Abstract
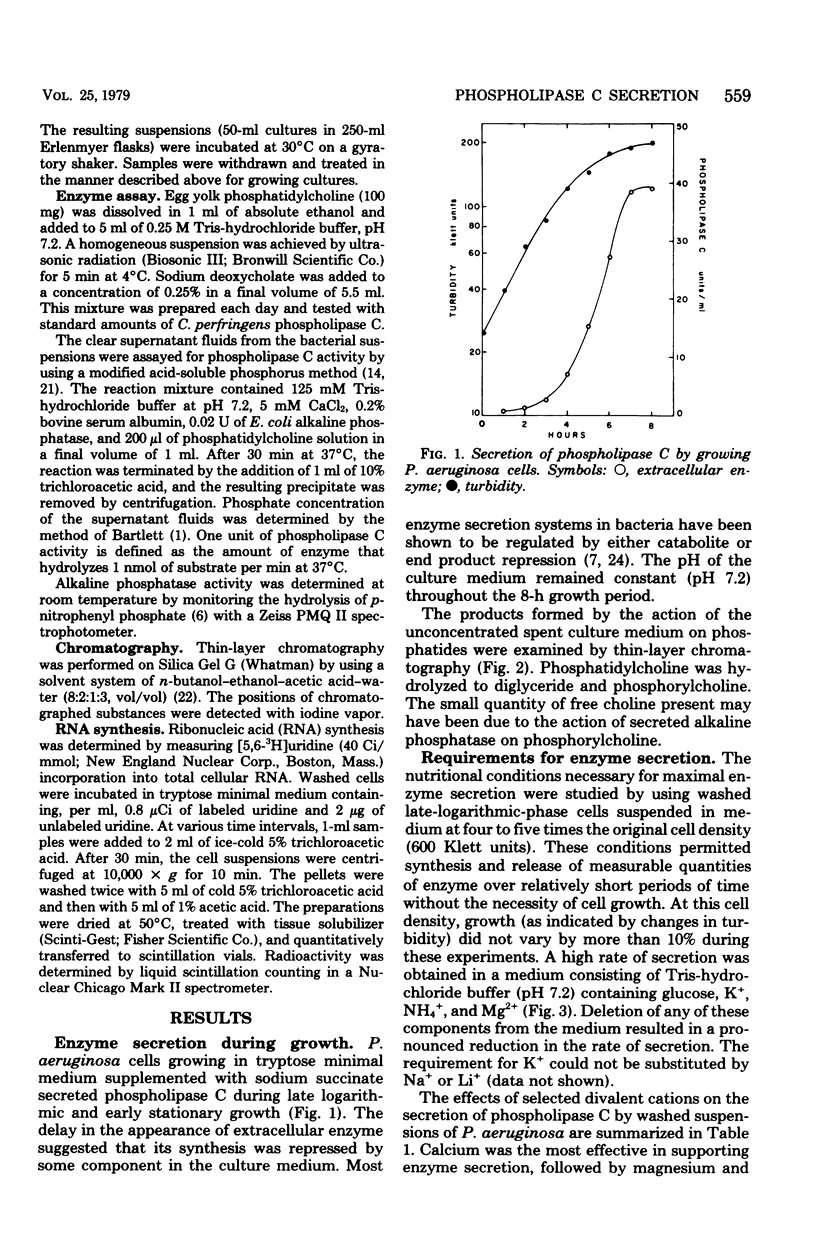
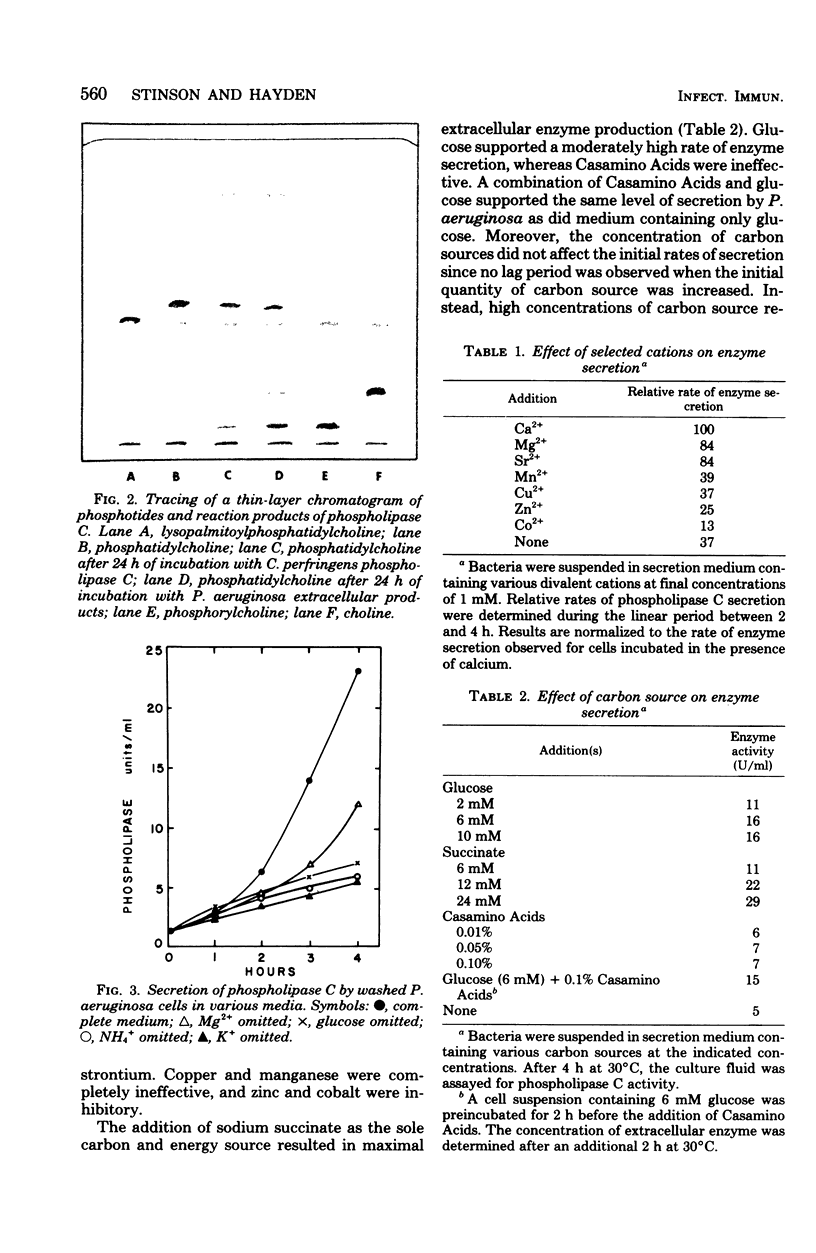
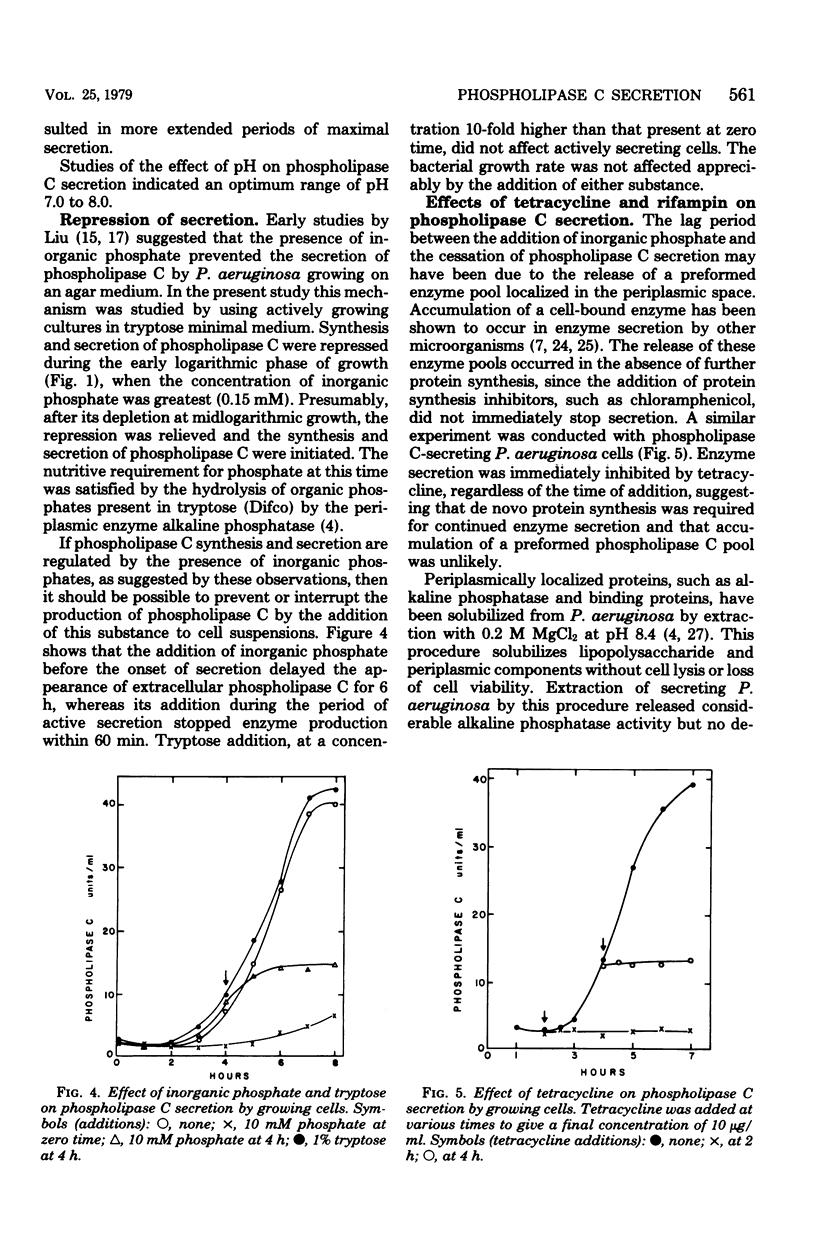
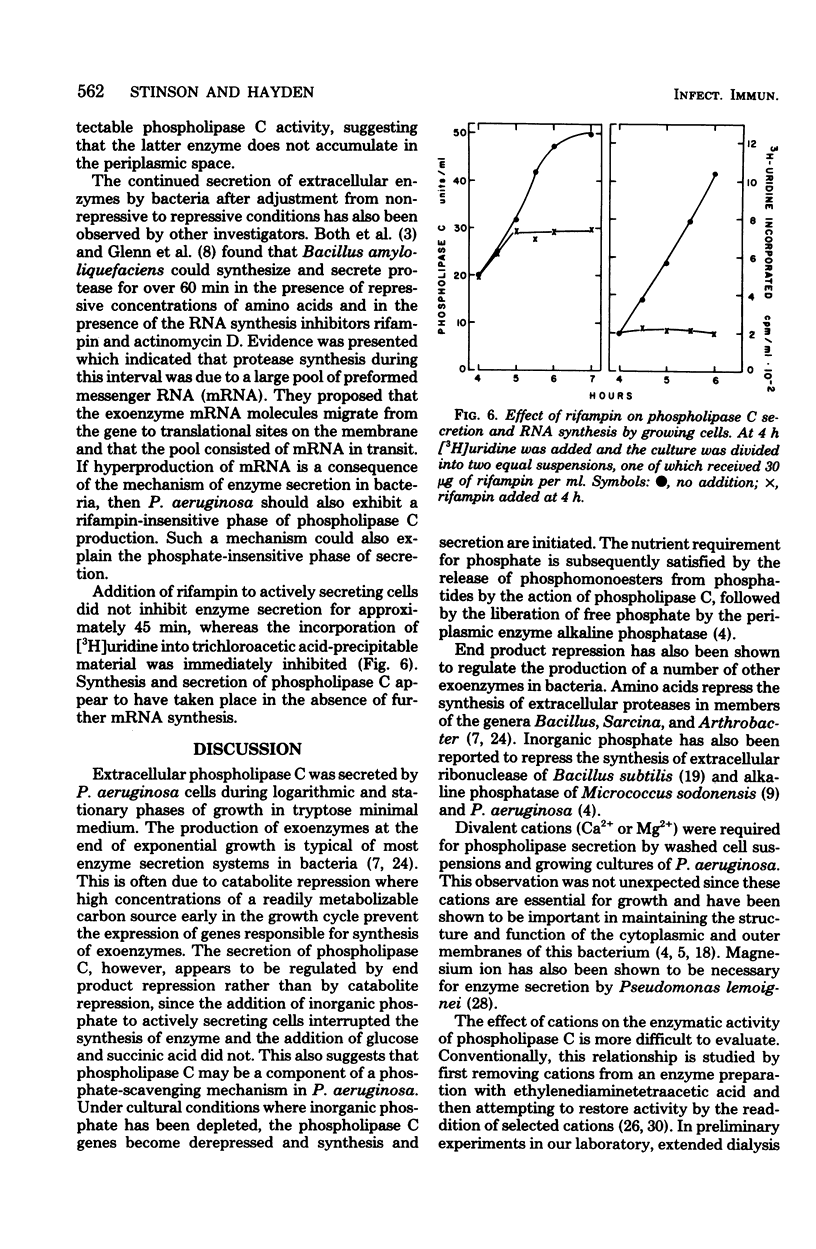
The conditions necessary for the secretion of phospholipase C (phosphatidylcholine cholinephosphohydrolase) by Pseudomonas aeruginosa were studied. Enzyme secretion by washed cell suspensions required a carbon source and ammonium, potassium, and calcium ions. The calcium requirement could be substituted by magnesium and strontium but not by copper, manganese, cobalt, or zinc. During growth in liquid medium, cells secreted phospholipase C during late logarithmic and early stationary phases. Secretion was repressed by the addition of inorganic phosphate but not by organic phosphates, glucose, or sodium succinate. Studies with tetracycline indicated that de novo protein synthesis was necessary for the secretion of phospholipase C and that the exoenzyme was not released from a preformed periplasmic pool. Similarly, extraction of actively secreting cells with 0.2 M MgCl2 at pH 8.4 solubilized large quantities of the periplasmic enzyme alkaline phosphatase but insignificant amounts of phospholipase C. Bacteria continued to secrete enzyme for nearly 45 min after the addition of inorganic phosphate or rifampin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Boethling R. S. Regulation of extracellular protease secretion in Pseudomonas maltophilia. J Bacteriol. 1975 Sep;123(3):954–961. doi: 10.1128/jb.123.3.954-961.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Stinnett J. D., Eagon R. G. Ultrastructural and chemical alteration of the cell envelope of Pseudomonas aeruginosa, associated with resistance to ethylenediaminetetraacetate resulting from growth in a Mg2+-deficient medium. J Bacteriol. 1974 Jan;117(1):302–311. doi: 10.1128/jb.117.1.302-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn A. R., Both G. W., McInnes J. L., May B. K., Elliott W. H. Dynamic state of the messenger RNA pool specific for extracellular protease in Bacillus amyloliquefaciens: its relevance to the mechanism of enzyme secretion. J Mol Biol. 1973 Jan 10;73(2):221–230. doi: 10.1016/0022-2836(73)90325-2. [DOI] [PubMed] [Google Scholar]
- Glenn A. R. Production of extracellular proteins by bacteria. Annu Rev Microbiol. 1976;30:41–62. doi: 10.1146/annurev.mi.30.100176.000353. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J., Bjorn M. J., Maxwell E. S. Pseudomonas aeruginosa exoenzyme S: an adenosine diphosphate ribosyltransferase distinct from toxin A. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3211–3215. doi: 10.1073/pnas.75.7.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Improved assay method for phospholipase C. Appl Microbiol. 1967 May;15(3):551–555. doi: 10.1128/am.15.3.551-555.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J Infect Dis. 1966 Feb;116(1):112–116. doi: 10.1093/infdis/116.1.112. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Adachi O., Shinagawa E., Ameyama M. Isolation and characterization of outer and inner membranes from Pseudomonas aeruginosa and effect of EDTA on the membranes. J Biochem. 1978 Jan;83(1):171–181. doi: 10.1093/oxfordjournals.jbchem.a131888. [DOI] [PubMed] [Google Scholar]
- May B. K., Walsh R. L., Elliott W. H., Smeaton J. R. Mechanism of the paradoxical stimulation of ribonuclease synthesis in Bacillus subtilis by actinomycin D. Biochim Biophys Acta. 1968 Nov 20;169(1):260–262. doi: 10.1016/0005-2787(68)90028-2. [DOI] [PubMed] [Google Scholar]
- O'Connor R., Elliott W. H., May B. K. Modulation of an apparent mRNA pool for extracellular protease in Bacillus amyloliquefaciens. J Bacteriol. 1978 Oct;136(1):24–34. doi: 10.1128/jb.136.1.24-34.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsaka A., Sugahara T. Quantitative determination of Clostridium welchii phospholipase C activity in aqueous reaction medium. J Biochem. 1968 Sep;64(3):335–345. doi: 10.1093/oxfordjournals.jbchem.a128900. [DOI] [PubMed] [Google Scholar]
- Okawa Y., Yamaguchi T. Studies on phospholipases from Streptomyces. III. Purification and properties of Streptomyces hachijoensis phospholipase C. J Biochem. 1975 Sep;78(3):537–545. doi: 10.1093/oxfordjournals.jbchem.a130938. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Shackelford A. H. Pseudomonas aeruginosa exotoxin in mice: localization and effect on protein synthesis. Infect Immun. 1974 Mar;9(3):540–546. doi: 10.1128/iai.9.3.540-546.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatzman A. R., Kosman D. J. Regulation of galactose oxidase synthesis and secretion in Dactylium dendroides: effects of pH and culture density. J Bacteriol. 1977 Apr;130(1):455–463. doi: 10.1128/jb.130.1.455-463.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoki S., Ikezawa H. Studies on phospholipase C from Pseudomonas aureofaciens. II. Further studies on the properties of the enzyme. J Biochem. 1976 Aug;80(2):361–366. doi: 10.1093/oxfordjournals.jbchem.a131284. [DOI] [PubMed] [Google Scholar]
- Stinson M. W., Cohen M. A., Merrick J. M. Isolation of dicarboxylic acid- and glucose-binding proteins from Pseudomonas aeruginosa. J Bacteriol. 1976 Nov;128(2):573–579. doi: 10.1128/jb.128.2.573-579.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi R., Ikezawa H. Hydrolytic action of phospholipases on bacterial membranes. J Biochem. 1977 Nov;82(5):1225–1230. doi: 10.1093/oxfordjournals.jbchem.a131809. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Ikezawa H. Phospholipase C from Clostridium novyi type A. II. Factors influencing the enzyme activity. J Biochem. 1977 Nov;82(5):1217–1233. doi: 10.1093/oxfordjournals.jbchem.a131808. [DOI] [PubMed] [Google Scholar]
- Taguchi R., Ikezawa H. Studies on the hemolytic and hydrolytic actions of phospholipases against mammalian erythrocyte membranes. Arch Biochem Biophys. 1976 Apr;173(2):538–545. doi: 10.1016/0003-9861(76)90290-3. [DOI] [PubMed] [Google Scholar]




