Synopsis
In recent years our knowledge and understanding of head and neck squamous cell carcinoma (HNSCC) has expanded dramatically. New high-throughput sequencing technologies have accelerated these discoveries since the first reports of whole exome sequencing of HNSCC tumors in 2011. In addition, the discovery of human papillomavirus (HPV) in relationship with oropharyngeal squamous cell carcinoma has shifted our molecular understanding of the disease. New investigation into the role of immune evasion in HNSCC has also led to potential novel therapies based on immune specific systemic therapies.
Keywords: Molecular biology, Targeted therapy, Immunology, Head and Neck Cancer
In recent years our knowledge and understanding of head and neck squamous cell carcinoma (HNSCC) has expanded dramatically. New high-throughput sequencing technologies have accelerated these discoveries since the first reports of whole exome sequencing of HNSCC tumors in 2011.1,2 In addition, the discovery of human papillomavirus (HPV) in relationship with oropharyngeal squamous cell carcinoma has shifted our molecular understanding of the disease.3 New investigation into the role of immune evasion in HNSCC has also led to potential novel therapies based on immune specific systemic therapies.
Distinct etiologic subsets of HNSCC
HNSCC forms after accumulation of genetic events which are accelerated by genomic instability related to carcinogen exposures, particularly tobacco and alcohol. These tumors may occur throughout the upper aerodigestive tract (oral cavity, oropharynx, larynx) and are found in older patients, usually with smoking or alcohol use history. They are also associated with p53 mutations and poor clinical outcomes with 5-year survival of 33.8–66.8%, depending on subsite.4,5
Recently, human papillomavirus (HPV) has been associated with a subset of HNSCC, chiefly in the oropharynx and primarily in younger, white, non-smokers.3,6 HPV is a double-stranded DNA virus which infects the squamous epithelium. High-risk subtypes, particularly HPV-16 and HPV-18, are associated with development of malignancy, both HNSCC and cervical cancer. The mechanism of oncogenesis is attributed to viral proteins E6 (which binds and degrades p53) and E7 (which inhibits retinoblastoma protein, a tumor suppressor gene that inhibits cell cycle progression).7,8 Patients with HPV-related HNSCC have improved prognosis with longer overall survival, decreased rate of recurrence, and improved response to chemoradiation.3, 9
Genetic alterations
In 2011, the first whole exome sequencing of HNSCC was published. 1,2 Recently, the Cancer Genome Atlas (TCGA) Research Network performed integrated genomic analysis including genome sequencing, copy number and loss of heterozygosity arrays, whole genome methylation and RNA sequencing on 279 head and neck cancers, constituting the largest of cohort of sequenced tumors studied.10
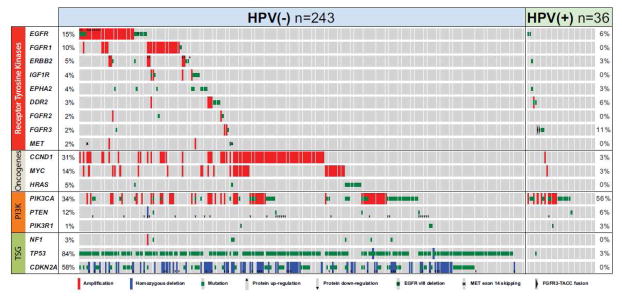
Gene mutations were segregated by HPV tumor status. HPV-positive tumors harbored fewer mutations compared to HPV-negative tumors. 1,10,11 TP53 mutations were found almost exclusively in HPV-negative tumors 1,10 while activating mutations and amplifications of PIK3CA were commonly seen in HPV-positive tumors (figure 1).10 This is consistent with prior data showing the same distinct genetic alterations.12
Figure 1.
Genetic alterations in key oncogenic pathways from TCGA. (From Hayes, N et al. The Cancer Genome Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature, in press. 2014, with permission.)
Beyond sequencing, gene promoter methylation of several genes including CDKN2A, CDH1, MGMT, DAPK1 has been established in oral squamous cell carcinoma.13 CDKN2A, a tumor suppressor gene, is one of the first genes in HNSCC to be associated with promoter methylation as a mechanism of downregulation. 14
Major pathways
TP53 and CDKN2A
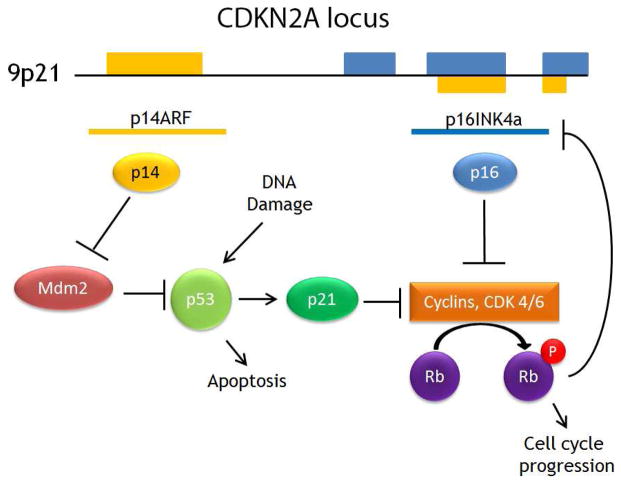
The TP53 gene encodes for the p53 protein, “guardian of the genome.” TP53 is one of the most frequently mutated genes in HNSCC1,2,10,15 and even premalignant lesions.16 The p53 protein acts as a tumor suppressor that accumulates in response to stress. including DNA damage.17 Accumulation of p53 induces cell cycle arrest to allow the cell to perform DNA repair. If damage is beyond repair, p53 induces apoptosis.15 The expression of p53 is regulated by MDM2, which inactivates and degrades p53.18 The CDKN2A locus at 9p21 codes for two alternatively spliced proteins p14ARF and p16INK4A, which both regulate p53 function.19 (Figure 2)
Figure 2. CDKN2A gene products and p53 regulation.
CDKN2A codes for alternatively spliced p14ARF and p16INK4a genes. The p14 protein inhibits MDM2, which ubiquitinates p53. Both p21, induced by p53, and p16 inhibit cyclins that promote cell cycle progression through phosphorylation of retinoblastoma protein (Rb). Rb feeds back to inhibit p16 production.
A majority (50–63%) of p53 mutations in HNSCC are missense mutations.1,2 Missense mutations in p53 can result in a stable protein with loss of key binding function or even act in a dominant negative fashion inactivating any remaining wildtype p53.15 Tobacco exposure is associated with increased rates of TP53 mutations.4,20 Mutations in TP53 have been associated with decreased overall survival,21 increased locoregional recurrence rates,22 and decreased response to therapy.23,24
In recent sequencing data, CDKN2A was found to be mutated in 9–12% of tumors.1,2 Loss of heterozygosity is frequently seen at the CDKN2A locus in HNSCC, including premalignant lesions.25 The p16 protein is also significant (overexpression is consistently seen in HPV-related oropharyngeal cancers).26 The mechanism is related to the inactivation of Rb by the E7 viral protein, resulting in unregulated overexpression of p16.27
EGFR, Ras and PI3K
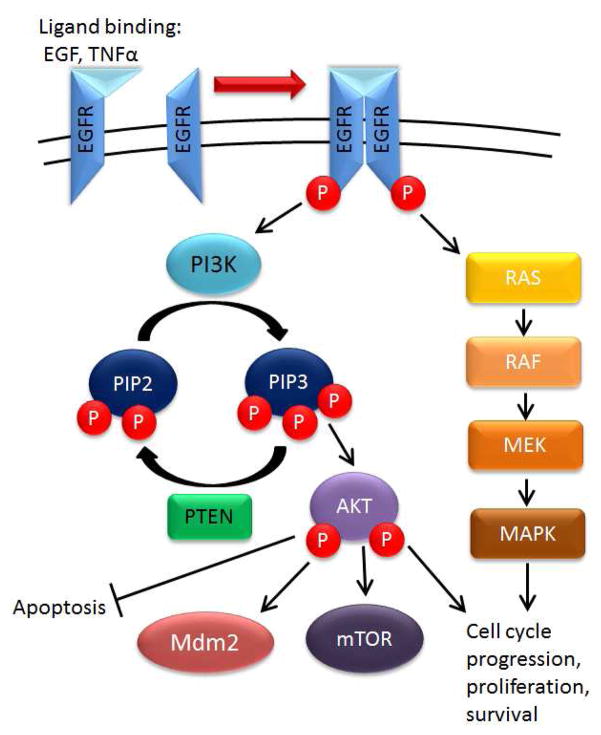
Epidermal growth factor receptor (EGFR) is part of the ErbB family of receptor tyrosine kinases. After ligand binding (EGF or TGF-α), activated EGFR forms a dimer and activtes downstream pathways. These include PI3K/Akt and Ras pathways that promote cell growth, proliferation and inhibit apoptosis (Figure 3). Activation of PI3K results in conversion of phosphatidylinositol biphosphate (PIP2) to phosphatidylinositol triphosphate (PIP3). PIP3 can then bind and phosphorylate Akt, triggering inhibition of apoptosis, mTOR activation and activation of MDM2.28 PTEN negatively regulates this signaling by dephosphorylating PIP3 to PIP2. thus preventing downstream signaling.
Figure 3. EGFR signaling and downstream pathways.
When an extracellular ligand binds to EGFR, dimerization occurs, promoting cross phosphorylation. This activates Ras signaling and activates PI3K to produce PIP3. PIP3 phosphorylates Akt which promotes mTOR signaling, promotes MDM2 (inhibits p53) and inhibits apoptosis.
In HNSCC, overexpression of the EGFR gene is seen in about 90% of tumors.29 Increased EGFR expression correlates with increased local recurrence30 and worse overall survival.30,31 EGFR overexpression plays a clear role in HNSCC; interestingly, few mutations have been observed in EGFR11,29 (Figure 1). HRAS, a target downstream of EGFR, is mutated in 4–5% of tumors.1,2,10
In HNSCC, PI3KCA (catalytic subunit of PI3 kinase) is mutated in 6–21% of tumors.1,2,32 Advanced stage HNSCC harbor increased mutations along the PI3K pathway.33 PI3K pathway genes are the main genes mutated in HPV-related tumors10,33 (Figure 1).
NOTCH Signaling
Notch is a cell surface receptor that binds to ligands on an adjacent cell surface, such as Jagged or Delta.28 Next, proteolytic cleavage releases an intracellular fragment that travels to the nucleus. affecting gene transcription. Downsteam targets include HES1 and HEY1, which promote cell cycle progression and survival.34
Inactivating mutations of NOTCH1 were found in 10–19% of head and neck tumors.1,2,10 This suggests that NOTCH1 acts as a tumor suppressor in HNSCC. In oral squamous cell carcinoma (SCC) cell lines, reactivation of the wild type NOTCH1 gene blocked cell proliferation.35 However, NOTCH1 also acts as an oncogene in hematologic malignancies.36 Within sequencing data of HNSCC, some mutations in NOTCH1 were not inactivating, suggesting that its role in HNSCC may be mixed.32,37 Recent data have shown that a subset of HNSCC tumors actually show downstream activation of NOTCH.37
Apoptotic pathways
Apoptotic pathways are regulated by intrinsic signals (such as p53) or extrinsic signals through cell surface receptors. Multiple signals such as p53 response to DNA damage, UV radiation, or influx of calcium ions can trigger apoptotic signaling. When the balance tips towards apoptosis, cytochrome c is released from the mitochondria, and the caspase cascade executes programmed cell death. Cell surface receptors, such as FAS and death receptors, may also trigger apoptosis through activation of caspase 8 and downstream caspases.28
With head and neck cancer, mutations in caspase 8 (CASP8) have been observed in 8–9% of tumors2 (TCGA data) with a majority occurring in oral cavity SCC.10 TRAF3, BIRC2 and FADD interact with the cell surface death receptors and were found to harbor mutations in head and neck cancer.10 TRAF3 mutations were noted primarily in HPV-positive tumors.
Implications for targeted therapy
Current chemotherapy treatments for head and neck cancer are not targeted, but instead platinum based treatments (primarily cisplatin and carboplatin) with concurrent radiation are the mainstay of treatment.38
Therapies targeting EGFR
The main targeted therapy currently available for HNSCC is Cetuximab, an anti-EGFR IgG1 antibody. Cetuximab was approved for the treatment of HNSCC after a study showed significantly improved progression-free and overall survival when concurrent Cetuximab with radiation was compared to radiation alone.39 Recent data have not shown improved outcomes when combining cetuximab with concurrent cisplatin in primary chemoradiation.40 Questions still remain regarding the mechanism of Cetuximab activity. Despite frequent EGFR overexpression, response rates to cetuximab as a single agent are around 10–15% in recurrent HNSCC41 and efficacy has not been found to correlate with EGFR expression.40,42
Other anti-EGFR antibodies (Panitumumab, Zalutumumab, Nimotuzumab) have shown promising results in preclinical studies.43 In phase II clinical trials, Panitumumab did not show improvement in overall survival,44 and the efficacy of the other antibodies has yet to be determined (Clinical trials: NCT01054625, NCT00401401, NCT01425736).
FDA approved small molecule tyrosine kinase inhibitors (TKI) of EGFR in other cancers include gefitinib, Erlotinib, Lapatinib, and Afatinib.43 In preclinical studies, these TKI treatments have been shown to increase cell death in response to radiation therapy, especially when combined with VEGF inhibitors.45,46 In clinical trials, they have not been shown to improve outcomes,47,48 but lapatinib may improve progression free survival in HPV-negative patients.49
VEGF and other tyrosine kinase inhibitors
Inhibition of VEGF may sensitize HNSCC to radiation treatment.50 However, treatment with Bevacizumab, an anti-VEGF antibody, has not shown improved outcomes. Importantly in clinical trials, it was associated with locoregional progression51 as well as increased incidence of osteoradionecrosis.52
Small molecule TKIs with multiple kinase targets approved for other cancers include vandetanib, sunitinib, sorafenib and dasatinib.43 Preclinical studies of these TKIs in HNSCC have shown promise, particularly in increasing radiation-induced cytotoxicity.53–55 Ongoing trials have not yet shown significant clinical results.56,57
PI3K and mTOR inhibitors
The PI3K/mTOR pathway is another potential target in HNSCC, highlighted in recent genomic data (Figure 1, TCGA). Preclinical studies of BEZ235, a small molecule inhibitor of PI3K and mTOR, have shown efficacy in head and neck cells harboring PIK3CA mutations33 and it may be more effective for HPV related tumors.58 Rapamycin and Everolimus, mTOR inhibitors that have been used for transplant immunosuppression, are currently under clinical trial investigation in HNSCC patients (NCT00935961, NCT01283334, NCT01195922).
Immunology
Recent studies have explored the role of immune system evasion in the progression of HNSCC.59 One major pathway of interest is the co-signaling of the programmed cell death protein 1 (PD-1) and its ligand (PD-L1). PD-L1 is normally expressed by antigen presenting cells and is overexpressed in solid tumor cells.60 The interaction between PD-1 and PD-L1 dampens the immune response.60 Currently, antibodies directed towards PD-1 and PD-L1 are in clinical trials for patients with other solid tumors. Recently Nivolumab, a IgG4 monoclonal anti-PD-1, has shown dramatic results in advanced melanoma and is undergoing review with the FDA.61 HNSCC tumors, especially HPV-positive tumors, show increased expression of PD-L1.62 However, PD-L1 expression in head and neck tumors has not been clearly associated with clinical prognosis.59 Clinical trials are beginning to investigate Pembrolizumab (anti PD-1) in head and neck cancer (NCT02255097, NCT02252042).
Another potential method of immune system modulation currently under investigation is with use of phosphodiesterase-5 (PDE5) inhibitors, such as tadalifil. PDE5 inhibitors act by increasing cGMP to inhibit myeloid derived suppressor cells (MDSC), which may dampen immune response to tumors.63 Preclinical studies showed that treatment with tadalifil reduced tumor growth.63 In head and neck patients, tadalifil has been shown to significantly decrease T-regulatory and MDSC in patient serum64 and phase II trials are currently accruing to ascess treatment efficacy (NCT01697800).
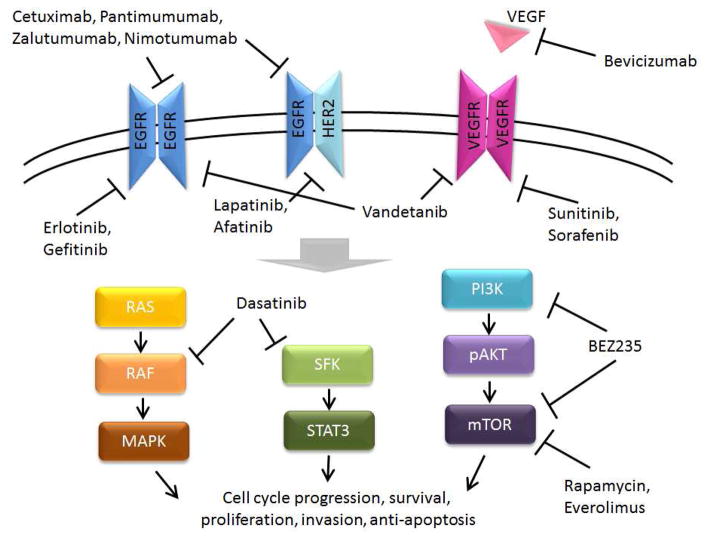
Figure 4. Potential targets for therapy in head and neck cancer.
SFK= Src family kinases.
(Adapted from Du Y, Peyser ND, Grandis JR. Integration of molecular targeted therapy with radiation in head and neck cancer. Pharmacology & therapeutics. Apr 2014;142(1):88–98, with permission.)
Key points.
Most head and neck squamous cell carcinomas are associated with smoking and alcohol, but an emerging subset of tumors is associated with human papillomavirus. These patients have improved clinical outcomes and distinct genetic profile
Genetic sequencing of head and neck cancer revealed mutations in key cancer pathways including p53, EGFR/Ras/PI3K, NOTCH, and apoptotic pathways
Therapies targeted towards these pathways are limited but under investigation
Head and neck cancers may progress by immune evasion, which is another targetable mechanism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrawal N, Frederick MJ, Pickering CR, et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011 Aug 26;333(6046):1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011 Aug 26;333(6046):1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010 Jul 1;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. The New England journal of medicine. 1995 Mar 16;332(11):712–717. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 5.Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. The oncologist. 2010;15(9):994–1001. doi: 10.1634/theoncologist.2009-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007 May 10;356(19):1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 7.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 8.Pagano M, Durst M, Joswig S, Draetta G, Jansen-Durr P. Binding of the human E2F transcription factor to the retinoblastoma protein but not to cyclin A is abolished in HPV-16-immortalized cells. Oncogene. 1992 Sep;7(9):1681–1686. [PubMed] [Google Scholar]
- 9.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomaviruspositive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008 Feb 20;100(4):261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 10.Hayes N, et al. The Cancer Genome Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2014 doi: 10.1038/nature14129. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Califano J. Sequencing the head and neck cancer genome-Impact on therapy. Ann N Y Acad Sci. 2014 doi: 10.1111/nyas.12599. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lechner M, Frampton GM, Fenton T, et al. Targeted next-generation sequencing of head and neck squamous cell carcinoma identifies novel genetic alterations in HPV+ and HPV-tumors. Genome medicine. 2013;5(5):49. doi: 10.1186/gm453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha PK, Califano JA. Promoter methylation and inactivation of tumour-suppressor genes in oral squamous-cell carcinoma. The Lancet Oncology. 2006 Jan;7(1):77–82. doi: 10.1016/S1470-2045(05)70540-4. [DOI] [PubMed] [Google Scholar]
- 14.Reed AL, Califano J, Cairns P, et al. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer research. 1996 Aug 15;56(16):3630–3633. [PubMed] [Google Scholar]
- 15.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 16.Califano J, van der Riet P, Westra W, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer research. 1996 Jun 1;56(11):2488–2492. [PubMed] [Google Scholar]
- 17.Carr AM. Cell cycle. Piecing together the p53 puzzle. Science. 2000 Mar 10;287(5459):1765–1766. doi: 10.1126/science.287.5459.1765. [DOI] [PubMed] [Google Scholar]
- 18.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997 May 15;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 19.Roussel MF. The INK4 family of cell cycle inhibitors in cancer. Oncogene. 1999 Sep 20;18(38):5311–5317. doi: 10.1038/sj.onc.1202998. [DOI] [PubMed] [Google Scholar]
- 20.Ronchetti D, Neglia CB, Cesana BM, et al. Association between p53 gene mutations and tobacco and alcohol exposure in laryngeal squamous cell carcinoma. Archives of otolaryngology--head & neck surgery. 2004 Mar;130(3):303–306. doi: 10.1001/archotol.130.3.303. [DOI] [PubMed] [Google Scholar]
- 21.Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2007 Dec 20;357(25):2552–2561. doi: 10.1056/NEJMoa073770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Jan 1;18(1):290–300. doi: 10.1158/1078-0432.CCR-11-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganly I, Soutar DS, Brown R, Kaye SB. p53 alterations in recurrent squamous cell cancer of the head and neck refractory to radiotherapy. British journal of cancer. 2000 Jan;82(2):392–398. doi: 10.1054/bjoc.1999.0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrone F, Bossi P, Cortelazzi B, et al. TP53 mutations and pathologic complete response to neoadjuvant cisplatin and fluorouracil chemotherapy in resected oral cavity squamous cell carcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010 Feb 10;28(5):761–766. doi: 10.1200/JCO.2009.22.4170. [DOI] [PubMed] [Google Scholar]
- 25.Ha PK, Chang SS, Glazer CA, Califano JA, Sidransky D. Molecular techniques and genetic alterations in head and neck cancer. Oral oncology. 2009 Apr-May;45(4–5):335–339. doi: 10.1016/j.oraloncology.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begum S, Gillison ML, Ansari-Lari MA, Shah K, Westra WH. Detection of human papillomavirus in cervical lymph nodes: a highly effective strategy for localizing site of tumor origin. Clin Cancer Res. 2003 Dec 15;9(17):6469–6475. [PubMed] [Google Scholar]
- 27.Li Y, Nichols MA, Shay JW, Xiong Y. Transcriptional repression of the D-type cyclin-dependent kinase inhibitor p16 by the retinoblastoma susceptibility gene product pRb. Cancer research. 1994 Dec 1;54(23):6078–6082. [PubMed] [Google Scholar]
- 28.Weinberg RA. The biology of cancer. New York: Garland Science; 2007. [Google Scholar]
- 29.Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Jun 10;24(17):2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- 30.Psyrri A, Yu Z, Weinberger PM, et al. Quantitative determination of nuclear and cytoplasmic epidermal growth factor receptor expression in oropharyngeal squamous cell cancer by using automated quantitative analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005 Aug 15;11(16):5856–5862. doi: 10.1158/1078-0432.CCR-05-0420. [DOI] [PubMed] [Google Scholar]
- 31.Chung CH, Ely K, McGavran L, et al. Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Sep 1;24(25):4170–4176. doi: 10.1200/JCO.2006.07.2587. [DOI] [PubMed] [Google Scholar]
- 32.Sun W, Gaykalova DA, Ochs MF, et al. Activation of the NOTCH pathway in head and neck cancer. Cancer research. 2014 Feb 15;74(4):1091–1104. doi: 10.1158/0008-5472.CAN-13-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer discovery. 2013 Jul;3(7):761–769. doi: 10.1158/2159-8290.CD-13-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ntziachristos P, Lim JS, Sage J, Aifantis I. From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer cell. 2014 Mar 17;25(3):318–334. doi: 10.1016/j.ccr.2014.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickering CR, Zhang J, Yoo SY, et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer discovery. 2013 Jul;3(7):770–781. doi: 10.1158/2159-8290.CD-12-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004 Oct 8;306(5694):269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 37.Song X, Xia R, Li J, et al. Common and complex Notch1 mutations in Chinese oral squamous cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Feb 1;20(3):701–710. doi: 10.1158/1078-0432.CCR-13-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Head and Neck Cancers. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) 2014. [Accessed October 24, 2014]. p. 2. [Google Scholar]
- 39.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. The New England journal of medicine. 2006 Feb 9;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 40.Ang KK, Zhang Q, Rosenthal DI, et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J Clin Oncol. 2014 Aug 25; doi: 10.1200/JCO.2013.53.5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vermorken JB, Herbst RS, Leon X, Amellal N, Baselga J. Overview of the efficacy of cetuximab in recurrent and/or metastatic squamous cell carcinoma of the head and neck in patients who previously failed platinum-based therapies. Cancer. 2008 Jun 15;112(12):2710–2719. doi: 10.1002/cncr.23442. [DOI] [PubMed] [Google Scholar]
- 42.Licitra L, Mesia R, Rivera F, et al. Evaluation of EGFR gene copy number as a predictive biomarker for the efficacy of cetuximab in combination with chemotherapy in the first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck: EXTREME study. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011 May;22(5):1078–1087. doi: 10.1093/annonc/mdq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Du Y, Peyser ND, Grandis JR. Integration of molecular targeted therapy with radiation in head and neck cancer. Pharmacology & therapeutics. 2014 Apr;142(1):88–98. doi: 10.1016/j.pharmthera.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Vermorken JB, Stohlmacher-Williams J, Davidenko I, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. The Lancet Oncology. 2013 Jul;14(8):697–710. doi: 10.1016/S1470-2045(13)70181-5. [DOI] [PubMed] [Google Scholar]
- 45.Huang SM, Li J, Armstrong EA, Harari PM. Modulation of radiation response and tumor-induced angiogenesis after epidermal growth factor receptor inhibition by ZD1839 (Iressa) Cancer research. 2002 Aug 1;62(15):4300–4306. [PubMed] [Google Scholar]
- 46.Chinnaiyan P, Huang S, Vallabhaneni G, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer research. 2005 Apr 15;65(8):3328–3335. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 47.Caponigro F, Romano C, Milano A, et al. A phase I/II trial of gefitinib and radiotherapy in patients with locally advanced inoperable squamous cell carcinoma of the head and neck. Anti-cancer drugs. 2008 Aug;19(7):739–744. doi: 10.1097/CAD.0b013e32830676a8. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez CP, Adelstein DJ, Rybicki LA, et al. Single-arm phase II study of multiagent concurrent chemoradiotherapy and gefitinib in locoregionally advanced squamous cell carcinoma of the head and neck. Head & neck. 2012 Nov;34(11):1517–1523. doi: 10.1002/hed.21971. [DOI] [PubMed] [Google Scholar]
- 49.Harrington K, Berrier A, Robinson M, et al. Randomised Phase II study of oral lapatinib combined with chemoradiotherapy in patients with advanced squamous cell carcinoma of the head and neck: rationale for future randomised trials in human papilloma virus-negative disease. European journal of cancer. 2013 May;49(7):1609–1618. doi: 10.1016/j.ejca.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 50.Hoang T, Huang S, Armstrong E, Eickhoff JC, Harari PM. Enhancement of radiation response with bevacizumab. Journal of experimental & clinical cancer research : CR. 2012;31:37. doi: 10.1186/1756-9966-31-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salama JK, Haraf DJ, Stenson KM, et al. A randomized phase II study of 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy compared with bevacizumab plus 5-fluorouracil, hydroxyurea, and twice-daily radiotherapy for intermediate-stage and T4N0-1 head and neck cancers. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2011 Oct;22(10):2304–2309. doi: 10.1093/annonc/mdq736. [DOI] [PubMed] [Google Scholar]
- 52.Yoo DS, Kirkpatrick JP, Craciunescu O, et al. Prospective trial of synchronous bevacizumab, erlotinib, and concurrent chemoradiation in locally advanced head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 Mar 1;18(5):1404–1414. doi: 10.1158/1078-0432.CCR-11-1982. [DOI] [PubMed] [Google Scholar]
- 53.Sano D, Matsumoto F, Valdecanas DR, et al. Vandetanib restores head and neck squamous cell carcinoma cells’ sensitivity to cisplatin and radiation in vivo and in vitro. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011 Apr 1;17(7):1815–1827. doi: 10.1158/1078-0432.CCR-10-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schueneman AJ, Himmelfarb E, Geng L, et al. SU11248 maintenance therapy prevents tumor regrowth after fractionated irradiation of murine tumor models. Cancer research. 2003 Jul 15;63(14):4009–4016. [PubMed] [Google Scholar]
- 55.Yadav A, Kumar B, Teknos TN, Kumar P. Sorafenib enhances the antitumor effects of chemoradiation treatment by downregulating ERCC-1 and XRCC-1 DNA repair proteins. Molecular cancer therapeutics. 2011 Jul;10(7):1241–1251. doi: 10.1158/1535-7163.MCT-11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Limaye S, Riley S, Zhao S, et al. A randomized phase II study of docetaxel with or without vandetanib in recurrent or metastatic squamous cell carcinoma of head and neck (SCCHN) Oral oncology. 2013 Aug;49(8):835–841. doi: 10.1016/j.oraloncology.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 57.Brooks HD, Glisson BS, Bekele BN, et al. Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer. 2011 May 15;117(10):2112–2119. doi: 10.1002/cncr.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sewell A, Brown B, Biktasova A, et al. Reverse-phase protein array profiling of oropharyngeal cancer and significance of PIK3CA mutations in HPV-associated head and neck cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 May 1;20(9):2300–2311. doi: 10.1158/1078-0432.CCR-13-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bauman JE, Ferris RL. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer. 2014 Mar 1;120(5):624–632. doi: 10.1002/cncr.28380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zandberg DP, Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck. Oral oncology. 2014 Jul;50(7):627–632. doi: 10.1016/j.oraloncology.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Topalian SL, Sznol M, McDermott DF, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014 Apr 1;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ukpo OC, Thorstad WL, Lewis JS., Jr B7-H1 expression model for immune evasion in human papillomavirus-related oropharyngeal squamous cell carcinoma. Head and neck pathology. 2013 Jun;7(2):113–121. doi: 10.1007/s12105-012-0406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Serafini P, Meckel K, Kelso M, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. The Journal of experimental medicine. 2006 Nov 27;203(12):2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weed DT, Vella JL, Reis I, et al. Tadalafil reduces myeloid derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with Head and Neck Squamous Cell Carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Oct 15; doi: 10.1158/1078-0432.CCR-14-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]