Abstract
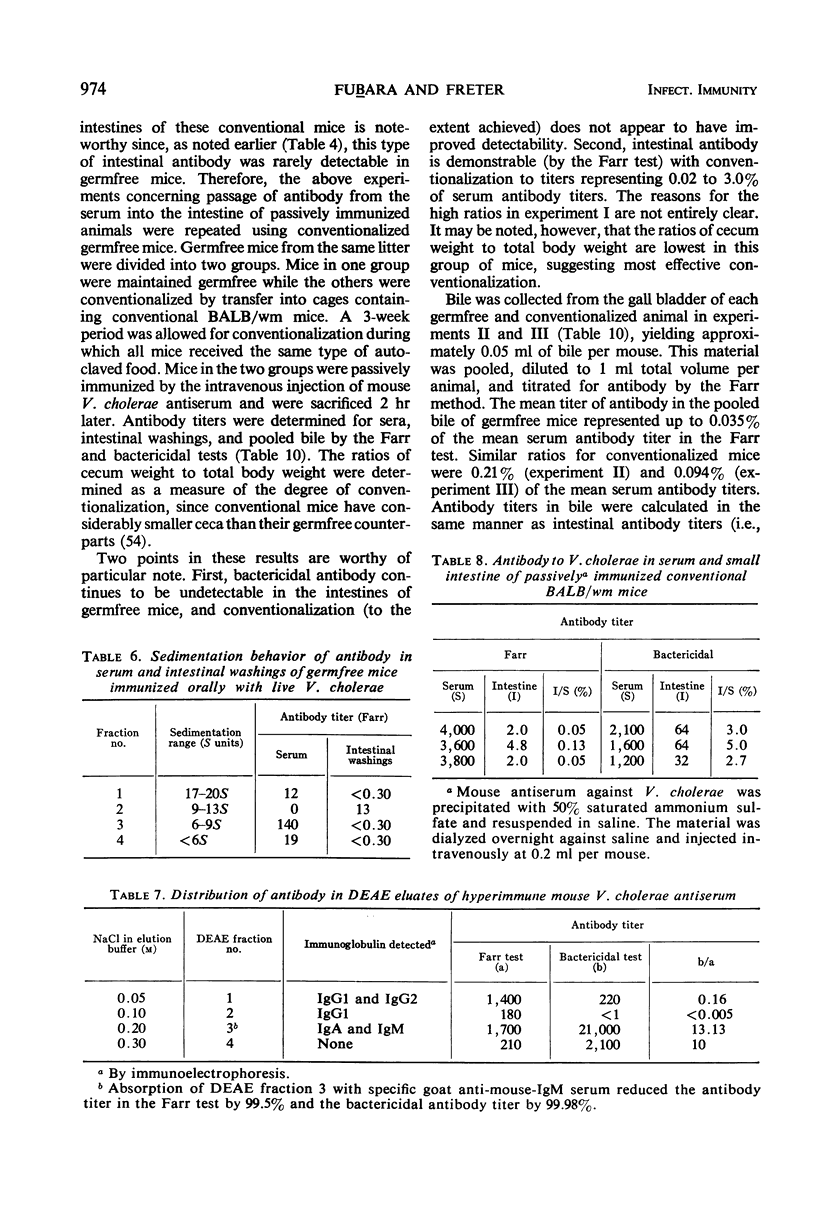
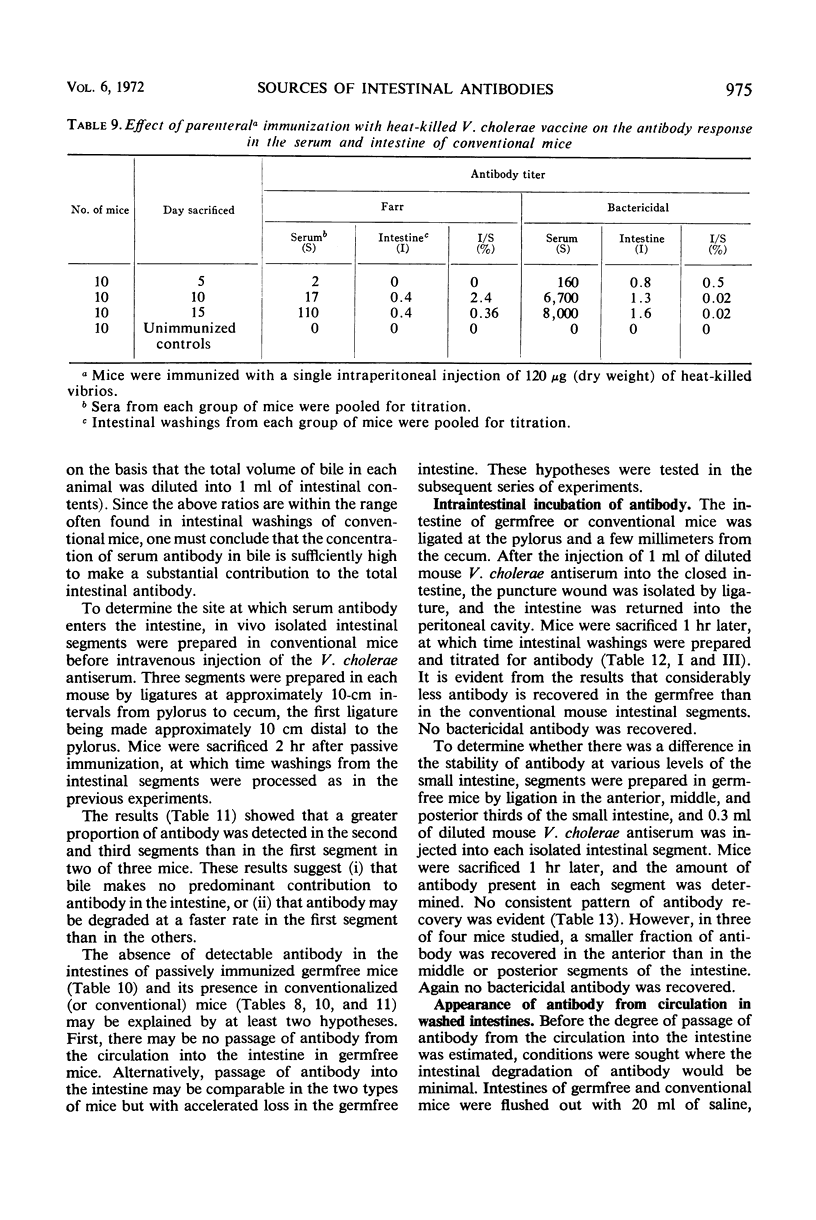
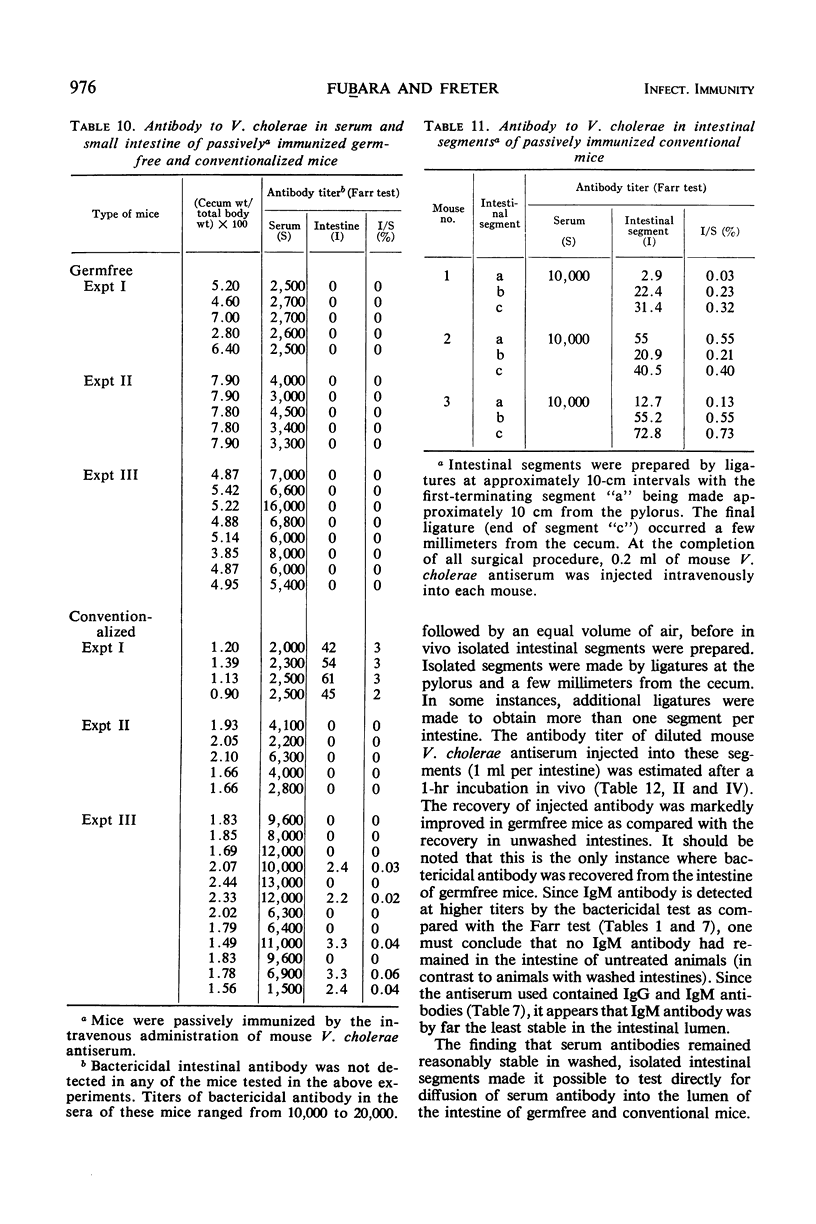
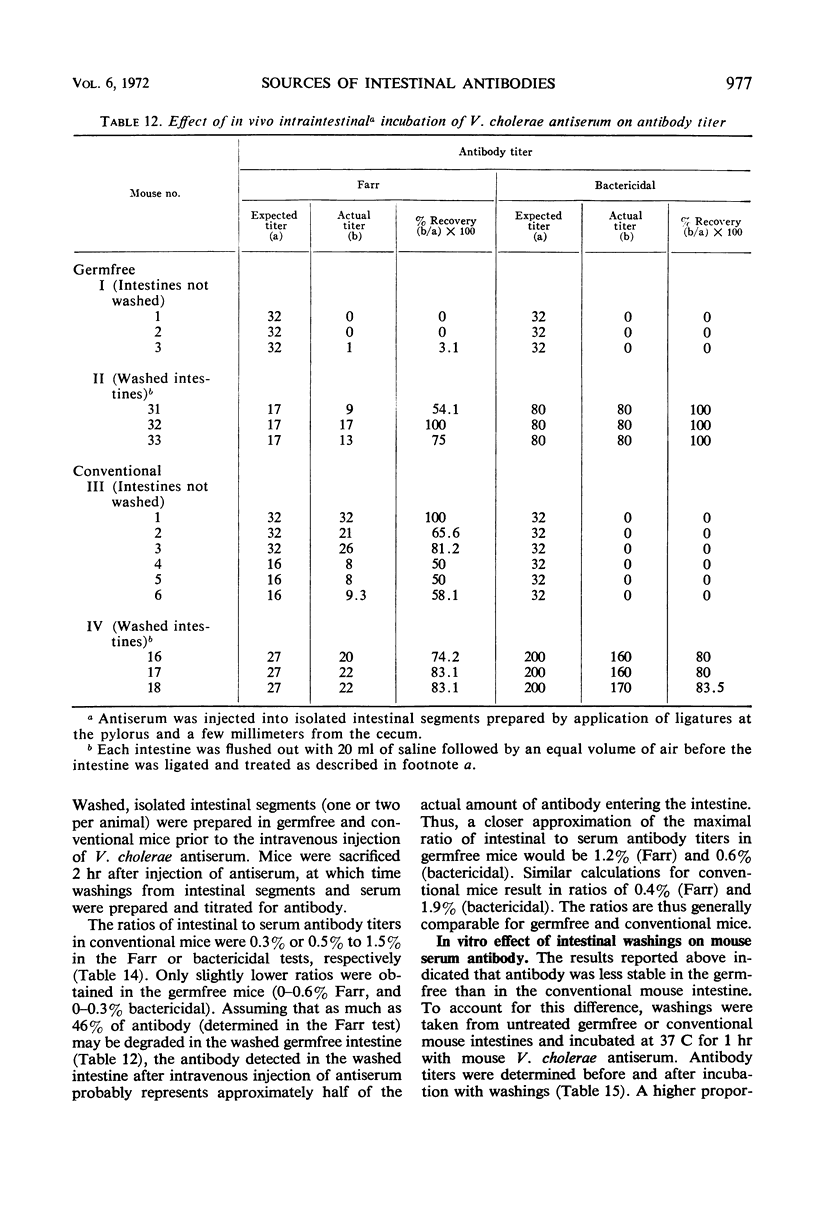
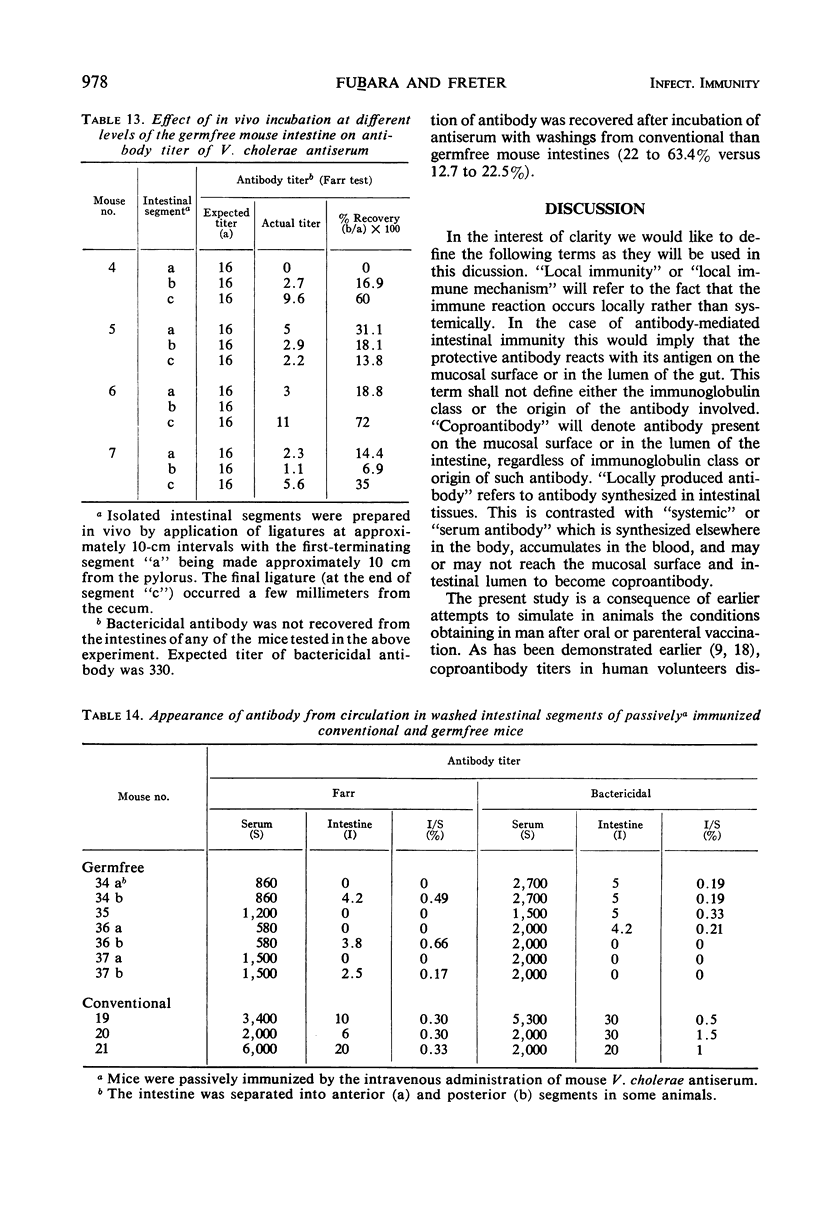
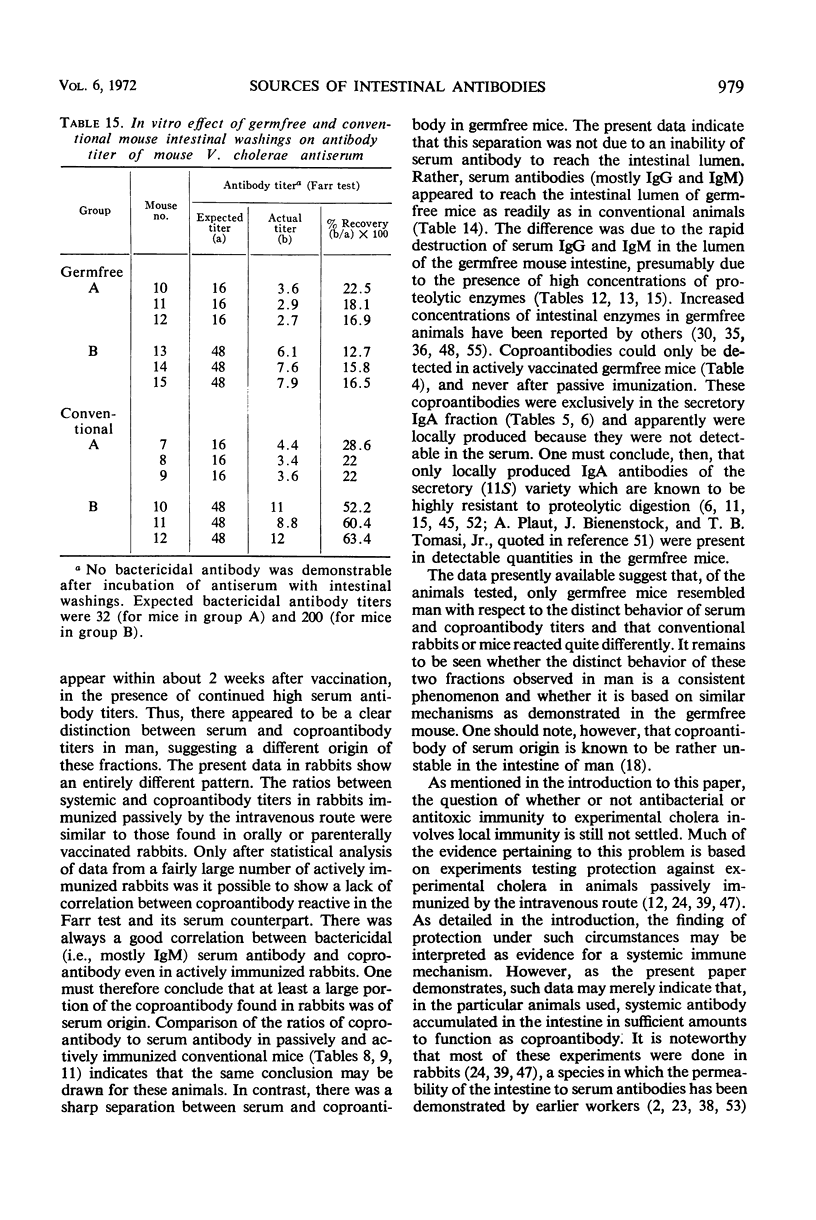
The present studies are concerned with the parameters which control the appearance of locally synthesized or serum-derived antibodies in the intestine. The data show that intestinal antibody may be found in rabbits as well as in conventional or germfree mice after active immunization with Vibrio cholerae. However, a large fraction of the intestinal antibody in rabbits and conventional mice originated from the serum as indicated by (i) analysis of correlation between serum and intestinal antibody titers, and (ii) the occurrence of intestinal antibody after parenteral administration of antiserum. In contrast, only locally synthesized 11S immunoglobulin A antibody was detected in the intestine of actively immunized germfree mice. No intestinal antibody was demonstrable in germfree mice after parenteral injection of V. cholerae antiserum. With respect to the appearance of serum antibody in the intestine, the response of conventionalized (ex-germfree) mice was intermediate between that of rabbits or conventional mice and germfree mice. The availability of serum-derived coproantibody in germfree and conventional mice was related to the rates of intestinal degradation of serum antibody. When enzymes were removed by prior washing of intestinal segments, serum antibodies entered the intestine of germfree or conventional mice at similar rates. Rates of entry of serum antibodies into the lumen were comparable at different levels of the small intestine. The presence of a normal enteric flora appeared to protect intestinal antibody from degradation by lowering the concentration or activity of intestinal enzymes. The results are discussed in relation to the question of whether antibacterial immunity to cholera involves local or systemic mechanisms.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BATTY I., BULLEN J. J. The permeability of the sheep and rabbit intestinal wall to antitoxin present in the circulation. J Pathol Bacteriol. 1961 Apr;81:447–458. doi: 10.1002/path.1700810218. [DOI] [PubMed] [Google Scholar]
- BERNFELD P., WAN J. ANTIGENS AND ENZYMES MADE INSOLUBLE BY ENTRAPPING THEM INTO LATTICES OF SYNTHETIC POLYMERS. Science. 1963 Nov 8;142(3593):678–679. doi: 10.1126/science.142.3593.678. [DOI] [PubMed] [Google Scholar]
- Benenson A. S., Mosley W. H., Fahimuddin M., Oseasohn R. O. Cholera vaccine field trials in east Pakistan. 2. Effectiveness in the field. Bull World Health Organ. 1968;38(3):359–372. [PMC free article] [PubMed] [Google Scholar]
- Brown W. R., Newcomb R. W., Ishizaka K. Proteolytic degradation of exocrine and serum immunoglobulins. J Clin Invest. 1970 Jul;49(7):1374–1380. doi: 10.1172/JCI106354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows W. Local immunity to cholera. Bull Soc Pathol Exot Filiales. 1971 Sep-Oct;64(5):589–596. [PubMed] [Google Scholar]
- Cebra J. J., Robbins J. B. Gamma-A-immunoglobulin from rabbit colostrum. J Immunol. 1966 Jul;97(1):12–24. [PubMed] [Google Scholar]
- Cederblad G., Johansson B. G., Rymo L. Reduction and proteolytic degradation of immunoglobulin A from human colostrum. Acta Chem Scand. 1966;20(9):2349–2357. doi: 10.3891/acta.chem.scand.20-2349. [DOI] [PubMed] [Google Scholar]
- Curlin G. T., Craig J. P., Subong A., Carpenter C. C. Antitoxic immunity in experimental canine cholera. J Infect Dis. 1970 May;121(5):463–470. doi: 10.1093/infdis/121.5.463. [DOI] [PubMed] [Google Scholar]
- Eddie D. S., Schulkind M. L., Robbins J. B. The isolation and biologic activities of purified secretory IgA and IgG anti-Salmonella typhimurium "O" antibodies from rabbit intestinal fluid and colostrum. J Immunol. 1971 Jan;106(1):181–190. [PubMed] [Google Scholar]
- FRETER R. COMPARISON OF IMMUNE MECHANISMS IN VARIOUS EXPERIMENTAL MODELS OF CHOLERA. Bull World Health Organ. 1964;31:825–834. [PMC free article] [PubMed] [Google Scholar]
- FRETER R. Coproantibody and bacterial antagonism as protective factors in experimental enteric cholera. J Exp Med. 1956 Sep 1;104(3):419–426. doi: 10.1084/jem.104.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman B. H., Herdman R., Hong R. Local immunomechanism of the urinary tract. Invest Urol. 1971 Mar;8(5):575–582. [PubMed] [Google Scholar]
- Freter R. Mechanism of Action of Intestinal Antibody in Experimental Cholera II. Antibody-Mediated Antibacterial Reaction at the Mucosal Surface. Infect Immun. 1970 Nov;2(5):556–562. doi: 10.1128/iai.2.5.556-562.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. K. Immunological observations on experimental cholera. J Med Microbiol. 1970 Aug;3(3):441–451. doi: 10.1099/00222615-3-3-441. [DOI] [PubMed] [Google Scholar]
- Gillmore J. D., Versage P. M., Phillips R. A. Specific and nonspecific passive immunity in infant rabbit cholera. J Infect Dis. 1966 Jun;116(3):313–318. doi: 10.1093/infdis/116.3.313. [DOI] [PubMed] [Google Scholar]
- HEREMANS J. F., VAERMAN J. P., VAERMAN C. STUDIES ON THE IMMUNE GLOBULINS OF HUMAN SERUM. II. A STUDY OF THE DISTRIBUTION OF ANTI-BRUCELLA AND ANTI-DIPHTHERIA ANTIBODY ACTIVITIES AMONG GAMMA-SS, GAMMA-IM AND GAMMA-1A-GLOBULIN FRACTIONS. J Immunol. 1963 Jul;91:11–17. [PubMed] [Google Scholar]
- Hoyer L. W., Borsos T., Rapp H. J., Vannier W. E. Heterogeneity of rabbit IgM antibody as detected by C'1a fixation. J Exp Med. 1968 Mar 1;127(3):589–603. doi: 10.1084/jem.127.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka K., Ishizaka T., Lee E. H., Fudenberg H. Immunochemical properties of human gamma-A isohemagglutinin. I. Comparisons with gamma-G and gamma-M-globulin antibodies. J Immunol. 1965 Aug;95(2):197–208. [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Borsos T., Rapp H. C'-1 fixation by human isoagglutinins: fixation of C'-1 by gamma-G and gamma-M but not by gamma-A antibody. J Immunol. 1966 Nov;97(5):716–726. [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. The importance of antibody in the prevention of experimental cholera in rabbits. Br J Exp Pathol. 1960 Feb;41:24–30. [PMC free article] [PubMed] [Google Scholar]
- JERVIS H. R., BIGGERS D. C. MUCOSAL ENZYMES IN THE CECUM OF CONVENTIONAL AND GERMFREE MICE. Anat Rec. 1964 Apr;148:591–597. doi: 10.1002/ar.1091480410. [DOI] [PubMed] [Google Scholar]
- KOSHLAND M. E., BURROWS W. Quantitative studies of the relationship between fecal and serum antibody. J Immunol. 1950 Jul;65(1):93–103. [PubMed] [Google Scholar]
- Kaur J., McGhee, Burrows W. Immunity to cholera: the occurrence and nature of antibody-active immunoglobulins in the lower ileum of the rabbit. J Immunol. 1972 Feb;108(2):387–395. [PubMed] [Google Scholar]
- Klinman N. R., Rockey J. H., Frauenberger G., Karush F. Equine anti-hapten antibody. 3. The comparative properties of gamma G- and gammaA-antibodies. J Immunol. 1966 Apr;96(4):587–595. [PubMed] [Google Scholar]
- LANDY M., MICHAEL J. G., WHITBY J. L. Bactericidal method for the measurement in normal serum of antibody to gramnegative bacteria. J Bacteriol. 1962 Mar;83:631–640. doi: 10.1128/jb.83.3.631-640.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MCINTYRE O. R., FEELEY J. C. PASSIVE SERUM PROTECTION OF THE INFANT RABBIT AGAINST EXPERIMENTAL CHOLERA. J Infect Dis. 1964 Dec;114:468–475. doi: 10.1093/infdis/114.5.468. [DOI] [PubMed] [Google Scholar]
- MICHAEL J. G., WHITBY J. L., LANDY M. Studies on natural antibodies to gram-negative bacteria. J Exp Med. 1962 Jan 1;115:131–146. doi: 10.1084/jem.115.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery J. L., Kraft S. C., Rothberg R. M. Demonstration of antibody in rabbit feces after active or passive parenteral immunization. Digestion. 1970;3(4):213–221. doi: 10.1159/000197034. [DOI] [PubMed] [Google Scholar]
- PANSE M. V., JHALA H. I., DUTTA N. K. PASSIVE IMMUNITY IN EXPERIMENTAL CHOLERA. J Infect Dis. 1964 Feb;114:26–30. doi: 10.1093/infdis/114.1.26. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Pleasants J. R., Wostmann B. S. Effect of dietary carbohydrates on intestinal disaccharidases in germfree and conventional rats. J Nutr. 1968 Jul;95(3):413–419. doi: 10.1093/jn/95.3.413. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. 3. Serologic studies and re-challenge experiments. J Infect Dis. 1969 Feb;119(2):158–164. doi: 10.1093/infdis/119.2.158. [DOI] [PubMed] [Google Scholar]
- Tomasi T. B., Jr, Bienenstock J. Secretory immunoglobulins. Adv Immunol. 1968;9:1–96. doi: 10.1016/s0065-2776(08)60441-1. [DOI] [PubMed] [Google Scholar]
- Wernet P., Breu H., Knop J., Rowley D. Antibacterial action of specific IgA and transport of IgM, IgA, and IgG from serum into the small intestine. J Infect Dis. 1971 Aug;124(2):223–226. doi: 10.1093/infdis/124.2.223. [DOI] [PubMed] [Google Scholar]
- Yolton D. P., Stanley C., Savage D. C. Influence of the indigenous gastrointestinal microbial flora on duodenal alkaline phosphatase activity in mice. Infect Immun. 1971 Jun;3(6):768–773. doi: 10.1128/iai.3.6.768-773.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]





