Abstract
1. Purpose of review
Several autoimmune lymphoproliferative syndromes have been described lately. We review here the main clinical and laboratory findings of these new disorders.
2. Recent findings
The prototypical autoimmune lymphoproliferative syndrome (ALPS) has had its diagnostic criteria modified; somatic mutations in RAS genes were found to cause an ALPS-like syndrome in humans; and mutations in a gene encoding a protein kinase C (PRKCD) were discovered to cause a syndrome of lymphoproliferation, autoimmunity and NK cell defect.
3. Summary
The recent discoveries shed light into the molecular pathways governing lymphocyte death, proliferation and immune tolerance in humans.
Keywords: autoimmune lymphoproliferative syndrome, apoptosis, genetics, RAS, PRKCD
INTRODUCTION
Apoptosis is crucial for immune system homeostasis, as lymphocytes undergo massive expansion during encounter with a pathogen and subsequently contract, leaving behind just a few memory cells [1]. It also helps maintain immunologic tolerance, as most self-reactive T and B cells undergo apoptosis during selection in the thymus or bone marrow, respectively [1]. The importance of these mechanisms is illustrated by the clinical findings of humans with genetic defects affecting different apoptotic pathways, here collectively called autoimmune lymphoproliferative syndromes. Although initially composed of one single disorder, the autoimmune lymphoproliferative syndrome (ALPS), caused by defects in genes of the FAS pathway of apoptosis (FAS, FASLG and CASP10), the group has significantly expanded recently, and now includes: RAS-associated Autoimmune Leukoproliferative Disorder (RALD), caused by somatic mutations in NRAS or KRAS; Caspase-Eight Deficiency Syndrome (CEDS); FADD deficiency; and PRKCD deficiency, the most recently described defect. Each disorder has its own clinical and laboratory particularities, which will be reviewed in this chapter.
Autoimmune Lymphoproliferative Syndrome
The clinical hallmarks of ALPS are chronic nonmalignant lymphadenopathy, splenomegaly, and multilineage cytopenias [2,3]. The diagnostic criteria for ALPS were revised in 2010 and are presented in Table 1. ALPS usually presents in early childhood, with a median age of onset of 3 years, although a minority of the patients manifest disease later in life, from 18 to 35 years of age [5]. The initial presentation of ALPS is often that of persistent lymphadenopathy and/or splenomegaly (69% of the patients) in an otherwise healthy child [5]. About a quarter of the patients present with an autoimmune disease, most often immune thrombocytopenic purpura (ITP) or hemolytic anemia, in addition to the lymphoproliferation. More rarely, patients present with pure autoimmunity or lymphoma as the initial manifestation of ALPS [5]. Recurrent infections are not a common finding, but can also occur due to neutropenia and/or blockage of nasopharyngeal passages due to lymphadenopathy. Recurrent fevers in the absence of infections are not seen in ALPS.
Table 1.
Diagnostic criteria for ALPS based on International ALPS Workshop 2009.
| Revised diagnostic criteria for ALPS based on First International ALPS Workshop 2009 |
|---|
|
|
| Required criteria |
|
|
| 1. Chronic (> 6 months), nonmalignant, noninfectious lymphadenopathy and/or splenomegaly |
| 2. Elevated CD3+ TCRαβ+CD4− CD8− DNT cells (> 1.5% of total lymphocytes or > 2.5% of CD3+ lymphocytes) in the setting of normal or elevated lymphocyte counts |
|
|
| Additional criteria |
| Primary |
| 1. Defective lymphocyte apoptosis in 2 separate assays |
| 2. Somatic or germline pathogenic mutation in FAS, FASLG, or CASP10 |
| Secondary |
| 3. Elevated plasma sFASL levels (> 200 pg/mL), plasma IL-10 levels (> 20 pg/mL), serum or plasma vitamin B12 levels (> 1500 ng/L) or plasma IL-18 levels > 500 pg/mL |
| 4. Typical immunohistologic findings as reviewed by a hematopathologist |
| 5. Autoimmune cytopenias (hemolytic anemia, thrombocytopenia, or neutropenia) with elevated IgG levels (polyclonal hypergammaglobulinemia) |
| 6. Family history of a nonmalignant/noninfectious lymphoproliferation with or without autoimmunity |
| Definitive diagnosis: Both required criteria plus one primary accessory criterion. |
| Probable diagnosis: Both required criteria plus one secondary accessory criterion. Treat as ALPS until genetics can be done. |
|
|
| Adapted from [4] |
Regardless of the opening symptoms, lymphadenopathy and/or splenomegaly will eventually be seen in 100% of the patients with ALPS, and are required for its diagnosis [4,5]. Hepatomegaly is found in about half the cases. Areas most commonly affected by lymphadenopathy are the neck, mediastinum, axillae, inguinal and pelvic regions, although virtually any lymph node in the body can be enlarged. The lymphoproliferation tends to improve over time, and after the age of 20 years as much as 66% of the patients will have had complete remission of these manifestations, and the remaining patients will have seen significant improvement [5].
Up to 72% of the patients develop autoimmune manifestations by the age of 30. Most present autoimmune cytopenias (52%), at a median age of 5 years [5]. Autoimmune hemolytic anaemia is the most frequent event, followed by autoimmune thrombocytopenia. Some patients (20%) also develop autoimmune diseases affecting other organs, such as autoimmune hepatitis, glomerulonephritis, uveitis, encephalomyelitis (Guillain-Barre syndrome), aplastic anemia, vasculitis, pancreatitis, angioedema and alopecia [5]. There may also be a family history of similar disorders, usually inherited in an autosomal dominant fashion with incomplete penetrance. Unlike the lymphoproliferation, however, the autoimmune disorders tend to persist in over 60% of the patients, mostly requiring continuous or intermittent immunosuppressive therapy [5].
Laboratory Findings
The defining laboratory finding in ALPS is the presence of an expanded population of CD3+TCR-αβ+CD4−CD8− lymphocytes, referred to as the double negative T (DNT) cells [6,7]. These are polyclonal, mature T cells, seen in the peripheral blood and secondary lymphoid tissue of ALPS patients [6,7]. A complete blood count (CBC) with differential may demonstrate lymphocytosis, reticulocytosis, thrombocytopenia, neutropenia, slight monocytosis, and/or eosinophilia.
Other common but less specific findings include polyclonal elevation of IgG and IgA, and the presence of autoantibodies directed to blood cell elements [8]. The most common autoantibodies occurring in ALPS patients are anti-erythrocyte antibodies detected by a Coombs direct antiglobulin test (DAT), and anti-platelet and anti-neutrophil antibodies [8,9]. Other abnormal laboratory findings that are commonly found in patients with ALPS include elevated serum levels of vitamin B12, IL-10, soluble FAS ligand, and IL-18 [10,11]. The combination of these markers proved to be a powerful predictor of the presence of FAS mutations in patients with an ALPS clinical phenotype [10]. Patients with a combination of high DNTs and IL10, Vitamin B12 or have a 97% chance of harboring a FAS mutation [10]. These markers were also very sensitive in detecting patients with somatic FAS mutations [12]. Thus, in the presence of elevated DNTs and vit. B12 or sFASL, a negative genetic screening for germline FAS mutations should prompt an investigation for somatic mutations in the sorted DNT population. These biomarkers were incorporated into the recently modified ALPS diagnostic criteria [4].
A lymph node biopsy can be very helpful to rule out other diagnosis, such as malignancy, and to diagnose ALPS. Findings typical of ALPS include follicular hyperplasia, often with focal progressive transformation of germinal centers, paracortical expansion with a mixed infiltrate containing DNT cells, and polyclonal plasmocytosis [13]. Additionally, up to 41% of the patients with FAS mutations may demonstrate hystiocitic proliferation, resembling sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) [14].
In patients with clinical and/or laboratory features consistent with a diagnosis of ALPS, molecular genetic testing of FAS (TNFRSF6), Fas Ligand (TNFSF6), and caspase-10 genes (CASP10) should be obtained. Based on their frequency, we recommend first testing for germline FAS mutations, followed by analysis of somatic FAS mutations in sorted DNT cells (specially if biomarkers are high). If both tests are negative, CASP10 and FASL should be tested, in any order. The location of specific gene mutation has been shown to be important in patient prognosis as certain mutation loci are associated with a higher risk of complications including lymphoma, and with a higher penetrance [15,16].
Genetics and Pathophysiology
ALPS can be caused by germline or somatic FAS mutations and by mutations in CASP10 and FASLG, as discussed below.
Germline FAS mutations
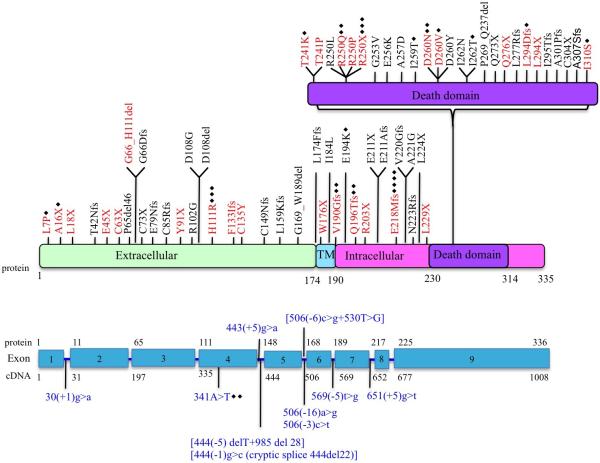
Most ALPS patients (62%) have germline mutations in TNFRSF6 (FAS) [17]. Mutations occur throughout the gene, either in coding regions or in splice sites, with the majority (~2/3) affecting the intracellular death domain (DD) region encoded by exon 9 (Figure 1) [5,16]. Most mutations are heterozygous, transmitted in an autosomal dominant fashion and exert a dominant-negative effect in the FAS pathway [19, 20]. A few ALPS cases with aggressive disease phenotype in early childhood caused by homozygous germline mutations in FAS have also been reported [21].
Fig 1.
Schematic representation of FAS mutations in ALPS patients. TM, transmembrane. Red text indicates mutations evaluated in this study. Blue text indicates complex mutations. Black diamonds represents the number of families with same mutation. Reproduced with permission from reference [18].
In contrast with the mutations located the intracellular death domain, mutations affecting the extracellular regions of the protein (about 25% of the total) commonly result in loss of protein expression from one allele leading to FAS haploinsufficiency, without a dominant negative effect [16]. These usually manifest by milder clinical disease and lower penetrance [16,22]. More recently, it has been described that up to 60% of ALPS patients with extracellular domain mutations that develop clinically important autoimmune disease present somatic mutations in the second allele of FAS [5,23,24]. These “second hits” developed later in life and either affected the death domain or caused loss of the healthy allele. This association of germline and somatic mutations in the same patient is unique and sheds light into the genetic mechanisms underlying disease severity and penetrance variability in ALPS.
Somatic FAS mutations
The second most common genetic cause of ALPS is somatic mutations in FAS [12,25]. These patients present with mutations in blood elements only, mostly affecting DNT cells and a small proportion (10-20%) of CD4, CD8, CD20 and CD34 (progenitor) cells. Given the low prevalence of mutant cells in total lymphocytes, these patients typically lack apoptosis defects as tested in vitro, and test negative for FAS mutations when evaluated in whole blood cells [12]. The clinical manifestations are similar to patients with germline FAS mutations.
Caspase-10 and FASLG mutations
CASP10 mutations were found in 10 patients thus far [26,27](Koneti Rao, personnal comuncation). These mutations were heterozygous and caused defective apoptosis in lymphocytes and dendritic cells [27]. The clinical phenotype was indistinguishable from that of patients with FAS mutations. To date, only 4 ALPS patients with FAS ligand (FASLG) mutations have been reported [28-31], including one with systemic lupus erythematosus (SLE)-like disease
Treatment and prognosis
ALPS management aims to control autoimmune cytopenias and to monitor for lymphoma. In a recent study, 74% of the patients required medical and/or surgical treatment at some point in life, with the median age of onset of immunosuppression being 7.5 years. [5]. Initial management for ALPS-related autoimmune cytopenias is similar to sporadic cytopenias in other patient populations, including parenteral high dose methylprednisolone and high dose intravenous immunoglobulin [32]. Some patients with autoimmune neutropenias who experience associated infection may be treated with low dose granulocyte colony stimulating factor. In patients with refractory autoimmune cytopenias requiring chronic steroid therapy, mycophenolate mofetil has been shown to be an effective steroid-sparing agent that maintains adequate blood cell counts and reduces the need for other immunosuppressive agents or splenectomy [18, 32, 33]. Finally, some ALPS patients with hypersplenism and associated cytopenias have shown significant improvement following treatment with sirolimus [18, 34]. Spleen guards should be considered for ALPS patients with massive splenomegaly, to help reduce the risk of traumatic splenic rupture. These patients with very large spleens should be discouraged from participating in contact sports. All ALPS patients who are asplenic should be treated with long-term antibiotic prophylaxis against pneumococcal sepsis using penicillin V. Patients that who have undergone surgical splenectomy are encouraged to wear medical alert
Most ALPS patients are expected to live a normal life span with few clinical complications. Patients with mutations affecting the intracellular domain of the FAS protein have more severe disease and are at increased risk for Hodgkin and Non-Hodgkin B-cell lymphomas [4,15,35]. Patients with mutations affecting the extracellular domain of FAS have also been reported to develop lymphomas, extending the spectrum of at-risk patients [5]. Although lymphoma is the most feared complication in ALPS, the most lethal complication is postsplenectomy sepsis. In a recent series, 6 out of 90 ALPS patients died over a 25 year follow up, being four of postsplenectomy infection, one of aplastic anemia and one of a stroke [5]. Based on these data, splenectomy is strongly disencouraged in ALPS.
ALPS-RELATED DISORDERS
There are several monogenic disorders that clinically resemble ALPS, but have distinctive findings that made them be classified separately. These entities are discussed below.
Caspase-8 deficiency
CASPASE-8 deficiency state (CEDS) is caused by autosomal recessive mutations in the gene encoding caspase-8 (CASP8). In 2002, Chun et al. described two siblings, a 12-yr-old female and an 11-yr-old male born to a distantly consanguineous family, with some features of the autoimmune lymphoproliferative syndrome (ALPS), such as lymphadenopathy, splenomegaly, slight elevation of the TCRαβ+CD4−CD8− T cells and defective lymphocyte apoptosis [36]. In addition, and unlike other ALPS patients, these patients also presented with mild recurrent sinopulmonary and cutaneous herpes simplex virus (HSV) infections [36]. The affected siblings carried homozygous mutations in the gene encoding caspase-8 (CASP8, p.R248W) associated with a combined immunodeficiency characterized by inverted CD4/CD8 ratios, slightly diminished IgG, IgA and IgM levels and poor responses to pneumococcal immunization. The authors noticed a defective activation of T, B and NK cells, underlying the immunodeficiency state.
Recently, two more patients from the same extended family have been identified (S. Rosenzweig and J. Oliveira, unpublished results). These patients had immunodeficiency, with recurrent sinopulmonary infections, warts, molluscum contagiosum, poor pneumococcal antibodies, and normal DNTs. Besides their splenomegaly and lymphocytosis, they also had accumulation of lymphocytes in multiple organs such as liver, spleen, lung, and brain. Patient 1 died from complications of a pulmonary transplant for interstitial lung disease of unknown etiology. Patient 2 died due to progressive pulmonary and neurological problems. The lymphocytic infiltration seen in these older patients is similar to the one seen in parenchymal organs of older mice lacking caspase-8 within their T cells [37]. Thus, CEDS in both humans and mice is characterized by a mild combined immunodeficiency with pronounced lymphocyte accumulation and infiltration but minimal autoimmunity.
Genetics and Pathophysiology
The clinical phenotype of immunodeficiency coupled to lymphoproliferation can be explained by the dual role of CASPASE-8 in signaling for both apoptosis and lymphocyte activation. Upon FAS death receptor stimulation, CASPASE-8 is recruited into the death-inducing signaling complex (DISC) for apoptosis induction. In contrast, upon immunoreceptor stimulation, CASPASE-8 assembles with the CARMA1-BCL10-MALT1 (CBM) and the IKKα/β complexes, activating the gene transcription factor NF-kB for lymphocyte activation [38,39].
FADD deficiency
Four related patients from a consanguineous family with FADD deficiency due to autosomal recessive mutations (p.C105W) were recently identified, who had an immunodeficiency phenotype [40]. The patients had invasive pneumococcal infections with functional hyposplenism, as well as repeated febrile episodes of encephalopathy with liver dysfunction that were associated with viral infections. One patient had increased DNT cells, a lymphocyte apoptosis defect, elevated biomarkers that are usually associated with ALPS-FAS, and DAT autoantibodies without reported autoimmune disease. Unlike CEDS or ALPS, none of the FADD-deficient patients had splenomegaly or lymphadenopathy. Taken together, FADD deficiency, like CEDS, clinically overlaps with ALPS but differs in featuring immunodeficiency prominently.
Pathophysiology
FADD has multiple roles as a signaling molecule [41]. It is an adapter molecule that links surface FAS to downstream caspases during apoptosis signaling (Figure 1), but can also associate with the CARMA1-BCL10-MALT1 complex (but not the IKKα/β complex) upon antigen receptor stimulation [38]. Additionally, it is also required for type I IFN antiviral immunity [42]. These different functions explain the broad phenotype of FADD deficient patients.
RAS-associated Autoimmune Leukoproliferative Disorder (RALD)
RALD patients have several clinical and laboratory features that overlap with ALPS [43-45]. They present with a generally mild degree of peripheral lymphadenopathy, significant splenomegaly, and autoimmunity including AIHA, ITP, and neutropenia age at ages varying from 1 to 47 years of life. In some patients, a history of recurrent mild upper and lower respiratory tract infections can be elicited [43]. Unlike ALPS, patients with RALD have transient or persistent elevation in granulocytes and monocytes. Some RALD patients have a clinical and laboratory phenotype very similar to juvenile myelomonocytic leukemia (JMML) early in life, with marked hepatosplenomegaly and monocytosis. However, unlike patients with JMML, the clinical outcome is chronic and benign [43-45].
Immunophenotyping in RALD reveals mild to no elevation in DNTs and an expansion of B cells. Total lymphocyte numbers can be normal or modestly decreased. In contrast, absolute or relative monocytosis is noted in all patients seen thus far. Autoantibodies are typically detected, including ANA, rheumatoid factor, anti-phospholipid, anti-cardiolipin anti-platelet, anti-neutrophil and/or anti-red blodd cell [43-45]. Serum soluble Fas ligand and vitamin B12 are normal, as well as in vitro FAS-induced apoptosis. By contrast, in RALD patients, the T cells are resistant to IL-2 withdrawal-induced cell death, pointing to a fundamentally different apoptotic defect [43-45]. The histopathological findings include nonspecific polyclonal plasmacytosis with reactive secondary follicles, but without the typical paracortical expansion caused by DNT cells seen in ALPS. Given the small number of patients diagnosed to date, it is not known whether these patients are at increased risk for hematological malignancy.
Genetics and Pathophysiology
RALD patients harbor somatic, gain-of-function mutations in KRAS or NRAS, which are present only in blood cells. These mutations disrupt the interaction of RAS with GTPase-activating proteins (GAPs), diminishing its GTPase activity by over 300-fold and locking the molecule in activated position [46]. This permanent activation state increases cell signaling through the RAS-ERK pathway, inducing the phosphorylation and destruction of the pro-apoptotic protein BIM [47,48]. Consequently, the cells become resistant to certain kinds of apoptotic stimuli, such as growth-factor (IL-2) withdrawal. Additionally, persistent ERK signaling decreases the intracellular levels of negative inhibitors of the cell cycle, namely p27kip1, allowing for increased proliferation in the face of limiting IL-2 levels [43]. Recent work has also suggested that adequate RAS signaling is important for B cell selection, potentially explaining the multiple antibody-mediated autoimmune manifestations seen in these patients [49,50].
PKCδ Deficiency
A novel benign lymphoproliferative disorder has been recently described in two patients from distinct ethnic backgrounds [51,52]. The patients presented with findings that resembled ALPS, such as chronic persistent splenomegaly, lymphadenopathy and autoimmune disorders, but did not fulfill criteria for this disease. The patient described by Kuehn et al. is a hispanic male with a clinical history including recurrent otitis with bilateral perforated tympanic membranes and sinusitis starting in early childhood [51]. He had persistent generalized lymphadenopathy and hepatosplenomegaly, accompanied by intermittent fevers starting at 3 years of age. Hepatosplenomegaly became very prominent by 5 years of age and marked mediastinal lymphadenopathy developed. He had an intermittent facial rash in a butterfly distribution and confluent erythematous macules over the trunk and extremities but no other evidence for vasculitis or glomerulopathy. Additionally, he had persistently detectable EBV copy numbers between 2000-8000 genomes/μl. These findings were reminiscent of the PKCδ−/− mouse [53].
Laboratory evaluation revealed a slight leukocytosis, normochromic normocytic anemia and borderline thrombocytopenia presumed to be secondary to splenic sequestration [51]. ANA and ENAs were positive, and flow cytometry revealed B cell lymphocytosis with the majority of B cells expressing CD5, with a decrease in class switched B cell memory subsets. NK cell cytolytic activity was very diminished, with normal numbers of NK cells. Lymph node histology showed open sinuses, expansion of the B cell areas with prominent B-follicles with ill-defined germinal centers, lacking polarization, and prominent mantles. Typical histological features of ALPS were not observed. This patient was treated with rapamycin, with near complete resolution of the lymphadenopathy.
The patient described by Salzer et al. was a 12 year-old boy from Turkish origin who had a clinical history of mild recurrent infections (otitis, gastroenteritis, sinusitis and pneumonia) until the age of 4, when he was started on IVIG [52]. He had multiple autoimmune disorders such as membranous glomerulonepritis early in life, relapsing polychondritis, hypothyroidism, and anti-phospholipid syndrome. He presented generalized lymphadenopathy and hepatosplenomegaly since the age of 3 years. Laboratory findings included positive ANA, anti-dsDNA, anti-cardiolipin and anti-collagen 4 antibodies. IgG weas slightly below the inferior normal range, with normal IgA and IgM. In contrast to the patient described by Kuehn et al., his total B-cell numbers were decreased, with low class-switched memory B cells and increased CD21low B cells. T cell immunophenotyping was normal. Lymph node histology demonstrated nonspecific reactive follicular hyperplasia, without ALPS features. The patient was initially treated with IVIG replacement for the infections and anti-CD20 for the autoimmunity. Lately, the patient has been treated with mycophenolate-mofetil and low dose steroids, with good disease control.
Genetics and Pathophysiology
Both patients harbored homozygous mutations in PRKCD, the gene encoding for the protein kinase C, isoform δ [51,52]. Protein kinase C (EC 2.7.11.13), also known as PKC, is a family of serine/threonine kinases that play a key role in the regulation of various cellular processes, including cell proliferation, apoptosis, and differentiation [54,55]. PKCδ has important roles in B cell signaling and autoimmunity, as well as regulation of growth, apoptosis, and differentiation of a variety of cell types [50,53-57]. The impressive lymphocyte accumulation seen in these patients could be explained by the excessive proliferation of the patient’s B cells demonstrated ex vivo, as well as by defective B cell apoptosis [51]. Interestingly, Kuehn et al. also noticed a striking oversecretion of IL-10 by the patient’s cultured B cells. As IL-10 is a B cell trophic factor, this could explain in part the cellular phenotype. The NK cell dysfunction reported serves as an explanation for the chronic, low grade EBV infection seen in one individual [51], but the role of PKCδ in NK cell function is currently unknown.
CONCLUSIONS
There is an ever-expanding group of human disorders characterized my marked benign lymphoproliferation and autoimmune phenomena. Despite their rarity, these monogenic disorders shed light into the role of specific proteins in human T cell proliferation, apoptosis and tolerance mechanisms.
KEYPOINTS.
The autoimmune lymphoproliferative syndrome (ALPS) is characterized by non-malignant lymphocyte accumulation, blood-element directed autoimmunity and elevation of circulating CD3+TCR-αβ+CD4−CD8− cells.
RAS-associate autoimmune leukoproliferative disease (RALD) is caused by somatic mutations in NRAS or KRAS, and manifested clinically by splenomegaly, mild lymphadenopathy, autoimmune phenomena and relative or absolute monocytosis.
Caspase-8 deficiency presents with findings of ALPS, such as mild lymphocyte accumulation, but also with a cellular immune deficiency, with defective function of T, B and NK cells.
FADD deficiency has been reported in only one family thus far, and presents mostly with as an immune deficiency, with recurrent bacterial and viral infections.
PKCδ deficiency is the newest addition to the group, and patients present with important lymphadenopathy and splenomegaly, autoimmune phenomena and clinical signs of NK cell deficiency, such as chronic EBV infection.
Acknowledgments
Research supported by a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq.
Footnotes
Conflics of interest
The author declares no conflicts of interest.
References
- 1.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 2.Bidere N, Su HC, Lenardo MJ. Genetic disorders of programmed cell death in the immune system. Annu Rev Immunol. 2006;24:321–352. doi: 10.1146/annurev.immunol.24.021605.090513. [DOI] [PubMed] [Google Scholar]
- 3.Sneller MC, Wang J, Dale JK, Strober W, Middelton LA, Choi Y, Fleisher TA, Lim MS, Jaffe ES, Puck JM, et al. Clincal, immunologic, and genetic features of an autoimmune lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Blood. 1997;89:1341–1348. [PubMed] [Google Scholar]
- 4.Oliveira JB, Bleesing JJ, Dianzani U, Fleisher TA, Jaffe ES, Lenardo MJ, Rieux-Laucat F, Siegel RM, Su HC, Teachey DT, et al. Revised diagnostic criteria and classification for the autoimmune lymphoproliferative syndrome (ALPS): report from the 2009 NIH International Workshop. Blood. 2010;116:e35–40. doi: 10.1182/blood-2010-04-280347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neven B, Magerus-Chatinet A, Florkin B, Gobert D, Lambotte O, De Somer L, Lanzarotti N, Stolzenberg MC, Bader-Meunier B, Aladjidi N, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118:4798–4807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 6.Bleesing JJ, Brown MR, Straus SE, Dale JK, Siegel RM, Johnson M, Lenardo MJ, Puck JM, Fleisher TA. Immunophenotypic profiles in families with autoimmune lymphoproliferative syndrome. Blood. 2001;98:2466–2473. doi: 10.1182/blood.v98.8.2466. [DOI] [PubMed] [Google Scholar]
- 7.Bleesing JJ, Brown MR, Dale JK, Straus SE, Lenardo MJ, Puck JM, Atkinson TP, Fleisher TA. TcR-alpha/beta(+) CD4(−)CD8(−) T cells in humans with the autoimmune lymphoproliferative syndrome express a novel CD45 isoform that is analogous to murine B220 and represents a marker of altered O-glycan biosynthesis. Clin Immunol. 2001;100:314–324. doi: 10.1006/clim.2001.5069. [DOI] [PubMed] [Google Scholar]
- 8.Kwon SW, Procter J, Dale JK, Straus SE, Stroncek DF. Neutrophil and platelet antibodies in autoimmune lymphoproliferative syndrome. Vox Sang. 2003;85:307–312. doi: 10.1111/j.0042-9007.2003.00374.x. [DOI] [PubMed] [Google Scholar]
- 9.Stroncek DF, Carter LB, Procter JL, Dale JK, Straus SE. RBC autoantibodies in autoimmune lymphoproliferative syndrome. Transfusion. 2001;41:18–23. doi: 10.1046/j.1537-2995.2001.41010018.x. [DOI] [PubMed] [Google Scholar]
- 10.Caminha I, Fleisher TA, Hornung RL, Dale JK, Niemela JE, Price S, Davis J, Perkins K, Dowdell KC, Brown MR, et al. Using biomarkers to predict the presence of FAS mutations in patients with features of the autoimmune lymphoproliferative syndrome. J Allergy Clin Immunol. 2010;125:946–949. doi: 10.1016/j.jaci.2009.12.983. e946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magerus-Chatinet A, Stolzenberg MC, Loffredo MS, Neven B, Schaffner C, Ducrot N, Arkwright PD, Bader-Meunier B, Barbot J, Blanche S, et al. FAS-L, IL-10, and double-negative CD4- CD8- TCR alpha/beta+ T cells are reliable markers of autoimmune lymphoproliferative syndrome (ALPS) associated with FAS loss of function. Blood. 2009;113:3027–3030. doi: 10.1182/blood-2008-09-179630. [DOI] [PubMed] [Google Scholar]
- 12.Dowdell KC, Niemela JE, Price S, Davis J, Hornung RL, Oliveira JB, Puck JM, Jaffe ES, Pittaluga S, Cohen JI, et al. Somatic FAS mutations are common in patients with genetically undefined autoimmune lymphoproliferative syndrome. Blood. 2010;115:5164–5169. doi: 10.1182/blood-2010-01-263145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim MS, Straus SE, Dale JK, Fleisher TA, Stetler-Stevenson M, Strober W, Sneller MC, Puck JM, Lenardo MJ, Elenitoba-Johnson KS, et al. Pathological findings in human autoimmune lymphoproliferative syndrome. Am J Pathol. 1998;153:1541–1550. doi: 10.1016/S0002-9440(10)65742-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maric I, Pittaluga S, Dale JK, Niemela JE, Delsol G, Diment J, Rosai J, Raffeld M, Puck JM, Straus SE, et al. Histologic features of sinus histiocytosis with massive lymphadenopathy in patients with autoimmune lymphoproliferative syndrome. Am J Surg Pathol. 2005;29:903–911. doi: 10.1097/01.pas.0000157997.61177.08. [DOI] [PubMed] [Google Scholar]
- 15.Straus SE, Jaffe ES, Puck JM, Dale JK, Elkon KB, Rosen-Wolff A, Peters AM, Sneller MC, Hallahan CW, Wang J, et al. The development of lymphomas in families with autoimmune lymphoproliferative syndrome with germline Fas mutations and defective lymphocyte apoptosis. Blood. 2001;98:194–200. doi: 10.1182/blood.v98.1.194. [DOI] [PubMed] [Google Scholar]
- 16.Kuehn HS, Caminha I, Niemela JE, Rao VK, Davis J, Fleisher TA, Oliveira JB. FAS haploinsufficiency is a common disease mechanism in the human autoimmune lymphoproliferative syndrome. J Immunol. 2011;186:6035–6043. doi: 10.4049/jimmunol.1100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behrmann I, Walczak H, Krammer PH. Structure of the human APO-1 gene. Eur J Immunol. 1994;24:3057–3062. doi: 10.1002/eji.1830241221. [DOI] [PubMed] [Google Scholar]
- 18.Teachey DT. New advances in the diagnosis and treatment of autoimmune lymphoproliferative syndrome. Curr Opin Pediatr. 2012;24:1–8. doi: 10.1097/MOP.0b013e32834ea739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegel RM, Frederiksen JK, Zacharias DA, Chan FK, Johnson M, Lynch D, Tsien RY, Lenardo MJ. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations. Science. 2000;288:2354–2357. doi: 10.1126/science.288.5475.2354. [DOI] [PubMed] [Google Scholar]
- 20.Wang L, Yang JK, Kabaleeswaran V, Rice AJ, Cruz AC, Park AY, Yin Q, Damko E, Jang SB, Raunser S, et al. The Fas-FADD death domain complex structure reveals the basis of DISC assembly and disease mutations. Nat Struct Mol Biol. 2010;17:1324–1329. doi: 10.1038/nsmb.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rieux-Laucat F, Le Deist F, Hivroz C, Roberts IA, Debatin KM, Fischer A, de Villartay JP. Mutations in Fas associated with human lymphoproliferative syndrome and autoimmunity. Science. 1995;268:1347–1349. doi: 10.1126/science.7539157. [DOI] [PubMed] [Google Scholar]
- 22.Hsu AP, Dowdell KC, Davis J, Niemela JE, Anderson SM, Shaw PA, Rao VK, Puck JM. Autoimmune lymphoproliferative syndrome due to FAS mutations outside the signal-transducing death domain: molecular mechanisms and clinical penetrance. Genet Med. 2012;14:81–89. doi: 10.1038/gim.0b013e3182310b7d. [DOI] [PubMed] [Google Scholar]
- 23.Magerus-Chatinet A, Neven B, Stolzenberg MC, Daussy C, Arkwright PD, Lanzarotti N, Schaffner C, Cluet-Dennetiere S, Haerynck F, Michel G, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121:106–112. doi: 10.1172/JCI43752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hauck F, Magerus-Chatinet A, Vicca S, Rensing-Ehl A, Roesen-Wolff A, Roesler J, Rieux-Laucat F. Somatic loss of heterozygosity, but not haploinsufficiency alone, leads to full-blown autoimmune lymphoproliferative syndrome in 1 of 12 family members with FAS start codon mutation. Clin Immunol. 2013;147:61–68. doi: 10.1016/j.clim.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Holzelova E, Vonarbourg C, Stolzenberg MC, Arkwright PD, Selz F, Prieur AM, Blanche S, Bartunkova J, Vilmer E, Fischer A, et al. Autoimmune lymphoproliferative syndrome with somatic Fas mutations. N Engl J Med. 2004;351:1409–1418. doi: 10.1056/NEJMoa040036. [DOI] [PubMed] [Google Scholar]
- 26.Zhu S, Hsu AP, Vacek MM, Zheng L, Schaffer AA, Dale JK, Davis J, Fischer RE, Straus SE, Boruchov D, et al. Genetic alterations in caspase-10 may be causative or protective in autoimmune lymphoproliferative syndrome. Hum Genet. 2006;119:284–294. doi: 10.1007/s00439-006-0138-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, Yao X, Puck JM, Straus SE, Lenardo MJ. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 28.Wu J, Wilson J, He J, Xiang L, Schur PH, Mountz JD. Fas ligand mutation in a patient with systemic lupus erythematosus and lymphoproliferative disease. J Clin Invest. 1996;98:1107–1113. doi: 10.1172/JCI118892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Del-Rey M, Ruiz-Contreras J, Bosque A, Calleja S, Gomez-Rial J, Roldan E, Morales P, Serrano A, Anel A, Paz-Artal E, et al. A homozygous Fas ligand gene mutation in a patient causes a new type of autoimmune lymphoproliferative syndrome. Blood. 2006;108:1306–1312. doi: 10.1182/blood-2006-04-015776. [DOI] [PubMed] [Google Scholar]
- 30.Bi LL, Pan G, Atkinson TP, Zheng L, Dale JK, Makris C, Reddy V, McDonald JM, Siegel RM, Puck JM, et al. Dominant inhibition of Fas ligand-mediated apoptosis due to a heterozygous mutation associated with autoimmune lymphoproliferative syndrome (ALPS) Type Ib. BMC Med Genet. 2007;8:41. doi: 10.1186/1471-2350-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magerus-Chatinet A, Stolzenberg MC, Lanzarotti N, Neven B, Daussy C, Picard C, Neveux N, Desai M, Rao M, Ghosh K, et al. Autoimmune lymphoproliferative syndrome caused by a homozygous null FAS ligand (FASLG) mutation. J Allergy Clin Immunol. 2013;131:486–490. doi: 10.1016/j.jaci.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118:5741–5751. doi: 10.1182/blood-2011-07-325217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao VK, Dugan F, Dale JK, Davis J, Tretler J, Hurley JK, Fleisher T, Puck J, Straus SE. Use of mycophenolate mofetil for chronic, refractory immune cytopenias in children with autoimmune lymphoproliferative syndrome. Br J Haematol. 2005;129:534–538. doi: 10.1111/j.1365-2141.2005.05496.x. [DOI] [PubMed] [Google Scholar]
- 34.Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, Manno C, Rappaport E, Schwabe D, Sheen C, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145:101–106. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, Dale JK, Fleisher TA, Middelton LA, Sneller MC, et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64:1002–1014. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun HJ, Zheng L, Ahmad M, Wang J, Speirs CK, Siegel RM, Dale JK, Puck J, Davis J, Hall CG, et al. Pleiotropic lymphocyte activation defects due to caspase-8 mutation cause human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 37.Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su H, Bidere N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S, Lenardo M. Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science. 2005;307:1465–1468. doi: 10.1126/science.1104765. [DOI] [PubMed] [Google Scholar]
- 39.Bidere N, Snow AL, Sakai K, Zheng L, Lenardo MJ. Caspase-8 regulation by direct interaction with TRAF6 in T cell receptor-induced NF-kappaB activation. Curr Biol. 2006;16:1666–1671. doi: 10.1016/j.cub.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 40.Bolze A, Byun M, McDonald D, Morgan NV, Abhyankar A, Premkumar L, Puel A, Bacon CM, Rieux-Laucat F, Pang K, et al. Whole-exome-sequencing-based discovery of human FADD deficiency. Am J Hum Genet. 2010;87:873–881. doi: 10.1016/j.ajhg.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park SM, Schickel R, Peter ME. Nonapoptotic functions of FADD-binding death receptors and their signaling molecules. Curr Opin Cell Biol. 2005;17:610–616. doi: 10.1016/j.ceb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Balachandran S, Venkataraman T, Fisher PB, Barber GN. Fas-associated death domain-containing protein-mediated antiviral innate immune signaling involves the regulation of Irf7. J Immunol. 2007;178:2429–2439. doi: 10.4049/jimmunol.178.4.2429. [DOI] [PubMed] [Google Scholar]
- 43.Niemela JE, Lu L, Fleisher TA, Davis J, Caminha I, Natter M, Beer LA, Dowdell KC, Pittaluga S, Raffeld M, et al. Somatic KRAS mutations associated with a human nonmalignant syndrome of autoimmunity and abnormal leukocyte homeostasis. Blood. 2011;117:2883–2886. doi: 10.1182/blood-2010-07-295501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliveira JB, Bidere N, Niemela JE, Zheng L, Sakai K, Nix CP, Danner RL, Barb J, Munson PJ, Puck JM, et al. NRAS mutation causes a human autoimmune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104:8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi M, Shinoda K, Piao J, Mitsuiki N, Matsuda K, Muramatsu H, Doisaki S, Nagasawa M, Morio T, Kasahara Y, et al. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood. 2011;117:2887–2890. doi: 10.1182/blood-2010-08-301515. [DOI] [PubMed] [Google Scholar]
- 46.de Vos AM, Tong L, Milburn MV, Matias PM, Jancarik J, Noguchi S, Nishimura S, Miura K, Ohtsuka E, Kim SH. Three-dimensional structure of an oncogene protein: catalytic domain of human c-H-ras p21. Science. 1988;239:888–893. doi: 10.1126/science.2448879. [DOI] [PubMed] [Google Scholar]
- 47.O'Reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DC, Strasser A. MEK/ERK-mediated phosphorylation of Bim is required to ensure survival of T and B lymphocytes during mitogenic stimulation. J Immunol. 2009;183:261–269. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- 49.Limnander A, Weiss A. Ca-dependent Ras/Erk signaling mediates negative selection of autoreactive B cells. Small Gtpases. 2011;2:282–288. doi: 10.4161/sgtp.2.5.17794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, Roose JP, Weiss A. STIM1, PKC-delta and RasGRP set a threshold for proapoptotic Erk signaling during B cell development. Nat Immunol. 2011;12:425–433. doi: 10.1038/ni.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, Hussey AA, Evbuomwan MO, Priel DA, Kuhns DB, et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121:3117–3125. doi: 10.1182/blood-2012-12-469544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salzer E, Santos-Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, Garncarz W, Mullauer L, Kain R, Boztug H, et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C delta. Blood. 2013;121:3112–3116. doi: 10.1182/blood-2012-10-460741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, et al. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002;416:865–869. doi: 10.1038/416865a. [DOI] [PubMed] [Google Scholar]
- 54.Berridge MJ, Irvine RF. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984;312:315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- 55.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 56.Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, Li L, Yuspa SH, Kazanietz MG. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- 57.Guo B, Su TT, Rawlings DJ. Protein kinase C family functions in B-cell activation. Curr Opin Immunol. 2004;16:367–373. doi: 10.1016/j.coi.2004.03.012. [DOI] [PubMed] [Google Scholar]
Bullets and annotations
- 57.Magerus-Chatinet A, Neven B, Stolzenberg MC, Daussy C, Arkwright PD, Lanzarotti N, Schaffner C, Cluet-Dennetiere S, Haerynck F, Michel G, et al. Onset of autoimmune lymphoproliferative syndrome (ALPS) in humans as a consequence of genetic defect accumulation. J Clin Invest. 2011;121:106–112. doi: 10.1172/JCI43752. *First paper demonstrating that the combination of somatic and germline mutations in FAS might cooperate to induce autoimmunity in patients with mild ALPS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niemela JE, Lu L, Fleisher TA, Davis J, Caminha I, Natter M, Beer LA, Dowdell KC, Pittaluga S, Raffeld M, et al. Somatic KRAS mutations associated with a human nonmalignant syndrome of autoimmunity and abnormal leukocyte homeostasis. Blood. 2011;117:2883–2886. doi: 10.1182/blood-2010-07-295501. *First description of somatic mutations in KRAS causing an ALPS-like syndrome in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi M, Shinoda K, Piao J, Mitsuiki N, Matsuda K, Muramatsu H, Doisaki S, Nagasawa M, Morio T, Kasahara Y, et al. Autoimmune lymphoproliferative syndrome-like disease with somatic KRAS mutation. Blood. 2011;117:2887–2890. doi: 10.1182/blood-2010-08-301515. *First description of somatic mutations in KRAS causing an ALPS-like syndrome in humans. [DOI] [PubMed] [Google Scholar]
- 51.Kuehn HS, Niemela JE, Rangel-Santos A, Zhang M, Pittaluga S, Stoddard JL, Hussey AA, Evbuomwan MO, Priel DA, Kuhns DB, et al. Loss-of-function of the protein kinase C delta (PKCdelta) causes a B-cell lymphoproliferative syndrome in humans. Blood. 2013;121:3117–3125. doi: 10.1182/blood-2012-12-469544. **First report demonstrating that mutations in PRKCD cause increased B-cell proliferation, tolerance breakdown and NK cell defects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salzer E, Santos-Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, Garncarz W, Mullauer L, Kain R, Boztug H, et al. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C delta. Blood. 2013;121:3112–3116. doi: 10.1182/blood-2012-10-460741. ** **First report demonstrating that mutations in PRKCD cause increased B-cell proliferation and tolerance breakdown. [DOI] [PMC free article] [PubMed] [Google Scholar]