Abstract
Purpose
Examine mediators of fatigue response to an exercise intervention for breast cancer survivors (BCS) in a pilot randomized controlled trial.
Methods
Postmenopausal BCS (n=46; ≤ Stage II), off primary treatment, and reporting fatigue and/or sleep dysfunction were randomized to a 3-month exercise intervention (160 minutes/week of moderate intensity aerobic walking, twice weekly resistance training with resistance bands) or control group. Six discussion group sessions provided behavioral support to improve adherence. Fatigue, serum cytokines, accelerometer physical activity, cardiorespiratory fitness, sleep dysfunction, and psychosocial factors were assessed at baseline and 3 months.
Results
Exercise intervention effect sizes for fatigue were: fatigue intensity d=0.30 (p=.34), interference d=−0.38 (p=.22), and general fatigue d=−0.49 (p=.13). Using Freedman-Schatzkin difference-in-coefficients tests, increase in fatigue intensity was significantly mediated by interleukin (IL)-6 (82%), IL-10 (94%), IL-6:IL-10 (49%), and tumor necrosis factor (TNF)-alpha:IL-10 (78%) with reduced sleep dysfunction increasing the relationship between intervention and fatigue intensity rather than mediating intervention effects (−88%). Decrease in fatigue interference was mediated by sleep dysfunction (35%) while IL-10 and pro:anti-inflammatory cytokine ratios increased the relationship between intervention and interference (−25% to −40%). The reduction in general fatigue was significantly mediated by minutes of physical activity (76%), sleep dysfunction (45%), and physical activity enjoyment (40%) with IL-10 (−40%) and IL-6:IL-10 (−11%) increasing the intervention-fatigue relationship. In the intervention group, higher baseline fatigue, anxiety, depression, and perceived exercise barriers interference predicted a greater decline in fatigue interference and/or general fatigue during the intervention.
Conclusions
Biobehavioral factors mediated and enhanced intervention effects on fatigue while psychosocial factors predicted fatigue response. Further study is warranted to confirm our results and improve understanding of relationships that mediate and strengthen the intervention-fatigue association.
Keywords: oncology, prevention, determinants, survivorship, predictors
Introduction
Recent meta-analyses support exercise as a treatment modality for fatigue after a cancer diagnosis (40). Nevertheless, half of exercise intervention trials have not demonstrated significant reductions in fatigue (40) and not all exercise trial participants report reduced fatigue with an exercise intervention (29). Inconsistent reports of exercise effects on fatigue may be due, in part, to differences in fatigue measures, exercise prescriptions, and baseline fatigue levels along with failure to tailor based on the multifactorial biobehavioral mechanisms underlying fatigue (1, 19, 28). Fatigue is described by patients as encompassing more than physical fatigue alone (13) and is sometimes assessed as “peripheral” or “central” (10). Two randomized exercise trials (one in breast cancer and one in hematologic cancer receiving bone marrow transplant) have demonstrated improvements in physical but not mental fatigue (16, 45). In cross-sectional studies, exercise demonstrated variable associations among different fatigue aspects in 58 head and neck cancer patients with the largest correlation being with average fatigue per day (r = −.18) and days per week fatigued (r = −.22) (30). Among 525 bladder cancer survivors, exercising at least 150 minutes per week was associated with less average fatigue and fatigue interference but was not associated with days per week fatigued or level of most intense fatigue (17). Additional data from randomized trials regarding how exercise effects may vary depending on the fatigue aspect assessed and measurement tool used is needed. These data will inform fatigue measurement choice for future exercise and cancer trials and facilitate tailoring of exercise recommendations based on the nature of the fatigue experienced by a cancer survivor.
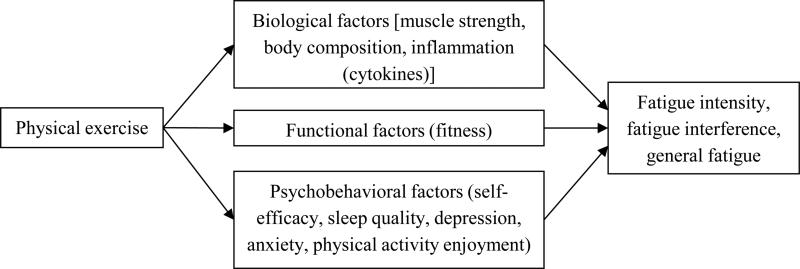
In addition to consideration of the fatigue outcome measure, exercise effectiveness as a treatment for fatigue could be improved by targeting the most important mediators responsible for exercise effects on fatigue. No prior prospective, randomized exercise and cancer trial has reported mediators of exercise effects on fatigue or attempted to examine mediators within a biobehavioral framework. Fatigue is often a significant and persistent symptom after cancer diagnosis and mediates the benefits of exercise on quality of life (4). Post-cancer fatigue may be caused by physical deconditioning (44), comorbid conditions (35), inflammation (39), psychosocial factors (25), neurotransmitter dysregulation (36), alterations in the hypothalamic-pituitary-adrenal axis function (36), and sleep disruption (36). Attempts to combine these multiple factors into theoretical models explaining the underlying mechanisms for fatigue after cancer diagnosis are limited, in part, by the lack of clear articulation of specific pathways linking factors to the patient-reported outcome (26). In contrast Al-Majid and Gray (1) have proposed a theoretical model articulating explicit pathways that are altered by cancer and its treatments but may also be potential targets improved with exercise. For example, cancer and its treatments lead to biological effects (e.g., decreased muscle strength, increases in pro-inflammatory cytokines), functional effects (e.g., decreased fitness), and psychobehavioral factors (e.g., sleep dysfunction, anxiety, depression) which contribute to cancer-related fatigue (Figure 1). Targeting these effects with exercise could potentially reverse the cancer and cancer treatment effects resulting in a reduction in fatigue.
Figure 1.
Hypothesized mediators of exercise effects on fatigue among breast cancer survivors based on Al-Majid and Gray (1)
Therefore, our specific aims were to obtain preliminary, pilot data that examines the: 1) effects of an exercise intervention for breast cancer survivors on three aspects of fatigue and 2) mediators of fatigue response to the intervention. We hypothesized that when compared with the control group, the intervention group would demonstrate a reduction in fatigue that varied based on the aspect measured. We also hypothesized that fatigue effects would be mediated by biological, functional, and biobehavioral factors as depicted in Figure 1. Although we focused on serum inflammatory markers during protocol design, the study outcomes were analyzed within a biobehavioral framework to be consistent with the rising interest in examining multifactorial mediators of intervention effects on cancer-related fatigue as a way to improve future interventions (2).
Methods
Setting, participants, and study design
The protocol was approved by the local institutional review board. Informed consent was obtained from all participants before beginning study activities. Study criteria were as follows:
Inclusion criteria
1) Female, 30 to 70 years of age, ductal carcinoma in situ (DCIS), Stage I or II breast cancer, 2) At least 4 weeks status post final primary treatment administration (longer term therapies such as aromatase inhibitors, estrogen receptor modulators were allowed), 3) ≥ 8 weeks post-surgical procedure, 4) English speaking, 5) Medical clearance for participation provided by physician, 6) Postmenopausal, 7) Average fatigue over the past week rated as ≥ 3 on a 1 to 10 Likert scale (11) or sleep dysfunction ≥ 1 on a 0 to 3 Likert scale (5), 8) Willing to abstain from “as needed” medications for 7 days prior to each blood draw. (Note: This pilot study was designed to collect preliminary results for both fatigue and sleep dysfunction. A comprehensive reporting of the sleep outcomes is beyond the scope of this report and is anticipated to be published separately. The inclusion of participants with either fatigue or sleep dysfunction was done to reduce the risk of a “floor effect” related to fatigue and sleep dysfunction while avoiding overly restrictive inclusion criteria that would have impeded our ability to recruit within the time frame limited by budgetary constraints.)
Exclusion criteria
1) Metastatic or recurrent breast cancer, 2) Unable to ambulate without assistance, 3) Unstable angina, 4) New York Heart Association class II, III, or IV congestive heart failure, 5) Uncontrolled asthma, 6) Interstitial lung disease, 7) Current use of steroids, 8) Having been told by a physician to only do exercise prescribed by a physician, 9) Dementia or organic brain syndrome, 10) Schizophrenia or active psychosis, 11) Connective tissue or rheumatologic disease [i.e., Systemic lupus erythematosus, rheumatoid arthritis, amyloidosis, Reiter's syndrome, psoriatic arthritis, mixed connective tissue disease, Sjögren's syndrome, progressive systemic sclerosis, CREST syndrome, polymyositis, dermatomyositis, vasculitis, polymyalgia rheumatic, temporal arteritis], 12) Participating, on average, in more than 20 minutes of physical activity on two or more days per week during the past six months, 13) Elective surgery planned for during the time of the intervention which would interfere with intervention participation (e.g., breast reconstructive surgery), 14) Live or work > 50 miles from the study site, 15) Lack of transportation to the study site, 16) Changes in usual medications expected during the study time period, 17) Plan to move residence out of the local area during the 5 months of study participation, 18) Plan to travel out of the local area for vacation during the first 4 weeks of the intervention or plan to travel out of the local area for more than a week during the last 8 weeks of the intervention, 19) Contraindication to participation in exercise (i.e., moderate intensity walking and strength training with resistance bands).
This two-arm randomized controlled pilot study took place at a Midwestern academic center in a small urban setting adjacent to a rural population. An exercise intervention was compared with control group with measurements obtained at baseline (pre-intervention; M0) and 3 months (post-intervention; M3). Participants were paid a small monetary incentive after completion of each assessment. Eligible participants were stratified by DCIS versus Stage I or II before randomization in blocks of four based on computer generated numbers. Participants were randomized in the order in which they completed baseline testing. Randomization numbers were kept in sealed, opaque envelopes so that study staff and participants were unaware of group allocation until all baseline testing was complete.
Exercise intervention
The exercise intervention combined aerobic walking with strength training using resistance bands. For the aerobic component, participants were gradually advanced by week 9 to 40-minute bouts of moderate intensity walking 4 times per week with no more than one day in between bouts (e.g., exercise on Monday, Wednesday, Thursday, Saturday each week or Tuesday, Wednesday, Friday, Sunday each week) resulting in a total weekly goal of 160 aerobic minutes. Moderate exercise intensity was based on the Karvonen method (i.e., 48% to 52% of heart rate reserve). Participants attended 26 individual supervised exercise sessions with an exercise specialist (three per week for the first 2 weeks and two per week for the last 10 weeks). Participants were also instructed to exercise at home (two walking sessions per week in the last 10 weeks of the intervention). The resistance training occurred twice weekly during the same sessions as the supervised aerobic walking (e.g., Monday/Thursday or Tuesday/Friday). The strength of the resistance bands were advanced as tolerated at intervals of ≥ 2 weeks. Eight different resistance exercises focused on the major muscle groups were included with up to 2 sets of 15 repetitions per exercise per exercise. To improve adherence, behavioral support was provided by six group meetings with a clinical psychologist or psychology intern under the supervision of a clinical psychologist (every other week) based on a prior successful behavior change intervention (32). Intervention participation occurred in cohorts or “waves” to facilitate the social support provided by the group meetings.
Control group instructions
The control group was instructed not to change their exercise behavior beyond what they were doing at time of study enrollment.
Measures: General
The following were measured by self-administered survey: age, race, ethnicity, education, annual household income, marital status, cancer stage, prior cancer treatment (chemotherapy, radiation), current anti-estrogen therapy, medical comorbidity score (14), smoking status, and prior receipt of physician exercise counseling. Participants kept a medication log for the seven days prior to each blood draw which was reviewed by a licensed physician on the investigative team (Rogers) for medication changes which might influence serum cytokine levels. Three-day diet records were collected (one weekend and two week days) and analyzed for carbohydrate differences which might act as a covariate (3). To facilitate consistency, the same staff member analyzed all diet records; diet data were analyzed using FoodWorks 13 (Long Valley, NJ). MTI/Actigraph accelerometer was worn for seven days; four valid days were required for analysis. Cutpoints for physical activity intensity were sedentary = 0-99 (9), inactive = 100-499, light activity = 500-1951, moderate activity = 1952-5724, vigorous = ≥ 5725 (12). Supervised session records completed by the supervising exercise specialists were used to determine adherence to resistance training and supervised aerobic exercise sessions.
Measures: Fatigue
Two different scales were used to measure fatigue. First, the Fatigue Symptom Inventory (15) was used to measure fatigue intensity (mean of 4 items, 1 to 10 scale) and fatigue interference (mean of 6 items, 1 to 10 scale). Patient Reported Outcomes Measurement Information System (PROMIS®) scale was used to measure general fatigue [seven items using a Likert scale (1 = rarely to 5 = always)] (http://www.nihpromis.org/default.aspx). Items were summed and then converted to a T score as provided on the PROMIS® website (http://www.nihpromis.org/Documents/PROMIS_Age_Gender_Comorbidity.pdf). For all measures used, a higher score indicated greater fatigue.
Measures: Potential mediators
For body composition, measured height and weight were used to calculate body mass index [BMI; (weight in pounds/height in inches squared) times 703] and circumferences were obtained for the calculation of the waist-to-hip ratio. Bioelectric impedance (i.e., Quantum X by RJL Systems) was used to assess percent body fat (i.e., performed same time of day for each measurement after a ≥ 4 hour fast). Extensor leg strength was assessed using a back and leg dynamometer (Takei, model T.K.K. 5002). The participant completed three trials with one-minute rest between trials. The maximum reading (best of the three efforts) provided the absolute strength measure. Cardiorespiratory fitness was estimated from a submaximal treadmill test using a modified Naughton protocol (43). Physical measures were obtained by individuals who were blinded to the participant's study group allocation.
Serum samples for interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor (TNF)-alpha were obtained after a 12-hour fast by an experienced phlebotomist between 7:45 AM and 10:00 AM. Participants were instructed to not take sporadic or “as needed” medications for seven days prior to the blood draw. During the 24 hours prior to the blood draw, participants abstained from exercise, smoking, and alcohol. Blood samples were collected, processed and stored using a standard operating procedure consistent with expert consensus recommendations (41). Samples were batch analyzed according to manufacturer's instructions by an investigator who was unaware of the participants’ group allocations. Luminex® technology was used to measure IL-6, IL-8, IL-10, and TNF-alpha using the High Sensitivity Human cytokine assay (Cat # HSCYTO-60SK, Millipore Corp. Billerica, MA). Detection limits were 0.1pg/mL for IL-6, 0.11 pg/mL for IL-8, 0.15pg/mL for IL-10, and 0.05 pg/mL for TNF-alpha. Cytokines were analyzed individually and as pro-inflammatory to anti-inflammatory ratios (i.e., IL-6:IL-10, IL-8:IL-10, and TNF-alpha:IL-10).
Patient Reported Outcomes Measurement Information System (PROMIS®) scale was used to measure depression (8 items), anxiety (7 items), and sleep/wake disturbances (16 items) with all items using a 1 to 5 Likert scale (http://www.nihpromis.org/default.aspx). Sums were converted to T scores for the analysis according to conversion tables published on the PROMIS® website. Higher scores indicate greater depressive symptoms, greater anxiety, or greater sleep/wake disturbances. Walking self-efficacy was measured using a 6 item scale asking participants to rate their confidence (0% to 100% in 10% increments) in their ability to walk at a moderately fast pace without stopping for 5 minutes up to 30 minutes in 5-minute increments (24). This scale has been validated based on significant associations with physical activity and functioning in cross-sectional and prospective studies (22, 23). Exercise social support was measured using 4 items asking the frequency with which family or friends had offered to exercise with the participant or given the participant encouragement to stick with their exercise program (38). The 5-point Likert responses (0 = rarely to 4 = very often) were summed for the analysis. Physical activity enjoyment was a single item asking the participant their agreement with the following statement “I enjoy engaging in regular physical activity” (1 = disagree to 5 = agree) (34).
Data analysis
Baseline characteristics for the intervention versus control group were compared with independent groups t-test or chi-square. Intent-to-treat analysis was performed (i.e., differences between the study groups were assessed with all data regardless of the participant's adherence to the exercise in the intervention group or self-initiation of exercise in the control group). Within group changes over time were tested with paired t-test. Between group differences were tested with independent groups t-tests. (Of note, non-parametric tests were performed and provided similar results to that of the parametric procedures. To allow expression of data in the unit of measure rather than the rank score, the parametric results are reported here.) Freedman and Schatzkin difference-in-coefficients test was used to test mediation of the intervention effects on fatigue. Freedman and Schatzkin test was chosen because of its increased study power when testing mediation in small randomized trials (7). This procedure also results in the most accurate Type I error rates when the relationship between the intervention and the potential mediator or between the mediator and the outcome are both null (21). Moreover, Freedman and Schatzkin does not require that both the relationship between the intervention and mediator and the relationship between the mediator and outcome be significant (21). In smaller pilot studies, important mediators may be missed using other methods because one of these relationships may lack statistical significance due to low study power. This was particularly important because of the pilot nature of the study described in this report. Mediation is considered statistically significant when including the potential mediator in the final model significantly reduces the relationship between the intervention and outcome (e.g., fatigue). In this case, mediation is reported as the proportion (or percentage) of the intervention effect that is due to the change in the mediator occurring during the intervention (reported as a positive percentage). When the final model which includes the mediator detects a stronger statistical relationship between the intervention and the outcome (i.e., negative percent change), then the change in the factor during the intervention is influencing the intervention-outcome relationship rather than mediating the relationship. Mediators were tested if they were statistically significant (or close to significant) on the between group differences or the paired t-test. Because of the pilot nature of the study and the need to generate hypotheses related to potential mediators warranting further study, we tested mediation using all fatigue outcomes and inflammatory markers regardless of significance of the intervention effect. For all statistical testing, a p value of < .05 was considered statistically significant.
Results
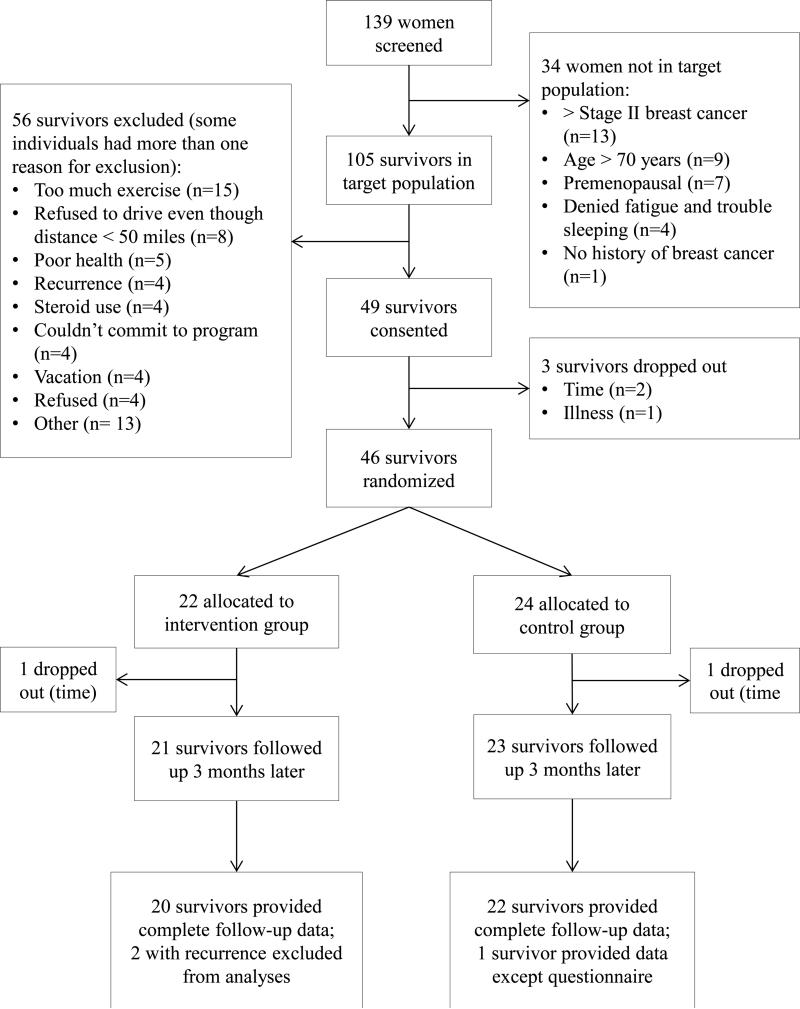
Participant flow is provided in Figure 2. The target population was defined based on cancer type and stage, fatigue and/or sleep dysfunction, age, and menopausal status with 34 (24%) excluded because they were not within the target population. Of the target population screened (n = 105), 56 (53%) were excluded with the most prevalent reasons for exclusion being: currently exercising more than 20 minutes on more than two days per week (n = 15), live or work > 50 miles from study site (n = 8), poor health (n = 5), not first time breast cancer diagnosis (i.e., recurrence) (n = 4), steroid use (n = 4), vacation plans (n = 4), unable to commit to the program (n = 4), and refused (n = 4). Of the 49 consented (47% of the target population), three dropped out before randomization due to illness (n = 1) or lack of time (n = 2). Of the 46 completing baseline testing and undergoing randomization, 22 were randomized to the intervention group and 24 to the control group with one in each study group dropping out before M3 testing (both due to time). Therefore, 44 (retention rate of 96% of randomized participants) completed the M3 assessment. However, two participants developed cancer recurrence during the trial (both in the intervention group). These two participants were dropped from the analysis for scientific reasons (i.e., the potential impact of the cancer recurrence on cytokine levels could erroneously skew the results farther from the reality of that which can be expected in survivors without recurrence). This conservative approach was acceptable because analyses results with and without the participants with recurrence were not substantially different confirming that removing the participants with recurrence did not manipulate the data for a more favorable result. We did not perform the sensitivity analysis because only two drop outs occurred with these being evenly distributed between the study groups and both being due to the same reason (i.e., time) thus removing any systematic bias that might be caused if both drop outs had occurred in the same study group or for different reasons. The less complex statistical approach is acceptable for this pilot study but future trials should consider performing sensitivity analysis as a method for dealing with the possibility that drop outs are not a random occurrence. Therefore, 42 participants (91% of the 46 randomized) were included in the final analyses reported here.
Figure 2.
Participant recruitment, allocation, and retention by study group.
No serious adverse events occurred. Of the related non-serious adverse events, two occurred in the intervention group (modification of resistance exercise required due to ongoing preexistent lymphedema symptoms and mild hematoma at site of blood draw) and two in the control group (both experienced elevated blood pressure during treadmill fitness test and were instructed to discuss with primary care physician). Two unrelated non-serious adverse events occurred (both in the intervention group) and included broken wrist due to a motor vehicle accident and new breast lump with negative mammogram.
Sample characteristics (combined and stratified by study group allocation) for all participants completing both baseline and follow-up assessments are provided in Table 1. The study groups differed significantly with regard to the percent who were never smokers (45% in control versus 74% in the intervention group, p = .04). Adjusting for smoking status did not significantly change our study results, therefore, the unadjusted results are presented in this report. At time of enrollment, 68% of the participants were fatigued and 93% reported sleep dysfunction with 64% reporting both symptoms. Medication changes during study participation reported on the medication log which may have influenced cytokine levels included changes in the following: nonsteroidal anti-inflammatory drugs (NSAIDs including aspirin) (n = 14), antihistamines (n = 5), HMG CoA reductase inhibitors (n = 3), selective serotonin receptor inhibiors (SSRIs) (n = 2), fish oils (n = 2), beta blocker (n = 1), and tamoxifen (n = 1) (note: participants may have had more than one medication change).
Table 1.
Baseline characteristics of participants overall and by group allocation [Mean ± SD (range) or n (%)]
| Variable | Overall (n = 44) | Control (n = 24) | Intervention (n = 20) | P value |
|---|---|---|---|---|
| Age, years | 56.2 ± 7.7 (32 - 69) | 55.2 ± 9.1 (32 - 67) | 57.2 ± 5.5 (45 - 69) | .38 |
| Race | ||||
| White | 42 (95.5%) | 22 (91.7%) | 20 (100.0%) | .49 |
| Other | 2 (4.5%) | 2 (8.3%) | 0 (0.0%) | |
| Education, years | 14.0 ± 2.2 (12 - 20) | 14.0 ± 2.4 (12 - 20) | 14.0 ± 1.9 (12 - 18) | .95 |
| Income | ||||
| < $10K | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | .65 |
| $10K to $20K | 1 (2.3%) | 0 (0.0%) | 1 (5.0%) | |
| $20K to $35K | 6 (13.6%) | 4 (16.7%) | 2 (10.0%) | |
| $35K to $50K | 5 (11.4%) | 3 (12.5%) | 2 (10.0%) | |
| ≥ $50K | 32 (72.7%) | 17 (70.8%) | 15 (75%) | |
| Marital status | ||||
| Married or living with sig other | 31 (70.5%) | 18 (75.0%) | 13 (65.0%) | .47 |
| Other | 13 (29.5%) | 6 (25.0%) | 7 (35.0%) | |
| Cancer stage | ||||
| DCIS | 8 (18.2%) | 5 (20.8%) | 3 (15.0%) | .88 |
| I | 21 (47.7%) | 11 (45.8%) | 10 (50.0%) | |
| II | 15 (34.1%) | 8 (33.3%) | 7 (35.0%) | |
| Received chemotherapy | 18 (40.9%) | 10 (41.7%) | 8 (40.0%) | .91 |
| Months since chemotherapy | 70.3 ± 65.5 (3 - 222) | 64.7 ± 51.0 (3 - 144) | 76.6 ± 82.2 (9 - 222) | .72 |
| Received radiation | 28 (63.6%) | 14 (58.3%) | 14 (70.0%) | .42 |
| Months since radiation | 44.1 ± 41.8 (1 - 168) | 37.6 ± 38.5 (1 - 132) | 51.2 ± 45.5 (12- 168) | .41 |
| Hormonal therapy | ||||
| Estrogen receptor modulator (yes) | 7 (15.9%) | 2 (8.3%) | 5 (25.0%) | .13 |
| Aromatase inhibitor (yes) | 16 (36.4%) | 9 (37.5%) | 7 (35.0%) | .86 |
| Months on hormonal therapy | 22.5 ± 18.7 | 16.9 ± 17.5 | 27.6 ± 18.9 | .18 |
| 1 - 59 | 1 - 53 | 1.3 - 59 | ||
| Beta blocker (yes) | 8 (18.2%) | 4 (16.7%) | 4 (20.0%) | 1.00 |
| Comorbidity score | 2.1 ± 1.6 | 2.0 ± 1.6 | 2.1 ± 1.7 | .91 |
| 0 - 6 | 0 - 6 | 0 - 6 | ||
| Smoker | ||||
| Never | 27 (61.4%) | 18 (75.0%) | 9 (45.0%) | .042 |
| Current or Ex | 17 (38.6%) | 6 (25.0%) | 11 (55.0%) | |
| Physician ever advised to exercise | 38 (86.4%) | 20 (83.3%) | 18 (90.0%) | .62 |
| Medication changes which may have effected cytokines (inflammation) | 20 (48.8%) | 11 (47.83%) | 9 (50.0%) | .89 |
| Possible effect of medication change on cytokines (inflammation) | ||||
| Decrease | 10 (24.4%) | 5 (21.7%) | 5 (27.8%) | .35 |
| No change (n=21) or both directions (n=4) | 25 (61.0%) | 13 (56.5%) | 12 (66.7%) | |
| Increase | 6 (14.6%) | 5 (21.7%) | 1 (5.6%) | |
| Carbohydrate (grams) | 200 ± 77 | 203 ± 79 | 198 ± 76 | .83 |
Excluding the two participants developing cancer recurrence during the trial, adherence to supervised aerobic exercise sessions was 91% and adherence to the resistance exercise sessions was 93% (based on session record sheets). Based on accelerometer monitoring, weekly minutes of ≥ moderate intensity exercise significantly increased from baseline to 3 months in the intervention compared with control group with the intervention group mean at 3 months being 294 ± 175 weekly minutes. Weekly minutes of ≥ moderate intensity exercise did not significantly increase in the control group (baseline mean = 148 ± 79; 3-month mean = 154 ± 75 minutes; paired t-test p value not significant). Based on exercise logs, the exercise goal with regard to total minutes of exercise done at home was met in 65% of the possible weeks. Discussion group attendance for all participants and waves combined was 94%. No protocol deviations occurred, however, DCIS was added to the inclusion criteria mid-trial to facilitate recruitment.
The preliminary effects of our exercise program on fatigue and possible mediators are provided in Tables 2 and 3. A medium negative effect size was noted for PROMIS® fatigue (d = −0.49) and fatigue interference (d = −0.38) with a small positive effect size noted for fatigue intensity (d = 0.30) (all p values >.10). Potential mediators with effect sizes that were statistically significant included accelerometer measured ≥ moderate intensity physical activity (d = 1.15, p<.01), walking self-efficacy (d = 0.66, p<.05), exercise social support (d = 0.85, p<.01), and physical activity enjoyment (d = 0.63, p<.05). A trend in fewer anxiety symptoms for intervention compared with control group was noted (d = −0.54, p<.10).
Table 2.
Preliminary effects of a walking program plus resistance exercise on sedentary activity, minutes of physical activity, and fatigue in breast cancer survivors post-primary treatment (participants with complete data, n = 42)
| Month 0 | Month 3 | Change over time | Between group difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | Mean | (SDa) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Effect Size | ||
| Weekly minutes sedentary behavior | Intervention | 7208.3 | (866.9) | 7141.4 | (536.2) | −84.4 | (788.2) | |||||
| Control | 7577.0 | (620.7) | 7549.2 | (628.4) | −55.2 | (435.3) | −29.2 | (619.5) | −0.05 | |||
| Weekly minutes ≥ moderate intensity physical activity | Intervention | 181 | (152) | 294 | (175) | 114 | *** | (109) | ||||
| Control | 148 | (79) | 154 | (75) | 10 | (70) | 103 | 89) | 1.15 | *** | ||
| Fatigue intensity | Intervention | 3.6 | (1.5) | 4.1 | (2.1) | 0.4 | (1.9) | |||||
| Control | 4.2 | (2.1) | 4.0 | (1.8) | −0.1 | (1.9) | 0.6 | (1.9) | 0.30 | |||
| Fatigue interference | Intervention | 2.2 | (1.4) | 1.8 | (0.8) | −0.4 | * | (1.1) | ||||
| Control | 2.5 | (1.7) | 2.6 | (1.9) | 0.1 | (1.8) | −0.6 | (1.5) | −0.38 | |||
| PROMIS® fatigue | Intervention | 51.1 | (5.3) | 47.6 | (5.4) | −3.8 | *** | (4.1) | ||||
| Control | 52.7 | (8.1) | 51.6 | (6.9) | −1.1 | (6.4) | −2.7 | (5.4) | −0.49 | |||
SD= standard deviation
p<.10 (trend only)
**p<.05
p<.01
Table 3.
Preliminary effects of a walking program plus resistance exercise on potential mediators of fatigue response to exercise in breast cancer survivors post-primary treatment (participants with complete data, n=42)
| Month 0 | Month 3 | Change over time | Between group difference | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Group | Mean | (SDa) | Mean | (SD) | Mean | (SD) | Mean | (SD) | Effect Size | ||
| Body mass index | Intervention | 29.8 | (4.8) | 29.6 | (5.0) | −0.2 | (0.9) | |||||
| Control | 32.6 | (6.6) | 32.2 | (6.7) | −0.3 | (1.1) | 0.1 | (1.0) | 0.14 | |||
| Waist-to-hip ratio | Intervention | 0.8 | (0.1) | 0.8 | (0.1) | 0.0 | (0.0) | |||||
| Control | 0.9 | (0.1) | 0.9 | (0.1) | 0.0 | (0.1) | −0.0 | (0.1) | −0.10 | |||
| Percent body fat | Intervention | 39.7 | (7.0) | 38.6 | (6.4) | −1.1 | ** | (2.2) | ||||
| Control | 42.2 | (7.6) | 41.5 | (7.4) | −0.6 | (2.4) | −0.4 | (2.3) | −0.19 | |||
| Back/leg extensor muscle strength | Intervention | 61.8 | (19.3) | 65.9 | (18.2) | 4.1 | (14.4) | |||||
| Control | 67.8 | (29.8) | 73.1 | (26.3) | 5.2 | (19.7) | −1.2 | (17.5) | −0.07 | |||
| ILb-6 (pg/ml) | Intervention | 2.6 | (1.8) | 2.8 | (2.2) | 0.0 | (1.5) | |||||
| Control | 7.8 | (16.2) | 7.3 | (11.6) | −0.7 | (6.1) | 0.8 | (4.7) | 0.16 | |||
| IL-8 (pg/ml) | Intervention | 6.8 | (6.3) | 5.4 | (2.2) | −1.7 | (5.6) | |||||
| Control | 6.1 | (3.1) | 6.3 | (4.7) | 0.1 | (2.8) | −1.7 | (4.3) | −0.40 | |||
| IL-10 (pg/ml) | Intervention | 5.5 | (4.2) | 4.7 | (3.6) | −1.0 | * | (2.4) | ||||
| Control | 8.4 | (12.8) | 7.4 | (9.9) | −0.4 | (4.9) | −0.7 | (4.0) | −0.17 | |||
| TNFc-alpha (pg/ml) | Intervention | 7.7 | (3.7) | 8.5 | (4.2) | 0.6 | (2.4) | |||||
| Control | 12.9 | (18.8) | 12.6 | (18.1) | −0.6 | (2.6) | 1.3 | (2.5) | 0.50 | |||
| IL-6:IL-10 | Intervention | 1.1 | (2.6) | 1.0 | (1.4) | −0.2 | (2.8) | |||||
| Control | 5.3 | (10.6) | 6.1 | (13.4) | 0.3 | (5.2) | −0.6 | (4.3) | −0.13 | |||
| IL-8:IL-10 | Intervention | 3.5 | (9.4) | 2.5 | (4.5) | −1.1 | (9.8) | |||||
| Control | 6.9 | (17.5) | 6.7 | (14.7) | −0.8 | (4.0) | −0.3 | (7.2) | −0.04 | |||
| TNF-alpha:IL-10 | Intervention | 5.6 | (15.7) | 4.2 | (7.0) | −1.5 | (15.9) | |||||
| Control | 11.9 | (26.7) | 15.2 | (31.5) | 2.2 | (17.1) | −3.8 | (16.6) | −0.23 | |||
| Fitness (ml/kg/min) | Intervention | 27.6 | (7.0) | 30.9 | (6.3) | 2.8 | ** | (4.9) | ||||
| Control | 24.0 | (5.7) | 25.1 | (5.0) | 1.1 | (4.2) | 1.7 | (4.5) | 0.37 | |||
| Depression | Intervention | 45.5 | (6.9) | 44.2 | (8.6) | −1.7 | (6.9) | |||||
| Control | 48.2 | (8.7) | 45.7 | (8.0) | −1.8 | (5.0) | 0.0 | (6.0) | 0.01 | |||
| Anxiety | Intervention | 48.9 | (7.9) | 45.6 | (8.9) | −4.0 | ** | (6.5) | ||||
| Control | 47.8 | (7.7) | 47.1 | (8.2) | −0.6 | (6.0) | −3.4 | (6.3) | −0.54 | * | ||
| Sleep dysfunction | Intervention | 49.4 | (7.1) | 46.2 | (8.0) | −3.7 | * | (8.6) | ||||
| Control | 53.4 | (9.2) | 51.1 | (7.4) | −1.9 | (7.6) | −1.8 | (8.1) | −0.22 | |||
| Walking self-efficacy | Intervention | 73.5 | (22.9) | 90.4 | (17.3) | 17.0 | *** | (20.5) | ||||
| Control | 62.8 | (31.5) | 67.3 | (32.1) | 4.2 | (18.5) | 12.8 | (19.5) | 0.66 | ** | ||
| Exercise social support | Intervention | 5.4 | (4.7) | 7.3 | (4.9) | 2.1 | ** | (3.2) | ||||
| Control | 3.9 | (3.7) | 3.1 | (3.2) | −0.8 | (3.5) | 2.9 | (3.4) | 0.85 | *** | ||
| Physical activity enjoyment | Intervention | 3.2 | (1.0) | 3.7 | (0.9) | 0.4 | (1.3) | |||||
| Control | 3.3 | (1.2) | 2.9 | (1.3) | −0.4 | (1.1) | 0.8 | (1.2) | 0.63 | ** | ||
SD= standard deviation
interleukin
tumor necrosis factor
p<.10 (trend only)
p<.05
p<.01
The results of the Freedman & Schatzkin analyses are provided in Table 4. The positive effect size increase in fatigue intensity was significantly mediated by IL-6 (82%), IL-10 (94%), IL-6:IL-10 (49%), and TNF-alpha:IL-10 (78%) with sleep dysfunction increasing the relationship between the intervention and fatigue intensity rather than mediating the intervention effects (−88%) (Table 4). The negative effect size decrease in fatigue interference for the intervention compared with control group was mediated by an improvement in sleep dysfunction (35%) while serum IL-10 and the pro:anti-inflammatory ratios increased the relationship between the intervention and fatigue interference (−25% to −40%) (Table 4). PROMIS® fatigue was significantly mediated by the positive intervention effects on weekly minutes of physical activity (76%), sleep dysfunction (45%), and physical activity enjoyment (40%) with IL-10 (−40%) and IL-6:IL-10 (−11%) increasing the statistical intervention-fatigue relationship (Table 4).
Table 4.
Potential mediators: Correlation of residualized change score with intervention and percent of intervention effect mediated (positive percent) or enhanced (negative percent) by the mediator
| Percent of intervention effect mediated | |||||
|---|---|---|---|---|---|
| Potential mediator | na | Correlation between mediator (residualized change score) and group allocation | Fatigue intensity | Fatigue interference | PROMIS® fatigue |
| Weekly minutes sedentary behavior | 42 | −0.23 | −46% | 13% | 11% |
| Weekly minutes ≥ moderate intensity physical activity | 42 | 0.53*** | −82% | 5% | 76%*** |
| Percent body fat | 42 | −0.17 | −22% | −1% | 5% |
| ILb-6 | 41 | −0.18 | 82%** | −3% | −9% |
| IL-8 | 42 | −0.20 | −7% | −6% | 9% |
| IL-10 | 38 | −0.21 | 94%** | −33%** | −40%*** |
| TNFc-alpha | 41 | 0.19 | −23% | −4% | −8% |
| IL-6:IL-10 | 38 | −0.02 | 49%** | −25%*** | −11%** |
| IL-8:IL-10 | 38 | −0.15 | 55%* | −30%*** | −8% |
| TNF-alpha:IL-10 | 38 | −0.17 | 78%** | −40%*** | −12% |
| Fitness | 42 | 0.35** | −64% | 4% | 24% |
| Anxiety | 41 | −0.24 | −42% | 7% | 5% |
| PROMIS® sleep dysfunction | 41 | −0.25 | −88%** | 35%** | 45%*** |
| Walking self-efficacy | 41 | 0.41*** | −33% | −2% | 24% |
| Exercise social support | 41 | 0.48*** | 29% | −53%* | −13% |
| Physical activity enjoyment | 41 | −0.37** | −62% | 22% | 40%** |
Total n varies due to missing survey on one participant and undetectable levels of IL-6 (n=1), IL-10 (n=3), and TNF-alpha (n=1)
interleukin
tumor necrosis factor
p<.10
p<.05
<.01
In a post hoc analysis, we examined the prevalence of non-responders (i.e., participants reporting greater fatigue after the intervention) and baseline factors that predicted the change in fatigue during the intervention (i.e., Pearson correlations between the change in fatigue and potential predictors measured at baseline). In the intervention group, fatigue intensity increased in 11/19 (58%), fatigue interference increased in 7/19 (37%), and PROMIS® fatigue increased in 3/19 (16%). No significant correlations with change in fatigue intensity were noted. A greater decline in fatigue interference was significantly associated with higher baseline fatigue intensity (r = −.50, p = .029), fatigue interference (r = −.84, p < .0001), anxiety (r = −.80, p < .0001), depression (r = −.81, p < .0001), and exercise barriers interference (r = −.46, p = .046). A greater decline in PROMIS® fatigue was significantly associated with higher baseline fatigue interference (r = −.56, p = .013), baseline PROMIS® fatigue (r = −.50, p = .03), anxiety (r = −.70, p < .001), and depression (r = −.69, p = .001). The following baseline factors were not predictive of the change in any of the fatigue measures used: age, education, cancer type, time since treatment, current hormonal therapy, breast cancer stage, number of comorbidities, self-efficacy, social support, enjoyment, pre-diagnosis physical activity, baseline physical activity.
Discussion
Although not statistically significant, the direction and magnitude of the effect sizes related to our exercise intervention varied depending on the fatigue measure used and aspect assessed. A non-significant small to medium effect size increase was noted for fatigue intensity with a non-significant small to medium effect size decrease noted for fatigue interference and PROMIS® fatigue. Only PROMIS® fatigue showed a within group statistically significant decline in the intervention group. Study power was limited by our small sample size which resulted from the budgetary and logistical constraints of a pilot study. Nevertheless, our effect size reductions in fatigue interference and general fatigue are consistent with that of prior studies (40) with our results suggesting an important finding relative to a possible increase in intermittent fatigue intensity. It is noteworthy that our data suggest important mediating relationships warranting further study. Specifically, inflammation, sleep quality, psychosocial factors (i.e., exercise social support and enjoyment), and minutes of weekly physical activity mediated the effects of our exercise intervention on fatigue in breast cancer survivors. Complex relationships that include both mediation and strengthening of the intervention-fatigue relationship exist. These relationships vary among the fatigue measures used and further study is needed to improve our understanding of these relationships. Inflammatory mediators of fatigue response to exercise may be due to beneficial reductions in the pro:anti-inflammatory ratios resulting from the dynamic response of the cytokine system to pro-inflammatory aspects of exercise training.
The National Comprehensive Cancer Network (NCCN) guidelines (a national resource used by U.S. medical oncologists as the “standard of care” for all cancer patients; http://www.nccn.org/index.asp) include exercise in its treatment algorithm for cancer-related fatigue. However, our study demonstrates that some cancer survivors will not experience reductions in their fatigue with an exercise intervention. Identifying reasons for this lack of response can be used to tailor future exercise interventions for improved effectiveness. To our knowledge, we are the first study to report predictors of fatigue response to an exercise intervention. Although preliminary in nature, our data suggest that breast cancer survivors with higher baseline anxiety, depression, and barriers interference may be more likely to experience a beneficial fatigue response to our intervention. Given the moderately complex nature of our intervention with respect to cost and staff time, our data suggest that targeting individuals with predictors of greater response could be used to better allocate financial and staff resources. It is also noteworthy that the factors that contribute to developing fatigue after cancer diagnosis have been well-studied but may not be the same factors that predict response to exercise. For example, affective state may increase fatigue prevalence (2) but, in contrast, predict a better response to our exercise intervention. Further research is needed to better understand predictors of fatigue response to exercise.
Our data further support the fact that exercise intervention effects on fatigue may vary when different fatigue measures are used and/or aspects assessed. The four Fatigue Symptom Inventory items used to measure fatigue intensity were fatigue on the day felt most fatigued, fatigue on day felt least fatigued, average fatigue, and current fatigue. In our study, it is conceivable that exercise may transiently increase fatigue intensity after an exercise bout which might increase the perception of fatigue by participants on the day they felt most fatigued and on average. However, the exercise training may have reduced fatigue interference because of improved participants’ physical ability to engage in their daily activities regardless of the transient increases in fatigue after exercise bouts. Also related, the seven PROMIS® fatigue items include three items asking about fatigue which interferes with work, thinking clearly, and bathing/showering, therefore it is not surprising that the intervention effects were similar to that noted with fatigue interference. Consistent with this, the change in PROMIS® fatigue was significantly correlated with the change in fatigue interference in the intervention group (r = .62, p<.01). However, the PROMIS® fatigue scale combines other aspects of fatigue with fatigue interference for a more general fatigue assessment which may explain the additional mediators related to minutes of physical activity and enjoyment. Future exercise and post-cancer fatigue research should measure multiple fatigue aspects (or dimensions), assess the pattern of fatigue intensity pre- and post-exercise bout, and compare how effects may differ for vigorous versus moderate intensity exercise training. Further study is needed to better understand and define the meaning of fatigue (a subjective patient-specific measure) while also determining fatigue aspects responsive to exercise that are most important for improving patient quality of life.
Also related to the definition of fatigue, prior research by other investigators (primarily in non-cancer populations) has differentiated between central and peripheral fatigue (10). We did not conceptualize fatigue in this manner because our goal was to examine fatigue as it might be reported by a patient in a clinical setting (i.e., patients usually do not differentiate central from peripheral fatigue). Nevertheless, interpretation of our data from the perspective of central versus peripheral fatigue warrants discussion and suggests future research directions. Peripheral fatigue is caused by neuromuscular abnormalities (e.g., excitation-contraction coupling, impaired calcium reuptake, etc.) that can be assessed with objective measures (10). Central fatigue results from a variety of possible central nervous system abnormalities (10) which include but are not limited to lack of self-motivation influenced by psychosocial factors such as depression or catastrophizing (8, 20). Although further study is needed, central fatigue has been reported to be the primary cause of fatigue in cancer patients and survivors (10). Exercise without psychosocial support is expected to improve peripheral more than central fatigue which may have contributed to the decline in fatigue interference and not fatigue intensity seen in our study. However, it is not possible to differentiate the effects of exercise alone from the effects of exercise plus the additional staff attention and group support in our study (e.g., support may have improved psychosocial factors that influence central fatigue). Therefore, it is possible that our intervention influenced both peripheral and central fatigue but to a different extent for each participant, thus explaining the variable rates of fatigue improvement and the greater effect on the more general measure of PROMIS® fatigue. Inclusion of measures that differentiate peripheral from central fatigue in future studies is warranted for improving our understanding of fatigue and its response to exercise interventions in cancer survivors.
Importantly, using consistent measures across studies would improve our ability to compare study results. PROMIS® (Patient Reported Outcomes Measurement Information System) is sponsored by the National Institutes of Health (NIH) and aims to develop a system of tools for patient-reported health status that can be used in multiple research and clinical populations and settings (http://www.nihpromis.org/about/abouthome) (6). Our results indicate that this scale shows change over time with the intervention and can be used to examine fatigue mediators. Our study is the first exercise and cancer trial to report the use of this scale (28) and supports the use of the, PROMIS® fatigue scale in future trials to facilitate collection of data that are comparable not only across cancer types but also with various chronic disease populations.
Identifying factors mediating the largest proportions of exercise intervention effects on fatigue will help prioritize and focus future interventions (exercise and otherwise) to treat cancer-related fatigue. This is particularly important with regard to the role of inflammation in fatigue due to the inconsistent associations related to cytokines and fatigue after cancer diagnosis reported in the literature (37). Our data suggest that the pro:anti-inflammatory balance plays a more consistent role in mediating exercise effects on fatigue when compared with individual cytokines alone. It is possible that individual variation in fatigue response to exercise may be due, in part, to genomic differences in inflammatory response (37). However, larger trials are needed to confirm our results while also examining moderators of the inflammatory response and the complex interaction between changes in inflammatory markers and intervention effects on fatigue beyond mediation alone.
In an effort to better understand the complex relationships among the individual cytokines and related ratios, post-hoc analyses examined the Pearson correlations among the raw difference scores for the intervention participants. Change in IL-6 and IL-10 were significantly associated with TNF-alpha (r = .55, p < .05 and r = .49, p < .05, respectively). None of the individual cytokines were significantly associated with the ratios with the highest correlation noted for IL-10 and each of the ratios (r = .20 to −.23, none significant). Although our small sample size precludes definitive conclusions related to specific mechanistic pathways, the direction of effect size changes and the correlations among the difference scores, support the theorized increase in anti-inflammatory cytokines (e.g., IL-10) as a result of higher levels of pro-inflammatory cytokines released during exercise (27).
Our data also provide additional support for the close association between sleep quality and fatigue previously reported in the literature (18). Mediation by social support and enjoyment, support continued investigation of the theorized biobehavioral models of fatigue (1). It is noteworthy that cross-sectional associations have suggested a relationship between self-efficacy and fatigue (25) but our study (the first to look at these relationships in a prospective design) did not detect a significant mediation effect by self-efficacy. Taken as a whole, our data suggest that combining exercise with interventions including sleep hygiene counseling, exercise social support, and exercise enjoyment has the potential to improve intervention effects on fatigue.
We originally published a pilot study evaluating a physical activity behavior change intervention effects on inflammatory markers of inflammation (31). Due to the small effects on cytokines, we attempted to reduce variability which would increase effect sizes by narrowing our study inclusion/exclusion criteria and by prescribing a more specific exercise dose (i.e., 40 minutes of aerobic exercise on four days per week with no more than two days lapsing between exercise sessions and resistance training on two non-consecutive days of the week). We also included participants with fatigue and/or sleep dysfunction in an effort to prevent the potential “floor effect” occurring when non-fatigued individuals are included (19). When the two studies are compared, fatigue effect sizes are higher in the study reported here. Also, the directions of cytokine-related effects were similar with the exception of TNF-alpha but the magnitude of the effects remained small to medium in size. Importantly, effect sizes in both studies, although small, suggest beneficial changes in the pro:anti-inflammatory ratios with chronic exercise participation.
Although extensor leg strength was assessed using a back and leg dynamometer, no significant change in this outcome was noted for the intervention compared with the control group (d = −0.07, p = .831). This differs from prior reports indicating an increase in strength with this measurement in response to a similar walking intervention (32). Several possible explanations exist. Prior study assessments were done by individuals who were not blinded to group allocation. Because of lack of blinding, assessors may have inadvertently provided increased encouragement during the testing for participants in the intervention group. Also, our intervention focused on general muscle strength and aerobic fitness rather than being specific to those muscle groups tested with the back/ and leg dynamometer. Lastly, our resistance protocol may not have been rigorous enough to result in significant improvements in muscle strength using the simple back/leg dynamometer. Future studies should assess mediation of fatigue using a strength measure sensitive to change with the intervention and/or a more intensive resistance training protocol before muscle strength is excluded as a possible mediator of fatigue response to exercise.
Also related to the adequacy of the exercise dose, the baseline mean weekly minutes of ≥ moderate intensity exercise exceeded the intervention goal of 160 weekly minutes which could conceivably threaten sufficient increases in exercise minutes due to a ceiling effect. Self-report of leisure-time exercise was used when determining study eligibility during the screening process but only the objective measure is reported here because it is generally considered to be a more accurate assessment of exercise behavior when compared with self-report. Nevertheless, accelerometers do not differentiate between leisure activity (i.e., volitional behavior that is more apt to change with an intervention) from non-leisure activities (e.g., occupation). Therefore, it is possible that some participants may have had greater amounts of physical activity when they wore the accelerometer (compared to self-report) because of non-leisure activities. The magnitude of the between group differences were similar for self-report and accelerometer (i.e., 110.5 minutes for self-report and 98.8 minutes for the accelerometer) and the baseline mean of the self-report was 17 ± 39 minutes for all participants combined. This suggests that the volitional (or leisure) exercise which would be anticipated to change the most during an exercise intervention was sufficiently low at baseline to limit a ceiling effect.
Furthermore, the standardized effect size for aerobic fitness (i.e., 0.37) is comparable to that reported by other studies including the weighted mean standardized effect size in a meta-analysis of exercise studies in cancer survivors (i.e., 0.32 for post-treatment cancer survivors) (40). In addition, the paired t-test of the within group change demonstrated a significant improvement in fitness for the intervention group participants from baseline to post-intervention. Therefore, limited study power due to the relatively large standard deviation of the between group difference is a more likely explanation of lack of statistical significance for aerobic fitness rather than an aerobic intervention that was “too mild” to cause improvement.
Discussion groups were included in our intervention to improve adherence to the exercise protocol. These groups may have inadvertently affected fatigue because they encouraged cognitive reframing (which may have influenced enjoyment) and social support relative to exercise. Therefore, conclusions about the effects of exercise independent of the group sessions cannot be made especially given the mediation by social support. However, the significant mediation of PROMIS® fatigue by the increase in physical activity minutes suggests that exercise independent of the groups plays a role. Also, our results suggest the importance of interventions that focus on multiple potential mediators. We also acknowledge the limited study power due to the small sample size. Nevertheless, the strong study design (e.g., randomized controlled trial), use of multiple fatigue measures, excellent retention, and report of mediators in a prospective study design significantly improve the usefulness of these study data. Also, we documented with accelerometer that time spent in sedentary behavior did not change for the intervention compared with control group. This is important because of the health risks associated with sedentary behavior (42), associations between sedentary behavior and fatigue in breast cancer survivors (33), and concerns about exercise training causing individuals to be less active during other times of day.
Our data suggest several important clinical and research implications. The correlation between the difference scores for change in fatigue intensity and interference in the intervention group was .44 (p<.10) suggesting that these constructs are different (account for only 19% of the variance of the other construct) yet overlap. It is possible that exercise that is too rigorous for an individual might increase fatigue intensity which worsens interference and could potentially act as an exercise barrier. Therefore, exercise recommendations for survivors with higher fatigue intensity should focus on adapting the exercise program that monitors for and avoids increases in fatigue intensity. In contrast, an individual with higher levels of anxiety and depressive symptoms at baseline can be advised to adapt an exercise training protocol similar to our intervention. Further research is needed to identify strategies for and usefulness of tailoring exercise counseling to the nature of the cancer survivor's fatigue.
Inflammation, sleep quality, and psychosocial factors may mediate or influence exercise intervention effects on fatigue in breast cancer survivors. Larger trials are needed to confirm our results and better understand the complex mediator and moderator relationships between biobehavioral factors and fatigue response to exercise. Inclusion of additional possible mediators such as catastrophizing, pain perception, neuropeptides, and catecholamines should be considered. Future studies should use several fatigue measures, including but not limited to the PROMIS® fatigue scale, to allow comparison with other studies and measurement of different fatigue aspects. If the biobehavioral mechanisms suggested in this study continue to be observed, interventions targeting these mechanisms can be developed to reduce fatigue in breast cancer survivors.
Acknowledgments
This project was supported by the National Cancer Institute 5R21CA135017. The results of the present study do not constitute endorsement by ACSM.
Grant support: This project was supported by the National Cancer Institute 5R21CA135017.
Footnotes
ClinicalTrials.gov identifier: NCT01147367
Disclosure statement: No personal or professional relationships that may represent a potential conflict of interest exist for this manuscript.
References
- 1.Al-Majid S, Gray DP. A biobehavioral model for the study of exercise interventions in cancer-related fatigue. Biological research for nursing. 2009;10(4):381–91. doi: 10.1177/1099800408324431. [DOI] [PubMed] [Google Scholar]
- 2.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–9. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 3.Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78(4):819–35. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- 4.Buffart LM, De Backer IC, Schep G, Vreugdenhil A, Brug J, Chinapaw MJ. Fatigue mediates the relationship between physical fitness and quality of life in cancer survivors. J Sci Med Sport. 2013;16(2):99–104. doi: 10.1016/j.jsams.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 6.Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerin E, Taylor LM, Leslie E, Owen N. Small-scale randomized controlled trials need more powerful methods of mediational analysis than the Baron-Kenny method. J Clin Epidemiol. 2006;59(5):457–64. doi: 10.1016/j.jclinepi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhuri A, Behan PO. Fatigue and basal ganglia. Journal of the neurological sciences. 2000;179(S 1-2):34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 9.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 10.Davis MP, Walsh D. Mechanisms of fatigue. J Support Oncol. 2010;8(4):164–74. [PubMed] [Google Scholar]
- 11.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36(5):480–7. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–81. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Support Care Cancer. 1996;4(2):82–96. doi: 10.1007/BF01845757. [DOI] [PubMed] [Google Scholar]
- 14.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 15.Hann DM, Jacobsen PB, Azzarello LM, et al. Measurement of fatigue in cancer patients: development and validation of the Fatigue Symptom Inventory. Qual Life Res. 1998;7(4):301–10. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 16.Heim ME, v d Malsburg ML, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor-related chronic fatigue. Onkologie. 2007;30(8-9):429–34. doi: 10.1159/000104097. [DOI] [PubMed] [Google Scholar]
- 17.Karvinen KH, Courneya KS, North S, Venner P. Associations between exercise and quality of life in bladder cancer survivors: a population-based study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16(5):984–90. doi: 10.1158/1055-9965.EPI-06-0680. [DOI] [PubMed] [Google Scholar]
- 18.Kim HJ, Barsevick AM, Fang CY, Miaskowski C. Common biological pathways underlying the psychoneurological symptom cluster in cancer patients. Cancer Nurs. 2012;35(6):E1–E20. doi: 10.1097/NCC.0b013e318233a811. [DOI] [PubMed] [Google Scholar]
- 19.Linden W, Satin JR. Avoidable pitfalls in behavioral medicine outcome research. Ann Behav Med. 2007;33(2):143–7. doi: 10.1007/BF02879895. [DOI] [PubMed] [Google Scholar]
- 20.Lukkahatai N, Saligan LN. Association of catastrophizing and fatigue: a systematic review. J Psychosom Res. 2013;74(2):100–9. doi: 10.1016/j.jpsychores.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McAuley E, Courneya KS, Lettunich J. Effects of acute and long-term exercise on self-efficacy responses in sedentary, middle-aged males and females. Gerontologist. 1991;31(4):534–42. doi: 10.1093/geront/31.4.534. [DOI] [PubMed] [Google Scholar]
- 23.McAuley E, Konopack JF, Morris KS, et al. Physical activity and functional limitations in older women: influence of self-efficacy. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):P270–7. doi: 10.1093/geronb/61.5.p270. [DOI] [PubMed] [Google Scholar]
- 24.McAuley E, Mihalko SL. Advances in sports and exercise psychology measurement. Fitness Information Technology, Inc.; Morgantown, WV: 1998. pp. 371–82. [Google Scholar]
- 25.McAuley E, White SM, Rogers LQ, Motl RW, Courneya KS. Physical Activity and Fatigue in Breast Cancer and Multiple Sclerosis: Psychosocial Mechanisms. Psychosom Med. 2010;72(1):88–96. doi: 10.1097/PSY.0b013e3181c68157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Payne JK. A neuroendocrine-based regulatory fatigue model. Biological research for nursing. 2004;6(2):141–50. doi: 10.1177/1099800404268280. [DOI] [PubMed] [Google Scholar]
- 27.Petersen AM, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57(Suppl 10):43–51. [PubMed] [Google Scholar]
- 28.Puetz TW, Herring MP. Differential effects of exercise on cancer-related fatigue during and following treatment: a meta-analysis. Am J Prev Med. 2012;43(2):e1–24. doi: 10.1016/j.amepre.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Rogers LQ. Using exercise to fight fatigue in breast cancer survivors: challenges and future directions. Expert review of pharmacoeconomics & outcomes research. 2012;12(3):251–4. doi: 10.1586/erp.12.20. [DOI] [PubMed] [Google Scholar]
- 30.Rogers LQ, Courneya KS, Rao K, et al. Physical activity, sleep, cognitive function, and fatigue in head and neck cancer survivors. Med Sci Sports Exerc. 2007;39(Suppl):S450. [Google Scholar]
- 31.Rogers LQ, Fogleman A, Trammell R, et al. Effects of a Physical Activity Behavior Change Intervention on Inflammation and Related Health Outcomes in Breast Cancer Survivors: Pilot Randomized Trial. Integrative cancer therapies. 2012 doi: 10.1177/1534735412449687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rogers LQ, Hopkins-Price P, Vicari S, et al. A randomized trial to increase physical activity in breast cancer survivors. Med Sci Sports Exerc. 2009;41(4):935–46. doi: 10.1249/MSS.0b013e31818e0e1b. [DOI] [PubMed] [Google Scholar]
- 33.Rogers LQ, Markwell SJ, Courneya KS, McAuley E, Verhulst S. Physical activity type and intensity among rural breast cancer survivors: patterns and associations with fatigue and depressive symptoms. Journal of cancer survivorship : research and practice. 2011;5(1):54–61. doi: 10.1007/s11764-010-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rogers LQ, Shah P, Dunnington G, et al. Social cognitive theory and physical activity during breast cancer treatment. Oncol Nurs Forum. 2005;32(4):807–15. doi: 10.1188/05.ONF.807-815. [DOI] [PubMed] [Google Scholar]
- 35.Romito F, Montanaro R, Corvasce C, Di Bisceglie M, Mattioli V. Is cancer-related fatigue more strongly correlated to haematological or to psychological factors in cancer patients? Support Care Cancer. 2008;16(8):943–6. doi: 10.1007/s00520-007-0357-1. [DOI] [PubMed] [Google Scholar]
- 36.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 37.Saligan LN, Kim HS. A systematic review of the association between immunogenomic markers and cancer-related fatigue. Brain Behav Immun. 2012;26(6):830–48. doi: 10.1016/j.bbi.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Prev Med. 1987;16(6):825–36. doi: 10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- 39.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immun. 2007;21(4):413–27. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Speck RM, Courneya KS, Masse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. Journal of cancer survivorship : research and practice. 2010;4(2):87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 41.Tuck MK, Chan DW, Chia D, et al. Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res. 2009;8(1):113–7. doi: 10.1021/pr800545q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Uffelen JG, Wong J, Chau JY, et al. Occupational sitting and health risks: a systematic review. Am J Prev Med. 2010;39(4):379–88. doi: 10.1016/j.amepre.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Wasserman K, Hansen JE, Sue DY, Casaburi R, Whipp BJ. Principles of exercise testing and interpretation including pathophysiology and clinical applications. 3rd ed. Lippincott Williams and Wilkins; Baltimore, MD: 1999. [Google Scholar]
- 44.Winters-Stone KM, Bennett JA, Nail L, Schwartz A. Strength, physical activity, and age predict fatigue in older breast cancer survivors. Oncol Nurs Forum. 2008;35(5):815–21. doi: 10.1188/08.ONF.815-821. [DOI] [PubMed] [Google Scholar]
- 45.Wiskemann J, Dreger P, Schwerdtfeger R, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011;117(9):2604–13. doi: 10.1182/blood-2010-09-306308. [DOI] [PubMed] [Google Scholar]