Abstract
Adenosine has been found to be cardioprotective during episodes of cardiac ischemia/reperfusion through activation of the A1 and possibly A3 receptors. Therefore, we have investigated whether activation of these receptors can protect also against apoptotic death induced by angiotensin II (Ang II) in neonatal rat cardiomyocyte cultures. Exposure to Ang II (10 nM) resulted in a 3-fold increase in programmed cell death (p < 0.05). Pretreatment with the A1 adenosine receptor agonist 2-chloro-N6-cyclopentyladenosine (CCPA, 1 μM), abolished the effects of Ang II on programmed cardiomyocyte death. Moreover, exposure of cells to the A1 adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (CPX) before pretreatment with CCPA, prevented the protective effect of the latter. Pretreatment with the A3 adenosine receptor agonist N6-(3-iodobenzyl) adenosine-5′-N-methyluronamide (IB-MECA, 0.1 μM), led to a partial decrease in apoptotic rate induced by Ang II. Exposure of myocytes to Ang II caused an immediate increase in the concentration of intracellular free Ca2+ that lasted 40–60 sec. Pretreatment of cells with CCPA or IB-MECA did not block Ang II-induced Ca2+ elevation. In conclusion, activation of adenosine A1 receptors can protect the cardiac cells from apoptosis induced by Ang II, while activation of the adenosine A3 receptors confers partial cardioprotection.
Keywords: A1 and A3 adenosine receptors, angiotensin II, apoptosis, cardiomyocytes, Feulgen and TUNEL stainings
Introduction
Apoptosis has been demonstrated to be an important mechanism in the remodeling process after acute myocardial infarction, and the development of either ischemic or non-ischemic cardiac failure. This phenomenon may contribute to the progression of cardiac dysfunction [1–4]. Angiotensin II (Ang II) was shown to induce programmed cardiomyocyte death, and therefore may be an important mediator of cellular loss in heart failure and after acute myocardial infarction [5–11]. Thus, identifying therapies that oppose Ang II-induced apoptosis has important investigative and clinical applications.
Cardiomyocytes and vascular cells readily form, transport, and metabolize the endogenous adenine nucleoside – adenosine, and regulate both interstitial and plasma adenosine concentrations. Four subtypes of membrane adenosine receptors have been identified, termed A1, A2A, A2B and A3 [12]. Adenosine has been found to be cardioprotective during episodes of cardiac hypoxia/ischemia [13–16]. It has been demonstrated that maximal preconditioning-induced cardioprotection requires activation of both A1 and A3 adenosine receptors [14, 16]. In addition, it has been shown that repeated activation of adenosine A1 receptors reduces infarct size [17]. These findings suggest that A1 adenosine receptor activation may hold promise as a new approach to long-term cardioprotection.
It has been demonstrated that adenosine and Ang II levels are increased in heart failure [18, 19] and the elevated adenosine levels may protect against progression of cardiac dysfunction [20]. Thus, increased adenosine levels in heart failure may protect against programmed myocyte death induced by high levels of Ang II. Therefore, we have investigated whether activation of A1 adenosine receptors, or A3 adenosine receptors, can protect against apoptotic death induced by Ang II in neonatal rat cardiomyocyte cultures. We also studied whether cardioprotection is related to intracellular Ca2+ changes.
Materials and methods
Cardiac cell cultures
Sprague-Dawley rat hearts (1–2 days old) were removed under sterile conditions and washed three times in phosphate buffered saline (PBS) to remove excess blood cells. The hearts were minced to small fragments and then agitated gently in a solution of proteolytic enzymes, RDB (Biological Institute, Ness-Ziona, Israel), which was prepared from a fig tree extract. The RDB was diluted 1:100 in Ca2+ and Mg2+-free PBS at 25°C for a few cycles of 10 min each, as described previously [21]. The mixture was centrifuged at 300 g for 5 min. The supernatant phase was discarded, and the cells were re-suspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% horse serum (Biological Industries, Kibbutz Beit Haemek, Israel) and 2% chick embryo extract (Biological Industries). The suspension of the cells was diluted to 1.0 × 106 cells/ml, and 1.5 ml were placed in 35-mm plastic culture dishes on collagen/gelatin-coated cover glasses. The cultures were incubated in a humidified atmosphere of 5% CO2, 95% air at 37°C. Confluent monolayers exhibiting spontaneous contractions were developed in culture within 2 days.
Before exposure to chemicals the growth medium was replaced with chemically defined medium based on DMEM supplemented with transferin (10 μg/ml), insulin (10 μg/ml), T4 (0.1 μmol/L) and Bovine Serum Albumin (100 μg/ml).
Experiments with Adenosine A1 and A3 receptor agonists and antagonists
CCPA – a selective A1 adenosine receptor agonist, IB-MECA – a selective A3 adenosine receptor agonist, CPX – a selective A1 adenosine receptor antagonist, and MRS1523 – a selective A3 adenosine receptor antagonist were added to cell cultures. Pretreatment with CCPA and IB-MECA was accomplished by adding these compounds 20 min before exposure of cells to Ang II. In additional experiments CPX and MRS1523 were added 20 min prior to pretreatment with CCPA and IB-MECA respectively. Apoptosis assays were performed after exposure of cardiac cells to chemicals for a period of 24 h.
α-Sarcomeric actin staining
In order to identify cardiomyocytes, cells on coverslips were stained for immunohistochemical demonstration of α-sarcomeric actin using mouse monoclonal anti α-sarcomeric actin (C-5) and goat anti-mouse biotinylated immunoglobulin conjugated with extrAvidin peroxidase. The chromogen 3-amino-9-ethylcarbazole (AEC) was used as described previously [21].
Feulgen procedure
Cells on coverslips were fixed in EFA (by volume, 75:20:5 of 96% ethanol: 40% neutral formol: acetic acid) for 20 min. Fixed samples were placed in 5N HCl for 60 min at 24°C to hydrolyze DNA and stained with the Schiff reagent as described previously [21].
In situ apoptosis assay
Apoptotic cells were identified in situ using the terminal deoxynucleotidyl transferase nick-end labeling (‘TUNEL’-like) assay as described previously [21]. After fixation in 10% neutral buffered formalin for 20 minutes at room temperature and a permeabilization in 70% ethanol for 30 min at −20°C, the assay was performed on cells on coverslips using the available TdT FragEL DNA fragmentation KIT, according to the manufacturer’s recommendations. Biotinylated nucleotides were detected using a streptavidin-horseradish peroxidase conjugate. Chromogenes AEC or DAB-Black (Zymed substrate KIT) reacted with the labeled sample to generate an insoluble colored substrate at the site of DNA fragmentation.
Image analysis of Fuelgen and TUNEL stained cells
The image analysis was performed with Scan-Array 2 Image Analyzer (Galai, Israel). The analyzer consisted of an Axiovert 135TV fluorescent microscope (Zeiss, Germany) and a black and white Sony video camera, interfaced to an image analysis computer. Morphonuclear parameters were computed as described in detail previously [21]. In the present work, the system described the following parameters: AREA – morphometric parameter, which corresponds to the area of the nuclear profile; IOD – the integrated optical density, a densitometric parameter, related to the total DNA content and apoptotic index – percent of apoptotic nuclei.
Fluorescence DNA stains
Cells were analyzed for apoptosis following visualization of the fluorescent DNA-binding dye Hoechst 33342 trihydrochloride trihydrate. The monolayers were rinsed with PBS and then incubated with 10 μg/ml H33342 for 30 min. Nuclei were visualized using fluorescent microscopy and analyzed for apoptotic morphology. An average of 1000 nuclei from random fields was analyzed for each slide. The apoptotic index was calculated as described by Wu [22]. At least three samples were scored per group.
Intracellular calcium measurements
Intracellular free calcium concentration was estimated from indo-1 fluorescence, using the ratio method described by Grynkiewicz et al. [23]. The cardiac cells grown on coverslips were transferred to a chamber on the stage of Zeiss inverted microscope filtered with UV epifluorescence illumination. Indo-1 was excited at 355 nm and the emitted light then split by a dichroic mirror to two photomultipliers (Hamamatsu, Japan) with input filters at 405 and 495 nm. The fluorescence ratio of 405/495 nm, which is proportional to Ca2+ concentration, was monitored. Assessment of changes in intracellular Ca2+ concentration was done in all experiments. In addition, intracellular Ca2+ concentration was measured after exposure of cardiac cells to Ang II in a Ca2+-free medium.
Chemicals
CCPA, IB-MECA and CPX were purchased from Research Biochemicals International (Natick, MA, USA). Apoptosis detection system (FragEL Kit) was purchased from Calbiochem (San Diego, CA, USA). Indo-1 was purchased from Teflabs (Texas Fluorescence Lab., USA). Other reagents were purchased from Sigma Chemicals (St. Louis, MO, USA).
Statistical analysis
Results are expressed as mean + S.E. ANOVA and Student’s t-test were used in statistical evaluation of the data. Values of p < 0.05 were considered significant.
Results
Characterization of neonatal cardiomyocytes in cell cultures after exposure to Ang II
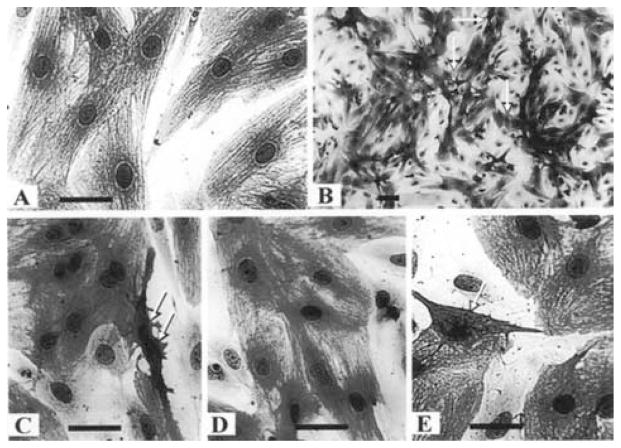
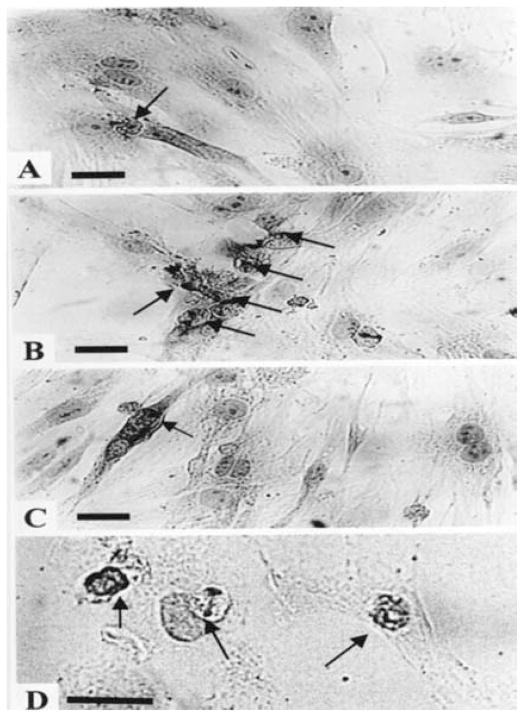
Primary cultures of neonatal rat myocytes maintained in serum-free medium are depicted in Fig. 1A. Myofibrils within these cells were elongated and uniformly distributed throughout the cell. Individual actin fibrils stained with α-sarcomeric actin antibody demonstrated the typical striated pattern. Each culture dish also contained a small number of dead cells, defined by a condensed granular cytoplasm and pyknotic nucleus. The cardiomyocytes of the Ang II (10 nmol/L) treated cultures showed an increase in myofibril content and moderate hypertrophy. In preliminary experiments myocytes were exposed to Ang II at concentrations of 1–1000 nmol/L for a period of 24 h (in serum-free medium) and only a weak dose-dependence response, was observed on apoptotic activity. Therefore, concentrations of 10–100 nmol/L of Ang II were employed in the present study. Morphological features of apoptosis in Ang II treated myocytes include nuclear condensation and fragmentation, blebbing of plasma membrane, shrinking, retraction and condensation of cytoplasm (Figs 1B and 1C).
Fig. 1.
Morphology of cardiocytes following Ang II treatment. Cardiac cells 3 days in culture were transferred to serum-free media for 2 days before treatment with Ang II (10 nmol/L) for 24 h. The cells were stained for α-saromeric actin and counterstained with hematoxylin. (A) Control cells. Myofibrils within these cells were uniformly distributed throughout the cell. Individual actin fibrils stained with α-sarcomeric actin antibody demonstrated the typical striated pattern (magnification × 660). (B) Ang II (10 nmol/L) treated cells (magnification × 170). (C) Ang II (10 nmol/L) treated cells (magnification × 660). (D) Pretreatment with CCPA (1 μmol/L) before Ang II (magnification × 660). (E) Pretreatment with IB-MECA (0.1 μmol/L) before Ang II. Arrows indicate apoptotic cardiomyocytes. Bars = 10 μm.
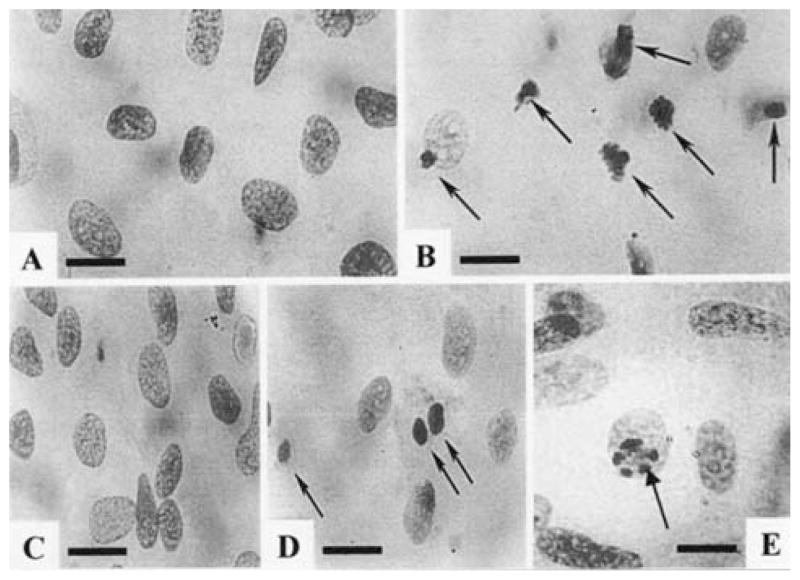
Fuelgen and Hoechst stainings demonstrated an increase in the number of apoptotic cells after exposure of cardiomyocytes to Ang II (10 nmol/L) for 24 h (Figs 2B and 3B).
Fig. 2.
Morphology of nuclei of Feulgen-stained cultured cardiomyocytes after exposure to Ang II (10 nmol/L). (A) Nuclei of control cells. A few small granules against a pale background characterizes chromatin. (B) Exposure of myocytes to Ang II for 24 h led to an increase in the number of apoptotic nuclei. Condensation, compacting and margination of nuclear chromatin were accompanied by disappearance of the structural framework of the nucleus and nuclear breakdown. (C) Pretreatment with CCPA (1 μmol/ L) before Ang II. (D) Ang II treated cells, exposed to CPX (1 μmol/L) before pretreatment with CCPA (1 μmol/L). (E) Pretreatment with IB-MECA (0.1 μmol/L) before Ang II. Arrows indicate apoptotic cardiomyocytes. Bars = 10 μm.
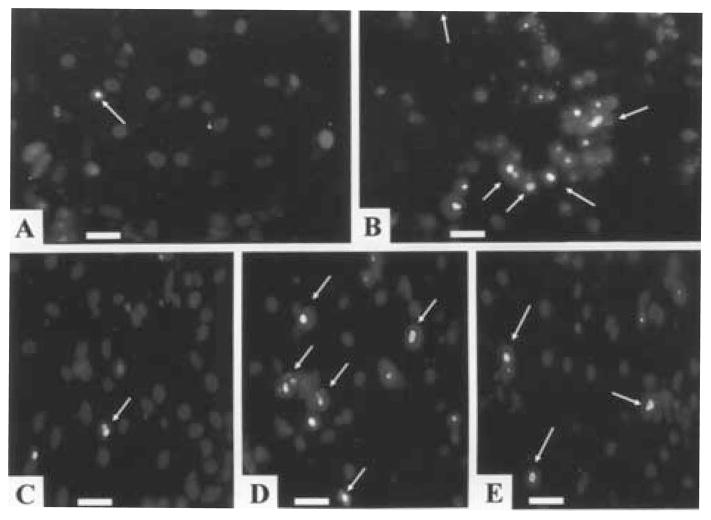
Fig. 3.
Hoechst staining. (A) Control cells. (B) After exposure of myocytes to Ang II (10 nmol/L) for 24 h. (C) Pretreatment with CCPA (1 μmol/L) before Ang II. (D) Ang II treated cells, exposed to CPX (1 μmol/L) before pretreatment with CCPA (1 μmol/L). (E) Pretreatment with IB-MECA (0.1 μmol/L) before Ang II. Arrows indicate apoptotic cardiomyocytes. Bars = 10 μm.
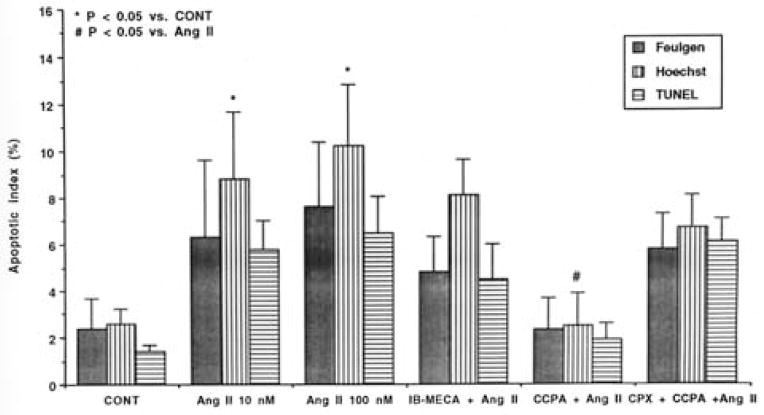
The apoptotic index (percent of apoptotic nuclei) of Feulgen-stained cells is shown in Fig. 4. Ang II (10–100 nmol/ L) caused a 3-fold increase in apoptotic cells (from 2.4 ± 1.3% in the control cells to 6.3 ± 3.3 and 7.6 ± 2.8% in the 10 and 100 nmol/L of Ang II treated cells respectively, p < 0.05).
Fig. 4.
Apoptotic index of Feulgen, Hoechst and TUNEL-stained myocytes after Ang II treatment. Cardiocytes were treated with the indicated drugs 15 min before application of Ang II (10 nmol/L). Feulgen Hoechst and TUNEL staining were performed 24 h later. CONT – control. IB-MECA –0.1 μmol/L. CCPA – 1 μmol/L. CPX – 1 μmol/L. Ang II was employed at a concentration of 10 nmol/L except when indicated.
Analysis of the fluorescent morphology of Hoechst stained cells shows that Ang II (10–100 nmol/L) caused a marked increase in apoptotic cell death (from 2.6 ± 0.65% in the control cells to 8.8 ± 2.9 and 10.2 ± 2.6% in the 10 and 100 nmol/ L of Ang II treated cells respectively, p < 0.05) (Fig. 4).
To further examine the cells that contained fragmented nuclear DNA typical of apoptosis, we performed an in situ assay based on end-labeling of DNA strand breaks by the TUNEL-like method (Fig. 5). TUNEL positive cells were obtained following Ang II treatment (Fig. 5B) confirming our results with the Fuelgen and Hoechst stainings. The apoptotic index of TUNEL-stained cells is shown in Fig. 4. Ang II (10–100 nmol/L) caused a 4-fold increase in apoptotic cell death (from 1.4 ± 0.28% in the control cells to 5.8 ± 1.24% and 6.5 ± 1.54% in the 10 and 100 nmol/L of Ang II treated cells respectively, p < 0.05).
Fig. 5.
Immunohistochemical demonstration of DNA breaks (TUNEL-like) following Ang II treatment. Cardiac cells 3 days in cultures were transferred to serum-free media for 2 days before treatment with Ang II (10 nmol/L) for 24 h. (A) Control. (B) Ang II (10 nmol/L) treated cells. (C) Pretreatment with CCPA (1 μmol/L) before Ang II (10 μmol/L). (D) Pretreatment with IB-MECA (0.1 μmol/L) before Ang II. Arrows indicate apoptotic cardiomyocytes. Bars = 1 μm.
Effects of adenosine A1 and A3 receptor ligands on cardiomyocyte death
We investigated the possible protective effects of adenosine A1 and A3 receptor activation on Ang II-induced apoptosis. Exposure of cardiac cells to CCPA (an adenosine A1 selective receptor agonist, 1 μmol/L), IB-MECA (an adenosine A3 selective receptor agonist, 0.1 μmol/L), CPX (an adenosine A1 receptor antagonist, 1 μmol/L) and MRS1523 (an adenosine A3 receptor antagonist, 1 μmol/L) alone had no effect on apoptosis (data not shown). Pretreatment with CCPA abolished the effects of Ang II on programmed cardiomyocyte death (Figs 1D, 2C, 3C, 4 and 5C). When the adenosine A1 receptor antagonist CPX was given to cells prior to pretreatment with CCPA, the protective effects of CCPA were not obtained (Figs 2D, 3D and 4). Pretreatment with IB-MECA, only partially blunted the apoptotic effects of Ang II (Figs 1E, 2E, 3E, 4 and 5D). Addition of MRS1523 prior to pretreatment with IB-MECA blunted the partial protection conferred by the adenosine A3 receptor antagonist (data not shown).
Effects of Ang II and adenosine agonists on intracellular Ca2+ concentrations
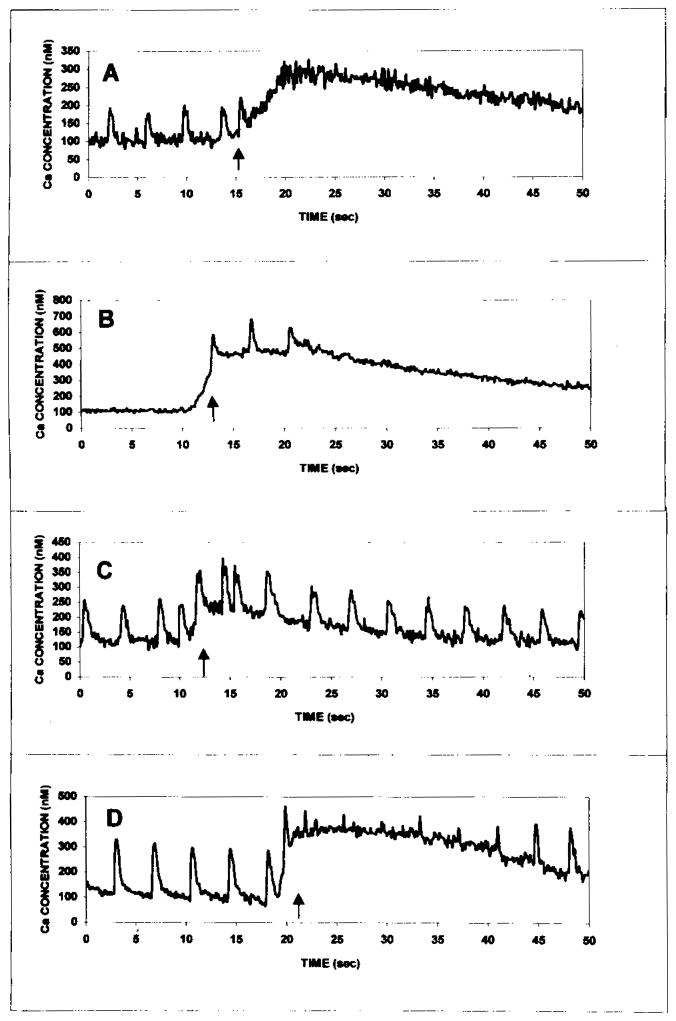
Control myocytes demonstrated spontaneous, regular beating activity and Ca2+ transients. Exposure of myocytes to Ang II (1–1000 nmol/L) caused an increase in the concentration of intracellular free Ca2+ in concentration-dependent manner. The drug produced a rapid rise followed by a sustained increase in Ca2+, that lasted 60–200 sec (Fig. 6A). Treatment of cells in a Ca2+-free medium did not abolish the intracellular Ca2+ rise induced by Ang II, indicating that Ca2+ elevation following response to Ang II arises from intracellular stores (Fig. 6D). The effect was not inhibited by pretreatment with either the adenosine receptor agonist CCPA (1 μmol/L) or the A3 adenosine receptor agonist IB-MECA (0.1 μmol/L) (Figs 6B and 6C).
Fig. 6.
Effect of Ang II on intracellular free calcium in cultured rat cardiomyocytes. (A) Effect of Ang II (10 nmol/L). (B) Effect of the selective adenosine A1 agonist CCPA (1 μmol/L) on the change in intracellular free calcium induced by Ang II (10 nmol/L). (C) Effect of the adenosine A3 agonist IB-MECA (0.1 μmol/L) on the change in intracellular free calcium induced by Ang II (10 nmol/L). (D) Effect of Ang II (10 nmol/L) in a calcium free buffer. Cultures were incubated with agonists for 5 min at room temperature prior to the addition of Ang II. Similar results were obtained in 5 different experiments.
Discussion
In the present study we have demonstrated that activation of the adenosine A1 receptor can significantly attenuate Ang II-mediated apoptosis in cardiocytes, while activation of the adenosine A3 receptor results in partial cardioprotection.
The vasopeptide Ang II acts on its target tissues via two subtypes membrane-bound receptors: AT1 and AT2 [24]. The AT1 receptor belongs to the seven membrane-domain G protein-coupled receptor family, bound to heterotrimaric Gq protein. The coupling of the AT2 receptor to the G protein family are less clearly established [25]. Recently, we have shown that stimulation of both receptor 54b types is required for the apoptotic effects of Ang II, while blockage of either the AT1 or AT2 receptor subtypes results in inhibition of Ang II-induced apoptosis [11]. Adenosine also exerts its biological effects via the activation of the G protein-coupled receptor family [12]. Evidence supports functional interaction between adenosine and AT1 receptors in the regulation of vascular resistance; stimulation of both receptors induces vasoconstriction [26]. Weihprecht et al. [27], demonstrated that blocking the A1 adenosine receptor with CPX attenuated the Ang II-induced afferent arteriolar renal vasoconstriction. Moreover, Liu et al. [28] showed that ischemic preconditioning in the rabbit heart is mediated through both AT1 and adenosine A1 receptors. Thus, in the vascular tree and in the process of ischemic preconditioning it seems that adenosine and Ang II cooperate in a mutually dependent and synergistic fashion. In contrast, we have demonstrated that in cardiac cells adenosine opposes the apoptotic effect of Ang II. The anti-apoptotic effect of adenosine does not involve the Ang II receptors, since pretreatment with either the A1 adenosine receptor antagonist – CPX or the A3 adenosine receptor antagonist – MRS1523 did not abolish Ang II induced programmed cardiomyocytes. Recently, it has been shown that activation of both adenosine receptor subtypes results in a decrease in intracellular energy supply (manifested by a decrease in the content of adenosine triphosphate (ATP) in cardiomyocytes), while simultaneously adequate amounts of ATP are preserved for maintenance of mitochondrial metabolism on a level sufficient for survival [29]. Apoptosis is an active, energy-consuming process, which is dependent on intracellular supply of ATP. Thus, a possible mechanism for the inhibition of the proapoptotic effect of Ang II by activation of adenosine receptors is a reduction of intracellular energy supply to a level insufficient for the apoptotic process. Our findings that adenosine protects against programmed myocyte death in heart cultures were confirmed in each apoptosis assay. Cardioprotective effect against apoptosis through activation of adenosine receptors was validated using the A1 and A3 receptors antagonists CPX and MRS1523 respectively.
Adenosine metabolism is changed in patients with heart failure and increases in adenosine levels may aid to reduce the severity of cardiac dysfunction [18, 19]. Since Ang II levels are also elevated in heart failure, it is possible that endogenous adenosine is increased to protect the cardiac cells from apoptosis in this condition.
We have attempted to determine whether the induction of programmed cardiomyocyte death by Ang II, and cardioprotection induced by adenosine agonists are related to changes in cytosolic Ca2+. The effect of Ang II on intracellular Ca2+ concentration was immediate and lasted 40–200 sec. Since treatment of cells in a calcium-free medium failed to prevent the intracellular Ca2+ elevation induced by Ang II, it can be concluded that the Ca2+ arises from intracellular stores e.g. –the sarcoplasmic reticulum. The calcium rise induced by Ang II was concentration-dependent and had an affect on the whole population of cultured myocytes. Pretreatment with the adenosine agonists had no effect on the Ang II-induced intracellular Ca2+ rise, while it blunted Ang II-induced apoptosis. Thus, it seems that prevention of Ang II-induced apoptosis is not mediated through changes in intracellular Ca2+.
Our findings present unequivocal data that adenosine confers cardioprotection against Ang II-induced apoptosis. This effect may play a role in pathological conditions in which both endogenous adenosine and Ang II levels are increased, e.g. heart failure and acute myocardial infarction. Recognition of the factors responsible for the initiation or prevention of programmed cell death may eventually lead to therapeutic interventions, as long acting adenosine A1 receptor agonists are currently undergoing clinical development.
Acknowledgments
We thank A. Isaac and T. Zinman for their valuable technical assistance and S. Victor for typing the manuscript. This study was supported in part by the Health Sciences Research Center at Bar-Ilan University.
References
- 1.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA. Apoptosis in myocytes in entage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- 2.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, DiLoreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 3.Moe GW, Naik G, Konig A, Lu X, Feng Q. Early and persistent activation of myocardial apoptosis, bax and caspases: Insights into mechanisms of progression of heart failure. Pathophysiology. 2002;8:183–192. doi: 10.1016/s0928-4680(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 4.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, Gambert SR, Cigola E, Anversa P. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 5.Cigola E, Kajstura J, Li B, Meggs LG, Anversa P. Angiotensin II activates programmed myocyte cell death in vitro. Exp cell Res. 1997;231:363–371. doi: 10.1006/excr.1997.3477. [DOI] [PubMed] [Google Scholar]
- 6.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 7.Diez J, Panizo A, Hemandez M, Vega F, Sola I, Fortuno MA, Pardo J. Cardiomyocyte apoptosis and cardiac angiotensin-converting enzyme in spontaneously hypertensive rats. Hypertension. 1997;30:1029–1034. doi: 10.1161/01.hyp.30.5.1029. [DOI] [PubMed] [Google Scholar]
- 8.Konstam MA, Kronenberg MW, Rousseau MF, Udelson JE, Melin J, Stewart D, Dolan N, Edens TR, Ahn S, Kinan D. Effects of the angiotensin converting enzyme inhibitor enalapril on the long-term progression of left ventricular dilatation in patients with asymptomatic systolic dysfunction. SOLVD (studies of left ventricular dysfunction) investigators. Circulation. 1993;88:2277–2283. doi: 10.1161/01.cir.88.5.2277. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B. Use of converting enzyme inhibitors in patients with asymptomatic left ventricular dysfunction. J Am Coll Cardiol. 1993;22:158A–161A. doi: 10.1016/0735-1097(93)90482-g. [DOI] [PubMed] [Google Scholar]
- 10.Cook JR, Glick HA, Gerth W, Kinosian B, Kostis JB. The cost and cardioprotective effects of enalapril in hypertensive patients with left ventricular dysfunction. Am J Hypertens. 1998;11:1433–1441. doi: 10.1016/s0895-7061(98)00180-0. [DOI] [PubMed] [Google Scholar]
- 11.Goldenberg I, Grossman E, Jacobson KA, Shneyvays V, Shainberg A. Angiotensin II-induced apoptosis in rat cardiomyocyte culture: A possible role of AT1 and AT2 receptors. J Hypertens. 2001;19:1681–1689. doi: 10.1097/00004872-200109000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shryock JC, Belardinelli L. Adenosine and adenosine receptors in the cardiovascular system: Biochemistry, physiology, and pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- 13.Lasley RD, Noble MA, Konyn PJ, Mentzer RM., Jr Different effects of an adenosine A1 analogue and ischemic preconditioning in isolated rabbit hearts. Ann Thorac Surg. 1995;60:1698–1703. doi: 10.1016/0003-4975(95)00717-2. [DOI] [PubMed] [Google Scholar]
- 14.Stambaugh K, Jacobson KA, Jiang JL, Liang BT. A novel cardioprotective function of adenosine A1 and A3 receptors during prolonged simulated ischemia. Am J Physiol. 1997;273:H501–H505. doi: 10.1152/ajpheart.1997.273.1.H501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Drake L, Sajjadi F, Firestein GS, Mullane KM, Bullough DA. Dual activation of adenosine A1 and A3 receptors mediates preconditioning of isolated cardiac myocytes. Eur J Pharmacol. 1997;320:241–248. doi: 10.1016/s0014-2999(96)00901-6. [DOI] [PubMed] [Google Scholar]
- 16.Safran N, Shneyvays V, Balas N, Jacobson KA, Nawrath H, Shainberg A. Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes. Mol Cell Biochem. 2001;217:143–152. doi: 10.1023/a:1007209321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dana A, Baxter GF, Walker JM, Yellon DM. Prolonging the delayed phase of myocardial protection: Repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J Am Coll Cardiol. 1998;31:1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 18.Funaya H, Kitakaze M, Node K, Minamino T, Komamura K, Hori M. Plasma adenosine levels increase in patients with chronic heart failure. Circulation. 1997;18:1363–1365. doi: 10.1161/01.cir.95.6.1363. [DOI] [PubMed] [Google Scholar]
- 19.Kitakaze M, Minamino T, Node K, Koretsune Y, Komamura K, Funaya H, Kuzuya T, Hori M. Elevation of plasma adenosine levels may attenuate the severity of chronic heart failure. Cardiovasc Drugs Ther. 1998;12:307–309. doi: 10.1023/a:1007726018470. [DOI] [PubMed] [Google Scholar]
- 20.Kitakaze M, Hori M. Adenosine therapy: A new approach to chronic heart failure. Expert Expert Opin Invest Drugs. 2000;9:2519–2535. doi: 10.1517/13543784.9.11.2519. [DOI] [PubMed] [Google Scholar]
- 21.Shneyvays V, Nawrath H, Jacobson A, Shainberg A. Induction of apoptosis in cardiac myocytes by an A3 adenosine receptor agonist. Exp Cell Res. 1998;243:383–397. doi: 10.1006/excr.1998.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C-F, Bishopric NH, Pratt RE. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J Biol Chem. 1997;272:14860–14866. doi: 10.1074/jbc.272.23.14860. [DOI] [PubMed] [Google Scholar]
- 23.Grynkiewicz G, Poenie M, Tsien TY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 24.Mukoyama M, Nakajima M, Horiuchi M, Sasamura H, Pratt RE, Dzau VJ. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J Biol Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- 25.Stoll M, Unger T. Angiotensin and its AT2 receptor: New insights into an old system. Regul Pept. 2001;99:175–182. doi: 10.1016/s0167-0115(01)00246-4. [DOI] [PubMed] [Google Scholar]
- 26.Traynor T, Yang T, Huang YG, Arend L, Oliverio MI, Coffman T, Briggs JP, Schnermann J. Inhibition of adenosine-1 receptor-mediated pre-glomerular vasoconstriction in AT1A receptor-deficient mice. Am J Physiol. 1998;275:F922–F927. doi: 10.1152/ajprenal.1998.275.6.F922. [DOI] [PubMed] [Google Scholar]
- 27.Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Synergistic effects of angiotensin and adenosine in the renal microvasculature. Am J Physiol. 1994;266:F227–F239. doi: 10.1152/ajprenal.1994.266.2.F227. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Tsuchida A, Cohen MV, Downey JM. Pretreatment with angiotensin II activates protein kinase C and limits myocardial infarction in isolated rabbit hearts. J Mol Cell Cardiol. 1995;27:883–892. doi: 10.1016/0022-2828(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 29.Shneyvays V, Mamedova L, Zinman T, Jacobson K, Shainberg A. Activation of A(3) adenosine receptor protects against doxorubicin-induced cardiotoxicity. J Mol Cell Cardiol. 2001;33:1249–1261. doi: 10.1006/jmcc.2001.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]