Abstract
Background
There is a large and persistent racial disparity in STI in the U.S. which has placed non-Hispanic-Blacks at disproportionately high risk. We tested a hypothesis that both individual-level risk factors (partner number, anal sex, condom use) and local-network features (concurrency and assortative mixing by race) combine to account for the association between race and chlamydia status.
Methods
Data from the Longitudinal Survey of Adolescent Health Wave III were used. Chlamydia status was determined using biomarkers. Individual-level risk behaviors were self-reported. Network location variables for concurrency and assortative mixing were imputed using egocentrically sample data on sexual partnerships.
Results
After controlling for demographic attributes including age, sex, marital status, education and health care access there remained a strong association between race and chlamydia status (OR = 5.23, 95% CI] 3.83–7.15], p < .001 for Non-Hispanic Blacks with Non-Hispanic Whites as the reference category). The inclusion of individual-level risk factors did not alter the association between race and chlamydia(OR = 5.23 for Non-Hispanic Blacks). The inclusion of concurrency and assortative mixing by race substantially reduced the association between race and chlamydia status (OR = 1.87, 95% CI [0.89–3.91] p > .05 for Non-Hispanic Blacks).
Keywords: Concurrency, Race, Sexual networks
1. Introduction
STIs are a significant cause of morbidity and mortality that have a disproportionate impact on minority populations within the U.S. Non-Hispanics Blacks (NHB), and in particular NHB females exhibit incidence and prevalence rates far greater than Non-Hispanic Whites (NHW). In 2010 the incidence rates of chlamydia (CT), syphilis, and HIV were roughly eight times greater among NHB than NHW and the incidence rate of gonorrhea was over 18 time greater among NHB than NHW (CDC, 2011, 2012a). These very different infections involve unique biological and immunological processes, but they are all transmitted via the same behaviors and traverse the same sexual network – so it is not surprising that we find similar patterns of infection. In this paper we provide a mechanism for the emergence and persistence of the racial disparity in STI incidence using a network theoretic perspective, and we use data from a nationally representative sample of young adults in the U.S. to empirically test our hypothesis.
There are numerous individual-level risk behaviors (ILRB) that directly affect the probability of acquiring an STI including partner number (Hallfors et al., 2007; Halpern et al., 2004; Laumann and Youm, 1999; Mayer and Anderson, 1995), anal sex (particularly receptive) (Mayer and Anderson, 1995; Lazzarin et al., 1992; Baldwin and Baldwin, 2000) and condom use. These STI-related behaviors have also been shown to vary significantly between different racial groups (Halpern et al., 2004; Baldwin and Baldwin, 2000; Leigh et al., 1993; Hamilton, 2011; Reece et al., 2010). The differences in behavior have not, however, been sufficient to account for the racial disparity in STI. The association between race and STI status persists even after controlling for partner number, anal sex, commercial sex, drug-related behaviors, education, income and healthcare access (Hallfors et al., 2007; Santelli et al., 2000; Tanfer et al., 1995). We hypothesize that ILRBs have been insufficient explanatory factors because they fail to capture the variation in risk associated with the local sexual network (LSN) environment which is defined by the partners to whom one is connected.
STI acquisition does not occur among isolated individuals; it occurs via an association with a partner. Consequently, the probability of acquiring an STI depends as much on the behavior of a partner as it does on one’s own behavior. Take, for example, the many cases of uninfected individuals that enter into a relationship with an uninfected partner and still acquire an STI despite remaining monogamous (Landman et al., 2008; Hugonnet et al., 2002). Because partners play a pivotal role in determining if someone acquires an STI the LSN should be taken into account when determining risk. This is the crux of the network hypothesis tested here – that risk for an STI is a function of both ILRBs and LSN and by extension the racial disparity observed at the population-level is the emergent outcome of aggregate differences in ILRB and LSN structure. Following the findings of Morris et al. (2009), the two components of the LSN that we explore as drivers of the disparity in STI are concurrency and assortative mixing by race.
The presence of concurrency in the sexual network transforms the cross-sectional landscape of a network from one of isolates and dyads, as would be present under serial monogamy, to a landscape that includes triads and potentially much larger connected components that can quickly and efficiently transmit an infection. The effects of concurrency on transmission are threefold. First, the time between acquiring infection and passing it on is reduced because one partnership does not have to end before another begins; second, the protective effect that comes from being earlier in the partner sequence is eliminated, because later partners can now pose an indirect risk to earlier partners when these partnerships overlap in time; and third, this “backwards path” of infection established by concurrent partnerships creates entirely new chains of infection (Moody, 2002).
The potential importance of concurrency for understanding the HIV epidemic was first suggested in the early 1990s (Watts and May, 1992). Since then research has demonstrated through simulation studies that even small differences in concurrency can have dramatic effects on connectivity in sexual networks, and there by the speed with which infection spreads and the number of people that can be infected (Morris et al., 2007; Morris and Kretzschmar, 2000). Empirical studies have also provided ecological evidence that concurrency may be a driver of epidemic transmission by linking high rates of concurrency with the severely HIV-impacted populations of Sub-Saharan Africa (SSA) (Morris and Kretzschmar, 1997; Lurie et al., 2003; Morris et al., 2010; Carter et al., 2007;Colvin et al., 1998; Mbizvo et al., 2001; Sandoy et al., 2010; Vissers et al., 2008) as well as by linking subgroup-level differences in concurrency with the prevalence of HIV (Morris et al., 2010; Carteret al., 2007; Colvin et al., 1998; Mbizvo et al., 2001; Sandoy et al., 2010; Vissers et al., 2008; Kenyon et al., 2009). Simulation studies, using empirically derived parameters, have also demonstrated that concurrency may be an important driver of the HIV epidemic in sub-Saharan Africa (Eaton et al., 2011; Goodreau et al., 2012; Johnson et al., 2009). Despite these findings, the extent to which concurrency has contributed to the HIV epidemic in SSA has remained a topic of debate (Sawers et al., 2011; Sawers and Stillwaggon, 2010;Epstein and Morris, 2011; Lurie and Rosenthal, 2010a,b; Tanser et al., 2011; Amelia and Martina, 2012; Morris et al., 2014). This is in large part due to the difficulty in collecting accurate data on sexual relationships including the duration of partnership overlap and the frequency and timing of sex acts across multiple partners. In addition there has historically been a reliance on prevalence as an epidemic outcome which is difficult to temporally match to the behaviors that may have contributed to transmission because HIV has a long duration of infection and the frequency of testing tends to be low.
In the U.S. context, a few previous studies have reported that NHB in the U.S. tend to have higher rates of concurrency (Hamilton, 2011; Santelli et al., 1998; Adimora et al., 2002, 2007) and we hypothesize that this is contributing to disparate incidence rates that in turn drive divergent prevalence. We further hypothesize that a high level of homophily in partner selection – assortative mixing – amplifies the effect of disparate concurrency rates by concentrating the additional connectivity within one group and that the higher prevalence levels that are generated among the group with higher concurrency rates can become a risk in and of themselves because selecting any partner from a population with a higher prevalence of infection is an additional risk.
In this analysis we test the hypothesis that ILRB differences in conjunction with second order risk derived from the LSN contributes to the observed racial disparity in STI.
2. Materials and methods
The data used in this study came from the National Longitudinal Survey of Adolescent Health (Add Health) Wave III (The National Longitudinal Study of Adolescent Health, 1998) which included 14,322 (75.7%) of the original sample (Harris et al., 2009). The subsample used here included heterosexual respondents who provided demographic and behavioral data about themselves and demographic information on at least one current sex partner between 18 and 26 years of age. Further details about these data are available elsewhere (The National Longitudinal Study of Adolescent Health, 1998; Harris et al., 2009).
The demographic variables we include in our model are sex, race (non-Hispanic White, non-Hispanic Black and other), marital status (married, not married) and age (18–22, 23–26). In addition we include two indicators of socio-economic status: education (did not complete high school, graduated high school or earned a GED, education beyond high school) and health insurance status (did not have health insurance in the last year, had health insurance for part of the last year, had health insurance all of the last year). The ILRBs are: number of partners in the last year, anal sex with a partner in the last year, and condom use at last sex. The LSN measures included in these analyses are concurrency and assortative mixing by race. The annual prevalence of concurrency in the sample population is shown in Table 1. In this population of young adults the annual prevalence of concurrency among NHB is approximately 20% while the prevalence among NHW is close to 11%. The pattern of assortative mixing is also shown in Table 1. Most relationships reported by both NHW and NHB – 74–89% – were with partners of the same race, with only 2–12% between NHW and NHB.
Table 1.
Annual prevalence of concurrency and assortative mixing by sex and race.
| Respondent | Concurrency prevalence | Partner race | |||
|---|---|---|---|---|---|
| White | Black | Other | |||
| Females | White | 11.22% | 79% | 5% | 16% |
| Black | 20.93% | 3% | 89% | 8% | |
| Other | 11.20% | 34% | 10% | 56% | |
| Males | White | 11.30% | 82% | 2% | 17% |
| Black | 19.08% | 12% | 74% | 14% | |
| Other | 12.50% | 33% | 3% | 64% | |
The inclusion of network characteristics in the predictive model presented unique challenges. Because the data were egocentrically sampled, respondent’s partners were not enrolled in the study, so there was no direct way to measure the partner’s concurrency. We adopted an imputation approach to address this limitation because it has the advantage of using only the data provided by the respondent. The imputation process uses what we know about each of the partner’s observable characteristics and the behavior of respondents with the same characteristics to impute what a partner’s behavior was likely to have been on average.
The imputation procedure is comprised of three steps. (1) For each of the partners identified by a respondent a subsample of the data was generated that included all of the respondents with the same demographic characteristics (age, sex, marital status and race) as the identified partner. (2) The subsample from step one was further refined to include only those respondents who reported a partner in the previous year with the same demographic attributes as the respondent for whom the partner attributes were being imputed – thus, there was a two-way matching. (3) Concurrency for each individual in the two-way matched subsample was coded either zero or one indicating no concurrency or at least 1 set of concurrent relationships respectively. From this subsample a weighted mean value was calculated using the number of partners with the same demographic characteristics as the index case reported by each member of the subsample as the sample weight. The result is the probability that a partnership selected from all partnerships in the subsample that are matched on the demographics of both the index and the partner is concurrent with another partnership. This mean value was then multiplied by 100 to represent the percentage of demographically matched partnerships in the population from which a partner is drawn that are concurrent with another partnership. If an index case reported more than one partner the mean prevalence across the multiple partners was used. The result is potentially an underestimation of concurrency exposure for those with multiple partners. As an alternative, the joint probability of exposure across all partners could be used, but given coital dilution in the context of multiple partners the joint probability would likely be an overestimate. Additionally, using the mean has the advantage of decoupling the number of partners an individual has from the estimated probability of their exposure to a concurrent partner. The impact of any bias introduced by using the mean across partnerships may also be negligible given a general regression toward the mean for all of the imputed values.
The variable for assortative mixing was calculated in a similar way. The functional mechanism by which assortative mixing impacts STI risk is via the variation in the underlying prevalence of STI across the different groups from which a partner may be selected. The non-random distribution of STI in the population was captured by calculating the prevalence of the STI of interest for each of the subsamples defined by the demographic characteristics of the respondent and the identified partner. In this case however, because both STI and disassortative mixing by race were rare events the imputation subsamples were less restrictive using only two demographic attributes of the respondent and the partner (race and sex) to define the subsample.
Several sensitivity analyses were also conducted. First, in order to impute the variables for each partner identified by a respondent, there had to be a sufficient number of other respondents who were demographically equivalent to each identified partner, who, in turn, identified at least one partner that was demographically equivalent to the respondent. This was limited by both the sample size and homogeneity in partner selection. All analyses where run twice, first only requiring a minimum subsample of five respondents in any imputation subsample and then with a more robust requirement of 10 respondents was run as a sensitivity analysis. The impact of these different requirements on the size and composition of the sample population are shown in Table 2. Second, the outcome variable of interest was CT, the most common STI reported. As an additional analysis a composite STI outcome that included CT, gonorrhea, and trichomonias is was also tested. STI status was determined using biomarkers. Third, the use of prevalence as the metric for both partner concurrency and assortative mixing assumes that there is a linear association between prevalence and CT on a log-odds scale. However, the impact of a 1% increase in prevalence at lower prevalence levels, e.g. from 3% to 4%, may be more important for determining differences in risk than a 1% increase in prevalence at higher prevalence levels, e.g. from 50% to 51%, due to the non-linear dynamics of epidemic transmission. Therefore the logs of the prevalence of each of the two LSNs are also used as independent variables.
Table 2.
Changes in sample size and chlamydia prevalence resulting from the sample reductions imposed by sample size requirement for imputing network location variables.
| Non-Hispanic White | Non-Hispanic Black | Other | Total cases dropped | ||
|---|---|---|---|---|---|
| All data 5349 | N | 3656 | 1078 | 745 | NA |
| N|CH+ | 86 (2.4%) | 120 (11.1%) | 27 (3.6%) | NA | |
| Five respondent minimum for imputation | ΔN | 57 (1.6%) | 65 (6.0%) | 8 (1.1%) | 130 (2.4%) |
| ΔN|CH+ | 1 (1.2%) | 9 (7.5%) | (0.0%) | 10 (4.3%) | |
| Ten respondent minimum for imputation | ΔN | 81 (2.2%) | 117 (10.9%) | 15 (2.0%) | 213 (3.9%) |
| ΔN|CH+ | 2 (2.3%) | 13 (10.8%) | 0 (0.0%) | 15 (6.4%) |
Each analysis followed a four iteration process utilizing logistic regression followed by a hierarchical variance partition. (1) Each of the demographic, ILRB and LSN variables were regressed on STI status to determine their univariate association. (2) The demographic variables were included in a multivariate model to provide a baseline association between race and STI status conditional on the other demographic attributes. (3) The ILRBs were then included in the model to determine if they altered the association between race and STI status. (4) The LSN variables were added to determine if local network location accounts for any residual association between race and STI not already explained by the differences in ILRB. Following the logistic regression analysis a hierarchical partitioning of the variance was performed using the ‘hier.part’ package in R to determine the percentage of the explained variance associated with race, the ILRB and the LSN.
3. Results
The results of the analyses are shown in Table 3. The unadjusted (bivariate) results in the first column show that odds of CT infection were significantly higher for NHB than NHW (OR = 5.79, 95% CI [4.28–7.84], p < .001), and this is the strongest single predictor of infection. Being unmarried was also significantly associated with CT. Respondents who had at least completed high school were significantly less likely to be CT+ compared to those with less than a high school education. Full-time medical insurance coverage during the previous year also had a significant protective effect, as did having part-time coverage. The number of partners reported in the last year was positively associated with CT (OR = 1.29, 95%CI [1.13–1.48], p < .001) but there was no significant association between reporting anal sex in the last year or condom use at last intercourse and CT. Both of the LSN variables were significantly associated with CT. The OR was 1.04 for a 1% change in the prevalence of concurrency among the two-way matched sample from which a partnership was drawn (95% CI [1.03–1.05], p < .001) and the OR was 1.16 for a 1% change in the prevalence of CT among the sample from which a partner was drawn (95% CI [1.13–1.19], p < .001)
Table 3.
Logistic regression results: dependent variable – chlamydia status.
| Variable class | Independent variables |
Exp(B) | |||
|---|---|---|---|---|---|
| Univariate | Demographic multivariate |
+ ILRB | + Network location |
||
| Female | 1.20 (0.90–1.60) | 1.44* (1.06–1.95) | 1.42* (1.05–1.92) | 1.61** (1.16–2.23) | |
| Not married | 1.77** (1.21–2.58) | 1.49* (1.01–2.21) | 1.39 (0.93–2.08) | 0.70 (0.38–1.29) | |
| Demographics and socio-economic indicators | Age (22–26) | 1.10 (0.82–1.47) | 1.22 (0.90–1.66) | 1.23 (0.91–1.67) | 1.18 (0.87–1.60) |
| Non-Hispanic Black | 5.79*** (4.28–7.84) | 5.23*** (3.83–7.15) | 5.23*** (3.83–7.16) | 1.87. (0.89–3.91) | |
| Other race | 1.33 (0.77–2.30) | 1.29 (0.74–2.24) | 1.30 (0.75–2.27) | 1.03 (0.57–1.86) | |
| High school | 0.51*** (0.36–0.74) | 0.53** (0.36–0.77) | 0.53** (0.36–0.78) | 0.54** (0.37–0.80) | |
| More than high school | 0.35*** (0.25–0.51) | 0.41*** (0.28–0.61) | 0.40*** (0.27–0.59) | 0.41*** (0.28–0.60) | |
| Insured part-time | 0.66 (0.43–1.00) | 0.85 (0.55–1.32) | 0.86 (0.55–1.33) | .89 (0.57–1.38) | |
| Insured full-time | 0.50*** (0.35–0.69) | 0.63* (0.44–0.90) | 0.65* (0.45–0.93) | 0.67*. (0.47–0.97) | |
| Individual behavior | Condom at last sex | .94 (0.84–1.04) | 0.93 (0.82–1.05) | 0.92 (0.82–1.04) | |
| Anal sex in last year | 1.30 (0.82–2.05) | 1.03 (0.63–1.68) | 1.07 (0.65–1.75) | ||
| Partners last year | 1.29*** (1.13–1.48) | 1.23** (1.05–1.44) | 1.24** (1.06–1.45) | ||
| Network location | Partner concurrency | 1.04*** (1.03–1.05) | 1.02** (1.01–1.04) | ||
| Partner STI status | 1.16*** (1.13–1.19) | 1.08* (1.01–1.15) | |||
| AIC | 1461.8 | 1458.7 | 1444.8 | ||
In the multivariate model with the demographic variables only (second column of coefficients), the OR for NHB declined slightly, from 5.79 to 5.23, but remained a significant predictor of CT infection. Adding the ILRB variables (third column) had a negligible impact on the association between the demographic attributes and CT with the exception of marital status which was no longer significant. Of the ILRB variables, only the number of partners in the previous year was significantly associated with CT (OR = 1.23, 95%CI [1.05–1.44] p < .01). But controlling for number of partners did not change the observed association between NHB and CT, which implies that this (and the other ILRBs), while significantly associated with CT risk, are not the driver of racial disparities in CT prevalence.
In the final model, which incorporated the LSN, both partner concurrency and the CT prevalence differentials in LSNs that are generated by assortative mixing were significantly associated with CT infection of respondents (OR = 1.02, 95% CI [1.01–1.04] p < .01; and OR = 1.08, 95% CI [1.01–1.15] p < .05 respectively). In contrast to the ILRBs, the inclusion of the LSN variables reduced the magnitude of the OR for NHB and CT from 5.23 to 1.87 and the residual effect was not statistically significant.
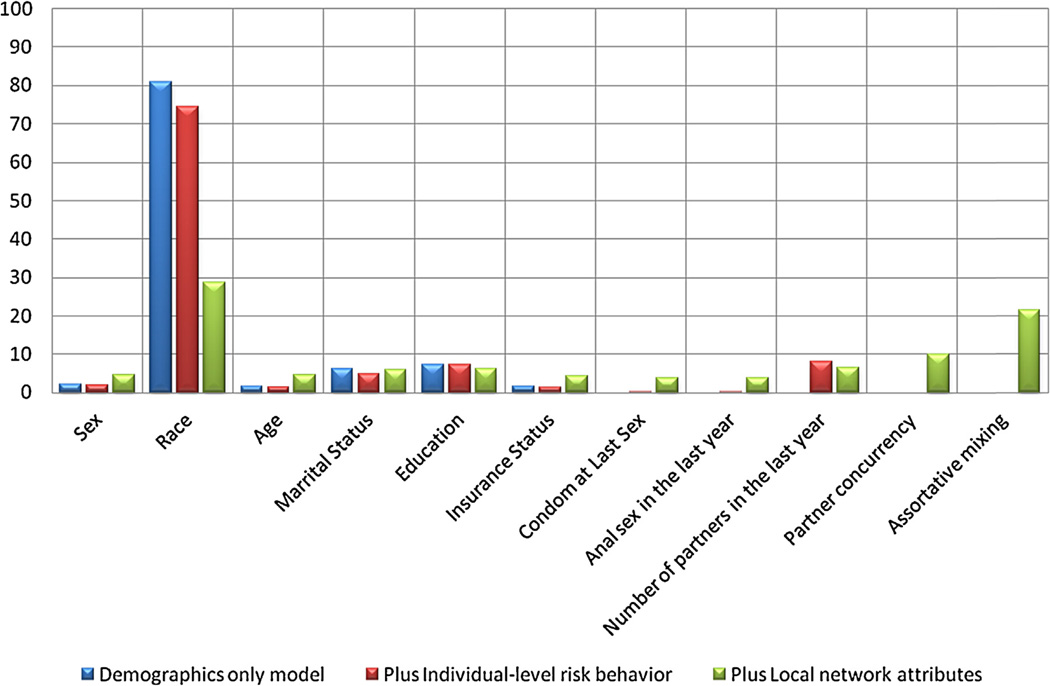
The hierarchical partitioning of the variance in the demographic model attributed 80.1% of the explained variance to race. Including ILRB only conferred a modest improvement in model fit based on AIC but the proportion of the explained variance attributable to race declined to 74.4%. The number of partners in the last year accounted for 8.0% of the explained variance. Including LSN reduced the fraction of the explained variance attributable to race to 28.7%, while partner concurrency accounted for 10.0% and assortative mixing accounted for 21.6%. The proportions of the explained variance attributable to each of the independent variable are shown in Fig. 1.
Fig. 1.
The attributable fractions of the explained variation in Chlamydia status in three models.
The results for the composite STI outcome were not significantly different from the results for CT alone, which is not surprising since CT accounted for the majority of STI cases. The sensitivity analyses performed by repeating the analyses using a more restrictive imputation that required 10 respondents for each imputation subsample also did not produce significantly different results. Similarly, the results did not substantially change when partner concurrency and assortative mixing were represented on the log scale. The full models under these additional scenarios are shown in Appendix A.
4. Discussion
There has been a long history of racial disparities in STI, and the disparities can be seen in the epidemiology of all reportable STIs: 2010 rate ratios comparing NHB to NHW for CT, syphilis, and HIV were approximately 8, and are higher, about 18, for gonorrhea (CDC, 2011, 2012a). There are certainly many factors contributing to these disparities but the results reported here provide support for the hypothesis that the structure of the local sexual network is a risk factor for STI and that variations in the network structure contribute more to the observed racial disparities in STI than do other demographic attributes and individual level risk behaviors. In this sample of young adults the odds of CT infection were 5.6 times higher for NHB than other groups. That association remained largely unchanged after controlling for ILRB like the number of sexual partners in the last year. However, after controlling for the probability of partner concurrency and a partner having a CT infection given assortative mixing, the variation in CT attributable to race declined by over two thirds, and was no longer statistically significant. As the CDC has reported “Race and ethnicity in the United States are population characteristics that also correlate with other fundamental determinants of health status (CDC, 2012b).” Our analysis suggests that in the case of STIs, concurrency and assortative mixing create a network structure that is associated with much of the racial disparity in risk. These network characteristics may in fact be a mechanism through which inequalities manifest themselves (Cooper et al., 2014; Adimora et al., 2013).
This research has several strength and weaknesses that should be noted. Fully observed sexual network data tend to be rare because enrolling a respondent’s partner is expensive, logistically difficult, and fraught with ethical and legal challenges. This paper presents a partial solution to this problem based on imputation from egocentrically sampled data and the reported characteristics of the respondent’s partners, data that are both easily collected and readily available. This approach assumes homogeneity in the risks associated with a partnership for all partnerships between any defined set of demographic groups. We can account for additional heterogeneity in the population by incorporating additional characteristics in to the imputation but we are ultimately limited by the sample size. However, despite this limitation, the method and the results presented here remain fundamentally grounded in the data. It should also be noted that the results of this analysis may also be subject to significant bias if there is a correlation between any of the categories used for imputation and a reporting bias.
This approach also allows us to test network properties within the generalized linear model framework. Logistic regression is a standard method in both sociology and epidemiology, and by creating individual level proxy variables for network location we are able to include local network information in a relatively straightforward way utilizing readily available data and standard analytic tools. As a caveat, when using a generalized linear model framework, it must be assumed that the relationships that are being modeled are linear in the log-odds of infection. Network processes often are non-linear, and the non-linear characteristics of transmission on a network are not taken into account using this method no rare the non-linear threshold properties often found in networks.
Our goal was to demonstrate, using the basic tools of epidemiological research and readily accessible data, that the results found in many simulation and modeling studies are supported by empirical correlations. Despite the limitations of this study it provides additional evidence that both concurrency and assortative mixing are playing a key role in generating and maintaining the racial disparity in STI. The impact of concurrency demonstrated here suggests that it may be a productive target for public health interventions.
Appendix A
See Table A1.
Table A1.
Logistic regression results.
| Independent variables | Dependent variable –chlamydia status | Dependent variable – combined chlamydia, gonorrhea and trichomoniasis | ||||
|---|---|---|---|---|---|---|
| Minimum five respondents in the pool for imputation |
Minimum five respondents in the pool for imputation |
Minimum 10 respondents in the pool for imputation |
||||
| Exp(B) | 95% CI | Exp(B) | 95% CI | Exp(B) | 95% CI | |
| Female | 1.77*** | 1.28–2.46 | 1.42* | 1.04–1.92 | 1.43* | 1.05–1.95 |
| Not married | 1.11 | 0.72–1.72 | 0.81 | 0.44–1.50 | 0.81 | 0.43–1.51 |
| Age (22–26) | 1.24 | 0.91–1.68 | 1.10 | 0.82–1.49 | 1.11 | 0.82–1.50 |
| Non-Hispanic Black | 0.68 | 0.27–1.71 | 1.49 | 0.71–3.10 | 1.33 | 0.61–2.91 |
| Other race | 0.58 | 0.30–1.14 | 0.92 | 0.51–1.66 | 0.92 | 0.51–1.67 |
| High school | 0.55** | 0.37–0.81 | 0.58** | 0.40–0.85 | 0.58** | 0.40–0.85 |
| More than high school | 0.41*** | 0.28–0.61 | 0.47*** | 0.32–0.69 | 0.48*** | 0.32–0.70 |
| Insured part-time | 0.89 | 0.57–1.38 | 0.81 | 0.52–1.25 | 0.80 | 0.52–1.23 |
| Insured full-time | 0.66* | 0.46–0.96 | 0.62** | 0.44–0.88 | 0.60** | 0.42–0.86 |
| Condom at last sex | 0.92 | 0.82–1.04 | 0.92 | 0.83–1.04 | 0.93 | 0.83–1.04 |
| Anal sex in the last year | 1.04 | 0.64–1.71 | 1.07 | 0.65–1.74 | 1.06 | 0.65–1.74 |
| Partner in the last year | 1.24** | 1.06–1.45 | 1.20* | 1.03–1.41 | 1.21* | 1.03–1.42 |
| Partner concurrency | 1.02* | 1.00–1.04 | 1.02* | 1.00–1.04 | ||
| Partner STI status | 1.10** | 1.03–1.18 | 1.11** | 1.04–1.20 | ||
| Log of partner concurrency | 1.25. | 0.98–1.60 | ||||
| Log of partner STI status | 2.81*** | 1.75–4.53 | ||||
References
- Adimora AA, Schoenbach VJ, Taylor EM, Khan MR, Schwartz RJ, Miller WC. Sex ratio, poverty, and concurrent partnerships among men and women in the United States: a multilevel analysis. Ann. Epidemiol. 2013 Nov;23(11):716–719. doi: 10.1016/j.annepidem.2013.08.002. http://dx.doi.org/10.1016/j.annepidem.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adimora AA, Schoenback VJ, Bonas DM, Martinson FE, Donaldson KH, Stancil TR. Concurrent sexual partnerships among women in the United States. Epidemiology. 2002;13:320–327. doi: 10.1097/00001648-200205000-00013. [DOI] [PubMed] [Google Scholar]
- Adimora AA, Schoenback VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am. J. Public Health. 2007:97. doi: 10.2105/AJPH.2006.099069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amelia K, Martina M. Lack of association between concurrency and HIV infection: an artifact of study design. J. Acquir. Immune Defic. Syndr. 2012;60:1. doi: 10.1097/QAI.0b013e3182460b79. [DOI] [PubMed] [Google Scholar]
- Baldwin JI, Baldwin JD. Heterosexual anal intercourse: an understudied, high-risk sexual behavior. Arch. Sex. Behav. 2000;29:357–373. doi: 10.1023/a:1001918504344. [DOI] [PubMed] [Google Scholar]
- Carter M, Kraft J, Koppenhaver T, Galavotti C, Roels T, Kilmarx P, et al. “A bull cannot be in a single kraal”: concurrent sexual partnerships in Botswana. AIDS Behav. 2007;11:822–830. doi: 10.1007/s10461-006-9203-6. [DOI] [PubMed] [Google Scholar]
- CDC. Sexually Transmitted Disease Surveillance 2010. Atlanta: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- CDC. HIV Surveillance Report, 2010. Prevention CfDCa. 2012a [Google Scholar]
- CDC. http://www.cdc.gov.std/stats12/minorities.htm.
- Colvin M, Abdol Karim S, Connolly C, Hoosen A, Ntuli N. HIV infection and asymptomatic sexually transmitted infections in a rural South African com-munity. Int. J. STD AIDS. 1998;9:548–550. doi: 10.1258/0956462981922683. [DOI] [PubMed] [Google Scholar]
- Cooper HL, Linton S, Haley DF, Kelley ME, Dauria EF, Karnes CC, Ross Z, Hunter-Jones J, Renneker KK, Del Rio C, Adimora A, Wingood G, Rothenberg R, Bonney LE. Changes in exposure to neighborhood characteristics are associated with sexual network characteristics in a cohort of adults relocating from public housing. AIDS Behav. 2014 Aug; doi: 10.1007/s10461-014-0883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton J, Hallett T, Garnett G. Concurrent sexual partnerships and primary HIV infection: a critical interaction. AIDS Behav. 2011;15:687–692. doi: 10.1007/s10461-010-9787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein H, Morris M. Concurrent partnerships and HIV: an inconvenient truth. J. Int. AIDS Soc. 2011:14. doi: 10.1186/1758-2652-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodreau S, Cassels S, Kasprzyk D, Montaño D, Greek A, Morris M. Concurrent partnerships, acute infection and HIV epidemic dynamics among young adults in Zimbabwe. AIDS Behav. 2012;16:312–322. doi: 10.1007/s10461-010-9858-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallfors DD, Bonita IJ, Miller WC, Bauer DJ. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am. J. Public Health. 2007;97:125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern CT, Hallfors D, Bauer DJ, Iritani B, Walker MW, Cho H. Implications of racial and gender differences in patterns of adolescent risk behavior for HIV and other sexually transmitted diseases. Perspect. Sex Reprod. Health. 2004:36. doi: 10.1363/psrh.36.239.04. [DOI] [PubMed] [Google Scholar]
- Hamilton DT. Sociology. Seattle: University of Washington; 2011. Racial disparities in STI related behavior: data from four studies. [Google Scholar]
- Harris KM, Halpern CT, Whitsel E, Hussey J, Tabor J, Entzel P, et al. The National Longitudinal Study of Adolescent Health. Research Design. 2009 [Google Scholar]
- Hugonnet S, Mosha F, Todd J, Mugeye K, Klokke A, Ndeki L, et al. Incidence of HIV infection in stable sexual partnerships: a retrospective cohort study of 1802 couples in Mwanza Region, Tanzania. J. Acquir. Immune Defic. Syndr. 2002;1:73–80. doi: 10.1097/00042560-200205010-00010. [DOI] [PubMed] [Google Scholar]
- Johnson L, Dorrington R, Bradshaw D, Wyk VP-V, Rehle T. Sexual behaviour patterns in South Africa and their association with the spread of HIV: insights from a mathematical model. Demogr. Res. 2009;21:289–340. [Google Scholar]
- Kenyon C, Dlamini S, Boulle A, White R, Badri M. A network-level explanation for the difference in HIV prevalence in South Africa’s racial groups. Afr. J. AIDS Res. 2009;8:243–254. doi: 10.2989/AJAR.2009.8.3.1.922. [DOI] [PubMed] [Google Scholar]
- Landman KZ, Ostermann J, Crump JA, Mgonja A, Mayhood MK, Itemba DK, et al. Gender differences in the risk of HIV infection among persons reporting abstinence, monogamy, and multiple sexual partners in Northern Tanzania. PLoS ONE. 2008;3:e3075. doi: 10.1371/journal.pone.0003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann E, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex. Transm. Dis. 1999;26:250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Lazzarin A, Saracco A, Musicco M, Nicolosi A. Man-to-woman sexual transmission of the human immunodeficiency virus. Risk factors related to sexual behavior, man’s infectiousness, and woman’s susceptibility. Arch. Intern. Med. 1992:152. [PubMed] [Google Scholar]
- Leigh BC, Temple MT, Trocki KF. The sexual behavior of US adults: results from a national survey. Am. J. Public Health. 1993;83:1400–1408. doi: 10.2105/ajph.83.10.1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie MN, Rosenthal S. Concurrent partnerships as a driver of the HIV epidemic in sub-Saharan Africa? The evidence is limited. AIDS Behav. 2010a;14 doi: 10.1007/s10461-009-9583-5. http://dx.doi.org/10.1007/s10461-009-9583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie MN, Rosenthal S. The concurrency hypothesis in Sub-Saharan Africa: convincing empirical evidence is still lacking. Response to Mah and Halperin, Epstein, and Morris. AIDS Behav. 2010b;14(1):34. doi: 10.1007/s10461-009-9640-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurie M, Williams B, Zuma K, Mkaya-Mwamburi D, Garnett G, Sweat M, et al. Who infects whom? HIV-1 concordance and discordance among migrant and non-migrant couples in South Africa. AIDS. 2003;17:2245–2252. doi: 10.1097/00002030-200310170-00013. [DOI] [PubMed] [Google Scholar]
- Mayer KH, Anderson DJ. Heterosexual HIV transmission. Infect. Agents Dis. 1995;4:273–284. [PubMed] [Google Scholar]
- Mbizvo E, Msuya S, Stray-Pederson B, Sundby J, Chirenje M, Hussain A. HIV seroprevalence and its associations with other reproductive tract infections in asymptomatic women in Harare, Zimbabwe. Int. J. STD AIDS. 2001;12:524–531. doi: 10.1258/0956462011923624. [DOI] [PubMed] [Google Scholar]
- Moody J. The importance of relationship timing for diffusion. Soc. Forces. 2002;81:25–56. [Google Scholar]
- Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11:641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- Morris M, Kretzschmar M. A micro-simulation study of the effect of concurrent partnerships on HIV spread in Uganda. Math. Popul. Stud.: Int. J. Math. Demogr. 2000;8:109–133. [Google Scholar]
- Morris M, Goodreau S, Moody J. Sexual networks, concurrency, and STD/HIV. In: Holmes KK, Sparling PF, Mardh PA, et al., editors. Sexually Transmitted Diseases. 4th ed. New York: McGraw-Hill; 2007. [Google Scholar]
- Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am. J. Public Health. 2009;99:1023–1031. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Epstein H, Wawer M. Timing is everything: international variations in historical sexual partnership concurrency and HIV prevalence. PLoS ONE. 2010:e14092. doi: 10.1371/journal.pone.0014092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Vu L, Leslie-Cook A, Akom E, Stephen A, Sherard D. Comparing estimates of multiple and concurrent partnerships across population based surveys: implications for combination HIV prevention. AIDS Behav. 2014;18:4. doi: 10.1007/s10461-013-0618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Condom use rates in a national probability sample of males and females ages 14 to 94 in the United States. J. Sex. Med. 2010;7:266–276. doi: 10.1111/j.1743-6109.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- Sandoy I, Dzekedzeke K, Fylkesnes K. Prevalence and correlates of sexual partnerships in Zambia. AIDS Behav. 2010;14:59–71. doi: 10.1007/s10461-008-9472-3. [DOI] [PubMed] [Google Scholar]
- Santelli JS, Brener ND, Lowry R, Bhatt A, Zabin LS. Multiple sexual partners among U.S. adolescents and young adults. Fam. Plan. Perspect. 1998:30. [PubMed] [Google Scholar]
- Santelli JS, Lowry R, Brener ND, Robin L. The association of sexual behavior with socioeconomic status, family structure and race/ethnicity among US adolescents. Am. J. Public Health. 2000;90:1582–1587. doi: 10.2105/ajph.90.10.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers L, Stillwaggon E. Concurrent sexual partnerships do not explain the HIV epidemics in Africa: a systematic review of the evidence. J. Int. AIDS Soc. 2010;13:34. doi: 10.1186/1758-2652-13-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers L, Isaac AG, Stillwaggon E. HIV and concurrent sexual partnerships: modelling the role of coital dilution. J. Int. AIDS Soc. 2011;14:44. doi: 10.1186/1758-2652-14-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanfer K, Cubbins LA, Billy JOG. Gender, race, class and self-reported sexually transmitted disease incidence. Fam. Plan. Perspect. 1995;27:196–202. [PubMed] [Google Scholar]
- Tanser F, Bärnighausen T, Hund L, Garnett G, McGrath N, Newell M-L. Effect of concurrent sexual partnerships on rate of new HIV infections in a high-prevalence, rural South African population: a cohort study. Lancet. 2011;378 doi: 10.1016/S0140-6736(11)60779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Longitudinal Study of Adolescent Health. Carolina Population Center at the University of North Carolina at Chapel Hill; 1998. [Google Scholar]
- Vissers D, Voeton H, Urassa M, Isingo R, Ndege M, Kumogola Y, et al. Separation of spouses due to travel and living apart raises HIV risk in Tanzanian couples. Sex. Transm. Dis. 2008;35:714–720. doi: 10.1097/OLQ.0b013e3181723d93. [DOI] [PubMed] [Google Scholar]
- Watts CH, May RM. The influence of concurrent partnerships on the dynamics of HIV/AIDS. Math. Biosci. 1992;108(1):89–104. doi: 10.1016/0025-5564(92)90006-i. [DOI] [PubMed] [Google Scholar]