Abstract
Objective
To assess treatment strategies for seven different scenarios for treating complex pelvic fracture urethral injury (PFUI), categorised as repeat surgery for PFUI, ischaemic bulbar urethral necrosis (BUN), repair in boys and girls aged ⩽12 years, in patients with a recto-urethral fistula, or bladder neck incontinence, or with a double block at the bulbomembranous urethra and bladder neck/prostate region.
Patients and methods
We retrospectively reviewed the success rates and surgical procedures of these seven complex scenarios in the repair of PFUI at our institution from 2000 to 2013.
Results
In all, >550 PFUI procedures were performed at our centre, and 308 of these patients were classified as having a complex PFUI, with 225 patients available for follow-up. The overall success rates were 81% and 77% for primary and repeat procedures respectively. The overall success rate of those with BUN was 76%, using various methods of novel surgical techniques. Boys aged ⩽12 years with PFUI required a transpubic/abdominal approach 31% of the time, compared to 9% in adults. Young girls with PFUI also required a transpubic/abdominal urethroplasty, with a success rate of 66%. In patients with a recto-urethral fistula the success rate was 90% with attention to proper surgical principles, including a three-stage procedure and appropriate interposition. The treatment of bladder neck incontinence associated with the tear-drop deformity gave a continence rate of 66%. Children with a double block at the bulbomembranous urethra and at the bladder neck-prostate junction were all continent after a one-stage transpubic/abdominal procedure.
Conclusion
An understanding of complex pelvic fractures and their appropriate management can provide successful outcomes.
Abbreviations: PFUI, pelvic fracture urethral injury; BUN, bulbar urethral necrosis; BMU, bulbomembranous urethra; RUG, retrograde urethrography; VCUG, voiding cysto-urethrography; BMG, buccal mucosal graft; OMF, oral mucosal flap; UVF, urethrovaginal fistula; BNP, bladder neck prostate
Keywords: Bulbar urethral necrosis, Female, Recto-urethral fistula, Bladder neck injury
Introduction
Complex situations of posterior urethral injuries associated with pelvic fracture (PFUI) require a greater understanding of all the techniques available to a reconstructive urologist. Of patients with a pelvic fracture, 10% have a posterior urethral injury. For many years the transpubic urethroplasty with omentoplasty described by Turner-Warwick [1] was considered the standard treatment for patients with a complex PFUI. In 1986, Webster et al. [2] described a new posterior urethroplasty using an elaborated perineal approach with excision of the inferior pubic bone. Due to the advances by Turner-Warwick and Webster, the success rate of primary anastomotic urethroplasty approached 90% [3,4].
Complicating factors can diminish the success rate, alter typical patient presentation, and require surgical adjustment to the standard primary posterior repair. As first described in 1977, Turner-Warwick [5] proposed that the condition can be regarded as complex in three main cases: (i) strictures >2 cm long and surrounded by dense fibrosis; (ii) strictures associated with extravasation, diverticula, false passages or fistulae; and (iii) extensive damage involving the bladder neck. Koraitim [6] expanded this definition to include patients in whom a urinoma formed. To further advance the knowledge of complicating factors, we suggest an additional four scenarios: (1) patients in whom a previous urethroplasty has failed; (2) those with long defects due to bulbar urethral ischaemic necrosis (BUN); (3) boys aged ⩽12 years; and (4) those with a double block at the bulbomembranous urethra (BMU) and at the bladder neck-prostate (BNP) junction. We also include our experience with three groups of established criteria for complexity: (5) girls aged ⩽12 years who might have a urethrovaginal fistula (UVF); (6) patients with a recto-urethral fistula (RUF); and (7) incontinence after a bladder neck injury.
Patients and methods
Our institute is a tertiary referral centre, and from 2000 to 2013, >550 PFUI procedures were performed in our centre. The data of patients operated outside our institution by the same surgeon (S.B.K.) were not included. Of the 550 PFUIs, 308 of the patients were classified as complex by the criteria listed above, and 225 of these patients were available for follow-up. The patients were subdivided into one of the seven categories of complex PFUI by their respective complicating factor. As a retrospective analysis, the methods were the same for all categories. Occasionally one patient met two criteria (i.e., boys aged ⩽12 years who also had a double block). Data from September 2013 onwards were not included, to ensure that the results were from patients with ⩾12 months of follow-up. In some cases the geographical distance from our centre prevented a practical and consistent follow-up, so these patients were followed up with the referring urologist and updates were given by using mobile phone software.
All patients were evaluated before surgery with voiding cysto-urethrography (VCUG) and retrograde urethrography (RUG). All patients received appropriate antibiotics and all had either a suprapubic catheter or urethral Foley catheter after surgery for ⩾4 weeks or until a successful VCUG showed no extravasation. No patient required a urethral catheter for >6 weeks. For patients with a PFUI it is our common practice to use urethroscopy to evaluate the bulbar urethra, and cystoscopy through the suprapubic tract to evaluate the bladder neck.
In all patients a tension-free anastomosis was made over six interrupted 4–0 polyglactin/5–0 polydioxanone sutures. At our institution a failure of the surgery was defined as the requirement of one postoperative dilatation, visual internal urethrotomy, or a repeat urethroplasty. These interventions were indicated for symptomatic and objectively documented poor flow rates.
Results
Multiple failed previous urethroplasties
Among the 308 PFUIs included, 126 patients had undergone previous failed multiple (more than one) urethroplasties. Most of the failed urethroplasties were referred from outside institutions. Only 5–20% of primary cases fail, and failure is an aberrant phenomenon, therefore increased complexity is implied. Furthermore, our centre is a tertiary referral centre in a country with a population of 1.2 billion, and 3000 urologists who regularly perform urethroplasty. The referred cases of repeat surgery imply complexity, otherwise the referring urologist would manage them.
The mean (range) age of these patients who underwent progressive perineal anastomotic urethroplasty was 26 (8–52) years and the mean follow-up was 56 (12–122) months. The number of surgical attempts before presentation to our institution was 1–5.
The data of these patients are shown in Table 1; 62% of the time the patients’ previous bulbar urethra mobilisation was inadequate (Fig. 1A and D). In PFUI, the posterior urethra is usually displaced upwards. If the bulbar urethra is properly mobilised the extension will add two-thirds of the original length to accommodate the gap to the proximal urethra [7]. If the bulbar urethra is inadequately mobilised then it leads to tension at the anastomotic site and failure of the anastomosis.
Table 1.
The extent of dissection and success rates comparing primary and repeat procedures.
| Procedure |
n (%) |
Success |
n (%) |
Success |
|---|---|---|---|---|
| Step | Primary | Rate (%) | Repeat | Rate (%) |
| 1 | 20 (11) | 95 | 23 (18) | 74 |
| 2 | 29 (16) | 86 | 13 (10) | 92 |
| 3 | 108 (59) | 82 | 75 (60) | 73 |
| 4 | 3 (2) | 1/3 | 5 (4) | 4/5 |
| 5 | 6 (3) | 4/6 | 5 (4) | 4/5 |
| 6 | 8 (4) | 2/8 | 5 (4) | 5/5 |
| Overall | 174 | 81 | 126 | 77 |
Step 1 Bulbar mobilisation. Step 2 Crural Separation. Step 3 Inferior pubectomy. Step 4 Supracrural re-routing. Step 5 Transpubic/abdominal approach with posterior pubectomy. Step 6 Transpubic/abdominal urethroplasty with posterior pubectomy and omentoplasty.
Figure 1.
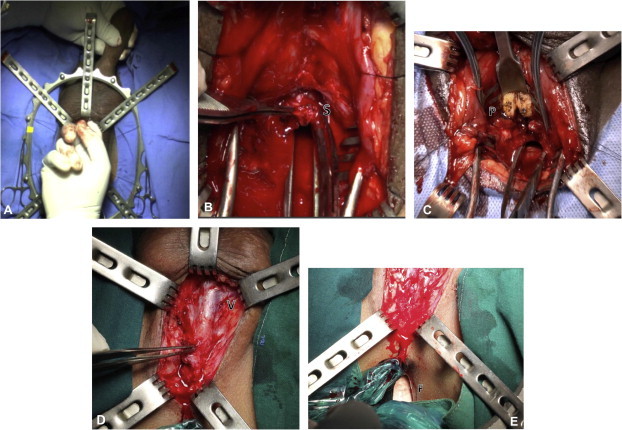
(A) Mobilisation of the bulbar urethra to the penoscrotal junction. (B) Excision of the scar (S). (C) Inferior pubectomy (P, inferior pubic bone). (D) Virgin tissue (V) seen near the distal bulbar urethra, suggesting no mobilisation by the previous surgeon. (E) A finger (F) in the rectum that can be used to palpate a sound passed through the posterior urethra.
The second cause of failure was inadequate excision of the scar. In almost all cases of previous failed attempts there was an extensive scar that required excision (Fig. 1B).
The third finding in repeat cases was a requirement for inferior pubectomy to obtain a tension-free anastomosis (Fig. 1C). We find that an inferior pubectomy is required in >60% of patients, and at essentially the same rate regardless of whether it is a primary or repeat procedure. This rate of inferior pubectomy is much higher than reported for the western experience [8]. We attribute this to anthropometric differences, including the pelvic structure and length of penis in different ethnic regions [9,10]. The mechanism of injury might also be a contributing factor in different parts of the world [11]. Most of our referred cases had no previous pubectomy and this could be a contributing factor to the failure. Overall, our success rates of 81% and 77% for primary and repeat cases, respectively, were comparable (P = 0.156).
One useful technique in repeat urethroplasty is to insert a finger into the patient’s rectum while dissecting the bulbar urethra from perineal body (Fig. 1E). The injury to the posterior urethra causes the prostate to retract away from the perineum [12]. In a patient with a high-riding prostate, the bougie cannot be felt in the perineum after transecting the bulbar urethra, and many urologists would proceed to an abdominal approach. We insert the left index finger into the rectum (with a second glove, of course) and the bougie through the suprapubic tract into the posterior urethra for palpation. When the direction of the dissection is known, and with a scalpel in the right hand, the posterior urethra can be opened perineally. This useful method can be applied under sterile conditions to avoid a transpubic approach.
The success of repeat surgery depends on an adequate blood supply. Repeated transection can cause shortening of the available urethra and a reduced blood flow to the anastomosis and distal urethra.
Long defects, BUN
The partial or complete loss of the bulbar urethra when there is an inadequate retrograde blood supply after bulbar urethral transection is termed BUN. This condition is rarely reported and has not been adequately described as a clinical entity. BUN was probably previously classified as ‘long gaps’ in the urethra, or ‘unsalvageable’ after failed urethroplasty [13,14]. It represents a complicated long defect with complete necrosis or obliteration (stenosis) of the bulbar urethra. BUN is a result of pelvic fracture and repeated transection attempts at repair. In 1986, Turner-Warwick [15] described spongionecrosis of the bulbar urethra.
When the bulbar urethral blood supply is transected, the blood supply to bulbar urethra relies upon retrograde blood flow from the normal bulbo-penile spongy tissue derived distally. Turner-Warwick described three situations that critically impair retrograde blood flow, i.e., over-mobilisation of the urethra distally, a coincidence of a hypospadiac deformity in which there is no continuity of the penile spongy urethra with that of the glans, and extensive spongiofibrosis resulting from previous urethritis or urethral surgery.
When the entire bulbar urethral plate has been obliterated by necrosis there is no bed upon which to perform augmentation urethroplasty with a buccal mucosal graft (BMG). The treatment of this ischaemic condition needs vascularised flaps either as a circumferential substitution in complete loss, or an augmentation in stenosis.
Specific procedures at our institution include:
-
(1)
A preputial tube (complete circumferential) [16] on a vascular pedicle mobilised subcutaneously to the perineum. (Fig. 2A–E) (in 25 patients).
-
(2)
An ingenious technique of oral mucosal flap (OMF) urethroplasty. An initial BMG is placed on scrotal skin for first stage, then a second-stage BMG is mobilised with neovascularity (Fig. 2F–H) (in eight patients).
-
(3)
The Turner-Warwick ‘scrotal drop back’ (in three patients).
-
(4)
A dorsal BMG with a ventral pedicle preputial flap in patients who did not have a pubectomy (two).
-
(5)
A pedicled preputial or penile skin flap (eight).
-
(6)
An entero-urethroplasty [13] with the use of re-tubularised sigmoid colon with its attendant mesentery (one).
Figure 2.
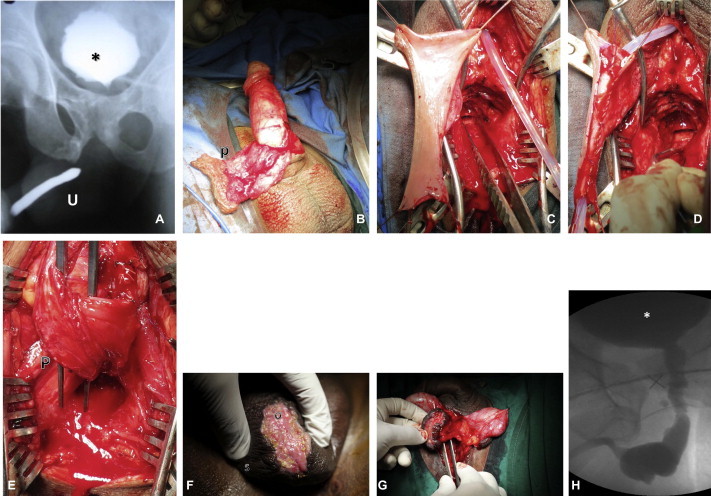
(A) A urethrogram of BUN (∗, bladder, U, penile urethra). (B) A pedicled preputial flap (p). C) The flap is transposed to the perineum. (D) Tubularisation over a 14 F catheter. (E) The completed proximal and distal anastomosis with pedicle (P). (F) Oral mucosa (O) quilted onto the scrotum (S). (G) The OMF is mobilised on the midline of the scrotal septum. (H) A follow-up VCUG after the OMF procedure.
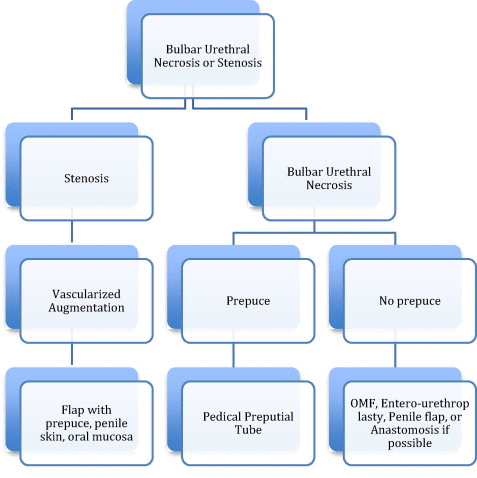
The choice of management option is variable, depending on the individual patient. Table 2 gives a general outline to our management. Generally, if the patient has a prepuce, our choice is to use a pedicled inner preputial tube.
Table 2.
Our algorithm for managing BUN or stenosis.
 |
After using the preputial tube, the patient rarely voids with a normal stream. The mean (range) urinary flow rate among those who were successful was 10.5 (3–26) mL/s. The preputial tube serves as a conduit, but never functions with the same viscoelastic properties as the normal urethra.
In all, 46 patients (mean age 28 years, range 12–55) were treated for BUN and followed up for a mean of 31 (12–132) months. The mean number of attempts of previous anastomotic urethroplasty was 2.2 (1–4).
In the OMF method the BMG is applied over the dartos of the scrotum through a wide elliptical incision in the first stage. At ≈ 6 months later, after graft uptake, and during a second stage, the OMF is mobilised with a midline scrotal septum and neovascularity. The OMF is then used as a flap transposed to the perineum and applied either as an onlay for partial loss or tubularised for complete loss.
Our first entero-urethroplasty was performed recently, but was not included in the analysis because there was insufficient follow-up information. The overall success rate for treating BUN was 76% (Table 3).
Table 3.
Success rates of different management options for BUN.
| Procedure | n (%) | Success rate |
|---|---|---|
| n/N or n (%) | ||
| Substitution: | ||
| Preputial tube | 25 (54) | 80 |
| OMF | 8 (17) | 4/8 |
| Scrotal drop back | 3 (6) | 1/3 |
| Augmentation: | ||
| Pedicled preputial | 8 (17) | 8/8 |
| or penile skin flap | ||
| Dorsal BMG | 2 (4) | 2/2 |
| w/ventral preputial flap | ||
| Overall | 46 | 76 |
The OMF was successful in half the patients. This is a versatile option for those with no prepuce, with poor penile skin, a history of pubectomy, and a long gap.
Boys aged ⩽12 years
The complexity in this group is due to two factors, i.e., the increased need for a transpubic approach, and the patient himself. In a young boy the best chance for success is during the first attempt, and the patient will depend for the rest of his life on the decisions the surgeon makes. Flynn et al. [17] reported that in prepubescent boys secondary repairs require more elaborate procedures, and prepubescent patients might have insufficient vascular connections in the glans, resulting in an inadequate retrograde blood flow.
The prostate in the young boy has yet to develop fully, and the same injury in an adult is less devastating to the posterior urethra. In adults, the location of the disruption is almost invariably the BMU.
Children can have urethral disruptions anywhere from the bladder neck through the entire posterior urethra. Common locations include supraprostatic, transprostatic, prostatomembranous, and BMU [18]. The puboprostatic ligaments of children are readily sheared off by sudden displacement of the fractured pubic rami, with a high incidence (44%) of proximal dislocation of the prostate, rendering repair of an ensuing stricture more difficult [19]. Awareness of these anatomical differences increases the index of suspicion for atypical anatomy, and preoperative imaging with VCUG and RUG must be more precise. Intraoperative decision-making should be meticulous in correctly aligning the bladder neck with the prostate and anterior urethra.
In all, 29 patients aged ⩽12 years were retrospectively reviewed. The mean (range) age at the time of repair was 10 (4–12) years, and they were evaluated for success after a mean follow-up of 35 (12–124) months.
In all cases a road traffic accident was the cause of the pelvic fracture. All patients presented with a suprapubic catheter. Nine of the 29 children were repeat cases and 20 had a primary repair.
The standard approach via the perineum should be attempted, with a low threshold for the transpubic/abdominal approach for those in whom the gap is too large to make a tension-free anastomosis. Children required the transpubic/abdominal approach 31% of the time, compared to 9% in adults (Table 4). For PFUI in children the surgeon should be prepared for the possible need for a transpubic/abdominal approach. We perform a posterior pubectomy, leaving a rim of anterior pubic bone intact (Fig. 3A and E). Leaving a portion of the bone avoids gait complications and herniation that occur with iatrogenic complete disruption of the pelvic ring. Despite this minor adjustment to our practice, our nomenclature maintains the term ‘transpubic/abdominal’.
Table 4.
Success rates for the steps to give a tension-free anastomosis, categorised by age groups. In those aged ⩽12 years the rate of transpubic/abdominal urethroplasty was significantly higher than in adults.
| Variable | Success rate (%) at age (years) |
||
|---|---|---|---|
| ⩽12 | 13–18 | ⩾19 | |
| N patients | 29 | 49 | 230 |
| Overall success | 83 | 78 | 80 |
| Step | |||
| 1,2 | 14 | 16 | 24 |
| 3 | 52 | 63 | 65 |
| 4 | 3 | 6 | 2 |
| 5,6 | 31 | 14 | 9 |
P = 0.006 for step 5,6. Steps as in Table 1.
Figure 3.
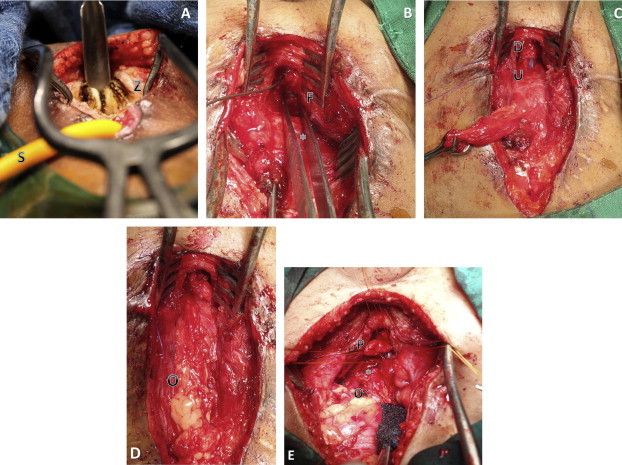
(A) A transabdominal view of a posterior pubectomy (Z, posterior pubic bone; S, the suprapubic catheter). (B) A transabdominal view of forceps highlighting the UVF (F) (∗, bladder). (C) The anastomosis of the proximal (U) and distal urethra (D), showing a Babcock clamp on the suprapubic tract. (D) The omental wrap (O) interposition. (E) The transpubic approach in a young boy (∗, bladder; O, omentum; P, prostate).
We could complete the anastomosis with the bulbar urethra and crural separation only 14% of the time, compared to 22% in adults (Table 4). The bulbar urethra has yet to develop the fibroelastic properties of the adult, and as such a simple bulbar mobilisation is unlikely to provide the length needed for a proper tension-free repair.
Double block PFUI
Some patients have a blockage at two levels, the BMU and BNP. Mundy and Andrich [20] described 15 patients with bladder neck injuries, of whom two had a (double-block) ‘sequestered prostate’, the ‘first transversely above the prostate combined with transverse rupture of the membranous urethra below the prostate causing a sequestered prostate’. With a complete double block the prostatic urethra is completely isolated from the bladder neck and bulbar region, and presents with a cystic lesion in the prostate (Fig. 4D–F). The cyst is the collection of seminal fluid in the isolated prostatic urethra. Preoperative counselling of the risks includes possible incontinence in such patients. The previous anatomical description explains why the injury is more common in children than adults.
Figure 4.
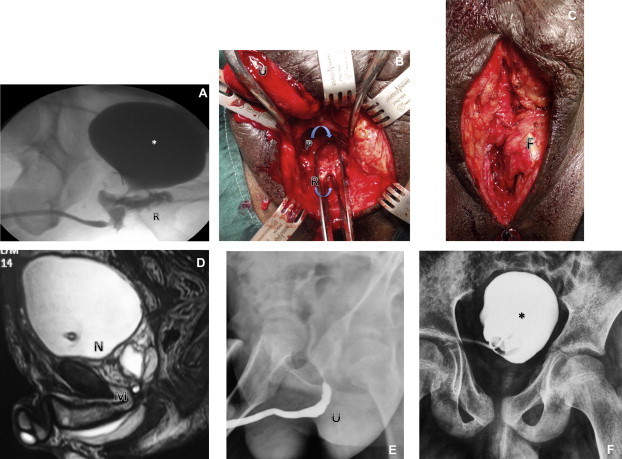
(A) VCUG and RUG showing a RUF (∗, bladder; R, RUF; U, urethra). (B) A RUF repair, with the bulbar (U) and top forceps in the prostatic urethra (P) and bottom forceps in the RUF (R). (C) Interposition of perineal fat (F). (D) MRI showing a block at the BNP junction (N) and bulbomembranous junction (M). (E) RUG showing a block at the bulbomembranous junction. (F) VCUG showing a block at the BNP junction.
We treated five patients with a double block, four of whom were children aged 5, 8, 11 and 12 years; one was an adult aged 36 years. All four children had been injured in a traffic accident, with a low-velocity crush injury to the pelvis. The man had fallen from a height.
These patients were treated with a progressive perineal approach followed by a transpubic/abdominal approach. The first step in the repair is a perineal incision, bulbar mobilisation and transection of the bulbar urethra at the level of scar. The second step is an abdominal incision and passage of a sound through the suprapubic site, and dissection of the obliterated anterior bladder neck. The scar around the bladder neck is excised. Once opened, a small-calibre cystoscope is passed into the anterior bladder neck and into the prostatic urethra to visualise the distal most membranous urethra. A bulbomembranous anastomosis is then made via the perineum. The BNP anastomosis is then made via the abdominal incision. All the children are currently continent and have a good flow rate, but have occasional nocturnal dribbling. The adult is incontinent and awaits insertion of an artificial urinary sphincter.
Girls aged ⩽12 years
PFUI in young girls is anecdotally reported as being rare, but the reports of an incidence of PFUI of 4–6% in women [21,22] are not dissimilar to the incidence of 10% of PFUI in men [23]. There is a suggestion of an increasing incidence in young girls compared to women. The diagnosis is missed on the initial assessment in up to 40% of patients, emphasising the need for a careful diagnostic evaluation [22]. In a review of 12 patients, Venn et al. [24] concluded that differing degrees of severity of pelvic trauma cause different types of urethral injury, but in general a more severe injury is needed to damage the female urethra than the male urethra.
In our centre, six girls (mean age 9 years, range 4–11), and with a mean follow-up of 56 (40–81) months, were treated for a PFUI. None of them were managed acutely in our centre, as all six were referred to our institute with a suprapubic catheter in situ. The cause was a road traffic accident in five patients and one was injured by a collapsed wall.
Antegrade cystoscopy is critical in assessing the bladder neck and detecting the presence of stones. Vaginoscopy is paramount for a proper evaluation of a UVF. Urethral transection was proximal in one and middle in five patients. An anastomotic urethroplasty was performed in all six patients. All patients required a transpubic/abdominal approach (Fig. 3B–D). Five patients had a concomitant UVF that was successfully closed at the time of urethroplasty. No patient had a rectal injury and all had daytime continence. Three girls have occasional nocturnal incontinence that might be attributed to a neurogenic injury to the bladder at the time of the accident. Four patients had a successful urethroplasty. One patient required a repeat transpubic/abdominal anastomotic urethroplasty, with no subsequent need for intervention. One patient with a distal injury developed vaginal stenosis and required a vaginotomy. She and her family were forewarned of the need for a repeat vaginotomy or pull-through vaginoplasty in the future.
Young girls require a transpubic/abdominal approach and proper evaluation at the time of the initial presentation. In a proximal injury a UVF is common, but can be treated successfully if recognised. A distal injury is associated with vaginal stenosis.
Patients with a RUF
Patients with a RUF are among most difficult cases to treat. The RUF is generally due to irradiation, after a radical prostatectomy, or to tuberculosis and malignancy of the rectum and prostate. A few reports cite a pelvic fracture as a rare cause of this urethral injury [25,26]. Among trauma patients, only 10% of RUF are associated with PFUI, and most RUFs in the trauma setting are associated with penetrating trauma [27]. The RUF can be a result of the initial injury or as a complication associated with an attempted repair. In a review of 573 patients with a pelvic fracture, Fu et al. [28] recognised an iatrogenic injury to the rectum in 5%. These were repaired primarily, with no fistula, but it highlights the risk of RUF in an unrecognised injury from a PFUI.
In our 308 patients, 10 (3%) presented with a RUF, of whom eight (2%) had a RUF due to the primary injury and two (0.6%) developed a RUF in association with the attempted repair of the urethra. The most common presentation for patients with a RUF is fecaluria or voiding through the rectum. Our approach is a three-stage method, with the first stage being a diverting colostomy (usually by the primary general surgeon). In the second stage, the anastomotic urethroplasty was performed with interposition of omentum in six patients and gracilis in two. In two patients with a small fistula, local perineal fat was used for interposition (Fig. 4A–C). In nine of the 10 patients the surgery was successful, with no recurrence or stricture. One patient required revision and was then lost to follow-up. In the third stage the colostomy was closed.
The principles of fistula repair for PFUI are similar to fistula repair for other causes, and include patency distal to the fistula, an adequate vascular supply, and non-overlapping suture lines. Interposition with omentum, fat, and gracilis also increases the success rate.
Bladder neck incontinence
There are three types of bladder neck condition after PFUI. The first is a normal anatomy and function. The second is a wide-open bladder neck at rest during cystography and antegrade cystoscopy (possibly due to neuropraxia). For those with an open bladder neck, anastomotic urethroplasty can be successful and patient might maintain continence [29]. The risk of incontinence requires appropriate patient education and preoperative counselling. The third condition is a tear-drop deformity, where there was trauma at the 12 o’clock (anterior) position of the bladder neck due to a splinter from a fractured pubic bone. Three of our patients had a tear-drop deformity, and we used a transpubic/abdominal approach with a median cystotomy and anatomical bladder-neck reconstruction. Two of the patients are continent.
In patients with a scar around the entire bladder neck, an anastomotic urethroplasty with excision of the scar and omentoplasty has not yielded favourable results. Such patients with incontinence should be considered for an artificial sphincter mechanism.
Conclusions
Successful surgery to repair a PFUI after repeat surgery requires adequate mobilisation of the bulbar urethra, excision of the scar, and can more often require inferior pubectomy.
Young boys aged ⩽12 years require a perineal abdominal approach with posterior pubectomy more often than in adults.
Young girls with a PFUI need a proper evaluation at presentation for concomitant injuries and might have an associated traumatic UVF. They require an abdominal approach for the repair.
BUN requires a substitution urethroplasty with vascularised flaps and tubes.
A double block is a complex PFUI with injury at the BMU and BNP, requiring two separate anastomoses to be made.
The repair of a RUF requires a three-staged approach with interposition of vascularised tissue.
Patients with a wide-open bladder neck on antegrade cystoscopy, perceived to lead to incontinence, still require an anastomotic urethroplasty for repair. Most maintain continence, and those who are incontinent might require subsequent bladder neck reconstruction or placement of an artificial urinary sphincter.
Conflict of interest
None.
Footnotes
Peer review under responsibility of Arab Association of Urology.
References
- 1.Turner-Warwick R. The use of the omental pedicle graft in urinary tract reconstruction. J Urol. 1976;116:341–347. doi: 10.1016/s0022-5347(17)58809-6. [DOI] [PubMed] [Google Scholar]
- 2.Webster G.D., Goldwasser B. Perineal transpubic repair: a technique for treating post-traumatic prostatomembranous urethral strictures. J Urol. 1986;135:278–279. doi: 10.1016/s0022-5347(17)45609-6. [DOI] [PubMed] [Google Scholar]
- 3.Koraitim M.M. The lessons of 145 posttraumatic posterior urethral strictures treated in 17 years. J Urol. 1995;153:63–66. doi: 10.1097/00005392-199501000-00024. [DOI] [PubMed] [Google Scholar]
- 4.Webster G.D., Ramon J., Kreder K.J. Salvage posterior urethroplasty after failed initial repair of pelvic fracture membranous urethral defects. J Urol. 1990;144:1370–1372. doi: 10.1016/s0022-5347(17)39744-6. [DOI] [PubMed] [Google Scholar]
- 5.Turner-Warwick R. Complex traumatic posterior urethral strictures. J Urol. 1977;118:564–574. doi: 10.1016/s0022-5347(17)58109-4. [DOI] [PubMed] [Google Scholar]
- 6.Koraitim M.M. Complex pelvic fracture urethral distraction defects revisited. Scand J Urol. 2014;48:84–89. doi: 10.3109/21681805.2013.817484. [DOI] [PubMed] [Google Scholar]
- 7.Da Silva E.A., Sampaio F.J. Urethral extensibility applied to reconstructive surgery. J Urol. 2002;167:2042–2045. doi: 10.1097/00005392-200205000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Kizer W.S., Armenakas N.A., Brandes S.B., Cavalcanti A.G., Santucci R.A., Morey A.F. Simplified reconstruction of posterior urethral disruption defects: limited role of supracrural rerouting. J Urol. 2007;177:1378–1381. doi: 10.1016/j.juro.2006.11.036. [DOI] [PubMed] [Google Scholar]
- 9.Krishnamoorthy V., Joshi P.B. Length of urethra in the Indian adult male population. Indian J Urol. 2012;28:297–299. doi: 10.4103/0970-1591.102706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohler T.S., Yadven M., Manvar A., Liu N., Monga M. The length of the male urethra. Int Braz J Urol. 2008;34:451–454. doi: 10.1590/s1677-55382008000400007. [DOI] [PubMed] [Google Scholar]
- 11.Barbagli G. History and evolution of transpubic urethroplasty: a lesson for young urologists in training. Eur Urol. 2007;52:1290–1292. doi: 10.1016/j.eururo.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Mundy A.R. Urethral trauma. Part I. Introduction, history, anatomy, pathology, assessment and emergency management. BJU Int. 2011;108:310–327. doi: 10.1111/j.1464-410X.2011.10339.x. [DOI] [PubMed] [Google Scholar]
- 13.Mundy A.R., Andrich D.E. Entero-urethroplasty for the salvage of bulbo-membranous stricture disease or trauma. BJU Int. 2010;105:1716–1720. doi: 10.1111/j.1464-410X.2009.09005.x. [DOI] [PubMed] [Google Scholar]
- 14.Erickson B.A., Breyer B.N., McAninch J.W. Single-stage segmental urethral replacement using combined ventral onlay fasciocutaneous flap with dorsal onlay buccal grafting for long segment strictures. BJU Int. 2012;109:1392–1396. doi: 10.1111/j.1464-410X.2011.10483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner-Warwick R. The Principles of Urethral Reconstruction. In: Rob C, Smith R, Scott McDougal W, editor, Rob and smith’s operative surgery, urology. Butterworths: London, 1986: p. 480–519.
- 16.Asopa H.S., Elhence I.P., Atri S.P., Bansal N.K. One stage correction of penile hypospadias using a foreskin tube. A preliminary report. Int Surg. 1971;55:435–440. [PubMed] [Google Scholar]
- 17.Flynn B.J., Delvecchio F.C., Webster G.D. Perineal repair of pelvic fracture urethral distraction defects: experience in 120 patients during the last 10 years. J Urol. 2003;170:1877–1880. doi: 10.1097/01.ju.0000091642.41368.f5. [DOI] [PubMed] [Google Scholar]
- 18.Boone T.B., Wilson W.T., Husmann D.A. Postpubertal genitourinary function following posterior urethral disruptions in children. J Urol. 1992;148:1232–1234. doi: 10.1016/s0022-5347(17)36869-6. [DOI] [PubMed] [Google Scholar]
- 19.Koraitim M.M. Posttraumatic posterior urethral strictures in children: a 20-year experience. J Urol. 1997;157:641–645. [PubMed] [Google Scholar]
- 20.Mundy A.R., Andrich D.E. Pelvic fracture-related injuries of the bladder neck and prostate: their nature, cause and management. BJU Int. 2010;105:1302–1308. doi: 10.1111/j.1464-410X.2009.08970.x. [DOI] [PubMed] [Google Scholar]
- 21.Perry M.O., Husmann D.A. Urethral injuries in female subjects following pelvic fractures. J Urol. 1992;147:139–143. doi: 10.1016/s0022-5347(17)37162-8. [DOI] [PubMed] [Google Scholar]
- 22.Black P.C., Miller E.A., Porter J.R., Wessells H. Urethral and bladder neck injury associated with pelvic fracture in 25 female patients. J Urol. 2006;175:2140–2144. doi: 10.1016/S0022-5347(06)00309-0. [DOI] [PubMed] [Google Scholar]
- 23.Jordan G.H., Virasoro R., Eltahawy E.A. Reconstruction and management of posterior urethral and straddle injuries of the urethra. Urol Clin North Am. 2006;33:97–109. doi: 10.1016/j.ucl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Venn S.N., Greenwell T.J., Mundy A.R. Pelvic fracture injuries of the female urethra. BJU Int. 1999;83:626–630. doi: 10.1046/j.1464-410x.1999.00001.x. [DOI] [PubMed] [Google Scholar]
- 25.Abdalla M.A. Posterior sagittal pararectal approach with rectal mobilization for repair of rectourethral fistula: an alternative approach. Urology. 2009;73:1110–1114. doi: 10.1016/j.urology.2008.11.061. [DOI] [PubMed] [Google Scholar]
- 26.Helmy T.E., Sarhan O.M., Dawaba M.E., Wadie B.S. Urethrorectal fistula repair in children: urologic perspective. J Trauma. 2010;69:1300–1303. doi: 10.1097/TA.0b013e3181e7934f. [DOI] [PubMed] [Google Scholar]
- 27.al-Ali M., Kashmoula D., Saoud I.J. Experience with 30 posttraumatic rectourethral fistulas. presentation of posterior transsphincteric anterior rectal wall advancement. J Urol. 1997;158:421–424. doi: 10.1016/s0022-5347(01)64493-8. [DOI] [PubMed] [Google Scholar]
- 28.Fu Q., Zhang J., Sa Y.L., Jin S.B., Xu Y.M. Recurrence and complications after transperineal bulboprostatic anastomosis for posterior urethral strictures resulting from pelvic fracture: a retrospective study from a urethral referral centre. BJU Int. 2013;112 doi: 10.1111/bju.12171. E358-63. [DOI] [PubMed] [Google Scholar]
- 29.Iselin C.E., Webster G.D. The significance of the open bladder neck associated with pelvic fracture urethral distraction defects. J Urol. 1999;162:347–351. [PubMed] [Google Scholar]


