without oxaliplatin) compared with leucovorin and fluorouracil, FOLFOX-4, or mFOLFOX-6 (leucovorin and fluorouracil with or without oxaliplatin). To assess the effect of oxaliplatin when combined with capecitabine, we compared outcomes between patients treated with XELOX versus capecitabine monotherapy.
We assessed the effect of oxaliplatin on post-relapse survival by comparing outcomes between the combined XELOX and FOLFOX groups and those who received leucovorin and fluorouracil monotherapy. The capecitabine and leucovorin and fluorouracil groups in X-ACT were not pooled because of potential differences in efficacy when these drugs are given as single agents in the adjuvant setting, based on a pre-planned multivariable analysis.9
All studies were done in accordance with the Declaration of Helsinki and investigations were undertaken after approval from local ethics committees or institutional review boards, as appropriate. All patients provided written informed consent.
Outcomes
The primary endpoint was disease-free survival, which was defined as the date of randomisation to the date of disease recurrence, new occurrence of colon cancer, or death from any cause. Secondary endpoints were relapse-free survival (defined in the same way as disease-free survival, but excluded deaths unrelated to treatment or colon cancer), overall survival (time from randomisation to the date of death, irrespective of the cause), and post-relapse survival (time from disease recurrence [relapse] until death from any cause; assessed only in patients who relapsed).
In the XELOXA and AVANT trials, the type of disease-free survival event (recurrence, new colon cancer, or death) was recorded, enabling direct identification of relapses. Because the definitions of disease-free survival differed between XELOXA and AVANT versus X-ACT and NSABP C-08, patients were identified as relapsing if a disease-free survival event occurred along with a censored death variable or death recorded at a subsequent date. Based on the definition of disease-free survival used in trials for the assigned treatment, the definition included new colon cancer and relapse. This rule was used to identify patients' distant (metastatic) relapses relevant to the scientific issue addressed by the analysis. In X-ACT and NSABP C-08, the types of disease-free survival event were not reported; hence, in order to replicate the identification process for patients with distant relapse in these trials, a disease-free survival event along with either a censored death or subsequent death would essentially identify which patients should be included the post-relapse survival analysis. This approach allowed for uniform identification of patients with distant relapse across trials. Post-relapse survival duration was then established as the time from relapse date until death or date last known to be alive.
For efficacy assessments, patients who did not have an event at the time of the trial database cutoff were censored on the date at which they were last known to be event-free.
In all four trials, adverse events were monitored during treatment and for 28 days after the final dose of study drug. All adverse events were encoded according to the Medical Dictionary for Regulatory Activities (MedDRA) version 13.1 and allocated a severity grade of 1–4 (mild, moderate, severe, or life-threatening or fatal). More than one occurrence of the same adverse event in the same patient was recorded as a single event at the greatest severity recorded. Incidences of serious adverse events were also observed. Since serious adverse events were not flagged in NSABP C-08, adverse events occurring during that study were recorded as serious if they were fatal or submitted to the Adverse Event Expedited Reporting System (AdEERS).
Data about treatments after relapse were collected for patients in X-ACT, XELOXA, and AVANT.
Statistical analyses
For each time-to-event assessment, we undertook Kaplan-Meier survival analyses and compared these using the log-rank test. We did assessments according to assigned treatment. We estimated hazard ratios (HRs) and associated 95% CIs using univariable Cox regression, and we used the Wald test in each subgroup to test its difference from the null value of 1. The same approach assessed treatment effects in subgroups stratified by sex, age (<70 years or ≥70 years), T stage (T1–2 or T3–4), and N stage (N1 or N2).
We used multiple Cox proportional hazards regression to test for independent effects of oxaliplatin after controlling for sex, age, T stage, and N stage. To assess whether the incremental effects caused by oxaliplatin were similar, irrespective of the fluoropyrimidine backbone, the oxaliplatin (yes/no) × fluoropyrimidine (leucovorin and fluorouracil or capecitabine) term was included in the model as a predictor and tested for significance. Additional covariables were considered but were removed from the final model, including colon cancer stage (IIIA or IIIB/C), number of positive lymph nodes (≤3 or >3), medical comorbidity measures (adapted Charlson Comorbidity Index/National Cancer Institute Combined Index), Eastern Cooperative Oncology Group Performance Status, and race. To focus the present analysis solely on patients with stage III colon cancer, the only patients from the four trials not included were those with stage II disease in NSABP C-08 (n=322) and in AVANT (n=584). As surgical, medical, and staging standards changed, and biological agents associated with survival benefit were introduced for metastatic disease during the course of the trials, we did sensitivity analyses to assess the potential effects of these changes. We also assessed surgical quality and staging by number of lymph nodes examined to control for patients enrolled in X-ACT in which this information was not collected. Date of randomisation (before 2004 or 2004 or later) was included to assess the effects of new treatments. Additionally, we did a direct comparison of XELOX versus FOLFOX in the full analytic cohort, adjusting for exposure to bevacizumab to supplement the assessment of oxaliplatin treatment effect.
Adjusted survival curves were created using the average covariate and corrected group prognosis methods;17,18 the latter approach is viewed as an improvement over the former because it derives the adjusted survival curve using a weighted average of the individual survival curves, calculated using a fitted Cox regression model. The weights used are proportional to the number of individuals at each level of the covariates in the entire sample at baseline.
In the XELOX or FOLFOX group, post-relapse survival was also compared between patients with documented use of therapies other than oxaliplatin after relapse and those who received oxaliplatin post relapse.
SAS version 8.2 was used for all statistical analyses. The four randomised controlled trials are registered with ClinicalTrials.gov, numbers NCT00009737, NCT00069121, NCT00096278, and NCT00112918.
Role of the funding source
F Hoffmann-La Roche and Genentech Inc, in conjunction with the authors, were involved in the analyses, data interpretation, and the decision to submit for publication. H-JS and CT had full access to all the study data and had final responsibility for the decision to submit for publication.
Results
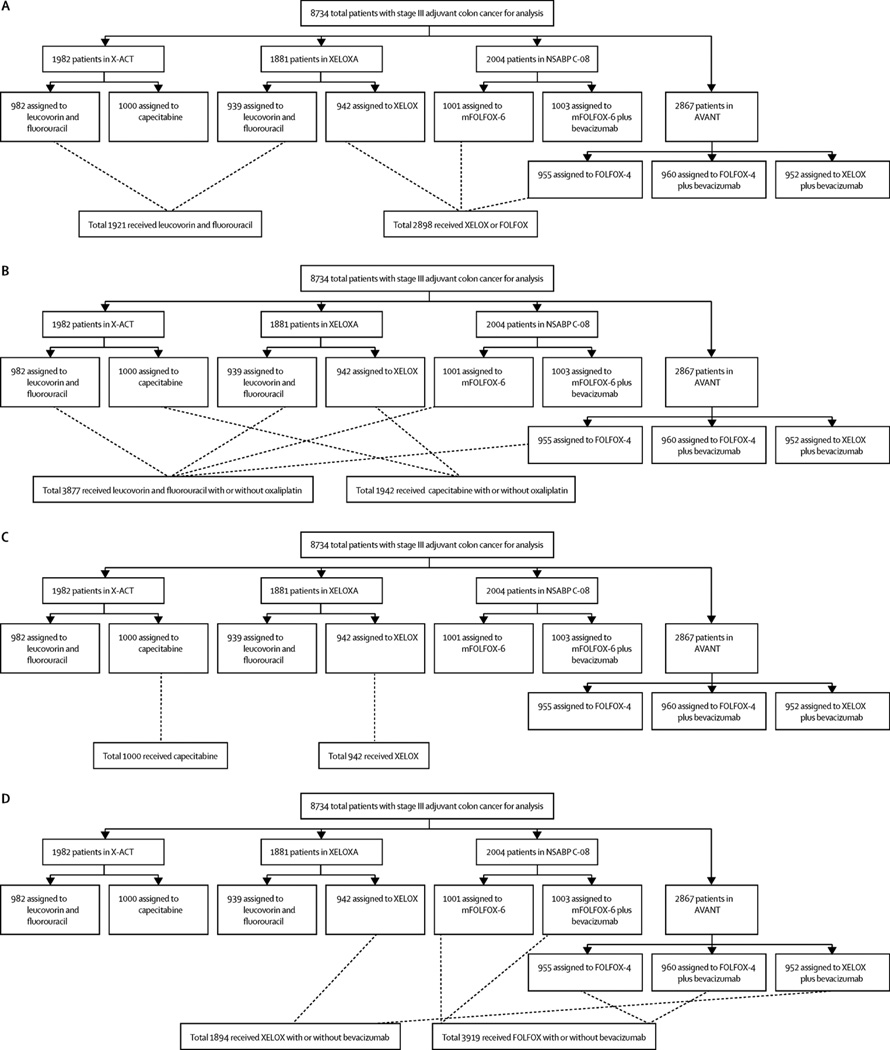
Figure 1 shows the compositions of the pooled analysis groups with respect to the individual trials. Baseline demographics and disease characteristics were, in general, balanced across the groups (table 1). The X-ACT trial did not gather data about numbers of examined lymph nodes, which resulted in a greater proportion of patients who received capecitabine with or without oxaliplatin with “unknown” number of nodes examined. Therefore, sensitivity analyses were done in patient subgroups with up to 12 and more than 12 nodes assessed. The median duration of follow-up was longer in XELOXA (84 months) and X-ACT (74 months) than in NSABP C-08 (36 months) and AVANT (50 months).
Figure 1. Summary description of the composition of pooled analytic groups with respect to the individual clinical trials.
(A) The XELOX or FOLFOX versus leucovorin and fluorouracil comparison, (B) the capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin comparison, (C) the XELOX versus capecitabine comparison, and (D) the sensitivity analysis directly comparing XELOX with or without bevacizumab versus FOLFOX with or without bevacizumab.
X-ACT=Xeloda in Adjuvant Colon Cancer Therapy. XELOXA=XELOX in Adjuvant Colon Cancer Treatment. NSABP=National Surgical Adjuvant Breast and Bowel Project. XELOX=capecitabine plus oxaliplatin. mFOLFOX-6=modified FOLFOX-6. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. AVANT=Avastin Adjuvant.
Table 1.
Baseline characteristics and demographics
| XELOX or FOLFOX vs leucovorin and fluorouracil monotherapy |
Capecitabine with or without oxaliplatin vs leucovorin and fluorouracil with or without oxaliplatin |
XELOX vs capecitabine | ||||
|---|---|---|---|---|---|---|
| Leucovorin and fluorouracil (n=1921)* |
XELOX or FOLFOX (n=2898)† |
Leucovorin and fluorouracil with or without oxaliplatin (n=3877)‡ |
Capecitabine with or without oxaliplatin (n=1942)§ |
Capecitabine (n=1000)¶ |
XELOX (n=942)‖ | |
| Age (years) | 62·0 (22–82) | 59·0 (19–85) | 60·0 (19–85) | 62·0 (22–83) | 62·0 (25–80) | 61·0 (22–83) |
| Sex | ||||||
| Women | 890 (46%) | 1374 (47%) | 1833 (47%) | 891 (46%) | 460 (46%) | 431 (46%) |
| Men | 1031 (54%) | 1524 (53%) | 2044 (53%) | 1051 (54%) | 540 (54%) | 511 (54%) |
| Race | ||||||
| Asian/Pacific Islander | 134 (7%) | 298 (10%) | 310 (8%) | 136 (7%) | 14 (1%) | 122 (13%) |
| Black | 30 (2%) | 97 (3%) | 113 (3%) | 19 (1%) | 5 (1%) | 14 (1%) |
| White | 1734 (90%) | 2447 (84%) | 3400 (88%) | 1756 (90%) | 975 (98%) | 781 (83%) |
| Hispanic | 14 (1%) | 32 (1%) | 28 (1%) | 19 (1%) | 1 (<1%) | 18 (2%) |
| Other | 9 (<1%) | 24 (1%) | 26 (1%) | 12 (<1%) | 5 (1%) | 7 (1%) |
| ECOG performance status | ||||||
| 0 | 1100 (57%) | 2338 (81%) | 2739 (71%) | 1084 (56%) | 385 (39%) | 699 (74%) |
| 1 | 336 (17%) | 551 (19%) | 652 (17%) | 367 (19%) | 132 (13%) | 235 (25%) |
| Unknown | 485 (25%) | 9 (<1%) | 486 (13%) | 491 (25%) | 483 (48%) | 8 (1%) |
| Disease stage | ||||||
| IIIA | 156 (8%) | 297 (10%) | 363 (9%) | 182 (9%) | 92 (9%) | 90 (10%) |
| IIIB | 1146 (60%) | 1505 (52%) | 2130 (55%) | 1124 (58%) | 603 (60%) | 521 (55%) |
| IIIC | 619 (32%) | 1093 (38%) | 1381 (36%) | 636 (33%) | 305 (31%) | 331 (35%) |
| Unknown | 0 | 3 (<1%) | 3 (<1%) | 0 | 0 | 0 |
| Primary tumour classification | ||||||
| T0 | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
| T1 | 36 (2%) | 101 (3%) | 110 (3%) | 39 (2%) | 12 (1%) | 27 (3%) |
| T2 | 161 (8%) | 263 (9%) | 346 (9%) | 168 (9%) | 90 (9%) | 78 (8%) |
| T3 | 1442 (75%) | 2124 (73%) | 2866 (74%) | 1461 (75%) | 761 (76%) | 700 (74%) |
| T4 | 282 (15%) | 407 (14%) | 552 (14%) | 274 (14%) | 137 (14%) | 137 (15%) |
| Carcinoma in situ | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
| Unknown | 0 | 1 (<1%) | 1 (<1%) | 0 | 0 | 0 |
| Regional lymph node classification | ||||||
| N1 | 1302 (68%) | 1805 (62%) | 2496 (64%) | 1306 (67%) | 695 (70%) | 611 (65%) |
| N2 | 619 (32%) | 1093 (38%) | 1381 (36%) | 636 (33%) | 305 (31%) | 331 (35%) |
| Number of lymph nodes examined | ||||||
| ≤12 | 456 (24%) | 1131 (39%) | 1128 (29%) | 459 (24%) | 0 | 459 (49%) |
| >12 | 483 (25%) | 1757 (61%) | 1765 (46%) | 475 (24%) | 0 | 475 (50%) |
| Unknown | 982 (51%) | 10 (<1%) | 984 (25%) | 1008 (52%) | 1000 (100%) | 8 (1%) |
| Time from surgery to relapse (years) | 1·46 (0·05–7·30)** | 1·42 (0·08–7·47)†† | 1·45 (0·05–7·30)‡‡ | 1·44 (0·06–7·47)§§ | -- | -- |
Data are median (range) or n (%) unless otherwise indicated. XELOX=capecitabine plus oxaliplatin. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. ECOG=Eastern Cooperative Oncology Group.
982 patients in X-ACT and 939 in XELOXA.
942 patients in XELOXA, 1001 in NSABP C-08, and 955 in AVANT.
982 patients in X-ACT, 939 in XELOXA, 1001 in NSABP C-08, and 955 in AVANT.
1000 patients in X-ACT and 942 in XELOXA.
X-ACT only.
XELOXA only.
N=744.
N=757.
N=1222.
N=659.
For our comparison of the efficacy of capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin, a total of 5819 patients were included in the primary analyses. Three patients receiving mFOLFOX-6 in the NSABP C-08 trial did not have the required information for stage derivation and were therefore excluded. In our analysis, median follow-up was 44 months (IQR 33–69 months) for leucovorin and fluorouracil with or without oxaliplatin, and 74 months (46–84 months) for capecitabine with or without oxaliplatin.
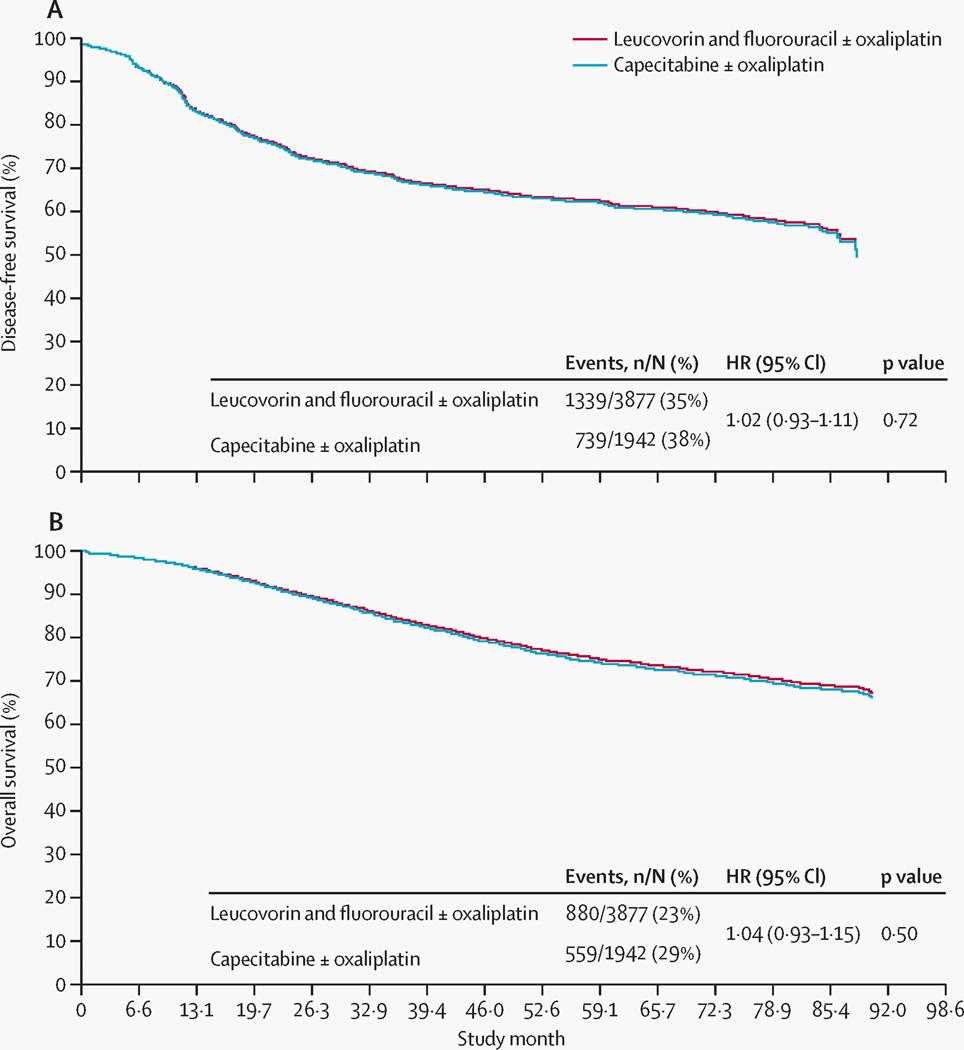
At 5 years' follow-up, 62·8% of patients in both groups were alive and disease free. The adjusted disease-free survival hazard ratio (HR) was 1·02 (0·93–1·11; p=0·72) (figure 2A). The unadjusted disease-free survival analysis (HR 1·01 [95% CI 0·92–1·10]; p=0·86) was consistent with the adjusted analysis, with the survival curves being almost identical.
Figure 2. Adjusted survival curves.
(A) Disease-free survival and (B) overall survival for capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin, adjusted for sex, age, tumour (T) stage, and nodal (N) stage. ±=with or without. HR=hazard ratio. No numbers at risk are presented because this figure displays adjusted survival curves derived from a Cox proportional hazards regression model (rather than Kaplan–Meier survival analysis); hence, those data are not directly used.
5-year relapse-free survival was 64·6% for patients in the capecitabine with or without oxaliplatin group and 64·7% for leucovorin and fluorouracil with or without oxaliplatin group. The adjusted 5-year relapse-free HR was 1·02 (0·93–1·12; p=0·72) (appendix p 3). As was the case for disease-free survival, the unadjusted 5-year relapse-free survival analysis (HR 1·01 [95% CI 0·92–1·11]; p=0·86) was consistent with the adjusted analysis, and the survival curves were similar.
5-year overall survival rates were 73·9% for patients in the capecitabine with or without oxaliplatin group and 75·2% for the leucovorin and fluorouracil with or without oxaliplatin group. The adjusted HR was 1·04 (95% CI 0·93–1·15; p=0·50) (figure 2B). Again, the unadjusted analysis was consistent with the adjusted analysis (HR 1·02 [95% CI 0·92–1·14]; p=0·65), and the survival curves were similar.
Sensitivity analyses showed consistent results between patients with up to 12 and more than 12 lymph nodes studied for disease-free, relapse-free, and overall survival (appendix p 4), and were consistent and similar to those in the primary analysis when the patients who were treated with bevacizumab were included (ie, total N=8734; table 2) or when the analysis was adjusted for study entry date (appendix p 5).
Table 2.
Sensitivity analyses of survival outcomes for XELOX versus FOLFOX in all treated patients
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| XELOX* versus FOLFOX* | ||
| Disease-free survival | 1·00 (0·90–1·11) | 0·98 |
| Relapse-free survival | 0·99 (0·89–1·10) | 0·85 |
| Overall survival | 1·12 (0·97–1·28) | 0·11 |
| XELOX* versus capecitabine monotherapy | ||
| Disease-free survival | 0·74 (0·64–0·86) | <0·0001 |
| Relapse-free survival | 0·73 (0·63–0·85) | <0·0001 |
| Overall survival | 0·75 (0·63–0·88) | 0·0007 |
Adjusted for use of bevacizumab.
XELOX=capecitabine plus oxaliplatin. FOLFOX=leucovorin and fluorouracil plus oxaliplatin.
Despite the oxaliplatin-by-fluoropyrimidine interactions not being significant at the 0·05 level for disease-free survival (p=0·17) or relapse-free survival (p=0·15; data not shown), the effects of oxaliplatin were estimated for the respective fluoropyrimidines. According to Cox regression modelling, the estimated disease-free survival HR for the effect of oxaliplatin was 0·75 (95% CI 0·60–0·93) with capecitabine and 0·70 (0·59–0·82) with leucovorin and fluorouracil. Further analyses that included additional covariables (study entry date [2004 or later vs before 2004]), and bevacizumab use [yes vs no]) showed a lessened degree of interaction (data not shown). HRs for relapse-free survival for capecitabine with or without oxaliplatin were 0·73 (95% CI 0·63–0·85) compared with leucovorin and fluorouracil with or without oxaliplatin 0·64 (0·57–0·71).
For overall survival, the multiple Cox regression analysis of the oxaliplatin-by-fluoropyrimidine interaction was significant (p=0·014). Therefore, the effects of oxaliplatin when used in combination with either capecitabine or leucovorin and fluorouracil were estimated in the same Cox regression analysis. The estimated HR for oxaliplatin was 0·75 (95% CI 0·63–0·88) with capecitabine, and 0·56 (0·48–0·65) with leucovorin and fluorouracil. Although the incremental benefit was slightly reduced with capecitabine versus leucovorin and fluorouracil, direct comparison of survival outcomes between XELOX-treated and FOLFOX-treated patients (adjusted for use of bevacizumab) showed that these did not differ significantly (table 2, appendix p 5), and 5-year disease-free survival (68·2% for XELOX and 66·6% for FOLFOX), relapse-free survival (70·1% and 68·4%), and overall survival (78·7% and 79·6%) were similar (table 3).
Table 3.
3-year, 4-year, and 5-year disease-free, relapse-free, and overall survival rates, by treatment group
| Patients still free of event |
Events | Censored cases |
Kaplan-Meier estimate (95% CI) for event-free rate |
|
|---|---|---|---|---|
| Disease-free survival | ||||
| FOLFOX with or without bevacizumab | ||||
| 3 years | 1978 | 964 | 977 | 0·74 (0·73–0·75) |
| 4 years | 648 | 1038 | 2233 | 0·70 (0·68–0·72) |
| 5 years | 71 | 1054 | 2794 | 0·67 (0·64–0·69) |
| XELOX with or without bevacizumab | ||||
| 3 years | 1204 | 486 | 204 | 0·73 (0·70–0·75) |
| 4 years | 848 | 528 | 518 | 0·70 (0·68–0·72) |
| 5 years | 555 | 548 | 791 | 0·68 (0·66–0·70) |
| Relapse-free survival | ||||
| FOLFOX with or without bevacizumab | ||||
| 3 years | 1972 | 913 | 1034 | 0·75 (0·74–0·76) |
| 4 years | 647 | 982 | 2290 | 0·71 (0·70–0·73) |
| 5 years | 71 | 996 | 2852 | 0·68 (0·66–0·71) |
| XELOX with or without bevacizumab | ||||
| 3 years | 1200 | 452 | 242 | 0·75 (0·73–0·77) |
| 4 years | 844 | 490 | 560 | 0·72 (0·70–0·74) |
| 5 years | 553 | 507 | 834 | 0·70 (0·68–0·72) |
| Overall survival | ||||
| FOLFOX with or without bevacizumab | ||||
| 3 years | 2476 | 421 | 1022 | 0·88 (0·87–0·89) |
| 4 years | 1014 | 499 | 2406 | 0·84 (0·83–0·85) |
| 5 years | 143 | 525 | 3251 | 0·80 (0·77–0·82) |
| XELOX with or without bevacizumab | ||||
| 3 years | 1537 | 229 | 128 | 0·87 (0·86–0·89) |
| 4 years | 1148 | 314 | 432 | 0·82 (0·80–0·84) |
| 5 years | 732 | 352 | 810 | 0·79 (0·77–0·81) |
Data are n, unless otherwise indicated. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. XELOX=capecitabine plus oxaliplatin.
For the comparison of XELOX versus capecitabine alone, a total of 1942 patients were analysed in the main analysis. Median follow-up was 70 months (IQR 45–82 months) for capecitabine and 80 months (47–84) for XELOX. Compared with capecitabine alone, XELOX was associated with significant improvements in disease-free survival (HR 0·76 [95% CI 0·66–0·88]; Wald test p=0·0003), relapse-free survival (0·75 [0·65–0·88]; p=0·0003), and overall survival (0·77 [0·65–0·91]; p=0·0025). Results were similar when an additional 952 patients treated with bevacizumab were included (table 2).
Overall, grade 3–4 adverse events of special interest were more frequent with leucovorin and fluorouracil with or without oxaliplatin (1731 [45%] in 3829 patients) than with capecitabine with or without oxaliplatin (690 [36%] in 1927 patients; table 4), whereas the incidence of serious adverse events was similar between the two treatments (620 [16%] vs 389 [20%], respectively).
Table 4.
Grade 3 or 4 adverse events of interest for oxaliplatin therapy
| Leucovorin and fluorouracil with or without oxaliplatin (n=3829) |
Capecitabine with or without oxaliplatin (n=1927) |
|
|---|---|---|
| All adverse events | 1731 (45%) | 690 (36%) |
| Neutropenia | 908 (24%) | 91 (5%) |
| Diarrhoea | 475 (12%) | 296 (15%) |
| Neuropathy | 295 (8%) | 113 (6%) |
| Vomiting or nausea | 203 (5%) | 141 (7%) |
| Stomatitis, all | 135 (4%) | 6 (<1%) |
| Hand–foot syndrome | 30 (<1%) | 228 (12%) |
| Febrile neutropenia | 80 (2%) | 7 (<1%) |
| Neutropenic fever or sepsis | 20 (<1%) | 2 (<1%) |
Data are n (%). The denominators are the numbers of patients who actually received the treatment regimens considered in this comparison.
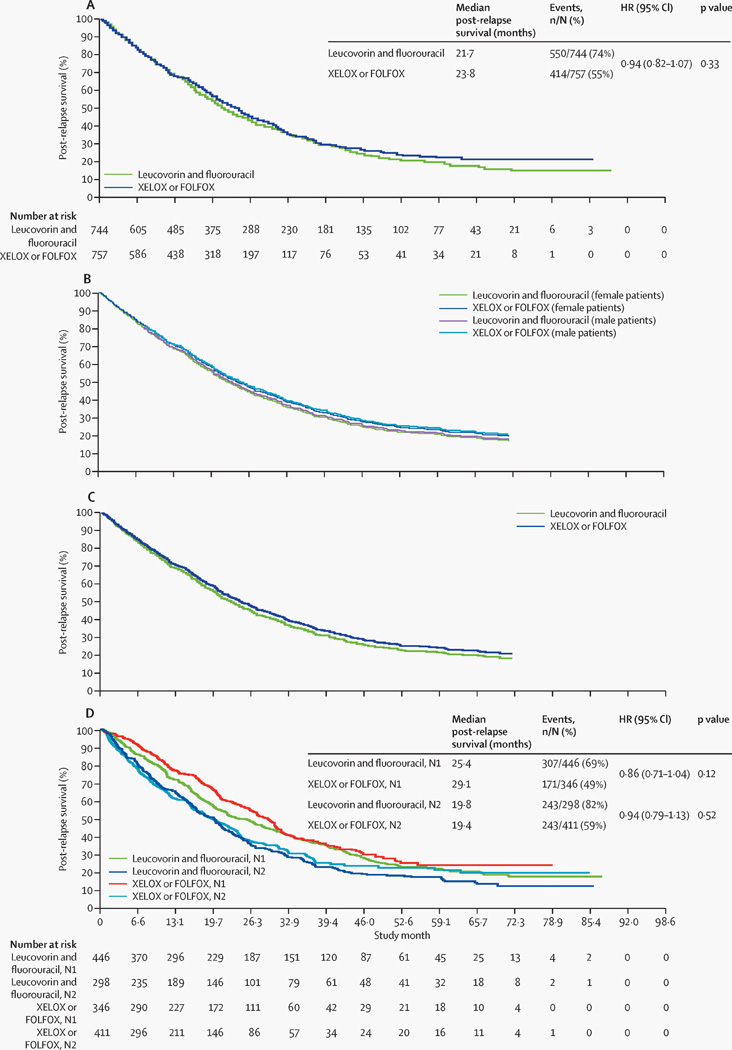
For our assessment of post-relapse survival, we first compared patients who received XELOX or FOLFOX versus those who received leucovorin and fluorouracil. A total of 757 patients in the XELOX or FOLFOX group and 744 in the leucovorin and fluorouracil group relapsed, and were analysed for post-relapse survival. Median follow-up for this analysis was 72 months (IQR 43–84 months) for leucovorin and fluorouracil, and 42 months (33–55 months) for XELOX or FOLFOX (IQR 33–55 months).
Unadjusted Kaplan-Meier analyses showed no significant difference in post-relapse survival between the XELOX or FOLFOX and the leucovorin and fluorouracil groups (HR 0·94 [95% CI 0·82–1·07]; p=0·33]; figure 3A). Adjusted analyses confirmed that there was no significant difference in post-relapse survival (HR 0·92 [95% CI 0·81–1·05]; p=0·23) (table 5). Adjusted survival curves generated using either the average covariate (figure 3B) or the corrected group prognosis method (figure 3C) support these results. Figure 3D shows the unadjusted Kaplan-Meier plots for the N1 and N2 subgroups from the XELOX or FOLFOX versus leucovorin and fluorouracil comparison.
Figure 3. Post-relapse survival curves.
(A) Unadjusted Kaplan-Meier plot of post-relapse survival in patients receiving adjuvant capecitabine plus oxaliplatin (XELOX) or leucovorin and fluorouracil plus oxaliplatin (FOLFOX) compared with those receiving leucovorin and fluorouracil alone. (B) Adjusted post-relapse survival in patients receiving adjuvant capecitabine plus oxaliplatin (XELOX) or leucovorin and fluorouracil plus oxaliplatin (FOLFOX), compared with those receiving leucovorin and fluorouracil alone (adjusted for sex, age, tumour [T] stage, and nodal [N] stage), generated by the average covariate method. (C) Adjusted post-relapse survival in patients receiving adjuvant capecitabine plus oxaliplatin (XELOX) or leucovorin and fluorouracil plus oxaliplatin (FOLFOX), compared with those receiving leucovorin and fluorouracil alone (adjusted for sex, age, T stage, and N stage), generated by the corrected group prognosis method. (D) Unadjusted Kaplan-Meier plot of post-relapse survival in patients receiving adjuvant capecitabine plus oxaliplatin (XELOX) or leucovorin and fluorouracil plus oxaliplatin (FOLFOX) compared with those receiving leucovorin and fluorouracil alone, by nodal stage. HR=hazard ratio. XELOX=capecitabine plus oxaliplatin. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. N=nodal stage. Please note that figures without numbers at risk display adjusted survival curves derived from a Cox proportional hazards regression model (rather than Kaplan–Meier survival analysis); hence, those data are not directly used.
Table 5.
Multiple Cox regressions for post-relapse survival
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| Randomized treatment: XELOX or FOLFOX vs leucovorin and fluorouracil | 0·92 (0·81–1·05) | 0·23 |
| Sex (women vs men) | 1·02 (0·90–1·16) | 0·71 |
| Age (<70 years vs ≥70 years) | 0·70 (0·60–0·81) | <0·0001 |
| T stage (T1–2 vs T3–4) | 0·82 (0·61–1·10) | 0·19 |
| N stage (N1 vs N2) | 0·73 (0·64–0·83) | <0·0001 |
XELOX=capecitabine plus oxaliplatin. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. T stage=tumour stage. N stage=nodal stage.
Adjusted analyses also showed that post-relapse survival was significantly longer for patients younger than 70 years than for those aged 70 years or older (table 5). In unadjusted analyses, the difference in post-relapse survival for stage N1 compared with stage N2 disease was consistent in the subgroups of patients with more than 12 nodes (HR 0·70 [95% CI 0·56–0·86]; p=0·0009) and 12 or fewer nodes examined (0·71 [0·56–0·89]; p=0·003).
As our next assessment of post-relapse survival, we compared this outcome in patients who received regimens containing capecitabine versus those containing fluorouracil. The analysis population comprised 659 patients who relapsed after receiving adjuvant capecitabine with or without oxaliplatin and 1222 patients who relapsed after receiving leucovorin and fluorouracil with or without oxaliplatin. Median follow-up for this analysis was 44 months (IQR 33–69 months) for leucovorin and fluorouracil with or without oxaliplatin, and 74 months (46–84 months) for capecitabine with or without oxaliplatin. Unadjusted Kaplan-Meier analysis (appendix p 6) showed that post-relapse survival was similar between the groups (HR 1·07 [95% CI 0·95–1·20]; p=0·26). Adjusted survival curves generated with use of either the average covariate or the corrected group prognosis method support these results (data not shown). Adjusted analyses again showed significantly longer post-relapse survival for patients aged younger than 70 years than for those aged 70 years and older (HR 0·72 [95% CI 0·63–0·83]; p=0·0001) and for stage N1 versus N2 disease (HR 0·75 [0·67–0·84]; p=0·0001).
In sensitivity analyses, post-relapse survival was similar between patients who received capecitabine and those who received XELOX (appendix p 7) (HR 0·94 [95% CI 0·78–1·13]; p=0·50)—confirming our pooling of patients treated with capecitabine with those treated with XELOX for our primary analyses—and for patients treated with capecitabine or leucovorin and fluorouracil monotherapy.
Patients who relapsed after less than 1 year had worse post-relapse survival than did those relapsing later in the overall population. However, we recorded no difference in post-relapse survival between the leucovorin and fluorouracil and oxaliplatin-based treatment groups when comparing patients with less than 1 year or 1 year or longer between surgery and relapse (appendix p 8).
Table 6 summarises post-relapse therapies in patients who relapsed (excluding participants from NSABP C-08, from whom data were not collected). In patients who received adjuvant XELOX or FOLFOX, no significant difference was recorded in post-relapse survival associated with the documented use of non-oxaliplatin therapies after relapse compared with those who received oxaliplatin after relapse (HR 1·03 [95% CI 0·77–1·38]; p=0·85).
Table 6.
Use of post-relapse therapies in patients who relapsed (excluding patients in NSABP C-08)
| XELOX or FOLFOX vs leucovorin and fluoruracil monotherspy |
Capecitabine with or without oxaliplatin vs leucovorin and fluorouracil with or without oxaliplatin |
XELOX vs capecitabine | ||||
|---|---|---|---|---|---|---|
| Leucovorin and fluorouracil (n=744) |
XELOX or FOLFOX (n=499) |
Leucovorin and fluorouracil with or without oxaliplatin (n=964) |
Capecitabine with or without oxaliplatin (n=659) |
Capecitabine (n=380) |
XELOX (n=279) | |
| Any listed post-relapse treatment | 542 (73%) | 361 (72%) | 707 (73%) | 475 (72%) | 279 (73%) | 196 (70%) |
| Fluoropyrimidine | 508 (68%) | 332 (67%) | 662 (69%) | 432 (66%) | 254 (67%) | 178 (64%) |
| Oxaliplatin | 357 (48%) | 86 (17%) | 390 (40%) | 209 (32%) | 156 (41%) | 53 (19%) |
| Irinotecan | 352 (47%) | 284 (57%) | 481 (50%) | 322 (49%) | 167 (44%) | 155 (56%) |
| Bevacizumab | 93 (13%) | 144 (29%) | 174 (18%) | 76 (12%) | 13 (3%) | 63 (23%) |
| EGFR inhibitor (cetuximab) or panitumumab) | 79 (11%) | 77 (15%) | 106 (11%) | 68 (10%) | 18 (5%) | 50 (18%) |
| Fluoropyrimidine, oxaliplatin, and irinotecan | 233 (31%) | 57 (11%) | 253 (26%) | 129 (20%) | 92 (24%) | 37 (13%) |
XELOX=capecitabine plus oxaliplatin. FOLFOX=leucovorin and fluorouracil plus oxaliplatin. EGFR=epidermal growth factor receptor.
Discussion
Our analysis has shown that combination therapy with oxaliplatin for stage III colon cancer provides consistently improved outcomes, irrespective of the fluoropyrimidine backbone (capecitabine or leucovorin and fluorouracil), and that the benefit of combining oxaliplatin with a fluoropyrimidine is not compromised by decreased survival following relapse. These data also suggest that the type of previous adjuvant therapy need not be a stratification factor for trials in metastatic colorectal cancer.
This analysis used pooled individual patient data from four large randomised controlled trials, which is to our knowledge the largest population of oxaliplatin-treated patients with stage III colon cancer studied so far (panel). This large population gave sufficient statistical power to record differences that it would not have been possible to detect in the individual trials. All four trials were well conducted, and were of uniform data quality, which allowed the data to be pooled. The trials also assessed regimens that are used frequently in clinical practice and are endorsed in clinical practice guidelines (leucovorin and fluorouracil, capecitabine, FOLFOX, and XELOX). The NSABP C-07 and MOSAIC trials were both excluded, mainly because they generated one of the hypotheses we wanted to test—namely, that adjuvant oxaliplatin might adversely affect post-relapse survival. Additionally, FLOX (which was assessed in NSABP C-07) is not used often, because it has both an unfavourable safety profile and no proven overall survival benefit.
The present analysis supports equivalent efficacy of XELOX and FOLFOX in the adjuvant setting. However, a significant oxaliplatin-by-fluoropyrimidine interaction for overall survival suggested that the relative contribution of oxaliplatin to this outcome differed depending on fluoropyrimidine choice. Consistent with this finding, individual HRs showed a slightly greater incremental benefit for oxaliplatin when combined with leucovorin and fluorouracil than when combined with capecitabine. These observations are likely related to the evolving standards between 1998 and 2007 when these studies accrued, with increased attention paid to the quality of surgery and staging, improved understanding of the importance of receiving all active agents in the metastatic setting, and the introduction of new agents with proven effects on overall survival in the metastatic setting, eg bevacizumab.19, 20, 21
Sensitivity analyses consistently showed improved outcomes (in disease-free, relapse-free, and overall survival) in patients randomly allocated to treatment groups in 2004 or later compared with those randomised before 2004. Owing to a desire to create a study population that was as homogeneous as possible, we excluded patients receiving XELOX in combination with bevacizumab from our primary analysis. This approach created an imbalance between the XELOX and FOLFOX analytic cohorts: most XELOX patients were recruited before 2004, whereas the entire FOLFOX population was recruited during or after 2004. When primary analyses were repeated on the entire stage III cohort, adjusted for use of bevacizumab, these differences were no longer statistically significant, and 3-year, 4-year, and 5-year disease-free, relapse-free, and overall survival rates were equivalent. This finding suggests that the observations made in the primary analyses were largely associated with this imbalance. It should also be noted that 5-year disease-free and overall survival outcomes are consistent with those reported in published studies for patients with stage III colon cancer who received oxaliplatin-based therapy.4, 6
The present analysis also showed significantly improved outcomes with XELOX versus capecitabine monotherapy. Since these two regimens have not been directly compared in a randomised trial, this is a salient finding that supports the use of XELOX in patients for whom combination therapy is appropriate.
The finding that post-relapse survival is not affected by previous use of oxaliplatin-containing adjuvant regimens is consistent with the established benefits of XELOX and FOLFOX, which significantly increase disease-free survival and overall survival compared with leucovorin and fluorouracil monotherapy.3, 4, 7 We found no evidence that suggested that the disease-free survival benefit of XELOX or FOLFOX is attenuated by shortened survival following recurrence; this result contrasts with those of the MOSAIC and NSABP C-07 trials. In MOSAIC, median post-relapse survival seemed to be shorter in patients randomly allocated to FOLFOX (21 months) compared with those assigned to bolus plus infusional leucovorin and fluorouracil (24 months), although no statistical comparison was done.3 In NSABP C-07, post-relapse survival was significantly shorter in patients with stage II and III colon cancer randomly assigned to FLOX compared with those assigned to bolus leucovorin and fluorouracil (HR 1·20 [95% CI 1·00–1·43]; p=0·0497);10 this finding might account for the improvement in overall survival with FLOX failing to reach statistical significance, despite a clear increase in disease-free survival.6
The reason for these apparent discrepancies in relation to the effect of adjuvant oxaliplatin on post-relapse survival is unclear. MOSAIC and NSABP C-07 post-relapse survival data have been reported only for the overall population of patients with both stage II and stage III disease.3, 5 By contrast, the present analysis focused solely on patients with stage III colon cancer. Similarly, leucovorin and fluorouracil were administered via different bolus or infusion regimens; however, this difference is unlikely to be important, since similar survival outcomes were shown with infusional and bolus intravenous fluoropyrimidine regimens in the GERCOR C96.1 trial.22 Moreover, there were also differences between the oxaliplatin-containing regimens: FOLFOX and XELOX in the present analysis and FLOX in NSABP C-07. We also cannot exclude differences in post-relapse therapy, but given the similarity in patient populations and timing of the studies, again major differences are unlikely. Finally, differences between the study populations in other prognostic or predictive molecular biomarkers 23 are unlikely but cannot be excluded. However, how these differences in patient populations and treatment might account for the apparent discrepancy with respect to post-relapse survival remains unclear. The two earlier studies that reported an adverse effect of adjuvant oxaliplatin on survival if patients relapse 3,10 did not have a sufficient number of events for a robust analysis, whereas the present analysis is sufficiently large to indicate this was most likely a chance finding and unlikely to be a true effect.
Previous results from the Adjuvant Colon Cancer End Points (ACCENT) project showed poorer post-relapse survival with node-positive versus node-negative disease in patients treated with fluorouracil-based adjuvant therapy or surgery alone.24 These findings are extended here by showing that, in node-positive stage III disease, a greater extent of nodal involvement (N2 vs N1) is prognostic for poorer post-relapse survival in patients receiving either oxaliplatin combinations or single-agent leucovorin and fluorouracil as adjuvant therapy. This suggests that both reduced disease-free and post-relapse survival might contribute to the reduction in overall survival that is associated with higher N stage.25 Greater baseline nodal involvement at diagnosis could well be associated with a more aggressive disease phenotype, and identification of the disease later in its natural history.
The observation that patients aged 70 years and older have worse post-relapse survival is again consistent with the initial ACCENT data, which show significantly shorter survival after recurrence with advancing age in patients with stage II and III colon cancer treated with fluorouracil-based adjuvant therapy.24 Along with the proportional increase in deaths from competing causes in older patients compared with younger individuals, a diminished functional reserve and accumulating comorbidity with age might limit delivery of otherwise optimum post-relapse therapy, thereby affecting post-relapse survival.26 The similar post-relapse survival recorded with capecitabine with or without oxaliplatin and leucovorin and fluorouracil with or without oxaliplatin adds to the large body of evidence showing at least equivalent outcomes between these two fluoropyrimidines in a range of settings.8, 11, 12, 13, 15
In terms of tolerability and safety, capecitabine-based treatment showed a favourable adverse event profile over leucovorin and fluorouracil-based regimens, with lower numbers of oxaliplatin-related grade 3–4 events (neuropathy, neutropenia, and febrile neutropenia) and overall grade 3–4 events, although grade 3 hand–foot syndrome was more frequent with capecitabine than with leucovorin and fluorouracil. This finding is not unexpected in view of the better safety profile of capecitabine monotherapy over bolus leucovorin and fluorouracil reported in X-ACT.8 Similar adverse event profiles of XELOX and FOLFOX in patients with metastatic colorectal cancer have been reported.11, 12, 13 One of the aims of the IDEA collaboration 27—a prospective combined analysis of phase 3 trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX-4 or mFOLFOX-6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer—is to reduce toxicities of oxaliplatin-based adjuvant therapy for stage III colon cancer, and results are eagerly anticipated.
Since this was a pooled analysis, the results are subject to the limitations of the original data sources. Duration of follow-up was imbalanced, and X-ACT did not provide numbers of lymph nodes assessed. However, sensitivity analyses suggested that the number of lymph nodes assessed was not a confounding factor. Access to data from one important trial (MOSAIC) was not possible. In current clinical practice, leucovorin and fluorouracil is often administered as a continuous infusion since this has been associated with improved tolerability compared with bolus administration,22, 28, 29, 30 but the leucovorin and fluorouracil monotherapy was administered as a bolus in the studies analysed, which was the standard of care when X-ACT and XELOXA were designed. Nonetheless, bolus leucovorin and fluorouracil versus bolus plus infusional leucovorin and fluorouracil show similar efficacy in trials of adjuvant therapy,22 which means that bolus leucovorin and fluorouracil is a reasonable comparator.
The main strengths of the dataset and analyses include the large patient population, high data quality, availability of patient-level data, and extended duration of follow-up of patients in the patient pool, which ensured that more than a third of patients in each group had experienced a disease-free survival event at data cutoff. The individual patient data approach allowed for adjustment with use of patient-level covariates to produce treatment effects with better precision. As such, power for comparisons of estimated treatment effects was increased, which also allowed us to address issues that were not the focus of the individual trials.
Taken together, these data add to the evidence that oxaliplatin plus capecitabine or leucovorin and fluorouracil is the standard of care for the adjuvant treatment of stage III colon cancer,2 offering physicians flexibility to treat patients according to the patients' overall physical performance and preference.
Supplementary Material
PANEL.
Research in context
Systematic review
Adjuvant therapy with oxaliplatin plus either intravenous leucovorin and fluorouracil or oral capecitabine is the standard of care for stage III colon cancer, and fluoropyrimidine monotherapy is recommended for some patients. No randomised studies have directly compared capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin in this setting. Therefore, we assessed the efficacy and safety of capecitabine with or without oxaliplatin versus leucovorin and fluorouracil with or without oxaliplatin using data pooled from four randomised controlled trials, which were selected on the basis of availability of access to complete patient-level data.
Interpretation
Combination therapy with oxaliplatin for stage III colon cancer provides consistently improved outcomes, irrespective of the fluoropyrimidine backbone (capecitabine or leucovorin and fluorouracil), and the benefit of combining oxaliplatin with a fluoropyrimidine is not compromised by decreased survival following relapse. These data also suggest that the type of previous adjuvant therapy need not be a stratification factor for trials in metastatic colorectal cancer. These data add to the evidence that oxaliplatin plus capecitabine or leucovorin and fluorouracil is the standard of care for the adjuvant treatment of stage III colon cancer, offering physicians flexibility to treat patients according to the patients' overall physical performance and preference.
Acknowledgments
This study was supported by Genentech Inc. Third-party writing assistance for this manuscript was provided by Daniel Clyde, support for which was provided by Genentech Inc. The NSABP C-08 study was supported by Public Health Service grants (U10 CA 12027, U10 CA 69651, U10-CA-37377, and U10 CA-69974) from the National Cancer Institute, the US Department of Health and Human Services, Sanofi-Synthelabo Inc, and Genentech Inc.
H-JS has an advisory role with honoraria and travel support for lectures from F Hoffmann-La Roche–Genentech Inc and Sanofi. CT has received honoraria from F Hoffmann-La Roche–Genentech Inc. TC has served as a consultant and participated in advisory boards for F Hoffmann-La Roche. EMcK is an employee of and has stock or stock options in Genentech Inc. MS has received speaker's bureau honoraria from Genentech Inc, Sanofi-Aventis, and Amgen. SL is an employee of Genentech Inc and owns Roche stock through the company's long-term compensation programme. DH has received honoraria from Genentech Inc.
Footnotes
Contributors
All authors reviewed the data analyses, contributed to the writing of the report, made final decisions about all parts of the report, and approved the final submitted version.
Declaration of interests
The other authors declare no competing interests.
Contributor Information
Hans-Joachim Schmoll, University Clinic, Martin Luther University, Halle (Saale), Germany.
Chris Twelves, Leeds Institute of Cancer and Pathology and St James’s University Hospital, Leeds, UK.
Weijing Sun, University of Pittsburgh Cancer Institute, Pittsburgh, PA, USA.
Michael J O'Connell, National Surgical Adjuvant Breast and Bowel Project (NSABP), Pittsburgh, PA, USA.
Thomas Cartwright, Florida Cancer Affiliates, New Port Richey, FL, USA.
Edward McKenna, Genentech Inc., South San Francisco, CA, USA.
Muhammad Saif, Tufts University School of Medicine, Boston, MA, USA.
Steve Lee, Genentech Inc., South San Francisco, CA, USA.
Greg Yothers, NSABP Biostatistical Center and University of Pittsburgh Graduate School of Public Health Department of Biostatistics, Pittsburgh, PA, USA.
Daniel Haller, Abramson Cancer Center at the University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer. [accessed Oct 2, 2012];Version 3. 2012 http://www.nccn.org/ [Google Scholar]
- 2.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 3.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 4.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 5.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 6.Yothers G, O'Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 8.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 9.Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol. 2012;23:1190–1197. doi: 10.1093/annonc/mdr366. [DOI] [PubMed] [Google Scholar]
- 10.Yothers GA, O'Connell MJ, Colangelo L, et al. Fluorouracil and leucovorin (Lv) with or without oxaliplatin (Ox) for adjuvant treatment of stage II and III colon cancer: long-term follow-up of NSABP C-07 with survival analysis. American Society for Clinical Oncology Gastrointestinal Cancers Symposium; Orlando, FL, USA. 2010. Jan, pp. 22–24. Abstract 401. [Google Scholar]
- 11.Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 12.Cassidy J, Clarke S, Díaz-Rubio E, et al. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br J Cancer. 2011;105:58–64. doi: 10.1038/bjc.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rothenberg ML, Cox JV, Butts C, et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/ folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. 2008;19:1720–1726. doi: 10.1093/annonc/mdn370. [DOI] [PubMed] [Google Scholar]
- 14.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol. 2012;13:1225–1233. doi: 10.1016/S1470-2045(12)70509-0. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [Google Scholar]
- 17.Ghali WA, Quan H, Brant R, et al. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. JAMA. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- 18.Nieto, FJ and Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 19.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 20.Bennouna J, Sastre J, Arnold D, et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML 18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29–37. doi: 10.1016/S1470-2045(12)70477-1. [DOI] [PubMed] [Google Scholar]
- 21.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 22.André T, Quinaux E, Louvet C, et al. Phase III study comparing a semimonthly with a monthly regimen of fluorouracil and leucovorin as adjuvant treatment for stage II and III colon cancer patients: final results of GERCOR C96.1. J Clin Oncol. 2007;25:3732–3738. doi: 10.1200/JCO.2007.12.2234. [DOI] [PubMed] [Google Scholar]
- 23.Tejpar S, Bertagnolli M, Bosman F, et al. Prognostic and predictive biomarkers in resected colon cancer: current status and future perspectives for integrating genomics into biomarker discovery. Oncologist. 2010;15:390–404. doi: 10.1634/theoncologist.2009-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Connell MJ, Campbell ME, Goldberg RM, et al. Survival following recurrence in stage II and III colon cancer: findings from the ACCENT data set. J Clin Oncol. 2008;26:2336–2341. doi: 10.1200/JCO.2007.15.8261. View in Article. [DOI] [PubMed] [Google Scholar]
- 25.Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22:1797–1806. doi: 10.1200/JCO.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 26.Biganzoli L, Aapro M. Adjuvant chemotherapy in the elderly. Ann Oncol. 2003;14:iii1–iii3. doi: 10.1093/annonc/mdg740. [DOI] [PubMed] [Google Scholar]
- 27.André T, Iveson T, Labianca R, et al. The IDEA (International Duration Evaluation of Adjuvant Chemotherapy) collaboration: prospective combined analysis of phase III trials investigating duration of adjuvant therapy with the FOLFOX (FOLFOX4 or Modified FOLFOX6) or XELOX (3 versus 6 months) regimen for patients with stage III colon cancer: trial design and current status. Curr Colorectal Cancer Rep. 2013;9:261–269. doi: 10.1007/s11888-013-0181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.André T, Colin P, Louvet C, et al. Semimonthly versus monthly regimen of fluorouracil and leucovorin administered for 24 or 36 weeks as adjuvant therapy in stage II and III colon cancer: results of a randomized trial. J Clin Oncol. 2003;21:2896–2903. doi: 10.1200/JCO.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 29.Chau I, Norman AR, Cunningham D, et al. A randomised comparison between 6 months of bolus fluorouracil/leucovorin and 12 weeks of protracted venous infusion fluorouracil as adjuvant treatment in colorectal cancer. Ann Oncol. 2005;16:549–557. doi: 10.1093/annonc/mdi116. [DOI] [PubMed] [Google Scholar]
- 30.Poplin EA, Benedetti JK, Estes NC, et al. Phase III Southwest Oncology Group 9415/Intergroup 0153 randomized trial of fluorouracil, leucovorin, and levamisole versus fluorouracil continuous infusion and levamisole for adjuvant treatment of stage III and high-risk stage II colon cancer. J Clin Oncol. 2005;23:1819–1825. doi: 10.1200/JCO.2005.04.169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.