Abstract
Background
Patients who smoke at the time of percutaneous coronary intervention (PCI) would ideally have a strong incentive to quit, but most do not. We sought to compare the health status outcomes of those who did and did not quit smoking after PCI with those who were not smoking prior to PCI.
Methods and Results
A cohort of 2,765 PCI patients from 10 US centers were categorized into never, past (smoked in the past, but had quit prior to PCI), quitters (smoked at time of PCI, but then quit), and persistent smokers. Health status was measured with the disease-specific Seattle Angina Questionnaire (SAQ) and the EuroQol 5 Dimensions (EQ-5D), adjusted for baseline characteristics. In unadjusted analyses, persistent smokers had worse disease-specific and overall health status as compared with other groups. In fully-adjusted analyses, persistent smokers showed significantly worse health-related quality of life as compared with never smokers. Importantly, of those who smoked at the time of PCI, quitters had significantly better adjusted SAQ angina frequency scores (mean difference=2.73; 95% CI, 0.13 to 5.33) and trends towards higher disease specific (SAQ quality of life mean difference=1.97; 95% CI, -1.24 to 5.18), and overall (EQ-5D VAS scores mean difference=2.45; 95% CI, -0.58 to 5.49) quality of life as compared with persistent smokers at 12 months.
Conclusions
While smokers at the time of PCI have worse health status at 1 year than those who never smoked, smokers who quit after PCI have less angina at 1 year than those who continue smoking.
Keywords: percutaneous coronary intervention, smoking, quality of life
Because cigarette smoking is a potent risk factor for coronary artery disease (CAD), over a quarter of patients presenting for percutaneous coronary intervention (PCI) are smoking at the time of treatment.1,2 In other diseases, such as vascular surgery, the benefits of treatment are severely undermined if patients continue to smoke.3,4 While available data suggest a survival benefit from smoking cessation after PCI,2 understanding the association of smoking cessation with patients' health status (their symptoms, function and quality of life) is of great importance because this is the primary benefit of PCI in most clinical settings, except reperfusion at the time of an ST-elevation myocardial infarction (STEMI).5 Several prior studies have suggested that smokers who stop smoking after PCI have better health status outcomes than those who continue to smoke,6,7 but these were conducted prior to the recent era of drug-eluting stents, aggressive secondary prevention and the emphasis on smoking cessation as a performance measure of quality.8
Understanding the health status outcomes of smokers as compared with non-smokers, and particularly the outcomes of those who do and do not quit smoking after PCI is important. First, smoking cessation is under the patient's, rather than the physician's, locus of control. Providing patients with additional insights into the likely impact of continued smoking may further motivate them to quit. Second, in an era of scarce medical resources, where it is no longer wise to provide therapies of little benefit, health systems may consider encouraging patients to stop smoking, prior to offering PCI for stable coronary disease, if they want their treatments to be maximally effective. Given the need to better clarify the association of persistent smoking on patients' health status outcomes, we studied a consecutive series of patients undergoing PCI in a 10-center study and compared the health status outcomes of those who did and did not quit smoking after their procedure with those who were not smoking prior to PCI.
Methods
Participants
To examine the health status outcomes after PCI as a function of smoking status, we leveraged the Outcomes of PCI Study (OPS)/Personalized Risk Information Services Manager™ (PRISM) study, a 10-center prospective PCI registry developed to test the benefits of a novel informed consent process using individualized, evidence-based estimates of procedural risks.9 Consecutive patients undergoing PCI were enrolled and those who completed 1-year clinical follow-up with the disease-specific health Seattle Angina Questionnaire (SAQ) and generic EuroQol 5 Dimensions (EQ-5D) were included in the present analysis. Each patient underwent a detailed, independent chart abstraction by trained study coordinators to collect demographic, comorbidity and disease severity information (Table 1). Patients also completed an interview at the time of their procedure and at 1, 6 and 12 months to qualify their health status and smoking. Institutional Review Board approval was obtained from all participating sites, and all patients provided written informed consent for baseline and follow-up assessments.
Table 1. Patient characteristics.
| 1-Year Smoking status | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristic | Never smokers (n = 935) | Past smokers (n = 1,326) | Quitter (n = 189) | Persistent smokers (n = 315) | P-Value (quitters vs. persistent smokers) | P-Value across all 4 groups |
| Demographic variables | ||||||
| Age | 65.9 ± 11.0 | 66.6 ± 9.8 | 58.5 ± 9.2 | 57.3 ± 9.4 | 0.162 | <0.001 |
| Male sex | 582 (62.2) | 1052 (79.3) | 133 (70.4) | 214 (67.9) | 0.568 | <0.001 |
| Race/ethnicity | 0.203 | 0.011 | ||||
| White/Caucasian | 843 (91.4) | 1209 (92.6) | 168 (90.8) | 281 (90.1) | ||
| Black/African-American | 47 (5.1) | 56 (4.3) | 12 (6.5) | 28 (9.0) | ||
| Other | 32 (3.5) | 40 (3.1) | 5 (2.7) | 3 (1.0) | ||
| Unknown | 13 | 21 | 4 | 3 | ||
| High school graduate | 872 (93.5) | 1205 (91.1) | 170 (89.9) | 263 (84.0) | 0.062 | <0.001 |
| Married | 653 (70.0) | 949 (71.8) | 112 (59.9) | 161 (51.1) | 0.056 | <0.001 |
| No insurance | 12 (1.3) | 16 (1.2) | 16 (8.6) | 25 (8.0) | 0.825 | <0.001 |
|
| ||||||
| Clinical and procedural variables | ||||||
| Body Mass Index | 30.3 ± 6.2 | 30.5 ± 5.8 | 30.4 ± 5.9 | 29.7 ± 6.5 | 0.233 | 0.186 |
| Pre-PCI creatinine level (mg/dL) | 1.1 ± 0.9 | 1.1 ± 0.8 | 1.0 ± 0.2 | 1.0 ± 0.6 | 0.951 | 0.002 |
| Diabetes | 298 (31.9) | 468 (35.3) | 46 (24.3) | 89 (28.3) | 0.337 | 0.004 |
| Dyslipidemia | 744 (79.6) | 1139 (85.9) | 139 (73.5) | 251 (79.7) | 0.111 | <0.001 |
| Chronic kidney disease | 85 (9.1) | 120 (9.0) | 7 (3.7) | 18 (5.7) | 0.314 | 0.021 |
| Prior MI | 202 (21.6) | 378 (28.5) | 56 (29.6) | 106 (33.7) | 0.349 | <0.001 |
| Prior PCI | 345 (36.9) | 581 (43.8) | 65 (34.4) | 135 (42.9) | 0.060 | 0.002 |
| Prior CABG | 154 (16.5) | 341 (25.7) | 28 (14.8) | 35 (11.1) | 0.224 | <0.001 |
| Chronic heart failure | 61 (6.5) | 159 (12.0) | 21 (11.1) | 37 (11.7) | 0.829 | <0.001 |
| LV Ejection Fraction | 55.4 ± 11.6 | 53.4 ± 12.3 | 52.0 ± 13.3 | 52.7 ± 11.4 | 0.612 | 0.003 |
| LV Systolic Function | 0.476 | 0.166 | ||||
| Normal | 498 (78.2) | 647 (73.8) | 97 (72.4) | 161 (71.2) | ||
| Mildly Depressed | 77 (12.1) | 127 (14.5) | 18 (13.4) | 41 (18.1) | ||
| Moderately Depressed | 41 (6.4) | 52 (5.9) | 10 (7.5) | 15 (6.6) | ||
| Severely Depressed | 21 (3.3) | 51 (5.8) | 9 (6.7) | 9 (4.0) | ||
| Moderate or severe LV dysfunction | 62 (9.7) | 103 (11.7) | 19 (14.2) | 24 (10.6) | 0.314 | 0.405 |
| CCS angina class | 0.847 | 0.082 | ||||
| No symptoms | 173 (24.6) | 225 (22.9) | 37 (23.9) | 70 (28.2) | ||
| class I | 57 (8.1) | 71 (7.2) | 9 (5.8) | 14 (5.6) | ||
| class II | 168 (23.9) | 238 (24.2) | 33 (21.3) | 48 (19.4) | ||
| class III | 132 (18.8) | 226 (23.0) | 26 (16.8) | 45 (18.1) | ||
| class IV | 174 (24.7) | 223 (22.7) | 50 (32.3) | 71 (28.6) | ||
| Unknown | 231 | 343 | 34 | 67 | ||
| PCI Indication | 0.831 | <0.001 | ||||
| Staged procedure | 67 (7.2) | 74 (5.6) | 11 (5.8) | 17 (5.4) | ||
| Stable angina | 338 (36.1) | 514 (38.8) | 39 (20.6) | 75 (23.8) | ||
| Unstable angina | 324 (34.7) | 437 (33.0) | 65 (34.4) | 103 (32.7) | ||
| NSTEMI | 152 (16.3) | 218 (16.4) | 57 (30.2) | 92 (29.2) | ||
| STEMI | 24 (2.6) | 19 (1.4) | 6 (3.2) | 15 (4.8) | ||
| Other | 30 (3.2) | 64 (4.8) | 11 (5.8) | 13 (4.1) | ||
| Acute coronary syndrome | 500 (53.3) | 674 (50.8) | 128 (67.7) | 210 (66.7) | 0.807 | <0.001 |
| STEMI | 24 (2.6) | 19 (1.4) | 6 (3.2) | 15 (4.8) | 0.388 | 0.004 |
| Use of DES | 780 (83.6) | 1,095 (82.6) | 156 (83.0) | 246 (78.1) | 0.186 | 0.169 |
| Number of diseased vessels | 1.5 ± 0.7 | 1.6 ± 0.8 | 1.5 ± 0.8 | 1.5 ± 0.7 | 0.469 | <0.001 |
| Number of treated vessels | 1.2 ± 0.5 | 1.2 ± 0.5 | 1.2 ± 0.5 | 1.2 ± 0.5 | 0.885 | 0.692 |
| Number of cardiac medications at discharge | 3.9 ± 1.0 | 4.0 ± 1.0 | 3.8 ± 1.0 | 3.9 ± 0.9 | 0.387 | 0.495 |
| Dual antiplatelet therapy | 904 (97.0) | 1,276 (96.3) | 181 (95.8) | 306 (97.1) | 0.407 | 0.686 |
| Post-procedure bleeding | 24 (2.6) | 36 (2.7) | 3 (1.6) | 6 (1.9) | 1.000 | 0.798 |
Data are reported as number (percentage) of patients unless otherwise indicated. CABG indicates coronary artery bypass grafting; CCS, Canadian Cardiovascular Society; DES, drug-eluting stents; LV, left ventricular; MI, myocardial infarction; NSTEMI, non-ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
Smoking status categorization
Smoking status was assessed by self-report using the recommendations of the Society for Nicotine Research and Tobacco to characterize smoking habits.10,11 Patients were asked, “Which of the following best describes your cigarette smoking status? 1) Have never smoked, not even a puff; 2) Have smoked in the past but less than 100 cigarettes total; 3) Stopped smoking more than 1 year ago; 4) Stopped smoking between 1 month and 1 year ago; 5) Have smoked (even a puff) in the past 30 days.” We categorized smoking status based on baseline and 1-year responses. At baseline, responses 1 and 2 were categorized as “never smokers.” Responses 3 and 4 were categorized as “past smokers (quit prior to PCI)”, and response 5 was categorized as “current smokers.” At 1 year, we reclassified “current smokers” into 2 groups including “quitters” and “persistent smokers” based on patient's self-reported smoking status at 1 year. Supplemental Table 1 provides more extensive details on our categorization.
Outcomes measures
While health status assessments were collected throughout the year of follow-up, the benefits of smoking cessation often take months to years to emerge.12-14 We thus focused upon patients' health status at 1 year after PCI to maximize the length of follow-up. Both disease-specific and generic health-related quality of life (HRQOL) questionnaires were assessed, as described below. HRQOL was defined as the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient.15
Disease-specific health status: Seattle Angina Questionnaire
The Seattle Angina Questionnaire (SAQ) is a disease-specific health status measure for patients with CAD that has previously been demonstrated to be valid, reliable, and sensitive to clinical changes.16-19 It is also associated with subsequent survival, rehospitalization rates and costs.20-22 For this study, we focused upon the SAQ Angina Frequency, Physical Limitation, and Quality of Life Domains as they were deemed, a priori, to be most relevant to the current analysis. Each domain score is transformed to a 0-100 scale, with higher scores indicating fewer symptoms, better functioning or greater quality of life. A score of 100 indicates no angina, no physical limitations due to angina and no impact of angina on patients' perceived quality of life.16,18,22
Generic health status: EuroQol 5 Dimensions Questionnaire
We also used the EQ-5D, developed by the EuroQol group, to qualify patients' overall health status.23,24 There is a 20-cm visual analog scale (VAS) with anchors of “best imaginable health state” (a score of 100) and “worst imaginable health state” (a score of 0).24 The EQ-5D VAS represents patients' overall views of their quality of life and has been previously validated for patients with acute coronary syndrome.25,26
Statistical Analysis
As described above, patients were initially categorized into 4 groups based upon their smoking status: never smokers, past smokers, quitters and persistent smokers. Baseline characteristics of patients across smoking categories were compared using chi-square tests for categorical variables and analysis of variance for continuous variables. To adjust for observed differences in 1-year health status, we used multivariable linear models to adjust for potential confounders at the time of PCI. Specifically, we adjusted for baseline health status in each model that included patients' baseline score for the domain of interest. In addition, we adjusted for clinically relevant and statistically significant factors that differed between smoking groups. These included demographic factors (age, gender, race, insurance status, marital status, and education level), medical history (hypertension, dyslipidemia, diabetes, chronic lung disease, prior MI, prior PCI, prior CABG, prior heart failure, atrial fibrillation/flutter, peripheral artery disease, cerebrovascular accident/transient ischemic attack, and chronic kidney disease (CKD)), disease severity (cardiogenic shock, LV ejection fraction <40%), angiographic and procedural characteristics (indication for PCI, use of drug-eluting stents, number of diseased/treated vessels and number of residual untreated vessels), treatment received (dual antiplatelet therapy, number of cardiac medications at discharge), as well as post-procedure bleeding. Statistical significance was defined as a 2-sided P value ≤ .05. All analyses were conducted using SAS v9.3 (SAS Institute Inc, Cary, NC).
Results
Baseline Patient Characteristics
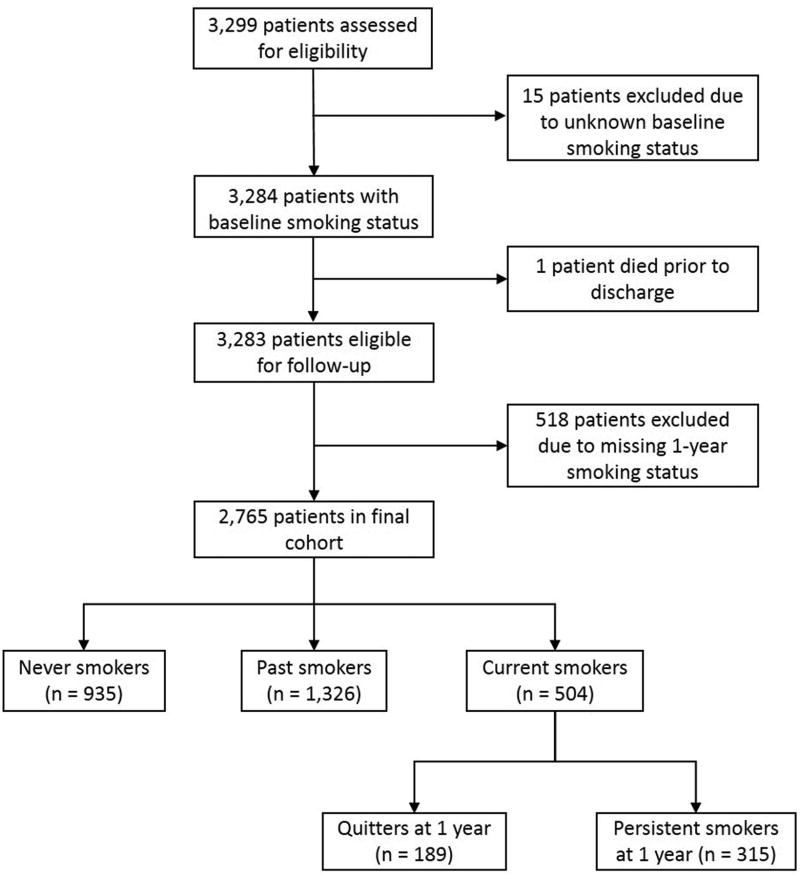
Of the 3,299 patients who completed the baseline HRQOL questionnaires at 10 hospitals, 15 patients were missing baseline smoking status and 1 patient died prior to discharge. Additionally, we excluded 518 patients without 1-year smoking status, leaving 2,765 (83.8%) for analysis (Figure 1). The differences in baseline demographics (age, sex, race, marital status) and clinical factors (diabetes, chronic kidney disease, prior MI, chronic heart failure) between included and excluded participants are described in Supplemental Table 2. At the time of the index PCI, 504 (18%) patients were current smokers, 1,326 (48%) were past smokers and 935 patients (34%) were nonsmokers. During the 1-year follow-up, 189 (37.5%) of the smokers quit and 315 (62.5%) persisted in smoking. There were marked differences in baseline demographic and clinical characteristics according to smoking status (Table 1). Persistent smokers and patients who quit smoking after PCI were, on average, 7 and 8 years younger than never smokers (57±9 years and 59±9 years old versus 66±11 years old; p<0.001). Fewer patients who were past smokers were female. There were also significant differences in other demographic and clinical characteristics, including that patients who never smoked and who were past smokers were more likely to have graduated from high school, to be married, to have a history of diabetes and CKD than those who were smoking at baseline. Patients who smoked at the time of their PCI were more likely to have presented with an acute coronary syndrome.
Figure 1.
Flowchart of inclusions and exclusions.
Association of smoking with health-related quality of life (HRQOL) outcomes after PCI
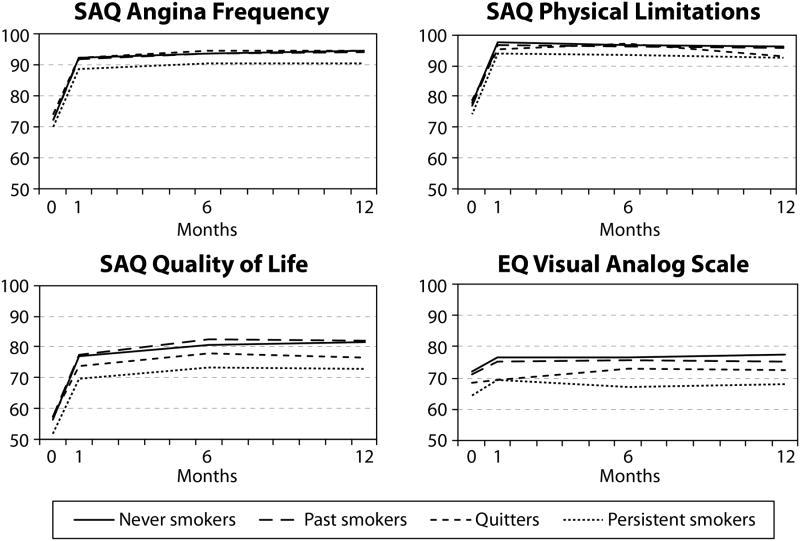
Table 2 presents the baseline and 1-year HRQOL of patients stratified by smoking status. At baseline, active smokers had significantly lower SAQ QOL and EQ-5D scores than past and non-smokers. One year after PCI, patients who never smoked and previously smoked had higher scores in all HRQOL domains than patients who continued to smoke after PCI (Figure 2). Quitters had health status scores that were either similar to non-smokers or intermediate between non-smokers and persistent smokers.
Table 2. Baseline and 12 month health status outcomes.
| 1-Year Smoking Status | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Never smokers n = 935 | Past smokers n = 1,326 | Quitters n = 189 | Persistent smokers n = 315 | P-Value (quitters vs. persistent smokers) | P-Value across all 4 groups | |
| Baseline | ||||||
| SAQ Angina Frequency | 72.4 ± 23.8 | 72.3 ± 25.1 | 74.1 ± 24.1 | 70.0 ± 25.1 | 0.076 | 0.306 |
| Any Angina – SAQAF, % | 695 (74.3) | 978 (73.8) | 136 (72.0) | 241 (76.5) | 0.225 | 0.680 |
| SAQ Physical Limitation | 77.7 ± 23.8 | 77.2 ± 23.5 | 78.8 ± 24.9 | 74.1 ± 27.5 | 0.063 | 0.120 |
| SAQ Quality of Life | 56.2 ± 25.5 | 57.3 ± 25.6 | 57.2 ± 26.2 | 51.7 ± 26.6 | 0.024 | 0.006 |
| EQ-5D: Visual analog scale | 71.8 ± 18.4 | 71.1 ± 20.0 | 68.3 ± 20.2 | 64.5 ± 21.0 | 0.044 | <0.001 |
| One year | ||||||
| SAQ Angina Frequency | 94.6 ± 13.7 | 94.1 ± 15.3 | 94.3 ± 13.3 | 90.4 ± 17.5 | 0.009 | <0.001 |
| Any Angina – SAQAF, % | 174 (18.6) | 241 (18.2) | 40 (21.2) | 96 (30.5) | 0.023 | <0.001 |
| SAQ Physical Limitation | 96.1 ± 11.6 | 95.8 ± 12.4 | 93.0 ± 16.7 | 92.7 ± 17.8 | 0.890 | <0.001 |
| SAQ Quality of Life | 81.6 ± 17.2 | 82.0 ± 18.7 | 76.5 ± 21.3 | 73.0 ± 23.9 | 0.097 | <0.001 |
| EQ-5D: Visual analog scale | 77.2 ± 17.3 | 75.2 ± 18.4 | 72.5 ± 18.9 | 68.0 ± 20.9 | 0.016 | <0.001 |
All continuous data are presented as mean ± standard deviation and categorical data are presented as number (percentage) of patients. EQ-5D indicates EuroQol 5 Dimension; SAQ, Seattle Angina Questionnaire; SAQAF, Seattle Angina Questionnaire angina frequency domain.
Data are shown as mean ± standard deviation or reported as number (percentage) of patients.
Figure 2.
Unadjusted changes in health-related quality of life (HRQOL) outcomes as measured by Seattle Angina Questionnaire (SAQ) domains and EuroQol 5 Dimensions visual analogue scale by smoking status categories over 12 months following percutaneous coronary intervention (PCI).
Specifically, the 1-year SAQ angina frequency scores of persistent smokers were significantly lower than those who never smoked, were past smokers, and who quit after their PCI (90.4±17.5 versus 94.6±13.7, 94.1±15.3, 94.3±13.3, respectively). The proportion of patients reporting any angina at 1 year (SAQ Angina Frequency Score <100) was significantly higher among persistent smokers group as compared with the other groups (30.5% versus 18.6% for never smokers, 18.2% for past smokers, 21.2% for quitters). SAQ physical limitation (92.7±17.8 versus 96.1±11.6 for never smokers, 95.8±12.4 for past smokers, 93.0±16.7 for quitters) and SAQ quality of life scores (73.0±23.9 versus 81.6±17.2 for never smokers, 82.0±18.7 for past smokers, 76.5±21.3 for quitters) were also significantly lower for patients who continued to smoke than other patients, as were these patients' EQ-5D VAS scores at 12 months (68.0±20.9 versus 77.2±17.3 for never smokers, 75.2±18.4 for past smokers, 72.5±18.9 for quitters).
Adjusted comparison of HRQOL outcomes according to smoking status
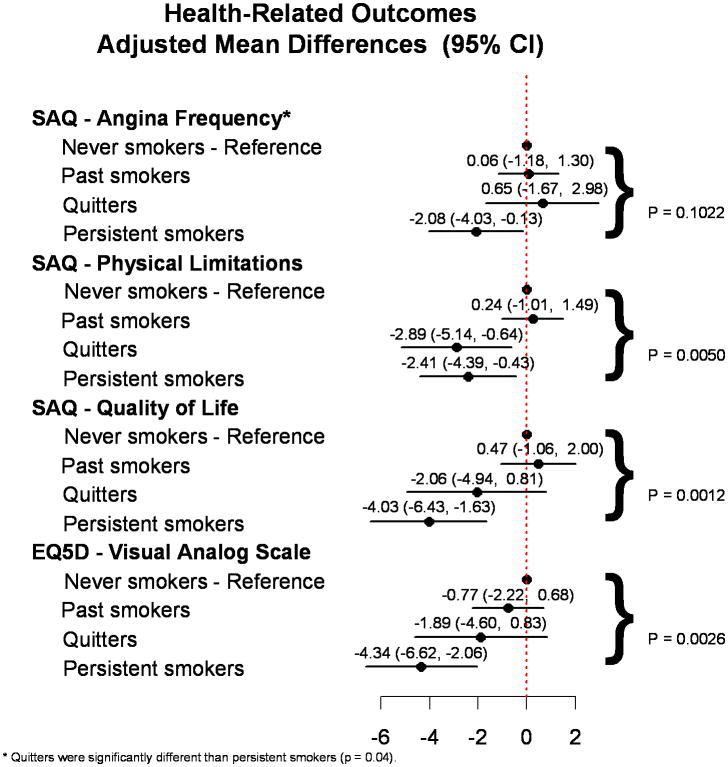
Figure 3 shows the fully-adjusted differences in HRQOL outcomes according to smoking status, with never smokers as the reference group. Persistent smokers were significantly worse in all aspects of HRQOL domains as compared with never smokers. In the multivariable adjusted model, patients who persistently smoked reported significantly lower SAQ angina frequency, physical limitation, quality of life and EQ-5D VAS scores as compared with never smokers at 1-year follow-up. Past smokers had similar health status scores as those who never smoked. Importantly, among those who smoked at the time of PCI, quitters had significantly better angina control (mean difference=2.73; 95% CI, 0.13 to 5.33) than persistent smokers (Figure 3). Moreover, their quality of life scores, both SAQ and EQ-5D VAS, trended towards being higher than persistent smokers at 1 year after their PCI (SAQ quality of life mean difference=1.97; 95% CI, -1.24 to 5.18 and EQ-5D VAS mean difference=2.45; 95% CI, -0.58 to 5.49).
Figure 3.
Multivariable adjusted analysis of the association of smoking status with health-related quality of life (HRQOL) outcomes at 1-year follow-up (reference group: never smokers).
Discussion
While smoking rates have continued to decline over time in the United States, those who smoke at the time of PCI are strongly urged to quit as a key component of secondary prevention. In this study, the largest observational study to date to evaluate the association of smoking cessation with health status outcomes, we found that persistent smokers had significantly worse HRQOL as compared with never smokers, but quitters did not. Among 504 patients who were smoking at the time of PCI, those who continued to smoke had significantly more angina and a trend towards poorer disease-specific and generic quality of life than those who quit.
These findings support and extend prior observational studies demonstrating improved health status after PCI among those who stopped smoking. For example, Haddock et al.27 investigated the impact of smoking status at the time of PCI in 271 consecutive patients and found that smokers has worse Short Form-12 Physical Component Scores, more angina and poorer disease-specific quality of life, as assessed by the SAQ 12 months after their procedure. Our work extends this observation be stratifying those who smoked at the time of PCI into those who quit and those who continued smoking and found much worse outcomes in the latter, which confirms the study of Taira et al.6 in 1,423 patients. While the exact pathophysiological mechanism underlying the association between smoking status and angina after PCI is unknown, it is congruent with basic science studies that demonstrate correlations between smoking and vascular reactivity,28 oxidative stress,29,30 endothelial damage,31 endothelial vasomotor dysfunction,32 platelet aggregation33,34 and adhesion.35 Regardless of the exact mechanism, and we acknowledge that no causal relationship has been established, the findings from this and prior studies can be shared with PCI patients who smoke to further motivate them to quit.
The benefits of smoking cessation have been clearly established. Patients with cardiovascular disease, including those who undergo PCI, will benefit from smoking cessation more than the general population.7 The higher likelihood of presenting with an acute coronary syndrome among patients who smoked at the time of PCI in our study suggests that they have more rupture-prone unstable plaques as compared with never smokers or past-smokers, as previously described.36-38 Although the reduction of cardiac event rate among the general population varies across studies,39,40 the risks of adverse cardiac events with cigarette smoking diminish relatively early after smoking cessation in patients with established CAD and these benefits continue to increase over time after quitting.41 In a meta-analysis of 20 cohort studies including 12,603 smoking patients with ischemic heart disease, cessation of smoking after AMI or cardiac surgery dramatically reduced mortality (relative risk, 0.64; 95% CI, 0.58–0.71).7 In our study, 50-70% of patients, across smoking categories, presented with an acute coronary syndrome, for whom substantial effort is often expended to encourage these smokers to quit. However only a small proportion of patients (37.5%) actually quit smoking after their PCI in our cohort, underscoring the limited effectiveness of current smoking cessation efforts. In this context, health care professionals need to further motivate patients to quit smoking. The findings from this study may support these efforts as we have shown that persistent smoking is associated with worse health status outcomes after PCI, the primary purpose of the procedure in stable coronary disease. Even though prior clinical trials of aggressive smoking cessation strategies, including nicotine replacement therapy for CAD patients, proved to be safe and effective,42 many clinicians have been reluctant to prescribe nicotine to patients with cardiovascular disease.43 One potential reason why clinicians are not effectively treating smoking habits is that they have grown numb to the mortality benefits and these data may further motivate them to use PCI as a ‘teachable moment’ to support their patients in stopping smoking.44
Although cessation of smoking rapidly reduces the risk of fatal cardiovascular events through several pathophysiologic mechanisms,14,34,45,46 there have been controversies how quickly and how much risk of adverse clinical outcomes will be affected by smoking cessation. Some authors have suggested substantial improvements occur within 2 to 3 years after smoking cessation,12,14 while others estimate that risk of adverse outcomes does not fall to the level of nonsmokers even after 20 years.47,48 In our study, it took only 1 year to see that smoking cessation was associated with better health status outcomes.
Limitations
There are several potential limitations to the current study that should be considered. First, there may have been misclassification of smoking status over time, as we did not use biological markers, such as cotinine levels, to verify smoking status. Such misclassification, however, would be expected to dilute the differences between groups and our results may therefore be a conservative estimate of the true benefit of smoking cessation. Second, we could not distinguish differences in smoking intensity among persistent smokers and past smokers even though the benefits of smoking cessation on cardiovascular outcomes might vary with the amount of cigarettes patients had smoked prior to their procedure. Third, unmeasured residual confounders might have contributed to differences in HRQOL scores at follow-up. Although we attempted to adjust for numerous clinical and socioeconomic factors, the possibility of residual unmeasured confounding remains. For example, we did not adjust for the quality of PCI, although we did not find significant differences in the number of diseased vessels or treated vessels between quitters and persistent smokers. Fourth, the sample size was relatively small, especially in quitters and persistent smokers. Although the lack of statistically significant differences in HRQOL outcomes between quitters and non-smokers might have been limited by statistical power, we did have sufficient power to demonstrate significantly less angina in smokers who quit as compared with smokers who continued to smoke. In addition, given our modest sample size and the number of comparisons made, our data should be interpreted with caution and confirmed in future studies. Moreover, clinicians performing PCI have no control over whether or not patients were smoking prior to treatment, but can influence their decision to stop smoking afterwards. Being able to share with patients the potential health status advantages of quitting might therefore be helpful in clinical practice. An additional limitation is that we were not able to report on benefits with respect to major adverse cardiac events over the year after PCI, as documented by others,2,7,49 and this important complementary information would help support the angina relief we found to be associated with quitting smoking. Finally, the follow-up period was only 1 year, precluding our ability to define longer-term benefits of smoking cessation.
Conclusions
Individuals who continue smoking after the PCI experienced significantly poorer HRQOL outcomes than patients who have never smoked. We also found that patients who quit smoking after PCI demonstrated significantly better angina control one year later as compared with persistent smokers. Whether sharing these additional insights with patients who smoke at the time of PCI would improve their success in quitting should be tested in future studies.
Supplementary Material
Acknowledgments
Sources of Funding: The Outcomes of PCI Study (OPS) was supported by an American Heart Association Outcomes Research Center grant (0875149N) and the Personalized Risk Information Services Manager™ (PRISM) study was supported by a grant from the National Heart Lung and Blood Institute (R01-HL096624). While the grants supported different aims, they employed the same patient population, study design, and data collection, and were combined for the purpose of these analyses. The funding agencies had no role in data collection, analysis, interpretation or the decision to submit the results. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Dr. Jae-Sik Jang was supported by a grant from Research year of Inje University in 2014 (20131465).
Dr. Ali Shafiq, Dr. Anna Grodzinsky, Dr. Timothy J. Fendler – received support from the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL110837.
Footnotes
Disclosures: Dr. John A. Spertus discloses that he owns the copyright to the Seattle Angina Questionnaire.
References
- 1.Detre K, Holubkov R, Kelsey S, Cowley M, Kent K, Williams D, Myler R, Faxon D, Holmes D, Jr, Bourassa M. Percutaneous transluminal coronary angioplasty in 1985-1986 and 1977-1981. The National Heart, Lung, and Blood Institute Registry. N Engl J Med. 1988;318:265–270. doi: 10.1056/NEJM198802043180501. [DOI] [PubMed] [Google Scholar]
- 2.Hasdai D, Garratt KN, Grill DE, Lerman A, Holmes DR., Jr Effect of smoking status on the long-term outcome after successful percutaneous coronary revascularization. N Engl J Med. 1997;336:755–761. doi: 10.1056/NEJM199703133361103. [DOI] [PubMed] [Google Scholar]
- 3.Koole D, Moll FL, Buth J, Hobo R, Zandvoort H, Pasterkamp G, van Herwaarden JA EUROSTAR collaborators. The influence of smoking on endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2012;55:1581–1586. doi: 10.1016/j.jvs.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Wiseman S, Kenchington G, Dain R, Marshall CE, McCollum CN, Greenhalgh RM, Powell JT. Influence of smoking and plasma factors on patency of femoropopliteal vein grafts. BMJ. 1989;299:643–646. doi: 10.1136/bmj.299.6700.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 6.Taira DA, Seto TB, Ho KK, Krumholz HM, Cutlip DE, Berezin R, Kuntz RE, Cohen DJ. Impact of smoking on health-related quality of life after percutaneous coronary revascularization. Circulation. 2000;102:1369–1374. doi: 10.1161/01.cir.102.12.1369. [DOI] [PubMed] [Google Scholar]
- 7.Critchley JA, Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: A systematic review. JAMA. 2003;290:86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 8.Fiore MC, Goplerud E, Schroeder SA. The joint commission's new tobacco-cessation measures--will hospitals do the right thing? N Engl J Med. 2012;366:1172–1174. doi: 10.1056/NEJMp1115176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spertus JA, Bach R, Bethea C, Chhatriwalla A, Curtis JP, Gialde E, Guerrero M, Gosch K, Jones PG, Kugelmass A, Leonard BM, McNulty EJ, Shelton M, Ting HH, Decker C. Improving the process of informed consent for percutaneous coronary intervention: Patient outcomes from the patient risk information services manager (ePRISM) study. Am Heart J. 2015;169:234–241.e1. doi: 10.1016/j.ahj.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 11.Conard MW, Haddock CK, Poston WS, Spertus JA Cardiovascular Outcomes Research Consortium. The impact of smoking status on the health status of heart failure patients. Congest Heart Fail. 2009;15:82–86. doi: 10.1111/j.1751-7133.2009.00053.x. [DOI] [PubMed] [Google Scholar]
- 12.Gordon T, Kannel WB, McGee D, Dawber TR. Death and coronary attacks in men after giving up cigarette smoking. A report from the Framingham study. Lancet. 1974;2:1345–1348. doi: 10.1016/s0140-6736(74)92214-4. [DOI] [PubMed] [Google Scholar]
- 13.Hirdes JP, Maxwell CJ. Smoking cessation and quality of life outcomes among older adults in the Campbell's Survey on Well-Being. Can J Public Health. 1994;85:99–102. [PubMed] [Google Scholar]
- 14.Lightwood JM, Glantz SA. Short-term economic and health benefits of smoking cessation: myocardial infarction and stroke. Circulation. 1997;96:1089–1096. doi: 10.1161/01.cir.96.4.1089. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH. Measurement of health-related quality of life in heart failure. J Am Coll Cardiol. 1993;22:185A–191A. doi: 10.1016/0735-1097(93)90488-m. [DOI] [PubMed] [Google Scholar]
- 16.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. doi: 10.1016/0002-9149(94)90555-x. [DOI] [PubMed] [Google Scholar]
- 17.Dougherty CM, Dewhurst T, Nichol WP, Spertus J. Comparison of three quality of life instruments in stable angina pectoris: Seattle Angina Questionnaire, Short Form Health Survey (SF-36), and Quality of Life Index-Cardiac Version III. J Clin Epidemiol. 1998;51:569–575. doi: 10.1016/s0895-4356(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 18.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: A new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12:142S–158S. doi: 10.1016/s0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 20.Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long-term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. doi: 10.1161/01.cir.0000020688.24874.90. [DOI] [PubMed] [Google Scholar]
- 21.Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. doi: 10.1016/S0002-8703(03)00436-8. [DOI] [PubMed] [Google Scholar]
- 22.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: Insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–353. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 23.EuroQol Group. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 24.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol group. Ann Med. 2001;33:337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 25.Ellis JJ, Eagle KA, Kline-Rogers EM, Erickson SR. Validation of the EQ-5D in patients with a history of acute coronary syndrome. Curr Med Res Opin. 2005;21:1209–1216. doi: 10.1185/030079905X56349. [DOI] [PubMed] [Google Scholar]
- 26.Schweikert B, Hahmann H, Leidl R. Validation of the EuroQol questionnaire in cardiac rehabilitation. Heart. 2006;92:62–67. doi: 10.1136/hrt.2004.052787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddock CK, Poston WS, Taylor JE, Conard M, Spertus J. Smoking and health outcomes after percutaneous coronary intervention. Am Heart J. 2003;145:652–657. doi: 10.1067/mhj.2003.67. [DOI] [PubMed] [Google Scholar]
- 28.Chan NN, Colhoun HM, Vallance P. Cardiovascular risk factors as determinants of endothelium-dependent and endothelium-independent vascular reactivity in the general population. J Am Coll Cardiol. 2001;38:1814–1820. doi: 10.1016/s0735-1097(01)01669-2. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi Y, Matsuno S, Kagota S, Haginaka J, Kunitomo M. Oxidants in cigarette smoke extract modify low-density lipoprotein in the plasma and facilitate atherogenesis in the aorta of watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 2001;156:109–117. doi: 10.1016/s0021-9150(00)00637-7. [DOI] [PubMed] [Google Scholar]
- 30.Guthikonda S, Sinkey C, Barenz T, Haynes WG. Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation. 2003;107:416–421. doi: 10.1161/01.cir.0000046448.26751.58. [DOI] [PubMed] [Google Scholar]
- 31.Michael Pittilo R. Cigarette smoking, endothelial injury and cardiovascular disease. Int J Exp Pathol. 2000;81:219–230. doi: 10.1046/j.1365-2613.2000.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita K, Tsukamoto T, Naya M, Noriyasu K, Inubushi M, Shiga T, Katoh C, Kuge Y, Tsutsui H, Tamaki N. Smoking cessation normalizes coronary endothelial vasomotor response assessed with 15O-water and PET in healthy young smokers. J Nucl Med. 2006;47:1914–1920. [PubMed] [Google Scholar]
- 33.Fusegawa Y, Goto S, Handa S, Kawada T, Ando Y. Platelet spontaneous aggregation in platelet-rich plasma is increased in habitual smokers. Thromb Res. 1999;93:271–278. doi: 10.1016/s0049-3848(98)00184-4. [DOI] [PubMed] [Google Scholar]
- 34.Morita H, Ikeda H, Haramaki N, Eguchi H, Imaizumi T. Only two-week smoking cessation improves platelet aggregability and intraplatelet redox imbalance of long-term smokers. J Am Coll Cardiol. 2005;45:589–594. doi: 10.1016/j.jacc.2004.10.061. [DOI] [PubMed] [Google Scholar]
- 35.Sawada M, Kishi Y, Numano F, Isobe M. Smokers lack morning increase in platelet sensitivity to nitric oxide. J Cardiovasc Pharmacol. 2002;40:571–576. doi: 10.1097/00005344-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Zaman AG, Helft G, Worthley SG, Badimon JJ. The role of plaque rupture and thrombosis in coronary artery disease. Atherosclerosis. 2000;149:251–266. doi: 10.1016/s0021-9150(99)00479-7. [DOI] [PubMed] [Google Scholar]
- 37.Burke AP, Farb A, Pestaner J, Malcom GT, Zieske A, Kutys R, Smialek J, Virmani R. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation. 2002;105:419–424. doi: 10.1161/hc0402.102952. [DOI] [PubMed] [Google Scholar]
- 38.Sambola A, Osende J, Hathcock J, Degen M, Nemerson Y, Fuster V, Crandall J, Badimon JJ. Role of risk factors in the modulation of tissue factor activity and blood thrombogenicity. Circulation. 2003;107:973–977. doi: 10.1161/01.cir.0000050621.67499.7d. [DOI] [PubMed] [Google Scholar]
- 39.Hjermann I, Velve Byre K, Holme I, Leren P. Effect of diet and smoking intervention on the incidence of coronary heart disease. Report from the Oslo Study Group of a randomised trial in healthy men. Lancet. 1981;2:1303–1310. doi: 10.1016/s0140-6736(81)91338-6. [DOI] [PubMed] [Google Scholar]
- 40.Hurt RD, Weston SA, Ebbert JO, McNallan SM, Croghan IT, Schroeder DR, Roger VL. Myocardial infarction and sudden cardiac death in Olmsted County, Minnesota, before and after smoke-free workplace laws. Arch Intern Med. 2012;172:1635–1641. doi: 10.1001/2013.jamainternmed.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samet JM. The 1990 report of the surgeon general: The health benefits of smoking cessation. Am Rev Respir Dis. 1990;142:993–994. doi: 10.1164/ajrccm/142.5.993. [DOI] [PubMed] [Google Scholar]
- 42.McRobbie H, Hajek P. Nicotine replacement therapy in patients with cardiovascular disease: Guidelines for health professionals. Addiction. 2001;96:1547–1551. doi: 10.1046/j.1360-0443.2001.961115472.x. [DOI] [PubMed] [Google Scholar]
- 43.Katz DA, Tang F, Faseru B, Horwitz PA, Jones P, Spertus J. Prevalence and correlates of smoking cessation pharmacotherapy in hospitalized smokers with acute myocardial infarction. Am Heart J. 2011;162:74–80. doi: 10.1016/j.ahj.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prignot J, Bartsch P, Vermeire P, Jamart J, Wanlin M, Uydebrouck M, Thijs J. Physician's involvement in the smoking cessation process of their patients. Results of a 1998 survey among 4,643 Belgian physicians. Acta Clin Belg. 2000;55:266–275. doi: 10.1080/17843286.2000.11754309. [DOI] [PubMed] [Google Scholar]
- 45.Hunter KA, Garlick PJ, Broom I, Anderson SE, McNurlan MA. Effects of smoking and abstention from smoking on fibrinogen synthesis in humans. Clin Sci (Lond) 2001;100:459–465. [PubMed] [Google Scholar]
- 46.Oren S, Isakov I, Golzman B, Kogan J, Turkot S, Peled R, Yosefy C. The influence of smoking cessation on hemodynamics and arterial compliance. Angiology. 2006;57:564–568. doi: 10.1177/0003319706293119. [DOI] [PubMed] [Google Scholar]
- 47.Negri E, La Vecchia C, D'Avanzo B, Nobili A, La Malfa RG. Acute myocardial infarction: Association with time since stopping smoking in Italy. GISSI-EFRIM investigators. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto. Epidemiologia dei Fattori di Rischio dell'Infarto Miocardico. J Epidemiol Community Health. 1994;48:129–133. doi: 10.1136/jech.48.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doll R, Peto R. Mortality in relation to smoking: 20 years' observations on male British doctors. Br Med J. 1976;2:1525–1536. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen T, Li W, Wang Y, Xu B, Guo J. Smoking status on outcomes after percutaneous coronary intervention. Clin Cardiol. 2012;35:570–574. doi: 10.1002/clc.22004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.