Abstract
Background
We determined the prevalence and incidence of liver dysfunction prior to and after initiation of combination antiretroviral therapy (cART) in the TREAT Asia Pediatric HIV Observational Database (TApHOD).
Methods
Data from children initiated on cART between 2–18 years of age with baseline alanine aminotransferase (ALT) available prior to and at least once after cART initiation in TApHOD between 2008–2012 were analyzed. Prevalence and incidence of liver dysfunction, and biomarkers including the aspartate aminotransferase (AST) to platelet ratio index (APRI) and FIB4 index were assessed.
Results
Data from 1930 children were included. Their median age was 6.9 years; 49% were male; 98% were perinatally infected; and 94% were initiated on non-nucleoside reverse transcriptase-based cART regimens. Prior to cART, the prevalence of ALT ≥ 3 times the upper limit of normal (*ULN) was 5.8%. There were 8.5% of children with APRI >1.5 (suggestive of liver fibrosis), and 2.7% with FIB4 index >1.3 (predictive of possible cirrhosis). Among the 1143 cases with normal baseline ALT (≤1*ULN), the incidence of ALT 3*ULN after cART was 1.19/1000 person-months (95% CI 0.93–1.51). Two of 350 with available tests (0.6%) met Hy’s law (ALT >3*ULN and total bilirubin >2*ULN). By multivariate analysis, baseline hemoglobin <7.5 g/dL was a predictor of ALT >3*ULN, while age 5–9 years at cART initiation was protective for liver dysfunction.
Conclusions
We demonstrated a low prevalence and incidence of liver dysfunction before and after cART initiation in children with normal baseline chemistries. In this population facing life-long cART, prospective surveillance for emergence of liver disease is warranted.
Keywords: liver, HIV, antiretroviral therapy, children, Asia
Introduction
Although the risk of AIDS-defining illnesses has decreased in the era of combination antiretroviral therapy (cART), this has been replaced by other long-term complications causing morbidity and mortality [1]. Despite hepatitis B and C being endemic in the Asia-Pacific, previous studies in children with HIV have reported lower prevalence of clinically diagnosed liver disease and hepatitis B or C co-infections compared to adults [2–5].
Interpreting whether an abnormal liver chemistry value is related to medications, comorbidities, or HIV infection itself can be difficult, and symptomatic children generally receive no further evaluation. This is a particular concern in resource-limited settings, where most children were initiated on non-nucleoside reverse transcriptase-based regimens with known hepatotoxicity [6,7].
When liver disease is suspected, non-invasive screening methods like the FibroScan® [8] may be beneficial, but in many circumstances are prohibitively expensive and/or not accessible for children. Combination biomarkers such as the aspartate aminotransferase (AST)/ALT ratio, AST-to-platelet ratio index (APRI) and FIB4 index, have been reported as potentially useful for predicting hepatic fibrosis in children with non-alcoholic fatty liver disease [9], chronic viral hepatitis [10], chronic liver disease from various etiologies [11], as well as among perinatally HIV-infected Latin-American children [12]. A recent US study has reported low level APRI elevations prior to cART, with increasing levels over time on ART in perinatally HIV-infected children [13].
The primary aim of this study was to determine the prevalence of liver dysfunction in a regional cohort of children and adolescents with HIV prior to cART. The secondary aims were to describe available biomarkers of liver disease, to determine the incidence of liver dysfunction, and to explore predictors of liver dysfunction after cART.
Patients and Methods
In this retrospective data analysis, the population included patients from 18 clinical sites in six Asian countries participating in the TREAT Asia Pediatric HIV Observational Database (TApHOD), which is a multicenter study of children and adolescents living with HIV in Asia, enrolled before 18 years of age. The cohort was established in 2008; both retrospective and prospective data have been collected [14]. Data transfer to a central data management and biostatistic analysis center is performed at 6-monthly intervals. Data transferred up to September 2012 were used for this analysis.
The following inclusion criteria were applied: 1) children and adolescents with confirmed diagnosis of HIV infection, 2) aged ≤ 18 years at the time of first-line cART initiation, 3) receiving cART - defined as a regimen of ≥ 3 antiretroviral agents, and 4) with baseline ALT tests within six months prior to cART. Children who had previously been treated with either mono- or dual-nucleoside reverse transcriptase inhibitor regimens were excluded.
Liver dysfunction was characterized as a pre-defined chemistry elevation above the upper limit of normal (ULN). For this analysis, we considered transaminase levels reflecting liver injury or inflammation as a marker of liver dysfunction. The baseline prevalence of liver dysfunction was presented as the frequency of abnormalities within six months prior to cART initiation, calculated by dividing the number of children with abnormal baseline lab values by the total number of children with available lab values. The incidence of new liver dysfunction after cART initiation was presented as the frequency of post-cART liver chemistry abnormalities among children with normal baseline ALT (<1*ULN) and at least one follow-up ALT test. The first increase in the specific parameters that met or exceeded threshold levels was counted, and the total follow-up time in person-months was used as the denominator. Person-months were applied instead of person-years, as the expected outcome could be seen within months after cART.
Normal ranges for liver chemistry values were referenced from the Harriet Lane Handbook 19th edition [15]. The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0 (December 2004) was used to assess the severity of abnormal laboratory parameters [16]. For ALT, elevations of ≥ 3*ULN at baseline and increases of ≥ 1*ULN, 3*ULN, and 5*ULN were evaluated. Calculating incidence by assessing fold changes from baseline values has been proposed by previous studies as a more sensitive indicator for liver injury [17,18]. For alkaline phosphatase (ALP) and total bilirubin (TBIL) we considered fold changes compared to absolute values. The number of cases who met Hy’s law, a validated predictor of serious drug-induced liver injury defined as ALT >3*ULN and TBIL >2*ULN [19,20], was also assessed. Other selected biomarkers included the AST/ALT ratio, APRI, and FIB4 index. AST/ALT ratios were calculated for each participant; a value of >0.7 denotes potential liver fibrosis [21]. The APRI was calculated by the formula ([AST/upper limit of normal]/platelet count [109/l]) × 100; a value of >1.5 suggests liver fibrosis [22,23]. The FIB4 Index was calculated by age × AST level/platelet count × √ALT; a value of ≤ 1.3 has been reported to have a 90% negative predictive value for cirrhosis [24].
Statistical analysis
Demographic characteristics of study participants were summarized by descriptive statistics. The following variables were included: age, sex, race, route of HIV acquisition, weight, height, body mass index, history of opportunistic infection, WHO staging/CDC category, CD4 lymphocyte count, HIV RNA level, cART regimen, duration on cART, viral hepatitis markers (HBsAg, HBeAg, anti-hepatitis C antibody), and hemoglobin. Liver-related laboratory results included ALT, AST, platelet count, ALP, and TBIL. Continuous variables were reported using means (standard deviation, SD) and/or medians (interquartile range; IQR), and categorical data were described in numbers and proportions. Age was categorized into four groups (<5, 5-<9, 9-<12, and 12–18 years). Height-for-age z-scores were calculated using the 2006 WHO child growth standards and macros reference for ages 2–5 years, and 2007 reference for ages 2–19 years [25,26]. Weight-for-age z-scores were calculated using the WHO child growth standards and macros for 1977 [27]. Stata version 12 (StataCorp. 2011. Stata Statistical Software: Release 12. College Station, TX: StataCorp LP) was used for all statistical analyses.
Predictors of severe liver chemistry abnormalities were analyzed by Cox survival analyses stratified by study site and Kaplan-Meier curves. ALT ≥ 3*ULN was used as a dependent outcome variable. Time-to-event was measured from cART start until the first occurrence of abnormal liver chemistries; in cases with no abnormal liver chemistries, time to censoring was measured from cART start until the date of the last available ALT test. Nevirapine (NVP) use was modeled as a time-updated variable with a lag time of 28 days after cessation to account for the drug’s long washout period. In order to improve the chance of retaining meaningful confounders, we selected potential predictors based on a significance level of <0.2 in the univariate analyses to be included in the multivariate models. Predictors were considered as significant in the multivariate model if they exhibited a p-value <0.05.
Results
Demographic data of overall study participants
Data from 1930 children who met the inclusion criteria were included as a prevalence population. Their characteristics are shown in Table 1. The median age was 6.9 years (IQR 4.5–9.4); 49% were male, and 46% were Thai. Almost all (98%) were perinatally infected. Prior to cART initiation, 58.3% were in WHO stage 3 or 4; their median CD4 lymphocyte count was 171 (IQR 41–389) cells/mm3, and 428 of 698 (61.3%) of children with HIV RNA results available had HIV RNA level ≥5.0 log10 copies/mL. Twenty-four of 506 tested for HBsAg were positive (4.7%), and 15 of 423 had positive hepatitis C antibody tests (3.5%). The first-line cART regimens used were NVP-based in 62%, efavirenz (EFV)-based in 32%, and protease inhibitor (PI)-based in 6%; varieties of dual-nucleoside reverse transcriptase inhibitor (NRTI) backbones were used. Sixty-six percent of all children received NVP as a part of their first line cART regimen.
Table 1.
Baseline characteristics of study participants prior to antiretroviral treatment
| Characteristics | Prevalence population n=1930 |
Incidence population n=1143 |
|---|---|---|
| Age (years) | ||
| mean (SD) | 7.2 (3.4) | 7.5 (3.3) |
| median (IQR) | 6.9 (4.5–9.4) | 7.3 (4.8–7.7) |
| 2– <5 years | 592 (30.7) | 304 (26.6) |
| 5– <9 years | 789 (40.9) | 489 (42.8) |
| 9– <12 years | 342 (17.7) | 216 (18.9) |
| ≥12 years | 207 (10.7) | 134 (11.7) |
| Male | 946 (49) | 557 (48.7) |
| Ethnicity | ||
| Thai | 889 (46) | |
| Vietnam | 428 (22) | |
| Indian | 93 (5) | |
| Malaysian | 77 (4) | |
| Indonesian | 73 (4) | |
| Chinese | 28 (2) | |
| Route of HIV acquisition | ||
| perinatal | 1844 (98.3) | 1097 (98.6) |
| others | 32 (1.7) | 16 (1.7) |
| Weight-for-age z-score | ||
| mean (SD) | −2.80 (2.09) | −2.63 (1.88) |
| median (IQR) | −2.52 (−3.86 – −1.40) | −2.38 (−3.49 – −1.39) |
| < −2.5 | 951 (50.9) | 530 (47.7) |
| −1.5 to −2.5 | 419 (22.4) | 277 (24.9) |
| ≥ −1.5 | 499 (26.7) | 305 (27.4) |
| Height-for-age z-score | ||
| mean (SD) | −2.40 (1.40) | −2.29 (1.33) |
| median (IQR) | −2.36 (−3.30 – −1.51) | −2.25 (−3.16 – −1.45) |
| < −2.5 | 828 (46.0) | 463 (42.9) |
| −1.5 to −2.5 | 526 (29.2) | 328 (30.4) |
| ≥ −1.5 | 446 (24.8) | 287 (26.6) |
| WHO category prior to cART initiation | ||
| 1 or 2 | 700 (41.6) | 793 (69.6) |
| 3 or 4 | 982 (58.3) | 346 (30.4) |
| Hemoglobin (g/dL) | ||
| <7.5 | 67 (4.1) | 33 (3.3) |
| ≥7.5 | 1553 (95.9) | 969 (96.7) |
| CD4 cell count (cells/mm3) | ||
| mean (SD) | 291 (448) | 314 (383) |
| median (IQR) | 171 (41–389) | 202 (54–436) |
| <200 | 875 (54.4) | 481 (49.6) |
| 200– <500 | 441 (27.5) | 288 (29.7) |
| ≥500 | 292 (18.2) | 201 (20.7) |
| Log10 VL (copies/mL) | ||
| mean (SD) | 4.93 (0.96) | 4.84 (0.99) |
| median (IQR) | 5.09 (4.70–5.53) | 5.03 (4.61–5.48) |
| VL ≥5.0 (log10 copies/mL) | 428 (61.3) | 271 (57.8) |
| VL <5.0 (log10 copies/mL) | 270 (38.7) | 198 (42.2) |
Data are in number (%) or mean (SD), or median (interquartile range) as appropriate.
SD-standard deviation; IQR-interquartile range; cART-combination antiretroviral treatment; VL-viral load.
Prevalence
The prevalence of liver dysfunction and abnormal biomarkers of liver disease prior to cART are shown in Table 2. The prevalence of liver chemistry abnormalities were 5.75% for ALT >3*ULN, 1.87% for ALT >5*ULN, and 0.27% for ALT >8*ULN. None met Hy’s law pre-cART. The prevalence of ALP >2*ULN was 1.76%. The median AST/ALT ratio was 1.51 (IQR 1.18–2.13). Almost all study participants (97.8%) had AST/ALT ratios >0.7. The median APRI prior to cART was 0.34 (IQR 0.18–0.63); the prevalence of APRI >1.5 was 8.5%. Twenty-two of 820 children (2.83%) had a FIB4 index >1.3.
Table 2.
Prevalence of liver dysfunction prior to cART
| Liver threshold/biomarker | na/nb or median (IQR) | % | |
|---|---|---|---|
| ALT≥1*ULN | 685/1930 | 35.49% | |
| ALT≥3*ULN | 111/1930 | 5.75% | |
| ALT≥5*ULN | 36/1930 | 1.87% | |
| ALT≥8*ULN | 16/1930 | 0.83% | |
| ALT≥3*ULN & Total Bilirubin≥2*ULN | 0/403 | 0.00% | |
| ALP≥3*ULN | 3/398 | 0.75% | |
| TBIL≥2*ULN | 7/403 | 1.74% | |
| AST/ALT ratio>0.7 | 1199/1226 | 97.80% | |
| AST/ALT ratio>1.0 | 1098/1226 | 89.56% | |
| Median (IQR) AST/ALT ratio | 1.51 (1.18–2.13) | ||
| APRI >0.5 | 322/988 | 32.59% | |
| APRI >1.5 | 84/988 | 8.50% | |
| Median (IQR) APRI score | 0.34 (0.18–0.63) | ||
| FIB4 index >1.3 | 28/988 | 2.83% | |
| Median (IQR) FIB4 index | 0.14 (0.06–0.25) | ||
Number of children with threshold enzyme elevations.
Total number of children with the laboratory tests obtained at baseline.
cART- combination antiretroviral therapy; IQR-interquartile range; ULN-upper limit of normal; ALT-alanine aminotransferase; AST-aspartate aminotransferase; ALP-alkaline phosphatase; TBIL-total bilirubin; APRI: AST to Platelet Ratio Index calculated from ([AST/upper limit of normal]/platelet count [109/l]) × 100; FIB4 index: calculated from age × AST level/platelet count ×√ALT.
Among the 24 cases known to be HBs Ag-positive, 16 of 24 (66.67%) had ALT ≥ 1*ULN, 2 of 24 (8.33%) had ALT ≥ 3*ULN, and 2 of 11 (18.18%) had APRI >1.5 prior to cART. Among the 15 children with positive anti-HCV tests, 5 of 15 (33.33%) had ALT ≥1*ULN, none had ALT ≥ 3*ULN, and 1 of 9 (11.11%) had APRI >1.5 prior to cART.
Incidence
The 1143 children with baseline ALT <1*ULN and follow-up ALT testing available after cART initiation were included as an incidence population. Incidence of liver dysfunction and abnormal biomarkers of liver disease after cART are shown in Table 3. The incidence of liver dysfunction after cART initiation was 1.19 (95% CI 0.93–1.51) per 1000 person-months for ALT 3*ULN, and 0.40 (95% CI 0.27–0.61) for ALT 5*ULN. The frequency of ALT >3*baseline value (259/1143, 22.7%) was almost four times higher than the frequency of ALT >3*ULN (66/1143, 5.8%) after cART initiation. The median time to ALT >3*baseline value was 37.8 months (IQR 16.0–63.3) and ALT >3*ULN was 45.3 months (IQR 24.2–70.2). Two of 350 cases with available ALT and TBIL (0.6%) had ALT ≥ 3*ULN and TBIL ≥ 2*ULN.
Table 3.
Incidence of liver dysfunction after cART initiation among participants with normal baseline values
| Liver threshold/biomarkers | Total patients in analysis | na/person-monthsb | Incidence per 1,000 person-months | 95%CI
|
|
|---|---|---|---|---|---|
| Lower | Upper | ||||
| ALT≥3*ULN | 1143 | 66/55560 | 1.19 | 0.93 | 1.51 |
| ALT≥5*ULN | 1143 | 23/56836 | 0.4 | 0.27 | 0.61 |
| ALT≥3*ULN & TBIL ≥2*ULN | 350 | 2/18407 | 0.11 | 0.03 | 0.43 |
| ALT≥3*baseline value | 1143 | 259/48512 | 5.34 | 4.73 | 6.03 |
| TBIL≥2*ULN | 350 | 9/18129 | 0.5 | 0.26 | 0.95 |
| ALP≥3*ULN | 349 | 8/19054 | 0.42 | 0.21 | 0.84 |
| AST/ALT ratio>0.7 | 852 | 845/13270 | 63.68 | 59.53 | 68.12 |
| AST/ALT ratio>1.0 | 852 | 811/14115 | 57.45 | 53.63 | 61.55 |
| APRI >1.5 | 822 | 27/34854 | 0.77 | 0.53 | 1.13 |
| FIB4 >1.3 | 820 | 6/13764 | 0.44 | 0.41 | 0.47 |
Number of children with threshold enzyme elevations.
person-months: total follow-up period of participants with normal baseline ALT.
cART- combination antiretroviral therapy; ALT-alanine aminotransferase; AST-Aspartate aminotransferase; ALP-alkaline Phosphatase; TBIL-total bilirubin; APRI: AST to Platelet Ratio Index calculated from([AST/upper limit of normal)/platelet count [109/l]) × 100; FIB4 index: calculated from age × AST level/platelet count ×√ALT.
Of the 852 children with available AST and ALT, 845 (36.3%) had AST/ALT ratios >0.7. Of these, 96% had AST/ALT ratios >1.0. The incidence of AST/ALT ratio >0.7 was 63.7 per 1000 person-months (95% CI 59.5–68.1). The incidence of APRI >1.5 was 0.77 per 1000 person-months (95% CI 0.53–1.13); among the 27 cases with APRI >1.5 after cART, 13 had HIV RNA results available within three months, of which 5 (38%) had HIV RNA levels >400 copies/mL. The incidence of FIB4 index >1.3 was 0.44 per 1000 person-months (95% CI0.41–0.47). There were five cases all with FIB4 scores >1.3, APRI scores >1.5, and AST/ALT ratios >1.0. Their ages ranged between 4.0–10.5 years, three were male, three were WHO stage 3 prior to cART, and hepatitis B infection status was negative in two (the other three had no hepatitis B testing reported).
Two cases met Hy’s law after cART initiation (2 of 1143, 0.17%; 0.11 per 1000 persons-months). One was a 3.7-year-old girl with CD4 cell count 507 cells/mm3 and 14.9%, hemoglobin (Hb) <7.5 g/dL, and in WHO stage 3 prior to cART. The second was a 6.4-year-old boy with CD4 cell count 756 cells/mm3 and 19%, and in WHO stage 2 prior to cART. Both had unknown hepatitis B serostatus, HIV RNA level >5.0 log10 copies/mL, and normal baseline ALT.
Predictors of elevated ALT after cART initiation
The univariate analysis identified multiple associations with ALT ≥ 3*ULN after cART initiation, including grade 3 or 4 anemia prior to cART (hazard ratio, HR 3.33, 95% CI 1.39–7.94, p=0.01), height-for-age z-score < −1.5 (HR 2.40, 95% CI 1.03–5.63, p= 0.04), and being female (HR 1.74, 95% CI 1.04–2.91, p=0.03). Being aged 5– <9 years at cART initiation was associated with a lower risk for ALT ≥ 3*ULN when compared to those aged 2– <5 years (HR 0.47, 95%CI 0.27–0.83, p=0.01). However, in multivariate analysis, only pre-cART grade 3 or 4 anemia remained a significant risk (HR 2.96, 95%CI 1.22–7.18, p=0.02), and age 5– <9 years at cART initiation remained a protective factor for ALT ≥ 3*ULN (HR 0.48, 95%CI 0.27–0.54, p=0.01). Older age was not protective: age 9-<12 years (HR 0.62, 95% CI 0.28–1.37, p=0.24), and age ≥ 12 years (HR 0.82–95% CI 0.28–2.41, p=0.72).
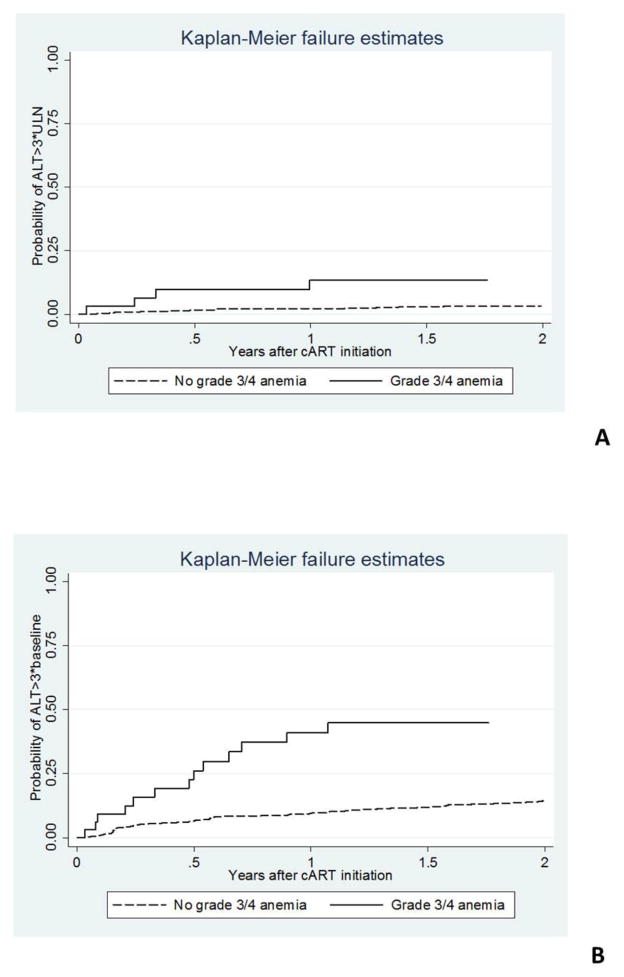
For ALT ≥ 3*baseline, both the univariate and multivariate analyses showed that grade 3 or 4 anemia prior to cART (HR 3.02, 95% CI 1.76–5.18, p<0.01) and being female (HR 1.41, 95% CI 1.09–1.82, p=0.01) were significant risk factors. NVP use analyzed as a time-updated variable was not associated with increased ALT after cART (HR 1.03, 95% CI 0.55–1.92, 9=0.93). Kaplan-Meier survival estimates for risk of ALT elevation by baseline anemia status are shown in Figure 1.
Figure 1. Kaplan-Meier curves for risk of severe liver dysfunction after cART initiation in participants with and without baseline grade 3/ 4 anemia. A) ALT >3*ULN as an outcome, and B) ALT >3*baseline as an outcome.
ALT-alanine aminotransferase; ULN-upper limit of normal; cART-combination antiretroviral treatment.
Discussion
In this study we described the overall prevalence of liver dysfunction prior to cART, the incidence of liver dysfunction after cART initiation, as well as patterns of liver disease biomarkers in HIV-infected children in our regional cohort. For perinatally HIV-infected children in resource-limited settings who are now growing up into adolescence, there remain concerns about the potential toxicities of life-long HIV and cART on organ function; especially when known hepatotoxic medicines have been used in the setting of severe immunosuppression. Liver chemistries and related biomarkers represent a more accessible surrogate method of assessing liver dysfunction when compared to other non-invasive methods (e.g., liver FibroScan® and ultrasonography), and merit additional study.
The prevalence of ALT elevations in our study were comparable to a previous report from HIV-infected children aged ≥ 18 years participating in pediatric HIV trials conducted by GlaxoSmithKline between 1995–2009 [28]. However, only 0.6% of 312 children included in their study were Asian. Children were included in that analysis regardless of their treatment status, and the prevalence of ALT elevations ≥ 3*ULN was 5.13% and ≥ 5*ULN was 2.24%. A study in South Africa reported a prevalence of grade 1 or 2 elevated ALT (1.25–5*ULN) in 32% of HIV-infected children prior to treatment, which may be related to relatively greater HIV disease severity, as 74% of them were in WHO stage 3 or 4 [29].
After cART initiation, only two children (0.17%) in our cohort met Hy’s law, which is a safety biomarker predicting serious hepatotoxicity [30]. The lower than expected incidence of ALT elevations may reflect the incomplete data capture of early transient liver enzyme elevations due to less frequent monitoring at some of the clinical sites. In general NVP-induced hepatotoxicity related to hypersensitivity reaction observed was around 10% [31], usually seen within the first six weeks.
We found ALP ≥ 2*ULN in 1.76% of all children prior to cART, which was lower than the 6.23% previously reported among HIV-infected children in a similar age group [28]. The difference may be related to extrahepatic sources of ALP, as a large proportion of HIV-infected children in our cohort had growth delay at the time of cART initiation. In addition, 1.74% of children had TBIL ≥ 2*ULN at baseline, which was more than four times higher than the 0.35% reported in the GlaxoSmithKline study [28]. Ineffective erythropoiesis due to chronic HIV or hemolytic disorders (e.g., hemoglobinopathies) could be possible explanations for increased bilirubin prior to cART in our predominantly Southeast Asian pediatric cohort [19].
Multivariate analysis showed that grade 3 or 4 anemia was significantly associated with ALT elevation ≥ 3*ULN after cART initiation. Nevertheless, in this study we had only 4% of children with grade 3–4 anemia. They might have had more advanced HIV disease, concomitant infection, and/or malnutrition making them less able to compensate for physiologic stressors associated with inflammatory responses to initial treatment; moreover they might also have received other hepatotoxic medications at the time of cART initiation. Although being aged between 5– <9 years at the time of cART initiation was likely to be a protective factor, this may not be a meaningful finding, as two-thirds of our study participants were in this age range and were likely to be slower disease progressors.
AST/ALT ratios >1 are highly suggestive of advanced liver disease in adult patients with chronic hepatitis [32]. We found that almost all of our study participants had AST/ALT ratios >1. An Italian study has shown that mean AST/ALT ratio was 0.9 ± 0.5 among HIV-uninfected children with steatohepatitis vs. 0.7 ± 0.3 among children without liver fibrosis (p=0.05) [21]. Although these tests are widely accessible and less costly methods of monitoring for liver dysfunction, an increased ratio may not be a reliable screening method due to its lower specificity; otherwise a higher cut-off may be needed to identify HIV-infected children with potential liver disease.
In a French study among children with chronic liver disease who underwent liver biopsy, the APRI was found to correlate significantly with METAVIR stages used in liver fibrosis assessment; the receiver operator curve indicated 73% diagnostic accuracy for cirrhosis [11]. In this study we found 8.5% of children had an APRI >1.5 pre-cART, with an incidence of 0.77 per 1000 person-months among those with a normal baseline ALT after starting treatment. Our baseline prevalence and incidence of elevated APRI after cART were higher than the levels reported in a perinatally HIV-infected US pediatric cohort (i.e., 0.8% and 0.5 per 100 persons-years, respectively) [13]. Our median APRI (0.34, IQR 0.18–0.63) was slightly higher than that reported from an observational cohort of 1012 children with HIV at a median age 5.4 years from five Latin-American countries (0.29, range 0.05–29.67) [12]. In their study 85% of children were on cART and only 3.2% of children had an APRI >1.5; less well-controlled HIV including significant higher mean HIV RNA, higher proportion of cases with non-suppressed HIV RNA, and lower mean CD4 count were reported among children with elevated APRI [12]. In this study we were unable to evaluate for an association between virologic control and APRI as only half the patients had HIV RNA results available within three months of the laboratory testing used in the APRI calculation.
The FIB4 index is a combination of several standard biochemical values (platelets, ALT, AST) and age that enables identification of patients with severe fibrosis and cirrhosis (F3–F4 in METAVIR scale for liver pathology), according to published ranges to define mild, moderate and advanced disease [33]. In this study we found 2.68% of children had FIB4 indexes >1.3 prior to cART. Among those with normal baseline ALT, there were six children who developed FIB4 indexes >1.3 during cART follow-up. A study by Yang et al., among children with non-alcoholic fatty liver disease at the median age of 12.2±2.3 years showed that FIB4 and APRI were the two markers which were significantly different between those with and without significant fibrosis [9]. To generalize its use among pediatric patients across the age and developmental spectrum, further study would be needed to justify the cut-off values of the FIB4 index.
There were several limitations in our study. This was a retrospective analysis of pooled data from multiple sites that had inconsistent laboratory monitoring and pharmacovigilance practices; with different treatment protocols, transient liver enzyme elevations may have been missed due to less frequent monitoring practices. The analysis cohort also likely represents a survivor cohort, as the median age at cART initiation was in early childhood, and those who were younger and severely ill may have died before cART could be initiated. Our findings serve as a baseline representation of our cohort’s liver biochemistry dysfunction and may be used to guide future hepatitis screening and liver function testing.
Acknowledgments
The TREAT Asia Pediatric HIV Observational Database is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907), and the AIDS Life Association. The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, The University of New South Wales.
Footnotes
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
References
- 1.Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group. Smith C, Sabin CA, Lundgren JD, et al. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS. 2010;24:1537–1548. doi: 10.1097/QAD.0b013e32833a0918. [DOI] [PubMed] [Google Scholar]
- 2.Zhou S, Zhao Y, He Y, Li H, Bulterys M, Sun X, et al. Hepatitis B and hepatitis C seroprevalence in children receiving antiretroviral therapy for human immunodeficiency virus-1 infection in China, 2005–2009. J Acquir Immune Defic Syndr. 2010;54:191–196. doi: 10.1097/QAI.0b013e3181c99226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aurpibul L, Lumbiganon P, Kolasaraksa P, et al. HIV and Hepatitis B coinfection among perinatally HIV-infected Thai adolescents. Pediatr Infect Dis J. 2012;31:943–947. doi: 10.1097/INF.0b013e31825eb0ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM. Hepatitis B and C virus co-infection in The TREAT Asia HIV Observational Database. J Gastroenterol Hepatol. 2007;22:1510–1518. doi: 10.1111/j.1440-1746.2007.05062.x. [DOI] [PubMed] [Google Scholar]
- 5.Law WP, Duncombe CJ, Mahanontharit A, Boyd MA, Ruxrungtham K, Lange JM, et al. Impact of viral hepatitis co-infection on response to antiretroviral therapy and HIV disease progression in the HIV-NAT cohort. AIDS. 2004;18:1169–1177. doi: 10.1097/00002030-200405210-00010. [DOI] [PubMed] [Google Scholar]
- 6.Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother. 2007;59:342–6. doi: 10.1093/jac/dkl524. [DOI] [PubMed] [Google Scholar]
- 7.Lumbiganon P, Kariminia A, Aurpibul L, Hansudewechakul R, Puthanakit T, Kurniati N, et al. TREAT Asia Pediatric HIV Observational Database (TApHOD) Survival of HIV-infected children: a cohort study from the Asia-Pacific region. J Acquir Immune Defic Syndr. 2011;56:365–71. doi: 10.1097/QAI.0b013e318207a55b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong VW1, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–462. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 9.Yang HR, Kim HR, Kim MJ, Ko JS, Seo JK. Noninvasive parameters and hepatic fibrosis scores in children with nonalcoholic fatty liver disease. World J Gastroenterol. 2012;18:1525–1530. doi: 10.3748/wjg.v18.i13.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGoogan KE, Smith PB, Choi SS, Berman W, Jhaveri R. Performance of the AST-to-platelet ratio index as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr. 2010;50:344–346. doi: 10.1097/MPG.0b013e3181aed725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Ledinghen V, Le Bail B, Rebouissoux L, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443–450. doi: 10.1097/MPG.0b013e31812e56ff. [DOI] [PubMed] [Google Scholar]
- 12.Siberry GK, Cohen RA, Harris DR, et al. NISDI PLACES Protocol. Prevalence and predictors of elevated aspartate aminotransferase-to-platelet ratio index in Latin American perinatally HIV-infected children. Pediatr Infect Dis J. 2014;33:177–182. doi: 10.1097/INF.0b013e3182a01dfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siberry GK, Patel K, Pinto JA, et al. Elevated Aspartate Aminotransferase-to-Platelet Ratio Index in Perinatally HIV-Infected Children in the United States. Pediatr Infect Dis J. 2014;33:855–7. doi: 10.1097/INF.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kariminia A, Chokephaibulkit K, Pang J, Lumbiganon P, Hansudewechakul R, Amin J, et al. Cohort profile: the TREAT Asia pediatric HIV observational database. Int J Epidemiol. 2011;40:15–24. doi: 10.1093/ije/dyp358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson J. Blood Chemistries and body fluids. In: Robertson J, Shilkofski N, editors. The Harriet Lane handbook; a manual for pediatric house officers/the Harriet Lane service, Children’s Medical and Surgical Center of the Johns Hopkins Hospital. 19. Philadelphia: Elsevier Mosby; 2012. pp. 661–672. [Google Scholar]
- 16.The Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events, Version 1.0. 2004 Dec; available at http://rsc.tech-res.com/Document/safetyandpharmacovigilance.
- 17.Cai Z, Christianson AM, Ståhle L, Keisu M. Reexamining transaminase elevation in phase I clinical trials: the importance of baseline and change from baseline. Eur J Clin Pharmaco. 2009;65:1025–1035. doi: 10.1007/s00228-009-0684-x. [DOI] [PubMed] [Google Scholar]
- 18.Katz DA, Isaacson JD, Han L, Drajesk JF, Comer GM, Heath-Chiozzi ME. Clinical observation of liver chemistry abnormalities in asthmatics. Curr Drug Saf. 2009;4:173–180. doi: 10.2174/157488609789006930. [DOI] [PubMed] [Google Scholar]
- 19.Abboud G, Kaplowitz N. Drug-induced liver injury. Drug Saf. 2007;30:277–294. doi: 10.2165/00002018-200730040-00001. [DOI] [PubMed] [Google Scholar]
- 20.Björnsson E, Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 21.Iacobellis A, Marcellini M, Andriulli A, et al. Non-invasive evaluation of liver fibrosis in paediatric patients with nonalcoholic steatohepatitis. World J Gastroenterol. 2006;12:7821–7825. doi: 10.3748/wjg.v12.i48.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, et al. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350–357. [PubMed] [Google Scholar]
- 23.DallaPiazza M, Amorosa VK, Localio R, Kostman JR, Lo Re V., 3rd Prevalence and risk factors for significant liver fibrosis among HIV-monoinfected patients. BMC Infect Dis. 2010;10:116. doi: 10.1186/1471-2334-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO child growth standards and macros (ages 2–5yrs) Available at: http://www.who.int/childgrowth/software/en/
- 26.WHO child growth standards and macros (ages 5–19yrs) Available at: http://www.who.int/growthref/en/
- 27.WHO child growth standards and macros. 1977 [Google Scholar]
- 28.Stirnadel HA, Bains C, Lakshmi M, et al. Background incidence of liver chemistry abnormalities in pediatric clinical trials for conditions with and without underlying liver disease. Regul Toxicol Pharmacol. 2012;62:329–335. doi: 10.1016/j.yrtph.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Gray D, Nuttall J, Lombard C, Davies MA, Workman L, Apolles P, Eley B, Cotton M, Zar HJ. Low rates of hepatotoxicity in HIV-infected children on anti-retroviral therapy with and without isoniazid prophylaxis. J Trop Pediatr. 2010;56:159–165. doi: 10.1093/tropej/fmp079. [DOI] [PubMed] [Google Scholar]
- 30.Temple R. Hy’s law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–3. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 31.van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN Study. Lancet. 2004;363:1253–63. doi: 10.1016/S0140-6736(04)15997-7. [DOI] [PubMed] [Google Scholar]
- 32.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 33.Park LS, Tate JP, Justice AC, Lo Re V, 3rd, Lim JK, Bräu N, et al. FIB4 index is associated with hepatocellular carcinoma risk in HIV-infected patients. Cancer Epidemiol Biomarkers Prev. 2011;20:2512–2517. doi: 10.1158/1055-9965.EPI-11-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]