Abstract
Asian Americans are one of the fastest-growing populations in the United States. A relatively large subset of this population carries a unique loss-of-function point mutation in aldehyde dehydrogenase 2 (ALDH2), ALDH2*2. Found in approximately 560 million people of East Asian descent, ALDH2*2 reduces enzymatic activity by approximately 60% to 80% in heterozygotes. Furthermore, this variant is associated with a higher risk for several diseases affecting many organ systems, including a particularly high incidence relative to the general population of esophageal cancer, myocardial infarction, and osteoporosis. In this review, we discuss the pathophysiology associated with the ALDH2*2 variant, describe why this variant needs to be considered when selecting drug treatments, and suggest a personalized medicine approach for Asian American carriers of this variant. We also discuss future clinical and translational perspectives regarding ALDH2*2 research.
Keywords: ALDH2, Asian, cancer, ischemia, alcohol, precision medicine, prediction in pharmacology
INTRODUCTION
Asian Americans represent 28% of all foreign-born people in the United States (1). By 2035, the US Census Bureau estimates the Asian American population will be close to 25 million (2). Hospital data for Asian Americans suggest their outcomes appear poorer compared to Caucasians; for example, Asian Americans have an increased risk for cardiac events during an inpatient stay (3). However, because in most clinical data the term Asian in a hospital chart describes any individual from the Asian subcontinent, it is hard for clinicians and scientists to evaluate these data meaningfully, as many ethnic populations and genetic backgrounds exist. One strategy could involve identifying Asian Americans by known genetic differences and using this information to examine specific outcomes and implement strategies for this genetic subpopulation to ultimately improve patient care. This is particularly relevant for common genetic variants.
The point mutation in aldehyde dehydrogenase 2 (ALDH2), identified as ALDH2*2, is the most frequent variant in humans and is present in 8% of the world’s population, or approximately 560 million people. The ALDH2*2 variant is more common than other highly studied human point mutations, including those leading to sickle cell anemia, cystic fibrosis, and glucose-6-phosphate dehydrogenase. ALDH2*2 has an identifiable clinical phenotype involving facial flushing and increased heart rate when alcohol is consumed. Importantly, ALDH2*2 affects human health beyond the response to alcohol and causes significant differences in outcome measures. Consequently, clinicians should also consider which pharmacological agents are chosen to treat Asian Americans with the ALDH2*2 variant.
In this review, we present findings regarding the possible clinical impact of the ALDH2*2 variant on the Asian American population. We include recommendations for clinicians and further potential directions of research regarding the impact of this variant on health.
WORLD AND UNITED STATES DISTRIBUTION
Genetic studies suggest the ALDH2*2 variant originated from a Han Chinese founder in central China and ultimately extended throughout many areas of East Asia. The Chinese Han-Changting population has the highest incidence of this variant, at 65% (4). Although the ALDH2*2 variant was initially genotyped in South American Indian tribes (5), a follow-up study refuted the conclusion that the variant occurs in this population (6). Thus, this variant is likely limited to those of East Asian descent.
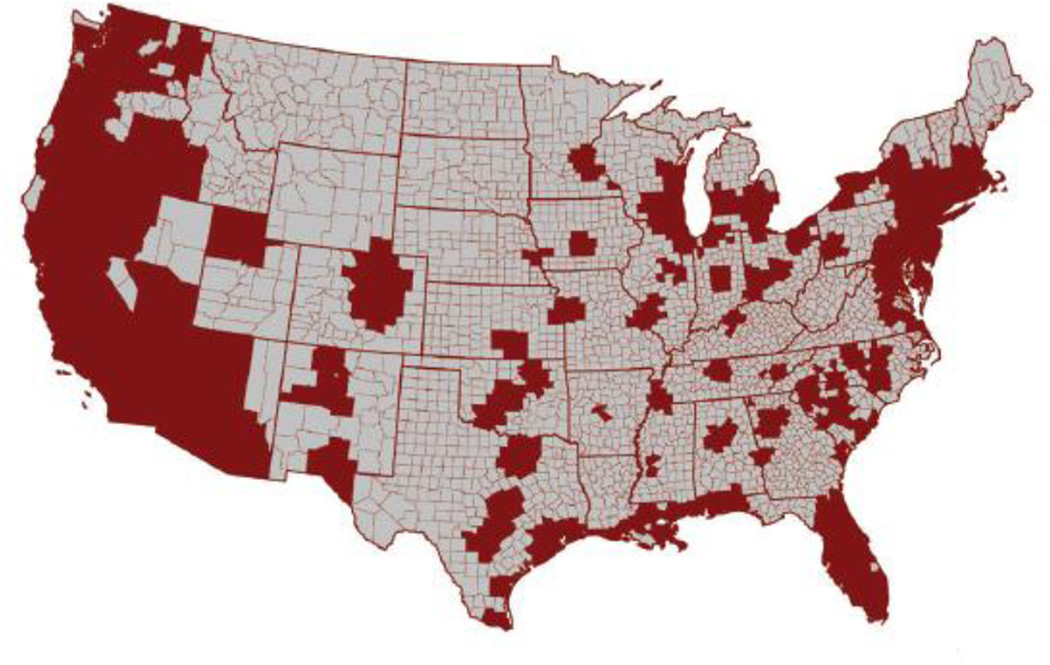
According to the Centers for Disease Control, Asian Americans reside mainly in California, New York, Texas, and Florida (Figure 1) (1). From 1820 to 1880, Chinese immigrants first arrived in the United States from the Guangdong province of China. Over the past century, Han Chinese have immigrated from Hong Kong, Taiwan, and mainland China, along with others immigrating from Japan, Korea, and Vietnam. ALDH2*2 variant incidence in these ethnicities ranged from 28% to 54% (7), and most of these immigrants settled in California (8). In the San Francisco Bay Area, the incidence of the ALDH2*2 variant among Chinese descendants is 48%, based on a sample population genotyped in 2009 (7).
Figure 1.
Asian American population distribution in the United States based upon Centers for Disease Control and Prevention census information collected from 2000 to 2006. These data were modified to estimate the geographic distribution of Asian Americans within the United States. Map and data are modified with permission from Reference 8a.
Other East Asian ethnicities in the United States may also carry the ALDH2*2 variant at a lower frequency, including the Hmong natives of Laos (4) and a small percentage of Filipino and Thai people (9). A small percentage of heterozygous ALDH2*2 genotypes has also been found in a Mongolian Chinese population that is not traditionally considered to possess the variant gene (7).
MOLECULAR BASIS FOR THE ALDH2*2 VARIANT
ALDH2, one of 19 ALDHs, is critical in catalyzing the oxidation of toxic cellular aldehydes. ALDH2 has the lowest Km for acetaldehyde at approximately 0.2 µM, making it the ALDH isozyme with the highest affinity for that substrate (10, 11). Located on chromosome 12q24, the ALDH2 gene codes for a 517–amino acid polypeptide. Through an N-terminal 17–amino acid targeting sequence, ALDH2 enters the mitochondrial matrix; after the targeting sequence is cleaved off, the mature 500–amino acid protein forms an active homotetramer (12).
Mitochondrial ALDH2 is better known for its role in detoxifying and removing acetaldehyde, a product of ethanol metabolism. However, ALDH2 also metabolizes other reactive aldehydes, including acrolein, malondialdehyde, and 4-hydroxynonenal (4-HNE). These aldehydes are either environmental pollutants or are products of polyunsaturated fatty acid oxidation, including arachidonic acid. Aldehydes are reactive moieties that cause damage by increasing cellular aldehydic load and altering cellular signaling, resulting in both DNA and protein damage by aldehydic adduct formation. The biochemical aspects of these insults on cellular functions have been reviewed extensively (13, 14).
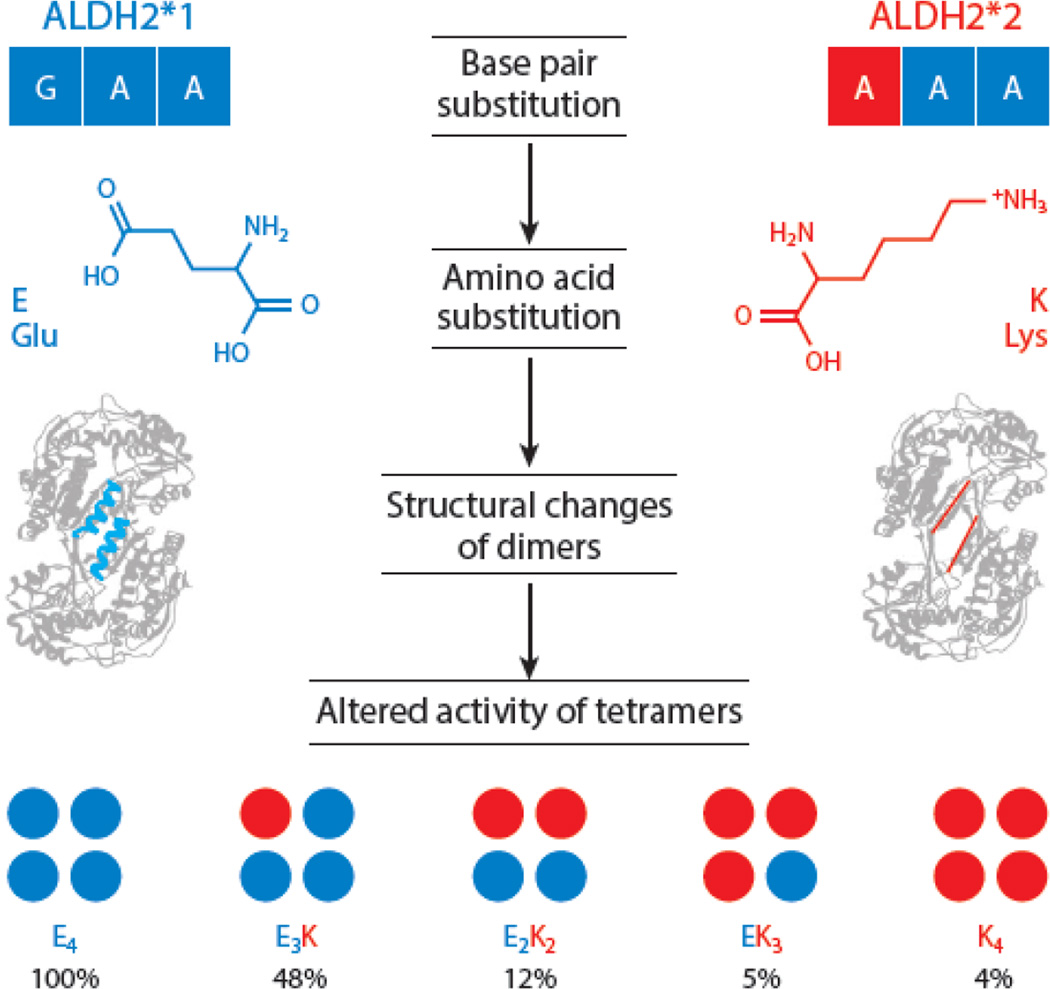
The ALDH2*2 point mutation occurs at nucleotide 1459, where an adenine is substituted for a guanine (rs671). The resultant change alters amino acid 487 of the mature protein, with lysine substituted for glutamic acid (15). This single amino acid substitution disorders an alpha helix structure, causing an allosteric disruption of the catalytic and coenzyme-binding sites. The widespread structural instability of the ALDH2*2 mutant results in reduced ALDH2 activity (16). When it forms a tetrameric complex with the wild-type ALDH2*1 enzyme, ALDH2*2 dominantly inhibits catalysis and increases ALDH2 enzyme turnover. Depending on the number of ALDH2*2 monomers present in a tetramer, the activity compared to an ALDH2*1 enzyme tetramer can be reduced from 48% to as little as 4% (Figure 2) (17). For heterozygous ALDH2*1/*2 individuals, the possible combinations reduce overall ALDH2 enzymatic activity by 60% to 80% compared to those of wild-type homozygous ALDH2*1/*1 individuals. Following decades of research involving this single point mutation and its widespread effects, many techniques have been developed to determine an individual’s ALDH2 genotype.
Figure 2.
The molecular basis for the ALDH2*2 variant. The ALDH2*2 mutation is a single point mutation of guanine (G) to adenine (A) at the first base pair of codon 487, resulting in an amino acid change from glutamic acid (Glu, E) to lysine (Lys, K). At physiological pH, glutamic acid is predominantly negatively charged, whereas lysine is largely positively charged. The mutation inhibits the normal formation of an integral alpha helix near the dimer-dimer interface of each monomer. If a tetramer contains just one K subunit, the activity of that tetramer drops by 52%. Enzymatic activity decreases substantially as the ratio of K monomers to normal E monomers increases, dropping to 4% in tetramers composed entirely of K monomers. The potential monomer combinations that occur in a heterozygote reduce enzymatic activity by 60% to 80% compared to the wild type.
SCREENING STRATEGIES FOR ALDH2*2
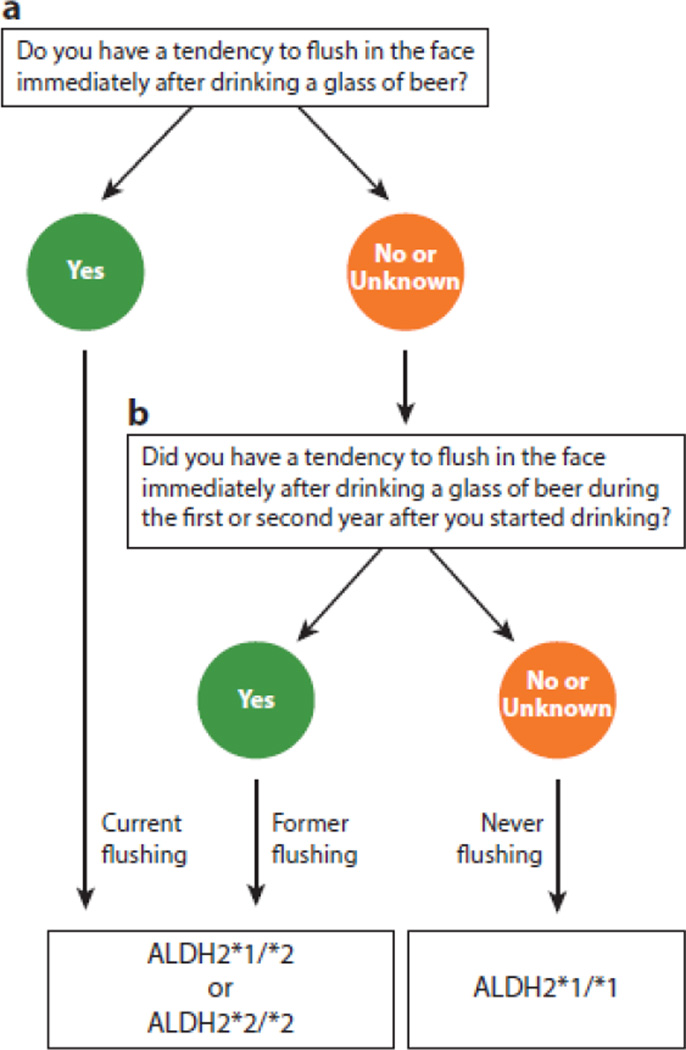
Testing for the ALDH2*2 genotype is quite feasible. A rapid genotyping method was recently described that can return results in less than 2 h at the cost of approximately $0.50 per test (18). Alternatively, a two-question screening tool predicted the ALDH2*2 variant with 90% sensitivity and 88% specificity in a general population of Japanese men (Figure 3) (19); this questionnaire could be used in locations with minimal genotyping resources. Screening by an ethanol patch test may be useful for specific pathologies such as alcohol-induced asthma (20). However, when compared to the questionnaire, the sensitivity and specificity of the ethanol patch test was much lower, and the test may perhaps be the least useful screening method for the ALDH2*2 variant (21).
Figure 3.
Questionnaire to predict ALDH2*2 genotype in Asian Americans. A two-step questionnaire predicted with 90% sensitivity and 88% specificity in East Asian Japanese cancer-free men whether a person was either homozygous for ALDH2*1 or heterozygous or homozygous for ALDH2*2. Current flushing and former flushing are indicative of people who are either homozygous or heterozygous for the ALDH2*2 variant (19).
IMPACT ON HUMAN HEALTH
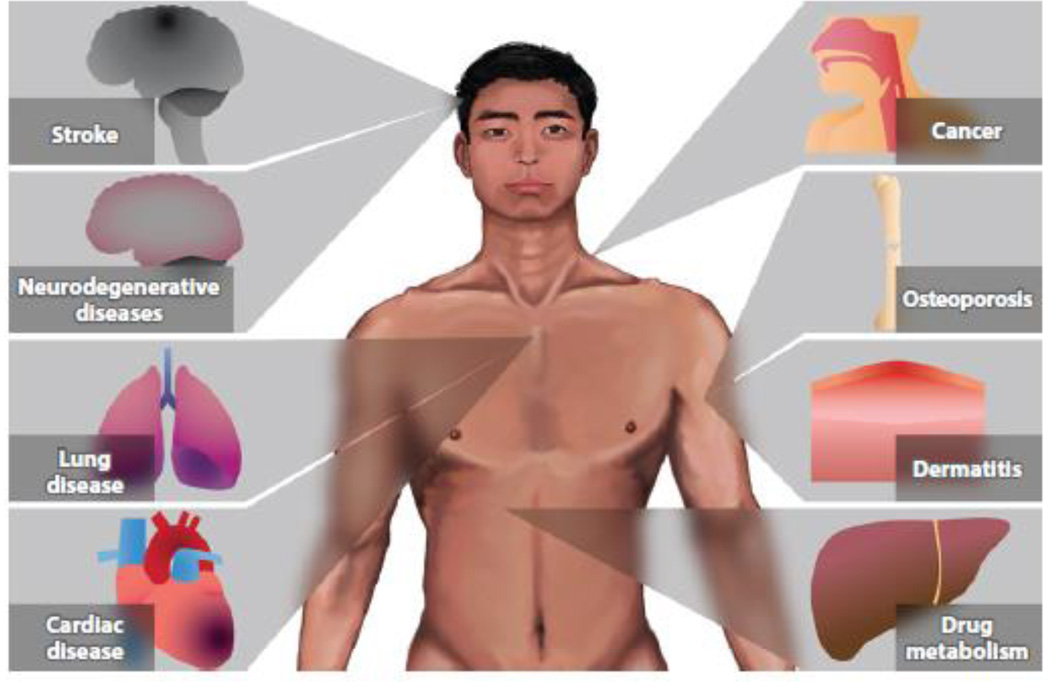
Although ALDH2*2 is classically known for its effect on limiting alcohol consumption and protection from developing alcoholism, this variant has only recently been recognized as having a major impact on human health and disease. Here we focus on clinical and translational studies that investigate what impact the ALDH2*2 variant may have on human health (Figure 4).
Figure 4.
Potential effects of the ALDH2*2 variant on human health. Patients with the ALDH2*2 mutation are at risk for a wide range of health concerns. In particular, clear associative risks with the ALDH2*2 variant exist for esophageal cancer, myocardial infarction, and osteoporosis.
Behavior
In addition to catalyzing acetaldehyde metabolism, ALDH2 converts aldehyde metabolites of amine neurotransmitters, including dopamine, norepinephrine, and serotonin, to less reactive forms (14). The ability of ALDH2 to affect behavior is believed to stem from this catalysis, as many behavioral deficits are linked to inefficient metabolism of aldehyde-derived neurotransmitter products. These include psychiatric disorders, addiction, cogitative disorders, Parkinson’s disease, and pain.
Psychiatric and personality disorders
Initial evidence suggests that ALDH2*2 may lead to a higher incidence of borderline personality and panic disorders among female alcoholics (22). Furthermore, higher novelty-seeking and lower harm-avoidance scores were noted among ALDH2*2 alcoholics compared to ALDH2*1 alcoholics (23). Heroin-dependent ALDH2*2 individuals also had higher risk-taking scores compared to controls with the same genotype (24). Another study suggested that ALDH2*2 may protect against developing bipolar disorder (25).
Addiction
After consuming alcohol, homozygotes and heterozygotes for the ALDH2*2 variant accumulate high acetaldehyde blood levels. Because of the subsequent unpleasant effects, alcoholism occurrence is reduced in ALDH2*2 individuals (26). A genome-wide association study (GWAS) of Japanese people found ALDH2 had the strongest association with drinking behavior (27). A similar association was also found in the Han Chinese population, with the association clustered around the ALDH2 gene location on chromosome 12q24 (28).
Chinese migration to San Francisco and New York in the early 1900s introduced opium and opium dens into Asian American and American culture. Interestingly, the tendency for Asians to use opium may be associated with the ALDH2*2 mutation. Genotyping of 250 heroin-dependent Han Chinese patients in Taiwan revealed that 60.8% were either ALDH2*1/*2 or ALDH2*2/*2, 10.2% higher than in population controls (29).
Cognitive disorders
Compared to wild-type mice, transgenic mice overexpressing ALDH2*2 exhibit increased neurodegeneration and impaired visual recognition memory and task learning, with age-dependent exacerbation of these behavioral deficits (30). Thus, human ALDH2*2 carriers may have a higher risk of cognitive impairment and decline, dementia, and Alzheimer’s disease by accumulating aldehyde-induced damage to brain cells.
Most initial studies in humans focused on how the ALDH2*2 variant affects Alzheimer’s disease. An initial case-control study in Japan suggested people with an ALDH2*1/*2 or ALDH2*2/*2 genotype had a greater tendency to develop late-onset Alzheimer’s disease (31). These findings were supported by a follow-up case-control study of Chinese patients (32). However, a Korean study failed to show an association between ALDH2*2 and Alzheimer’s disease (33). The conflicting findings suggest additional genetic differences in apolipoprotein E or environmental exposures to neurotoxins, for example, may contribute to the phenotype associated with ALDH2*2.
Parkinson’s disease
ALDH1A1 and ALDH2 are the ALDH enzymes most highly expressed in the substantia nigra. An ALDH1A1 and ALDH2 double knockout mouse exhibited a Parkinson’s-like phenotype, suggesting the importance of ALDH enzymes in protecting neurons in the substantia nigra from cellular damage (34). Furthermore, a recent clinical investigation using patients enrolled in the Parkinson’s, Genes & Environment Study showed that exposure to pesticides that inhibit ALDH increase Parkinson’s disease risk by 2- to 6-fold (35). Although rs671 single nucleotide polymorphism (SNP) incidence was too low to study, the haplotypes that increased Parkinson’s disease risk 2–5-fold in patients who had been exposed to pesticides all possessed an ALDH2 minor allele variant rs737280 (35) that reduces ALDH2 enzymatic activity (36).
Pain
Asians are more responsive to painful stimuli compared to other ethnicities (37–39). Exogenous administration of acetaldehyde or 4-HNE in rodents produces pain-like behavior (40, 41). Thus, a deficiency in ALDH2 enzymatic activity could be a possible genetic basis for the increased pain sensitivity seen in Asians.
Recently, our group discovered that ALDH2*1/*2 knock-in mice, which have 20% of the ALDH2 activity compared to wild-type littermates, exhibited a more profound response when subjected to acute inflammatory pain models. Nociception was tightly correlated with ALDH2 activity at the site of insult (R2 = 0.90). ALDH2*2/*1 knock-in mice also accumulated more toxic aldehydes such as 4-HNE compared to wild-type mice. Although further studies are needed in humans, these rodent studies provide evidence that the ALDH2*2 variant contributes to nociception, providing a potential genetic basis for the differences in pain behavior observed in humans.
Endocrine Disease
Evidence suggests ALDH2 also impacts endocrine diseases including diabetes and osteoporosis. These findings are described below.
Diabetes
The ALDH2*2 variant may modify blood glucose control, affecting the development of noninsulin-dependent diabetes mellitus. Individuals with the ALDH2*2 variant who consume alcohol have higher hemoglobin A1C values compared to those with the ALDH2*1/*1 enzyme (42). In Han Chinese patients with coronary artery disease, the ALDH2*2 variant is a potential risk factor for diabetes in females (43).
Osteoporosis
Among a cohort of Japanese, a genetic screen identified ALDH2*2 as being associated with an increased osteoporosis risk (44). People homozygous for ALDH2*2 had a higher morbidity rate, with a 3.33 odds ratio, compared with ALDH2*1 individuals. Females with osteoporosis had an even higher morbidity rate, with a 4.31 odds ratio.
Cardiovascular Disease
As discussed below, an increased incidence of myocardial infarction and coronary artery disease is strongly associated with the ALDH2*2 variant. Recent research has suggested associations between the enzyme and stroke.
Cardiac ischemia
A Japanese study of 342 men with a myocardial infarction and 1,820 cardiac disease–free men identified a 1.56 higher odds ratio for myocardial infarction in those homozygous for the ALDH2*2 variant (45). This finding was further supported by studies in Korean and Han Chinese populations (46, 47). A GWAS in Japanese men also found the ALDH2*2 variant increased the risk of coronary artery disease and myocardial infarction (48). A recent meta-analysis of nine case-control studies further supported the hypothesis that the ALDH2*2 variant increases patients’ odds ratios for coronary artery disease and myocardial infarction (49).
Remote ischemic conditioning, consisting of brief ischemic periods followed by reperfusion, reduces both cardiac troponin release and all-cause mortality after cardiac bypass surgery (50). Although the remote conditioning mechanism is not entirely clear, remote pharmacological activation of epsilon protein kinase C, an upstream mediator ALDH2 activator (51), can reduce myocardial infarct size by remote conditioning in rodents (52). Thus, the ALDH2*2 variant, with reduced enzymatic activity, may mitigate the remote ischemic conditioning benefits during cardiac bypass surgery. People with the ALDH2*2 variant showed reductions in forearm blood flow response to acetylcholine after remote preconditioning followed by ischemia-reperfusion that were less than those seen in people with the ALDH2*1/*1 genotype (53).
Unexpectedly, a prospective cohort study of ALDH2*2-variant Han Chinese children 6–17 months old undergoing cardiac bypass surgery for tetralogy of Fallot repair had less troponin release, needed less inotropic support, and had shorter hospital stays compared to ALDH2*1 children. Biopsies of myocardial tissue in children with cyanotic heart disease, including tetralogy of Fallot, transposition of the great arteries, and double outlet right ventricle, showed less ALDH2 activity for either the wild type or ALDH2*2 variant compared to noncyanotic controls. The ALDH2*2 variant may require compensatory measures to improve outcomes when cyanosis is present at birth (54).
Stroke
One animal model commonly used in stroke research is the stroke-prone spontaneously hypertensive rat. Interestingly, these rodents have approximately 30% less ALDH2 protein expression compared to the spontaneously hypertensive rat strain (55). Furthermore, in rodent models of stroke, increasing ALDH2 activity by the small molecule Alda-1 improved reactive aldehyde clearance and reduced cerebral infarct size (55, 56). These findings suggest ALDH2 is an important mediator in reducing cellular damage from a stroke.
In a Finnish population study, investigators noted a 2.33-fold increased risk of stroke for men consuming alcohol who reported more than one hangover annually (57). Because people with the ALDH2*2 variant limit or abstain from alcohol use because of the side effects such as facial flushing and increased heart rate, the variant may protect them against alcohol-induced stroke. A study in Japanese men reported those with the ALDH2*2 variant had fewer multiple lacunar infarcts compared to ALDH2*1 individuals (58). The ALDH2*2 variant also associates with other factors that may contribute to a stroke, including hypertension and alcohol-induced increases in triglyceride levels (59, 60). Interestingly, a case report demonstrated two heterozygous ALDH2*2 individuals had a stroke hours after excessive alcohol consumption, perhaps because of rapid aldehyde accumulation (61). Further supporting this hypothesis, a study of 51 ischemic stroke patients showed elevated plasma 4-HNE levels when measured at either 3 days or 6 months after a stroke compared to 30 matched healthy patients (55).
Lung Disease
Many inhaled aldehydes, including perfumes and pollutants from smoke, smog, and chemicals, are detoxified by ALDH2 present in the lung. ALDH2 is also important in stem cell–induced self-renewal of epithelium in human airways (62). As discussed below, the ALDH2*2 variant may also influence outcomes related to reactive airway disease and lung cancer.
Reactive airway disease
ALDH2 likely influences asthma and chronic obstructive pulmonary disease development or disease progression because aldehydes such as malondialdehyde and acrolein are significantly higher in exhaled breath and sputum compared to healthy nonsmokers (63). Compared to the Asian ALDH2*1/*1 genotype, Asian patients with an ALDH2*2 variant have a greater tendency to trigger an asthma exacerbation after alcohol consumption. Moreover, when alcohol triggered an asthma reaction, blood acetaldehyde levels were twice as high compared to when alcohol did not trigger asthma (64). This suggests that alcohol consumption and the resulting higher levels of acetaldehyde in Asian Americans with ALDH2*2 may be an unrecognized trigger for asthma attacks in patients. Furthermore, researchers have yet to determine whether ALDH2*2 is an independent risk factor for asthma regardless of alcohol consumption.
Lung cancer
The risk of lung cancer may be directly linked to environmental exposures to aldehydes, including acetaldehyde and acrolein derived from cigarette smoke. A case-control study of 718 patients with lung cancer in Japan identified an association between a history of smoking more than 15 packs per year and a higher risk of lung cancer for those with the homozygous ALDH2*2 mutation compared to patients with the ALDH2*1/*1 genotype (65). Furthermore, 9.75% of the patients were homozygous for ALDH2*2, which exceeds the prevalence of the homozygous ALDH2*2 variant at 4.20% for the study’s general population (7).
Oral and Gastrointestinal Cancer
Acetaldehyde is recognized by the International Agency for Research on Cancer as a known Group I human carcinogen (66). People with the ALDH2*2 variant who are exposed to both alcohol and cigarettes are at the highest risk for developing cancer (67). Interestingly, the reported activities of ALDH2 in the digestive tract show the lowest activity at the esophagus and the highest change in the ratio of alcohol dehydrogenase (ADH) to ALDH2 activity (Table 1). The activity ratios may suggest why patients with the ALDH2*2 variant have a clear risk for esophageal cancer.
Table 1.
ALDH2 activity reported in various human gastrointestinal tissuesa
| Tissue | ALDH2 activity (milliunit/g human tissue) |
Calculated ADH:ALDH2*1/*1 activity ratio |
Estimated ADH:ALDH2*1/*2 activity ratio |
Estimated ADH:ALDH2*2/*2 activity ratio |
|---|---|---|---|---|
| Esophagusb | 29.9 | 20.0 | 50.6 | 505.9 |
| Stomach | 132.0 | 1.8 | 4.5 | 45.1 |
| Pancreas | 213.0 | 0.3 | 0.8 | 7.5 |
| Liver | 1060.0 | 2.7 | 6.8 | 68.4 |
| Colon | 40.2 | 4.6 | 11.4 | 113.8 |
| Rectum | 41.8 | 7.3 | 18.2 | 182.4 |
Abbreviations: ADH, alcohol dehydrogenase; ALDH2, aldehyde dehydrogenase 2.
The table is based on values presented in Reference 76. ADH:ALDH2*1/*2 ratios were calculated assuming a 60% reduction in ALDH2 activity compared to ALDH2*1 and a 96% reduction in ALDH2*2/*2 activity.
The lowest activity for ALDH2 and highest activity ratio for ADH:ALDH2 were found in the esophagus, suggesting acetaldehyde buildup in the digestive tract is highest in the esophagus.
Upper digestive tract
An initial study in alcoholic Japanese males showed the ALDH2*2 genotype was 4.1 times more frequent in the esophageal cancer group compared to the alcoholic males in the study without esophageal cancer. Additionally, although these data are from a small study, the ALDH2*2 heterozygotes had an odds ratio of 7.6 in alcoholics and 12.1 in nonalcoholics to develop esophageal cancer (68). The effect is also only specific to East Asians, as a recent Dutch Caucasian patient study showed no association between the Caucasian ALDH2*1/*1 and cancer (69).
In 2011, a meta-analysis of four cohort studies and nine case-control studies confirmed a strong association between alcohol consumption and increased esophageal cancer risk in the ALDH2*2 population (70). For East Asians, four factors increase esophageal cancer risk: (a) a history of smoking cigarettes, (b) a history of drinking alcohol, (c) the presence of the ALDH2*2 mutation, or (d) the presence of a mutation in alcohol dehydrogenase (ADH1B, rs1229984) that causes a rapid conversion of alcohol to acetaldehyde. When all four risk factors are present, the odds ratio of developing esophageal cancer is a staggering 189 (67).
Gastrointestinal and associated organs
Unlike the clear association with esophageal cancer, conflicting results have made it difficult to determine if an association exists between ALDH2*2 and other gastrointestinal cancers. Stomach cancer development among individuals with the ALDH2*2 variant may depend highly on smoking and drinking habits (71, 72). For pancreatic cancers, ALDH2*2 males—but not females— have a higher risk compared to case controls (73). Another study suggested that both alcohol and the ALDH2*2 variant may contribute to an increased risk for pancreatic cancer (74). No association between liver cancer and ALDH2*2 alone was initially reported (68); however, a risk may be associated with hepatitis C infection (75). Conflicting results have also been found for colon cancer (76, 77).
Fanconi Anemia
Acetaldehyde-induced DNA damage in Fanconi anemia animal models, particularly in the hematopoietic system, was recently described (78, 79) and reviewed (14). In 2013, the association between the ALDH2*2 genotype and Fanconi anemia symptom severity was examined in 64 Japanese patients. Heterozygotes or homozygotes for ALDH2*2 with Fanconi anemia developed bone marrow failure more quickly and had a higher frequency of kidney, cardiovascular, and skeletal malformations (80).
Dermatitis
Case reports describe dermatitis or skin irritation after alcohol application for people of Asian descent, with a possible link to the ALDH2*2 variant (81, 82). Whether ethanol exposure causes contact dermatitis, particularly for homozygous ALDH2*2 individuals, is important to consider. In addition to a potential for alcohol-induced dermatitis, repeated ethanol hand washing can also raise peak blood acetaldehyde levels to 0.5 mg/L 30 min after multiple ethanol hand washes (in individuals presumed to be ALDH2*1/*1, although no genotyping was performed) (83). Researchers should also consider how hand washing with ethanol affects blood acetaldehyde levels, particularly in ALDH2*2/*2 individuals.
Furthermore, radiation dermatitis appears in approximately 85% of patients receiving radiation therapy for cancer treatment (84). Research on an animal model demonstrated that increasing ALDH2 activity with a selective ALDH2 activator, Alda-1, significantly reduced radiation dermatitis (85).
DRUG CONSIDERATIONS FOR PEOPLE WITH THE ALDH2*2 VARIANT
An excellent review regarding ALDH inhibitors was recently published (86). As discussed below, ALDH2 contributes a critical role in the metabolism of nitroglycerin, acyclovir, and 5-nitrofuran. Many natural products, environmental exposures, and perhaps acetaminophen also alter ALDH2 activity. The ALDH2 enzyme may also determine methotrexate toxicity (87). Here we discuss why the ALDH2*2 variant should be considered when selecting drug treatments.
Nitroglycerin
ALDH2 converts glyceryl trinitrate (nitroglycerin) to nitric oxide through an esterase-mediated mechanism requiring Cys302 at the enzymatic catalytic core (88, 89). Subjects with an ALDH2*1/*2 genotype compared to the ALDH2*1/*1 genotype had 33% less vasodilation, as assessed by forearm blood flow, when nitroglycerin was infused at 1 µg/min (90). The human ALDH2*2 variant has a catalytic efficiency 10-fold less for nitroglycerin compared to wild-type ALDH2 (91). ALDH2 also contributes to the nitroglycerin tolerance mechanism (92).
Nitroglycerin is commonly used intraoperatively for vasodilation and during an acute myocardial infarction. During an acute hypertensive stroke, the England-based Rapid Intervention with Glyceryl trinitrate in Hypertensive Stroke (RIGHT) clinical trial showed improved functional outcome when a nitroglycerin infusion was given to reduce blood pressure en route to the hospital (93). However, researchers need to examine whether nitroglycerin administration is beneficial during ischemia for patients with the ALDH2*2 variant; it may be detrimental, as shown by a rodent myocardial ischemia study (94). The conversion of either isosorbide mononitrate or isosorbide dinitrate to nitric oxide does not appear to require ALDH2 (95), providing an alternative treatment to nitroglycerin in ALDH2-deficient subjects.
Acyclovir
Acyclovir is primarily eliminated by renal excretion. However, approximately 8.5–14.1% of the drug is metabolized by ADH to acyclovir aldehyde, which in turn is metabolized through ALDH to 9-carboxymethoxymethylguanine (CMMG) (96, 97). With impaired renal function, acyclovir renal elimination is limited, and the drug is primarily metabolized. When 1,000 mg of the acyclovir prodrug valaciclovir was given orally in Japanese patients with end-stage renal disease, the acyclovir half-life was significantly altered by the ALDH2 genotype; patients with an ALDH2*2/*2 genotype had a prolonged half-life 8.6 h longer on average than those with an ALDH2*1/*1 genotype (26.7 h versus 18.1 h). These data suggest that metabolism of acyclovir and valaciclovir is mediated by ALDH2 (98).
5-Nitrofuran
Nifurtimox, or 5-nitrofuran, is a treatment used for Chagas disease and African sleeping sickness to cause trypanosome-induced cellular death. However, 5-nitrofuran has considerable human toxicity (99). Interestingly, 5-nitrofuran directly binds to and dose-dependently inhibits human ALDH2 activity, suggesting 5-nitrofuran prodrug activation depends on ALDH2 in humans (100). Zebra fish and yeast model systems have also been used to show ALDH2 inhibition reduces 5-nitrofuran toxicity (100). Because 5-nitrofuran is being investigated for treating medulloblastoma and neuroblastoma (101), further studies will be needed to investigate whether this drug is effective in treating these diseases in patients with the ALDH2*2 variant.
Acetaminophen
Acetaminophen is the most commonly used antipyretic and analgesic in the world. When metabolized, acetaminophen produces several metabolites, including the electrophile N-acetyl-p-benzoquinonimine (NAPQI), which is hepatotoxic when acetaminophen is used in excess. The acetaminophen interaction with ALDH2 was identified by peptide sequencing using an affinity-purified pull-down assay (102). More recently, acetaminophen (0.5 mM) decreased ALDH2 enzymatic activity in a dose-dependent manner from 0.2 to 1.0 mM (from 8.3% to 31%) (103). ALDH2 activity was reduced in mice by 75% 1 h after acetaminophen administration (350 mg/kg intraperitoneal), an inhibition that persisted for 4 h after drug administration (104). This leads to the question of whether acetaminophen administration may be detrimental to preserving tissue following ischemic injury, particularly in patients with the ALDH2*2 variant.
Natural Products
Many natural products affect ALDH2-mediated enzymatic activity. Products including citral, present in lemon and lime; daidzin, found in the kudzu plant (Japanese arrowroot); gossypol; areca nuts; and betel nuts and leaves have been reported to alter ALDH2 enzymatic activity to varying degrees (42).
Environmental Exposures
Aldehydes such as acrolein and acetaldehyde are present in frequently encountered environmental toxins, including cigarette smoke, pesticides, biofuels, and exhaust from motorized vehicles. ALDH2 has the highest catalytic efficiency for acetaldehyde, acrolein, and 4-HNE compared to other ALDH enzymes (105). For individuals with the ALDH2*2 variant, exposure to environmental aldehydes could lead to a higher risk of developing various pathologies relative to wild-type individuals.
Pesticides
Recently, 26 pesticides were screened for their effects on ALDH enzyme activity using substantia nigra neurons from newborn rats (35). Five pesticides—captan, folpet, thiram, ziram, and benomyl—resulted in at least a 15% reduction in ALDH activity compared to baseline values (35). Although the inhibition of ALDH activity and not specific ALDH2 activity was measured, potentially underestimating the pesticide inhibitory effect, data regarding benomyl support an irreversible ALDH2 inhibition in mice (106).
Solvents
Many glycol ether–based solvents are commonly used in items such as paints, cosmetics, sunscreens, and dyes. Most glycol compounds, once ingested, absorbed, or inhaled, convert ether to an acetaldehyde. Glycol ether exposure may reduce sperm count in men (107). Knockout ALDH2 mice are more protected from ethylene glycol monoethyl ether–induced sperm toxicity compared to wild-type mice (108). Thus, the ALDH2*2 variant may protect against the effects of some types of organic solvents.
However, chronic exposure to ethyl tertiary-butyl ether (ETBE), a common gasoline additive, may be more damaging for people with the ALDH2*2 variant. Because metabolism by cytochrome P450 enzymes oxidizes ETBE to tertiary-butyl alcohol and acetaldehyde, chronic exposure may lead to acetaldehyde accumulation. In an ALDH2*2 knockout mouse, chronic exposure to ETBE resulted in lower sperm motility and more frequent DNA damage compared to wild-type mice (109). These data suggest chronic exposure to certain organic solvents may lead to pathophysiology related to acetaldehyde accumulation. Additionally, methyl tertiary-butyl ether and tertiary-amyl methyl ether are both oxidized to an alcohol and formaldehyde.
A CLINICAL AND TRANSLATIONAL VISION
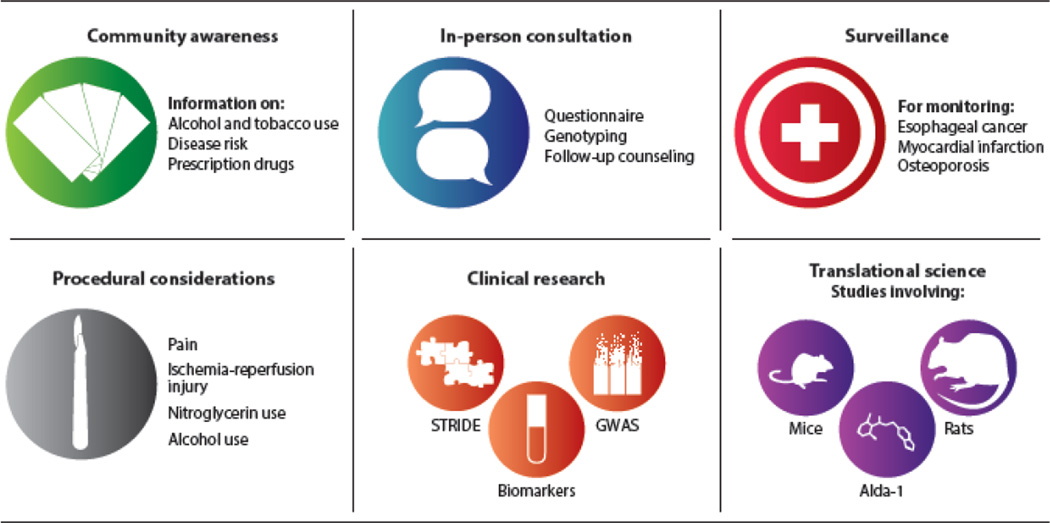
Clearly, the ALDH2*2 variant is not benign. Considering the increasing number of Asian Americans of East Asian descent in the United States, implementing a personalized approach to medicine based on an ALDH2*2 genotype will improve health by categorizing potential disease risk and medication efficacy. A plan consisting of preventative care through education and counseling, medical surveillance and management, and translation of basic and clinical research into new practices may improve outcomes for this large population (Figure 5).
Figure 5.
A personalized medicine plan for Asian Americans with the ALDH2*2 variant. The goal is to create a focus on preventative care, surveillance, and management while incorporating a clinical-translational approach for further research. Preventative care should focus on raising community and physician awareness in addition to implementing one-to-one patient counseling. Surveillance measures such as screening for high-risk diseases should occur, and awareness when physicians perform surgical procedures for those with the ALDH2*2 variant should be stressed. Additional scientific studies are needed that use translational research tools with platforms like the Stanford Translational Research Integrated Database Environment (STRIDE), biomarkers, and additional genome-wide association studies (GWAS), particularly for Asian Americans. In addition, translational basic science studies are needed in relevant models such as the ALDH2*2 knock-in mouse and how chemical compounds, including Alda-1, mechanistically modify disease.
Preventative Care
Measures to reduce environmental exposure risk for the ALDH2*2 population should involve education of the community and healthcare providers. Community awareness can be created through outreach programs by distributing information through medical websites, social media, and brochures in many languages. Physician education is also needed to effectively relay information to patients and adapt treatments to suit patients with reduced ALDH2 activity.
Physicians can use the two-part questionnaire to generate a discussion regarding the ALDH2*2 variant during an annual physical exam (Figure 3). If the ALDH2*2 variant is suspected, genotyping and counseling should be offered. Physicians can then identify individuals with the ALDH2*2 variant, particularly among low-incidence ethnicities in which the variant is less known. For patients with the ALDH2*2 variant, physicians should conduct a more detailed family history of diseases commonly associated with the variant. Medications that may interact with the ALDH2*2 variant can be reviewed and changed if possible. Physicians should also consider initiating a discussion regarding alcohol-based hand sanitizers, sunscreen, and mouthwash (81). The physical exam should also focus in particular on identifying specific diseases that are more common among those with the ALDH2*2 variant.
Surveillance and Management
Clinicians should outline guidelines for monitoring ALDH2*2 patients for disease development, particularly for esophageal cancer, coronary artery disease, and osteoporosis. For example, esophagogastroduodenoscopy should be used routinely for esophageal cancer screening, particularly for patients with additional risk factors. These screening recommendations could mirror the present guidelines established for colon cancer screening, which increases surveillance measures once a positive family history is identified (110).
For surgical or procedural interventions, genotyping Asian Americans for the ALDH2*2 variant may eventually become standard of care. Because nitroglycerin is often emergently used intraoperatively to treat myocardial ischemia, for uterine relaxation after delivery of a child, and to reduce blood pressure after a hypertensive stroke, the potential efficacy should be determined before the treatment is administered. Patients also receive alcohol injections during procedures such as septal ablation for hypertrophic obstructive cardiomyopathy, embolization surgeries, and neurolysis for pain control. The acetaldehyde syndrome previously described for patients with ALDH2*2 undergoing a celiac plexus block with alcohol (111) highlights the importance of identifying patients at risk for this response.
A Clinical-Translational Approach
A seamless clinical and translational effort, housed under one center, could maximize the health and wellness of the Asian American population by joining together clinicians and scientists who share a common interest in improving Asian American health. Animal models with specific variants seen in the Asian American population, such as the transgenic knock-in mouse for ALDH2*2 our group has created (14), can be used to test observations witnessed in the clinic. Additionally, the small molecule Alda-1, which creates a molecular patch restoring enzymatic activity to the ALDH2*2 enzyme, could reverse many of the effects responsible for pathophysiology in the ALDH2*2 population (51). Agents such as Alda-1 could be used to both prevent disease and modify existing disease in subjects with ALDH2*2.
Additional clinical treatments and biomarkers are also needed. Preventative treatments, such as using cysteine tablets to scavenge aldehydes and potentially reduce aldehydic load in the oral cavity and saliva, require further clinical study (112). The development of sensitive biomarkers, such as for breath testing (113) or erythrocyte aldehydic adduct formation (114), may function to further stratify disease and assess disease-modifying therapeutics. Defining, identifying, and treating unique genetic polymorphisms such as ALDH2*2 that exist at high frequencies among Asian Americans will ultimately improve the care and health of this population.
SUMMARY POINTS.
Asian Americans are one of the fastest-growing populations in the United States and will consist of 25 million people by 2035. However, their diverse ethnic populations and genetic backgrounds make it difficult to implement a personalized medicine treatment strategy.
Approximately 560 million people in the world of East Asian descent have a genetic variant in ALDH2 called ALDH2*2 that reduces enzymatic activity. This phenotype is commonly identified as Asian flush after alcohol consumption.
People with the ALDH2*2 genetic variant clearly have a higher associative risk for diseases such as esophageal cancer, coronary artery disease, myocardial infarction, and osteoporosis. Other health problems, including addiction, Alzheimer’s disease, pain, diabetes, stroke, Fanconi anemia, and dermatitis, may also be associated with the variant.
The ALDH2*2 variant causes altered responses to commonly used drugs, including nitroglycerin, acyclovir, and 5-nitrofuran. Physicians may need to reconsider drug selections for this particular patient population.
An ALDH2 activator, Alda-1, improves outcomes in animal models of myocardial infarction, stroke, radiation dermatitis, and pain. Alda-1 also creates a molecular patch restoring enzymatic activity to the ALDH2*2 enzyme and has tremendous therapeutic potential.
A personalized medicine approach based on genotyping Asian Americans for the ALDH2*2 genetic variant should be implemented and will ultimately improve the care and health of this large population.
FUTURE ISSUES.
As many behavioral associations exist for the ALDH2*2 variant, how does ALDH2*2 affect human behavior?
What role does the ALDH2*2 variant play in cardiac disease, particularly regarding remote conditioning and in general outcomes after cardiac bypass surgery and myocardial infarction in adults and children?
How do blood acetaldehyde levels change, and what dermatological reactions occur, in homozygous ALDH2*2 individuals after using ethanol-based hand sanitizers or mouthwash?
Is nitroglycerin use during acute ischemic events such as myocardial infarction or stroke less effective or detrimental for patients with the ALDH2*2 variant?
Is acute renal toxicity associated with acyclovir in ALDH2*2-variant patients with normal renal function?
Given the common use of acetaminophen, does this drug alter the activity of the ALDH2*2 variant? Do the variant’s structural differences alter the drug’s ability to inhibit ALDH2?
How does environmental exposure to aldehydes such as pesticides, solvents, cigarette smoke, and air pollution alter the risk of developing or accelerating disease processes in people with the ALDH2*2 variant?
What biomarkers and surveillance measures can be developed to successfully monitor aldehydic load to prevent, identify, and monitor treatment strategies for diseases caused by aldehyde accumulation?
ACKNOWLEDGMENTS
E.R.G., V.O.Z., and J.C.B.F. received fellowship support from HL-109212, FAPESP 2012/05035-4, and 20112/05765-2, respectively. All additional work from this manuscript was supported by a National Institutes of Health (NIH) MERIT Award AA11147 to D.M-R.
Terms and Definitions
- ALDH2
aldehyde dehydrogenase 2
- ALDH2*2
the East Asian variant of ALDH2, resulting from a base pair substitution of guanine to adenine
- ALDH2*1/*1
a homozygous genotype for ALDH2 that does not possess the East Asian variant
- ALDH2*1/*2
a heterozygous genotype for the East Asian variant of ALDH2 that reduces enzymatic activity of the enzyme by approximately 60% to 80%
- ALDH2*2/*2
a homozygous genotype for the East Asian variant of ALDH2 that reduces ALDH2 activity to approximately 4% compared to wild type
- rs671
SNP for the ALDH2 East Asian variant; can be homozygous (A;A) or heterozygous (G;A) for the variant or may not possess it (G;G)
- ADH
alcohol dehydrogenase
- GWAS
genome-wide association study
- Fanconi anemia
a disease associated with a mutation in one of 15 proteins mediating DNA repair
- Radiation dermatitis
an inflammatory reaction of the skin secondary to ionizing radiation
- CMMG
9-carboxymethoxymethylguanine, a metabolite of acyclovir
- NAPQI
N-acetyl-p-benzoquinonimine, a metabolite of acetaminophen
Footnotes
DISCLOSURE STATEMENT
D.M.-R. and C.-H.C. are founders of ALDEA Pharmaceuticals. However, none of the work described here is supported by or done in collaboration with the company.
LITERATURE CITED
- 1.Grieco EM, Acosta YD, de la Cruz GP, Cambino C, Gryn T, et al. Am. Community Surv. Rep. ACS-19. Washington, DC: US Census Bur.; 2012. The foreign-born population in the United States: 2010. http://www.census.gov/prod/2012pubs/acs-19.pdf. [Google Scholar]
- 2.US Census Bur. 2012 Natl. Popul. Proj. Summ. Tables. US Census Bur., Popul. Div.; Washington, DC: 2012. Table 4: Projections of the population by sex, race and Hispanic origin for the United States: 2015 to 2060 (NP2012-T4) ( http://www.census.gov/population/projections/data/national/2012/summarytables.html. [Google Scholar]
- 3.Qian F, Ling FS, Deedwania P, Hernandez AF, Fonarow GC, et al. Care and outcomes of Asian-American acute myocardial infarction patients: findings from the American Heart Association Get With The Guidelines-Coronary Artery Disease program. Circ. Cardiovasc. Qual. Outcomes. 2012;5:126–133. doi: 10.1161/CIRCOUTCOMES.111.961987. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Borinskaya S, Yoshimura K, Kal’ina N, Marusin A, et al. Refined geographic distribution of the oriental ALDH2*504Lys (nee 487Lys) variant. Ann. Hum. Genet. 2009;73:335–345. doi: 10.1111/j.1469-1809.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goedde HW, Agarwal DP, Harada S, Rothhammer F, Whittaker JO, Lisker R. Aldehyde dehydrogenase polymorphism in North American, South American, and Mexican Indian populations. Am. J. Hum. Genet. 1986;38:395–399. [PMC free article] [PubMed] [Google Scholar]
- 6.Novoradovsky AG, Kidd J, Kidd K, Goldman D. Apparent monomorphism of ALDH2 in seven American Indian populations. Alcohol. 1995;12:163–167. doi: 10.1016/0741-8329(94)00086-7. [DOI] [PubMed] [Google Scholar]
- 7.Luo HR, Wu GS, Pakstis AJ, Tong L, Oota H, et al. Origin and dispersal of atypical aldehyde dehydrogenase ALDH2*487Lys. Gene. 2009;435:96–103. doi: 10.1016/j.gene.2008.12.021. [DOI] [PubMed] [Google Scholar]
- 8.Palaniappan LP, Araneta MR, Assimes TL, Barrett-Connor EL, Carnethon MR, et al. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Cent. Dis. Control Prev. [assessed online February 5, 2014];Heart Disease Death Rates, 2000–2006; Asians and Pacific Islanders Ages 35+, by County. http://www.cdc.gov/dhdsp/maps/national_maps/hd_api.htm.
- 9.Goedde HW, Agarwal DP, Fritze G, Meier-Tackmann D, Singh S, et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum. Genet. 1992;88:344–346. doi: 10.1007/BF00197271. [DOI] [PubMed] [Google Scholar]
- 10.Parrilla R, Okawa K, Lindros KO, Zimmerman UJ, Kobayashi K, Williamson JR. Functional compartmentation of acetaldehyde oxidation in rat liver. J. Biol. Chem. 1974;249:4926–4933. [PubMed] [Google Scholar]
- 11.Eriksson CJ, Marselos M, Koivula T. Role of cytosolic rat liver aldehyde dehydrogenase in the oxidation of acetaldehyde during ethanol metabolism in vivo. Biochem. J. 1975;152:709–712. doi: 10.1042/bj1520709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun T, Bober E, Singh S, Agarwal DP, Goedde HW. Evidence for a signal peptide at the amino-terminal end of human mitochondrial aldehyde dehydrogenase. FEBS Lett. 1987;215:233–236. doi: 10.1016/0014-5793(87)80152-7. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien PJ, Siraki AG, Shangari N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health. Crit. Rev. Toxicol. 2005;35:609–662. doi: 10.1080/10408440591002183. [DOI] [PubMed] [Google Scholar]
- 14.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D. Targeting aldehyde dehydrogenase 2: new therapeutic opportunities. Physiol. Rev. 2014;94:1–34. doi: 10.1152/physrev.00017.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc. Natl. Acad. Sci. USA. 1984;81:258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larson HN, Zhou J, Chen Z, Stamler JS, Weiner H, Hurley TD. Structural and functional consequences of coenzyme binding to the inactive Asian variant of mitochondrial aldehyde dehydrogenase: roles of residues 475 and 487. J. Biol. Chem. 2007;282:12940–12950. doi: 10.1074/jbc.M607959200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner H, Wei B, Zhou J. Subunit communication in tetrameric class 2 human liver aldehyde dehydrogenase as the basis for half-of-the-site reactivity and the dominance of the oriental subunit in a heterotetramer. Chem. Biol. Interact. 2001;130–132:147–156. doi: 10.1016/s0009-2797(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 18.Yuan XW, Zhou WJ, Shang X, Li L, Liu YH, Xu XM. Rapid simultaneous genotyping of polymorphisms in ADH1B and ALDH2 using high resolution melting assay. Clin. Chem. Lab. Med. 2013;51:e75–e77. doi: 10.1515/cclm-2012-0509. [DOI] [PubMed] [Google Scholar]
- 19.Yokoyama T, Yokoyama A, Kato H, Tsujinaka T, Muto M, et al. Alcohol flushing, alcohol and aldehyde dehydrogenase genotypes, and risk for esophageal squamous cell carcinoma in Japanese men. Cancer Epidemiol. Biomarkers Prev. 2003;12:1227–1233. [PubMed] [Google Scholar]
- 20.Matsuse H, Shimoda T, Fukushima C, Mitsuta K, Kawano T, et al. Screening for acetaldehyde dehydrogenase 2 genotype in alcohol-induced asthma by using the ethanol patch test. J. Allergy Clin. Immunol. 2001;108:715–719. doi: 10.1067/mai.2001.118791. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama A, Muramatsu T, Ohmori T, Kumagai Y, Higuchi S, Ishii H. Reliability of a flushing questionnaire and the ethanol patch test in screening for inactive aldehyde dehydrogenase-2 and alcohol-related cancer risk. Cancer Epidemiol. Biomarkers Prev. 1997;6:1105–1107. [PubMed] [Google Scholar]
- 22.Kimura M, Miyakawa T, Matsushita S, So M, Higuchi S. Gender differences in the effects of ADH1B and ALDH2 polymorphisms on alcoholism. Alcohol. Clin. Exp. Res. 2011;35:1923–1927. doi: 10.1111/j.1530-0277.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 23.Kimura M, Sawayama T, Matsushita S, Higuchi S, Kashima H. Association between personality traits and ALDH2 polymorphism in Japanese male alcoholics. Alcohol. Clin. Exp. Res. 2009;33:799–803. doi: 10.1111/j.1530-0277.2009.00898.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SY, Wang TY, Chen SL, Huang SY, Tzeng NS, et al. Interaction between novelty seeking and the aldehyde dehydrogenase 2 gene in heroin-dependent patients. J. Clin. Psychopharmacol. 2013;33:386–390. doi: 10.1097/JCP.0b013e3182900fb3. [DOI] [PubMed] [Google Scholar]
- 25.Wang YS, Lee SY, Chen SL, Chang YH, Wang TY, et al. Role of DRD2 and ALDH2 genes in bipolar II disorder with and without comorbid anxiety disorder. Eur. Psychiatry. 2013;29:142–148. doi: 10.1016/j.eurpsy.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Harada S, Agarwal DP, Goedde HW. Aldehyde dehydrogenase polymorphism and alcohol metabolism in alcoholics. Alcohol. 1985;2:391–392. doi: 10.1016/0741-8329(85)90100-4. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi F, Isono M, Nabika T, Katsuya T, Sugiyama T, et al. Confirmation of ALDH2 as a major locus of drinking behavior and of its variants regulating multiple metabolic phenotypes in a Japanese population. Circ. J. 2011;75:911–918. doi: 10.1253/circj.cj-10-0774. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Lu X, Wang L, Chen S, Li J, et al. Common variants at 12q24 are associated with drinking behavior in Han Chinese. Am. J. Clin. Nutr. 2013;97:545–551. doi: 10.3945/ajcn.112.046482. [DOI] [PubMed] [Google Scholar]
- 29.Wang TY, Lee SY, Chen SL, Chen SH, Chu CH, et al. The aldehyde dehydrogenase 2 gene is associated with heroin dependence. Drug Alcohol Depend. 2012;120:220–224. doi: 10.1016/j.drugalcdep.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 30.Ohsawa I, Nishimaki K, Murakami Y, Suzuki Y, Ishikawa M, Ohta S. Age-dependent neurodegeneration accompanying memory loss in transgenic mice defective in mitochondrial aldehyde dehydrogenase 2 activity. J. Neurosci. 2008;28:6239–6249. doi: 10.1523/JNEUROSCI.4956-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamino K, Nagasaka K, Imagawa M, Yamamoto H, Yoneda H, et al. Deficiency in mitochondrial aldehyde dehydrogenase increases the risk for late-onset Alzheimer’s disease in the Japanese population. Biochem. Biophys. Res. Commun. 2000;273:192–196. doi: 10.1006/bbrc.2000.2923. [DOI] [PubMed] [Google Scholar]
- 32.Wang B, Wang J, Zhou S, Tan S, He X, et al. The association of mitochondrial aldehyde dehydrogenase gene (ALDH2) polymorphism with susceptibility to late-onset Alzheimer’s disease in Chinese. J. Neurol. Sci. 2008;268:172–175. doi: 10.1016/j.jns.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Kim JM, Stewart R, Shin IS, Jung JS, Yoon JS. Assessment of association between mitochondrial aldehyde dehydrogenase polymorphism and Alzheimer’s disease in an older Korean population. Neurobiol. Aging. 2004;25:295–301. doi: 10.1016/S0197-4580(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 34.Wey MC, Fernandez E, Martinez PA, Sullivan P, Goldstein DS, Strong R. Neurodegeneration and motor dysfunction in mice lacking cytosolic and mitochondrial aldehyde dehydrogenases: implications for Parkinson’s disease. PLOS ONE. 2012;7:e31522. doi: 10.1371/journal.pone.0031522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fitzmaurice AG, Rhodes SL, Cockburn M, Ritz B, Bronstein JM. Aldehyde dehydrogenase variation enhances effect of pesticides associated with Parkinson disease. Neurology. 2014;82:419–426. doi: 10.1212/WNL.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson PA, James MR, Heath AC, Montgomery GW, Martin NG, et al. Effects of variation at the ALDH2 locus on alcohol metabolism, sensitivity, consumption, and dependence in Europeans. Alcohol. Clin. Exp. Res. 2006;30:1093–1100. doi: 10.1111/j.1530-0277.2006.00128.x. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh AY, Tripp DA, Ji LJ, Sullivan MJ. Comparisons of catastrophizing, pain attitudes, and cold-pressor pain experience between Chinese and European Canadian young adults. J. Pain. 2010;11:1187–1194. doi: 10.1016/j.jpain.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Watson PJ, Latif RK, Rowbotham DJ. Ethnic differences in thermal pain responses: a comparison of South Asian and White British healthy males. Pain. 2005;118:194–200. doi: 10.1016/j.pain.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 39.Woodrow KM, Friedman GD, Siegelaub AB, Collen MF. Pain tolerance: differences according to age, sex and race. Psychosom. Med. 1972;34:548–556. doi: 10.1097/00006842-197211000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur. J. Neurosci. 2007;26:2516–2523. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- 41.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murata C, Suzuki Y, Muramatsu T, Taniyama M, Atsumi Y, et al. Inactive aldehyde dehydrogenase 2 worsens glycemic control in patients with type 2 diabetes mellitus who drink low to moderate amounts of alcohol. Alcohol. Clin. Exp. Res. 2000;24:5S–11S. [PubMed] [Google Scholar]
- 43.Xu F, Chen Y, Lv R, Zhang H, Tian H, et al. ALDH2 genetic polymorphism and the risk of type II diabetes mellitus in CAD patients. Hypertens. Res. 2010;33:49–55. doi: 10.1038/hr.2009.178. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi J, Hasegawa Y, Kawasaki M, Masui T, Kanoh T, et al. ALDH2 polymorphisms and bone mineral density in an elderly Japanese population. Osteoporos. Int. 2006;17:908–913. doi: 10.1007/s00198-006-0077-2. [DOI] [PubMed] [Google Scholar]
- 45.Takagi S, Iwai N, Yamauchi R, Kojima S, Yasuno S, et al. Aldehyde dehydrogenase 2 gene is a risk factor for myocardial infarction in Japanese men. Hypertens. Res. 2002;25:677–681. doi: 10.1291/hypres.25.677. [DOI] [PubMed] [Google Scholar]
- 46.Jo SA, Kim EK, Park MH, Han C, Park HY, et al. A Glu487Lys polymorphism in the gene for mitochondrial aldehyde dehydrogenase 2 is associated with myocardial infarction in elderly Korean men. Clin. Chim. Acta. 2007;382:43–47. doi: 10.1016/j.cca.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 47.Xu F, Chen YG, Xue L, Li RJ, Zhang H, et al. Role of aldehyde dehydrogenase 2 Glu504lys polymorphism in acute coronary syndrome. J. Cell. Mol. Med. 2011;15:1955–1962. doi: 10.1111/j.1582-4934.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeuchi F, Yokota M, Yamamoto K, Nakashima E, Katsuya T, et al. Genome-wide association study of coronary artery disease in the Japanese. Eur. J. Hum. Genet. 2012;20:333–340. doi: 10.1038/ejhg.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q, Zhou S, Wang L, Lei M, Wang Y, et al. ALDH2 rs671 polymorphism and coronary heart disease risk among Asian populations: a meta-analysis and meta-regression. DNA Cell Biol. 2013;32:393–399. doi: 10.1089/dna.2013.1995. [DOI] [PubMed] [Google Scholar]
- 50.Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. 2013;382:597–604. doi: 10.1016/S0140-6736(13)61450-6. [DOI] [PubMed] [Google Scholar]
- 51.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gross ER, Hsu AK, Urban TJ, Mochly-Rosen D, Gross GJ. Nociceptive-induced myocardial remote conditioning is mediated by neuronal gamma protein kinase C. Basic Res. Cardiol. 2013;108:381. doi: 10.1007/s00395-013-0381-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Contractor H, Stottrup NB, Cunnington C, Manlhiot C, Diesch J, et al. Aldehyde dehydrogenase-2 inhibition blocks remote preconditioning in experimental and human models. Basic Res. Cardiol. 2013;108:343. doi: 10.1007/s00395-013-0343-3. [DOI] [PubMed] [Google Scholar]
- 54.Zhang H, Gong DX, Zhang YJ, Li SJ, Hu S. Effect of mitochondrial aldehyde dehydrogenase-2 genotype on cardioprotection in patients with congenital heart disease. Eur. Heart J. 2012;33:1606–1614. doi: 10.1093/eurheartj/ehs061. [DOI] [PubMed] [Google Scholar]
- 55.Guo JM, Liu AJ, Zang P, Dong WZ, Ying L, et al. ALDH2 protects against stroke by clearing 4-HNE. Cell Res. 2013;23:915–930. doi: 10.1038/cr.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fu SH, Zhang HF, Yang ZB, Li TB, Liu B, et al. Alda-1 reduces cerebral ischemiareperfusion injury in rat through clearance of reactive aldehydes. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014;387:87–94. doi: 10.1007/s00210-013-0922-8. [DOI] [PubMed] [Google Scholar]
- 57.Rantakomi SH, Laukkanen JA, Sivenius J, Kauhanen J, Kurl S. Hangover and the risk of stroke in middle-aged men. Acta Neurol. Scand. 2013;127:186–191. doi: 10.1111/j.1600-0404.2012.01696.x. [DOI] [PubMed] [Google Scholar]
- 58.Nagasawa H, Wada M, Arawaka S, Kawanami T, Kurita K, et al. A polymorphism of the aldehyde dehydrogenase 2 gene is a risk factor for multiple lacunar infarcts in Japanese men: the Takahata Study. Eur. J. Neurol. 2007;14:428–434. doi: 10.1111/j.1468-1331.2007.01700.x. [DOI] [PubMed] [Google Scholar]
- 59.Hui P, Nakayama T, Morita A, Sato N, Hishiki M, et al. Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens. Res. 2007;30:585–592. doi: 10.1291/hypres.30.585. [DOI] [PubMed] [Google Scholar]
- 60.Kato N, Takeuchi F, Tabara Y, Kelly TN, Go MJ, et al. Meta-analysis of genome-wide association studies identifies common variants associated with blood pressure variation in east Asians. Nat. Genet. 2011;43:531–538. doi: 10.1038/ng.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lai CL, Liu MT, Yin SJ, Lee JT, Lu CC, Peng GS. Heavy binge drinking may increase risk of stroke in nonalcoholic hypertensives carrying variant ALDH2*2 gene allele. Acta Neurol. Taiwanica. 2012;21:39–43. [PubMed] [Google Scholar]
- 62.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, et al. Isolation and in vitro characterization of basal and submucosal gland duct stemprogenitor cells from human proximal airways. Stem Cells Transl. Med. 2012;1:719–724. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corradi M, Pignatti P, Manini P, Andreoli R, Goldoni M, et al. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur. Respir. J. 2004;24:1011–1017. doi: 10.1183/09031936.04.00002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Takao A, Shimoda T, Kohno S, Asai S, Harda S. Correlation between alcohol-induced asthma and acetaldehyde dehydrogenase-2 genotype. J. Allergy Clin. Immunol. 1998;101:576–580. doi: 10.1016/S0091-6749(98)70162-9. [DOI] [PubMed] [Google Scholar]
- 65.Park JY, Matsuo K, Suzuki T, Ito H, Hosono S, et al. Impact of smoking on lung cancer risk is stronger in those with the homozygous aldehyde dehydrogenase 2 null allele in a Japanese population. Carcinogenesis. 2010;31:660–665. doi: 10.1093/carcin/bgq021. [DOI] [PubMed] [Google Scholar]
- 66.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, et al. A review of human carcinogens—Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10:1033–1034. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 67.Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. 2009;137:1768–1775. doi: 10.1053/j.gastro.2009.07.070. [DOI] [PubMed] [Google Scholar]
- 68.Yokoyama A, Muramatsu T, Ohmori T, Higuchi S, Hayashida M, Ishii H. Esophageal cancer and aldehyde dehydrogenase-2 genotypes in Japanese males. Cancer Epidemiol. Biomarkers Prev. 1996;5:99–102. [PubMed] [Google Scholar]
- 69.Dura P, Berkers T, van Veen EM, Salomon J, te Morsche RHM, et al. Polymorphisms in alcohol-metabolizing enzymes and esophageal carcinoma susceptibility: a Dutch Caucasian case-control study. J. Hum. Genet. 2013;58:742–748. doi: 10.1038/jhg.2013.95. [DOI] [PubMed] [Google Scholar]
- 70.Oze I, Matsuo K, Wakai K, Nagata C, Mizoue T, et al. Alcohol drinking and esophageal cancer risk: an evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2011;41:677–692. doi: 10.1093/jjco/hyr026. [DOI] [PubMed] [Google Scholar]
- 71.Matsuo K, Oze I, Hosono S, Ito H, Watanabe M, et al. The aldehyde dehydrogenase 2 (ALDH2) Glu504Lys polymorphism interacts with alcohol drinking in the risk of stomach cancer. Carcinogenesis. 2013;34:1510–1515. doi: 10.1093/carcin/bgt080. [DOI] [PubMed] [Google Scholar]
- 72.Cao HX, Li SP, Wu JZ, Gao CM, Su P, et al. Alcohol dehydrogenase-2 and aldehyde dehydrogenase-2 genotypes, alcohol drinking and the risk for stomach cancer in Chinese males. Asian Pac. J. Cancer Prev. 2010;11:1073–1077. [PubMed] [Google Scholar]
- 73.Miyasaka K, Kawanami T, Shimokata H, Ohta S, Funakoshi A. Inactive aldehyde dehydrogenase-2 increased the risk of pancreatic cancer among smokers in a Japanese male population. Pancreas. 2005;30:95–98. doi: 10.1097/01.mpa.0000147084.70125.41. [DOI] [PubMed] [Google Scholar]
- 74.Kanda J, Matsuo K, Suzuki T, Kawase T, Hiraki A, et al. Impact of alcohol consumption with polymorphisms in alcohol-metabolizing enzymes on pancreatic cancer risk in Japanese. Cancer Sci. 2009;100:296–302. doi: 10.1111/j.1349-7006.2008.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tomoda T, Nouso K, Sakai A, Ouchida M, Kobayashi S, et al. Genetic risk of hepatocellular carcinoma in patients with hepatitis C virus: a case control study. J. Gastroenterol. Hepatol. 2012;27:797–804. doi: 10.1111/j.1440-1746.2011.06948.x. [DOI] [PubMed] [Google Scholar]
- 76.Chiang CP, Jao SW, Lee SP, Chen PC, Chung CC, et al. Expression pattern, ethanol-metabolizing activities, and cellular localization of alcohol and aldehyde dehydrogenases in human large bowel: association of the functional polymorphisms of ADH and ALDH genes with hemorrhoids and colorectal cancer. Alcohol. 2012;46:37–49. doi: 10.1016/j.alcohol.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Miyasaka K, Hosoya H, Tanaka Y, Uegaki S, Kino K, et al. Association of aldehyde dehydrogenase 2 gene polymorphism with pancreatic cancer but not colon cancer. Geriatr. Gerontol. Int. 2010;10(Suppl. 1):S120–S126. doi: 10.1111/j.1447-0594.2010.00616.x. [DOI] [PubMed] [Google Scholar]
- 78.Garaycoechea JI, Crossan GP, Langevin F, Daly M, Arends MJ, Patel KJ. Genotoxic consequences of endogenous aldehydes on mouse haematopoietic stem cell function. Nature. 2012;489:571–575. doi: 10.1038/nature11368. [DOI] [PubMed] [Google Scholar]
- 79.Langevin F, Crossan GP, Rosado IV, Arends MJ, Patel KJ. Fancd2 counteracts the toxic effects of naturally produced aldehydes in mice. Nature. 2011;475:53–58. doi: 10.1038/nature10192. [DOI] [PubMed] [Google Scholar]
- 80.Hira A, Yabe H, Yoshida K, Okuno Y, Shiraishi Y, et al. Variant ALDH2 is associated with accelerated progression of bone marrow failure in Japanese Fanconi anemia patients. Blood. 2013;122:3206–3209. doi: 10.1182/blood-2013-06-507962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilkin JK, Fortner G. Ethnic contact urticaria to alcohol. Contact Dermat. 1985;12:118–120. doi: 10.1111/j.1600-0536.1985.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 82.Wong JW, Harris K, Powell D. Alcohol urticaria syndrome. Dermatitis. 2011;22:350–354. doi: 10.2310/6620.2011.11067. [DOI] [PubMed] [Google Scholar]
- 83.Kramer A, Below H, Bieber N, Kampf G, Toma CD, et al. Quantity of ethanol absorption after excessive hand disinfection using three commercially available hand rubs is minimal and below toxic levels for humans. BMC Infect. Dis. 2007;7:117. doi: 10.1186/1471-2334-7-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salvo N, Barnes E, van Draanen J, Stacey E, Mitera G, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr. Oncol. 2010;17:94–112. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ning S, Budas GR, Churchill EN, Chen CH, Knox SJ, Mochly-Rosen D. Mitigation of radiation-induced dermatitis by activation of aldehyde dehydrogenase 2 using topical Alda-1 in mice. Radiat. Res. 2012;178:69–74. doi: 10.1667/rr2861.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Koppaka V, Thompson DC, Chen Y, Ellermann M, Nicolaou KC, et al. Aldehyde dehydrogenase inhibitors: a comprehensive review of the pharmacology, mechanism of action, substrate specificity, and clinical application. Pharmacol. Rev. 2012;64:520–539. doi: 10.1124/pr.111.005538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aslibekyan S, Brown EE, Reynolds RJ, Redden DT, Morgan S, et al. Genetic variants associated with methotrexate efficacy and toxicity in early rheumatoid arthritis: results from the treatment of early aggressive rheumatoid arthritis trial. Pharmacogenomics J. 2013;14:48–53. doi: 10.1038/tpj.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayer B, Beretta M. The enigma of nitroglycerin bioactivation and nitrate tolerance: news, views and troubles. Br. J. Pharmacol. 2008;155:170–184. doi: 10.1038/bjp.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ferreira JC, Mochly-Rosen D. Nitroglycerin use in myocardial infarction patients. Circ. J. 2012;76:15–21. doi: 10.1253/circj.cj-11-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mackenzie IS, Maki-Petaja KM, McEniery CM, Bao YP, Wallace SM, et al. Aldehyde dehydrogenase 2 plays a role in the bioactivation of nitroglycerin in humans. Arterioscler. Thromb. Vasc. Biol. 2005;25:1891–1895. doi: 10.1161/01.ATV.0000179599.71086.89. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Zhang D, Jin W, Shao C, Yan P, et al. Mitochondrial aldehyde dehydrogenase-2 (ALDH2) Glu504Lys polymorphism contributes to the variation in efficacy of sublingual nitroglycerin. J. Clin. Invest. 2006;116:506–511. doi: 10.1172/JCI26564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ. Res. 2005;97:618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 93.Ankolekar S, Fuller M, Cross I, Renton C, Cox P, et al. Feasibility of an ambulance-based stroke trial, and safety of glyceryl trinitrate in ultra-acute stroke: the rapid intervention with glyceryl trinitrate in Hypertensive Stroke Trial (RIGHT, ISRCTN66434824) Stroke J. Cereb. Circ. 2013;44:3120–3128. doi: 10.1161/STROKEAHA.113.001301. [DOI] [PubMed] [Google Scholar]
- 94.Sun L, Ferreira JC, Mochly-Rosen D. ALDH2 activator inhibits increased myocardial infarction injury by nitroglycerin tolerance. Sci. Transl. Med. 2011;3:107ra11. doi: 10.1126/scitranslmed.3002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, et al. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc. Natl. Acad. Sci. USA. 2005;102:12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Miranda P, Blum MR. Pharmacokinetics of acyclovir after intravenous and oral administration. J. Antimicrob. Chemother. 1983;12(Suppl. B):29–37. doi: 10.1093/jac/12.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- 97.de Miranda P, Good SS, Laskin OL, Krasny HC, Connor JD, Lietman PS. Disposition of intravenous radioactive acyclovir. Clin. Pharmacol. Ther. 1981;30:662–672. doi: 10.1038/clpt.1981.218. [DOI] [PubMed] [Google Scholar]
- 98.Hara K, Suyama K, Itoh H, Nagashima S. Influence of ALDH2 genetic polymorphisms on aciclovir pharmacokinetics following oral administration of valaciclovir in Japanese end-stage renal disease patients. Drug Metab. Pharmacokinet. 2008;23:306–312. doi: 10.2133/dmpk.23.306. [DOI] [PubMed] [Google Scholar]
- 99.Castro JA, de Mecca MM, Bartel LC. Toxic side effects of drugs used to treat Chagas’ disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- 100.Zhou L, Ishizaki H, Spitzer M, Taylor KL, Temperley ND, et al. ALDH2 mediates 5-nitrofuran activity in multiple species. Chem. Biol. 2012;19:883–892. doi: 10.1016/j.chembiol.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saulnier Sholler GL, Bergendahl GM, Brard L, Singh AP, Heath BW, et al. A phase 1 study of nifurtimox in patients with relapsedrefractory neuroblastoma. J. Pediatr. Hematol. Oncol. 2011;33:25–30. doi: 10.1097/MPH.0b013e3181f47061. [DOI] [PubMed] [Google Scholar]
- 102.Landin JS, Cohen SD, Khairallah EA. Identification of a 54-kDa mitochondrial acetaminophen-binding protein as aldehyde dehydrogenase. Toxicol. Appl. Pharmacol. 1996;141:299–307. doi: 10.1006/taap.1996.0287. [DOI] [PubMed] [Google Scholar]
- 103.Lee YP, Liao JT, Cheng YW, Wu TL, Lee SL, et al. Inhibition of human alcohol and aldehyde dehydrogenases by acetaminophen: Assessment of the effects on first-pass metabolism of ethanol. Alcohol. 2013;47:559–565. doi: 10.1016/j.alcohol.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Lu Z, Bourdi M, Li JH, Aponte AM, Chen Y, et al. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity. EMBO Rep. 2011;12:840–846. doi: 10.1038/embor.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yoval-Sánchez B, Rodríguez-Zavala JS. Differences in susceptibility to inactivation of human aldehyde dehydrogenases by lipid peroxidation byproducts. Chem. Res. Toxicol. 2012;25:722–729. doi: 10.1021/tx2005184. [DOI] [PubMed] [Google Scholar]
- 106.Staub RE, Quistad GB, Casida JE. Mechanism for benomyl action as a mitochondrial aldehyde dehydrogenase inhibitor in mice. Chem. Res. Toxicol. 1998;11:535–543. doi: 10.1021/tx980002l. [DOI] [PubMed] [Google Scholar]
- 107.Cherry N, Moore H, McNamee R, Pacey A, Burgess G, et al. Occupation and male infertility: glycol ethers and other exposures. Occup. Environ. Med. 2008;65:708–714. doi: 10.1136/oem.2007.035824. [DOI] [PubMed] [Google Scholar]
- 108.Wang RS, Ohtani K, Suda M, Kitagawa K, Nakayama K, et al. Reproductive toxicity of ethylene glycol monoethyl ether in Aldh2 knockout mice. Ind. Health. 2007;45:574–578. doi: 10.2486/indhealth.45.574. [DOI] [PubMed] [Google Scholar]
- 109.Weng Z, Ohtani K, Suda M, Yanagiba Y, Kawamoto T, et al. Assessment of the reproductive toxicity of inhalation exposure to ethyl tertiary butyl ether in male mice with normal, low active and inactive ALDH2. Arch. Toxicol. 2014;88:1007–1021. doi: 10.1007/s00204-014-1192-z. [DOI] [PubMed] [Google Scholar]
- 110.Qaseem A, Denberg TD, Hopkins RH, Jr, Humphrey LL, Levine J, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann. Intern. Med. 2012;156:378–386. doi: 10.7326/0003-4819-156-5-201203060-00010. [DOI] [PubMed] [Google Scholar]
- 111.Noda J, Umeda S, Mori K, Fukunaga T, Mizoi Y. Acetaldehyde syndrome after celiac plexus alcohol block. Anesthes. Analg. 1986;65:1300–1302. [PubMed] [Google Scholar]
- 112.Salaspuro V, Hietala J, Kaihovaara P, Pihlajarinne L, Marvola M, Salaspuro M. Removal of acetaldehyde from saliva by a slow-release buccal tablet of L-cysteine. Int. J. Cancer J. Int. Cancer. 2002;97:361–364. doi: 10.1002/ijc.1620. [DOI] [PubMed] [Google Scholar]
- 113.Alkhouri N, Cikach F, Eng K, Moses J, Patel N, et al. Analysis of breath volatile organic compounds as a noninvasive tool to diagnose nonalcoholic fatty liver disease in children. Eur. J. Gastroenterol. Hepatol. 2014;26:82–87. doi: 10.1097/MEG.0b013e3283650669. [DOI] [PubMed] [Google Scholar]
- 114.Takeshita T, Morimoto K. Accumulation of hemoglobin-associated acetaldehyde with habitual alcohol drinking in the atypical ALDH2 genotype. Alcohol. Clin. Exp. Res. 2000;24:1–7. [PubMed] [Google Scholar]