Summary
Changes in behavioral state modify neural activity in many systems [1–5]. In some vertebrates such modulation has been observed and interpreted in the context of attention [2] and sensorimotor coordinate transformations [3]. Here we report state-dependent activity modulations during walking in a visual-motor pathway of Drosophila. We used two-photon imaging to monitor intracellular calcium activity in motion-sensitive lobula plate tangential cells (LPTCs) in head-fixed Drosophila walking on an air-supported ball. Cells of the horizontal system (HS)—a subgroup of LPTCs—showed stronger calcium transients in response to visual motion when flies were walking rather than resting. The amplified responses were also correlated with walking speed. Moreover, HS neurons showed a relatively higher gain in response strength at higher temporal frequencies, and their optimum temporal frequency was shifted toward higher motion speeds. Walking-dependent modulation of HS neurons in the Drosophila visual system may constitute a mechanism to facilitate processing of higher image speeds in behavioral contexts where these speeds of visual motion are relevant for course stabilization.
Results
Moving animals analyze optic flow—the patterns of retinal image shift caused by relative motion between the eyes and visual contrasts in the surroundings—to stabilize their locomotor path, balance, and gaze [6]. Horizontal system (HS) neurons are necessary elements of the optic-flow-processing and optomotor pathways in flies [7, 8] and have been thoroughly studied in blowflies [9] and, recently, in Drosophila [10]. HS neuron receptive fields consist of large overlapping areas, stacked along the dorsal-ventral axis; the three HS neurons in each optic lobe are accordingly referred to as HS-North (HSN), HS-Equatorial, and HS-South [10]. HS-North and HS-Equatorial respond best to simultaneous horizontal front-to-back motion on the ipsilateral eye and back-to-front optic flow on the contralateral eye, a pattern of image motion that occurs during yaw rotations of the body and head [11]. The neurons are also stimulated during translation of the fly [12, 13].
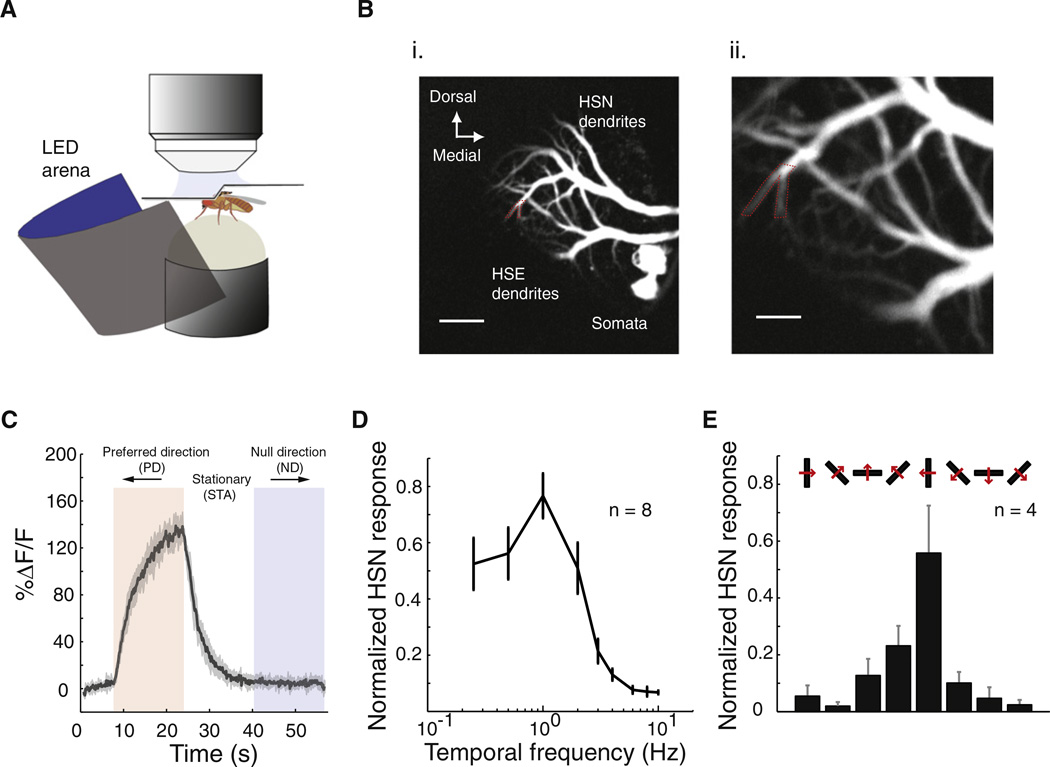
We used two-photon imaging to record transient changes in intracellular calcium concentration from dendrites of HSN neurons in flies walking on an air-supported ball [14] (Figure 1A). We expressed the genetically encoded calcium indicator GCaMP3.0 [15] in HS neurons by using the R27B03-Gal4 driver from the Rubin laboratory [16] (see Supplemental Experimental Procedures, available online). HS neurons were identified on the basis of their characteristic morphology: large somata, broad and stereotyped dendrites in the lobula plate (Figure 1B and Figure S1), and axons projecting to the protocerebrum toward the midline [17]. We presented the fly with large-field vertical gratings moving horizontally, approximating the optic flow that occurs during pure yaw rotation of the fly, while monitoring both walking and HSN calcium transients [14] (see Supplemental Experimental Procedures). HSN neurons responded to horizontal motion in their preferred direction (PD) with slow increases of the GCaMP signal (Figure 1C), in agreement with previous studies using synthetic calcium indicators in the blowfly [18]. When the fly was stationary, HSN dendrites displayed temporal frequency tuning and direction-selective responses similar to those recently measured in Drosophila with the use of somatic whole-cell patch-clamp recordings [10] (Figures 1D and 1E, respectively).
Figure 1. Two-Photon Calcium Imaging of the Motion-Sensitive HSN Neuron in Drosophila.
(A) Schematic of the walking fly recording setup.
(B) We recorded from the HSN’s dendrite branch, outlined in dotted red (from fly 6). HSE: HS-Equatorial. Scale bars represent 20 µm (i) and 5 µm (ii).
(C) Canonical responses of the HSN neuron (from fly 6) to a horizontally rotating vertical grating pattern moving at a temporal frequency of 1 Hz in trials in which the fly was standing still. We always recorded from the left half of the brain; therefore, a front-to-back preferred direction (PD) for the neuron corresponds to counterclockwise rotations of the pattern.
(D) Temporal frequency tuning of HSN dendrites. Shown are the mean normalized peak responses across eight different flies. Error bars indicate mean ± standard error of the mean (SEM).
(E) Directional tuning of HSN dendrites. The figure shows the mean normalized peak response (error bars indicate mean ± SEM) across four different flies, during 10 s of grating motion of different orientations (indicated by red arrows) moving at 1 Hz. In all cases, the peak response was calculated as the mean value from a 0.5 s time window centered at the peak of the response and was normalized to the neuron’s maximum peak response.
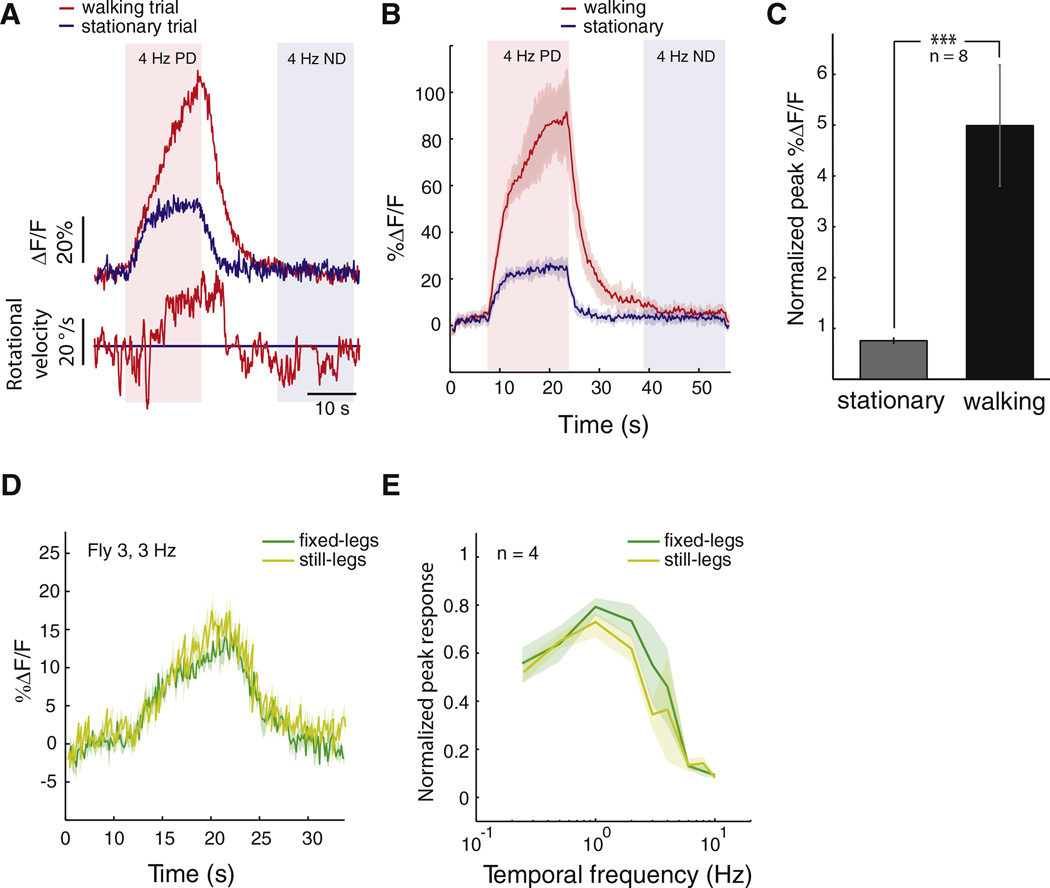
Recent electrophysiological studies in the visual system of tethered mice and tethered flies have shown enhanced responses to visual stimuli during walking and flying, respectively [4, 5]. Recordings in restrained flies from motion-sensitive neurons exposed to a neuromodulator that is known to be released during flight [19] have also suggested the existence of context-dependent response modulation in these neurons. To examine whether HSN responses are modified during walking, we compared its responses to moving vertical gratings in walking and stationary trials of the same fly. In stationary flies, the strongest motion-induced physiological responses are observed at temporal frequencies of about 1 Hz; responses at higher temporal frequencies are significantly reduced [10] (Figure 1D). To stay within the nonsaturating range, we presented moving stimuli at temporal frequencies of 3–4 Hz, where the calcium transients are about 20%–40% of those found at 1 Hz (Figure 1D), thereby making potential increases in HSN activity during behavior more visible. We found that the neuron’s response amplitude was greatly increased when the fly walked (Figure 2A). This response gain, measured as the ratio of peak HSN response in walking flies to that in stationary flies, was seen across trials for the same neuron (Figure 2B, mean response gain = 2.96, n = 5 trials) and across different walking flies (Figure 2C, mean response gain = 6.5 ± 4.4; range: 2.7–16; n = 8 flies).
Figure 2. HSN Responses Depend on the Walking State of the Fly.
(A) Single trials (upper traces, calcium signals; lower traces, rotational velocities calculated over 0.5 s) from the HSN neuron and fly shown in Figure 1C during stationary (trial 7, blue) and walking (trial 1, red) conditions. For all examples in this figure, the fly was stimulated with a large-field, horizontal grating moving at a temporal frequency of 4 Hz.
(B) Mean ± standard deviation (SD) response of the neuron during walking (red) and stationary (blue) trials (n = 5 trials per condition).
(C) Mean normalized peak response during walking (black bar) and stationary (gray bar) trials across all flies (Mann-Whitney test, ***p < 0.001, n = 8).
(D). Comparison of HSN responses in the two different nonwalking conditions. Shown are the mean ± SD of three different trials of HSN responses to a vertical grating moving horizontally at a temporal frequency of 3 Hz when the fly was standing still on the ball (”still-legs”) or when the same fly had its legs waxed (“fixedlegs”).
(E) We repeated the same comparison across different temporal frequencies for four different flies to obtain temporal frequency tuning curves under the two nonwalking conditions (curves comparison: Mann-Whitney test; p values ranged from 0.2 to 0.9).
We noticed that grooming also modulated HSN response to moving gratings, but to a lesser extent than walking (data not shown). In some experiments, flies displayed long periods of walking interrupted only by grooming. In such cases, we achieved the stationary condition by waxing the fly’s legs (the “fixed-legs” condition). We carried out control experiments to test whether this manipulation resulted in HSN responses and tuning curves different from those of the more natural stationary condition (“still-legs”) and found that HSN responses in the fixed-legs condition matched those in the still-legs condition (Figure 2D). Moreover, the similarity was maintained across all temporal frequencies (Figure 2E), suggesting that active walking was required for gain modulation to take effect.
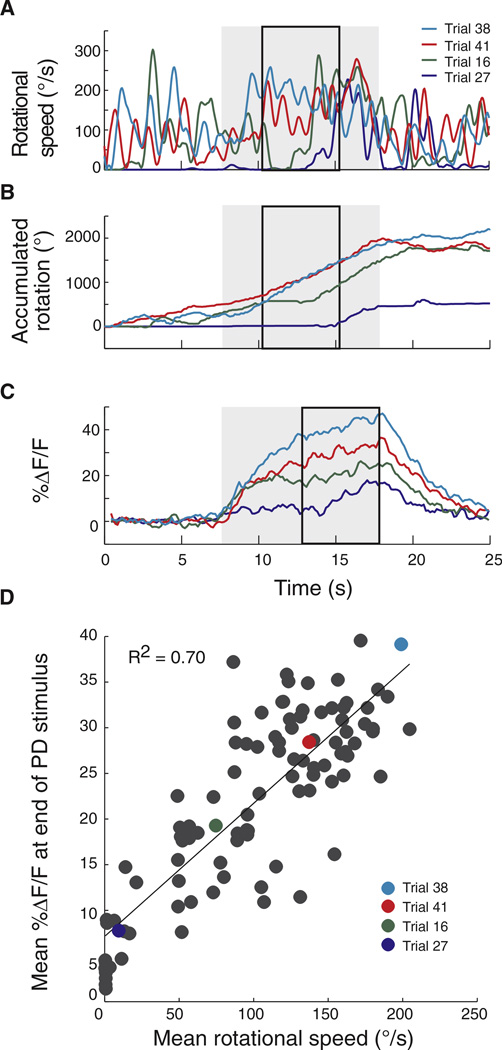
We then evaluated the dependence of gain modulation on walking speed. We performed 100 successive imaging trials on individual flies presented with a PD motion stimulus of constant temporal frequency (4 Hz). Flies walked at a range of speeds (Figure 3A) and for different distances (Figure 3B) during these trials. Comparing calcium transients during the same period of the response but under different walking conditions, we found that trials in which the fly walked more (as measured by increases in turning) were often the same trials that featured the larger calcium signals (compare calcium and rotational responses for the four highlighted trials in Figures 3A–3C). We quantified this across 100 trials by comparing the net calcium accumulation at the end of PD stimulation (period outlined in black boxes, Figure 3C) to the mean rotational speed in the preceding 5 s (period outlined in black boxes, Figure 3A) and found a moderate but significant correlation (Figure 3D; R2 = 0.70, 0.11, 0.18, 0.65, and 0.39; p < 0.001 for all five flies). We obtained similar results when we compared net calcium levels to overall walking speed (including translational components) during the same time period (data not shown). Thus, a significant component of the increased HSN response that we saw could be explained by walking speed alone. The direction in which the fly turned did not, however, appear to modify responses (data not shown).
Figure 3. HSN Responses Are Correlated with Walking Speed.
(A) Rotational walking velocities during periods of PD stimulation (gray box) for four different trials (indicated in different colors) for a single fly.
(B) Trial-to-trial variability in accumulated rotation caused by the fly walking on the ball for the same trials.
(C) Calcium transients (i.e., fluorescence transients, %DF/F) in response to PD stimulation in the same trials.
(D) Correlation between mean calcium transients during the last 5 s of PD stimulus presentation (black box) and mean rotational walking speed over 100 trials for the same fly. Mean walking speed calculated over the 5 s periods shown in black boxes in (B): periods used to compute calcium transients are later in order to account for the delay in the transients.
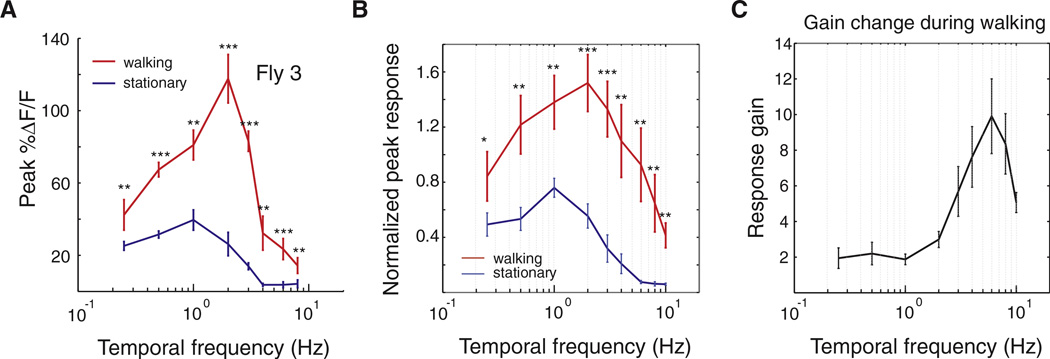
The magnitude of the walking-induced amplification of the response of the HSN neuron was also highly dependent on the temporal frequency of the stimulus. We tested temporal frequencies ranging from 0.25 to 10 Hz. During walking trials, the mean HSN peak response was significantly amplified at all frequencies (Figures 4A and 4B), although at frequencies < 1 Hz, the modulation was less reproducible (5/8 flies, Figure S2). Interestingly, this response gain—the ratio of peak HSN responses in walking relative to stationary conditions—was dependent on the temporal frequency of the stimulus, with an approximately constant gain at lower speeds (< 2 Hz) and a maxim um at 6 Hz (Figure 4C). Furthermore, in six out of eight flies, the peak of the tuning curve was shifted toward higher temporal frequencies and was significantly different from the peak of the temporal tuning curve in stationary trials (Figure 4A and Figure S2, Mann-Whitney test, p < 0.001). We plotted walking speed against temporal frequency and found that walking speed did not increase at higher temporal frequencies (Figure S3). Thus, the temporal-frequency-dependent neuronal response gains we observed could not be explained by walking speed differences alone.
Figure 4. HSN Temporal Frequency Tuning Curve Is Significantly Amplified and Its Peak Shifts toward Higher Frequencies during Walking.
(A) Temporal frequency tuning curves for HSN dendrites in walking and stationary conditions for a single fly (fly 3; walking: n = 10 trials; stationary: n = 5 trials).
(B) Mean temporal frequency tuning curve of HSN under walking and stationary conditions across all flies. The responses of HSN were normalized to the maximal responses for each fly under stationary conditions and averaged across flies (for 0.25 Hz and 10 Hz, n = 6 flies; other frequencies, n = 8 flies). *p < 0.05; **p < 0.01; ***p < 0.001.
(C) Mean HSN response gain across temporal frequencies (number of flies per frequency same as in B). The gain is defined as the maximum calcium transient measured when the fly was walking divided by the maximum calcium transient obtained in stationary flies.
Discussion
We used a tethered fly-on-a-ball imaging setup [14] to record Drosophila HSN neuron calcium transients in response to motion stimuli. We found walking-dependent gain modulation in HS neurons, similar to recent results in vertical system (VS) lobula plate tangential cells (LPTCs) during tethered flight [4]. Such state-dependent regulation of neural sensitivity has been suggested to reduce energy consumption [20], increasing the system’s gain only when the animal requires it most. In the Maimon et al. 2010 study in flying flies [4], LPTC membrane potentials were more depolarized and their synaptic responses stronger during flight. Assuming that voltage-dependent calcium currents underlie the calcium transients that we observe [21], the changes seen in flying flies likely apply to the walking fly as well. However, other sources of calcium transients may include release from internal stores.
The origin of behavior-dependent gain modulation of HS neurons remains unclear. Flies with fixed legs have HSN responses that closely resemble those from stationary flies. This does not support the idea that response amplification is initiated by a walking command signal alone [22] and instead suggests that active proprioceptive feedback produced by leg movement is also required to increase HS neuron response. Numerous neuronal mechanisms can account for gain modulation, including changes in background synaptic activity [23], inhibition [24], and release of neuromodulators such as octopamine [19], and additional experiments will be required to identify the specific mechanisms involved.
When presented with constant spatial frequency grating patterns over a range of temporal frequencies, insects display optomotor turning reactions that give rise to a bell-shaped tuning curve, featuring a maximum response at some intermediate, temporal frequency optima (TFO) with reduced amplitude responses for lower and higher temporal frequencies [25, 26]. The published TFO measured for Drosophila optomotor turning behavior in response to rotatory motion by walking tethered flies is in the range of 1–4 Hz [27, 28], and in tethered flight it is reported to peak within the range of 5–10 Hz [29, 30] (but see [31]). However, the electrophysiological responses of LPTCs to moving gratings show a lower temporal frequency peak at approximately 1 Hz in restrained Drosophila (for VS neurons [32] and HS neurons [10]). The results summarized in Figure 4 may partly resolve this paradox. In our calcium imaging data, the TFO measured in HS neurons for quiescent flies parallels the electrophysiological measurements, peaking at approximately 1 Hz, while the responses for the actively walking flies feature a TFO that is shifted toward higher speeds, closer to the 3 Hz peak previously reported for walking flies [27]. It remains to be seen whether this result can be generalized to other LPTCs and to other motion-sensitive neurons that have not yet been recorded from. However, given the strong agreement of our fixed-leg data with recent electrophysiological results [10] and several recent results demonstrating gain modulation [4, 19, 22], it appears quite likely that the behavioral state of the fly will, in general, affect the speed tuning of motion-sensitive neurons. Perhaps if these experiments were repeated in a flying fly [4], the TFO would be shown to shift even further toward the behaviorally observed range for flight.
Changes in the tuning of visual neurons have been observed across species during adaptation to repeated presentations of the same stimulus [33, 34]. In our experiments, flies are head-fixed and are presented with identical visual stimuli independent of behavioral state—a very different situation. However, some of the cellular mechanisms underlying the tuning shift that we observe during walking may be shared with those involved in adaptation-related tuning changes. In fly motion vision, it has been proposed that time constants of filters that make up elementary motion detectors might be modified during adaptation [35–37], but evidence for this idea is mixed [38].
The gain modulation and TFO shift that we report is also reminiscent of modulations that have been observed in visual responses under increased temperatures. In the behaviorally relevant temperature range, blowfly photoreceptors have been shown to be more sensitive at elevated temperatures, with an amplitude increase of over 50% and temporal responses that are more than twice as fast [39]. Additionally, the responses of motion-sensitive H1 neurons in the blowfly lobula plate were shown to exhibit an increase in TFO from 2 to 4 Hz [40]. The authors of the H1 study concluded that the increased sensitivity of photoreceptors is the most likely source of the increased sensitivity in the H1 neuron. In both studies, these dramatic changes required an increase of 10°C or more. However, in our experiments, the brain was under perfusion that was temperature controlled to 21°C, and so any potential heating of the brain was clamped by the bath.
Our data demonstrate that when flies are actively walking, HS neurons show both an upward shift in response amplitude and a prominent increase in gain at higher temporal frequencies. What role might the enhanced sensitivity to higher image motion speeds serve? When flies are stationary, responses of motion-sensitive neurons to high image speeds are attenuated, as seen in temporal frequency tuning curves (Figure 1D) [9, 10]. This may be a strategy that stabilizes optomotor responses, whereby flies do not respond with a high behavioral gain to motion that they cannot ultimately track [41]. However, when animals walk, the retina is exposed to a higher range of image speeds because of self motion. The increased response gain to high temporal frequencies that we observed (Figures 4A–4C) may enable the detection of the faster retinal image shifts that the fly experiences when walking through its visual environment.
Experimental Procedures
We mounted a tethered and dissected fly under a two-photon microscope objective, leaving its legs free to move an air-supported ball that is tracked at high resolution [14]. We used a modular LED arena [42] to present horizontally moving vertical gratings with a constant spatial period of 22.5° to the fly at varying temporal frequencies (the angular velocity of the pattern divided by the spatial period) and imaged HS neuron dendrites at 8 Hz in frame scan mode. Experiments were conducted at 21°C. Data analysis was performed in MATLAB (The MathWorks, Natick, MA, USA). Further details of the data analyses and experimental procedures are provided in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank Janelia’s Fly Core and particularly K. Hibbard, D. Hall, and G. Zheng for stock construction and maintenance. We thank Janelia’s Instrumentation Design & Fabrication group for important contributions to optical and mechanical design. We received generous advice and support from others at Janelia, including K. Svoboda and L. Petreanu. We thank G. Turner for comments on the manuscript, and we thank the anonymous referees for their thoughtful suggestions. This research was funded by the Howard Hughes Medical Institute.
Footnotes
Supplemental Information
Supplemental Information includes three figures and can be found with this article online at doi:10.1016/j.cub.2010.06.072.
References
- 1.Homberg U. Flight-Correlated Activity Changes in Neurons of the Lateral Accessory Lobes in the Brain of the Locust Schistocerca-Gregaria. J. Comp. Physiol. [A] 1994;175:597–610. [Google Scholar]
- 2.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu. Rev. Neurosci. 2004;27:611–647. doi: 10.1146/annurev.neuro.26.041002.131039. [DOI] [PubMed] [Google Scholar]
- 3.Bradley DC, Maxwell M, Andersen RA, Banks MS, Shenoy KV. Mechanisms of heading perception in primate visual cortex. Science. 1996;273:1544–1547. doi: 10.1126/science.273.5281.1544. [DOI] [PubMed] [Google Scholar]
- 4.Maimon G, Straw AD, Dickinson MH. Active flight increases the gain of visual motion processing in Drosophila. Nat. Neurosci. 2010;13:393–399. doi: 10.1038/nn.2492. [DOI] [PubMed] [Google Scholar]
- 5.Niell CM, Stryker MP. Modulation of visual responses by behavioral state in mouse visual cortex. Neuron. 2010;65:472–479. doi: 10.1016/j.neuron.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor GK, Krapp HG. Sensory systems and flight stability: What do insects measure and why? Adv Insect Physiol. 2007;34:231–316. [Google Scholar]
- 7.Hausen K, Wehrhahn C. Microsurgical Lesion of Horizontal Cells Changes Optomotor Yaw Responses in the Blowfly Calliphora-Erythrocephala. P Roy Soc Lond B Biol. 1983;219:211–216. [Google Scholar]
- 8.Heisenberg M, Wonneberger R, Wolf R. Optomotor-Blind - Drosophila Mutant of Lobula Plate Giant Neurons. J. Comp. Physiol. 1978;124:287–296. [Google Scholar]
- 9.Borst A, Haag J. Neural networks in the cockpit of the fly. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:419–437. doi: 10.1007/s00359-002-0316-8. [DOI] [PubMed] [Google Scholar]
- 10.Schnell B, Joesch M, Foerstner F, Raghu SV, Otsuna H, Ito K, Borst A, Reiff DF. Processing of Horizontal Optic Flow in Three Visual Interneurons of the Drosophila Brain. J Neurophysiol. 2010;103:1646–1657. doi: 10.1152/jn.00950.2009. [DOI] [PubMed] [Google Scholar]
- 11.Krapp HG, Hengstenberg R, Egelhaaf M. Binocular contributions to optic flow processing in the fly visual system. J. Neurophysiol. 2001;85:724–734. doi: 10.1152/jn.2001.85.2.724. [DOI] [PubMed] [Google Scholar]
- 12.Kern R, van Hateren JH, Michaelis C, Lindemann JP, Egelhaaf M. Function of a fly motion-sensitive neuron matches eye movements during free flight. PLoS Biol. 2005;3:e171. doi: 10.1371/journal.pbio.0030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karmeier K, van Hateren JH, Kern R, Egelhaaf M. Encoding of naturalistic optic flow by a population of blowfly motion-sensitive neurons. J. Neurophysiol. 2006;96:1602–1614. doi: 10.1152/jn.00023.2006. [DOI] [PubMed] [Google Scholar]
- 14.Seelig JD, Chiappe ME, Lott GK, Dutta A, Osborne JE, Reiser MB, Jayaraman V. Two-photon calcium imaging from head-fixed Drosophila during optomotor walking behavior. Nat Methods. 2010;7:535–540. doi: 10.1038/nmeth.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian L, Hires SA, Mao T, Huber D, Chiappe ME, Chalasani SH, Petreanu L, Akerboom J, McKinney SA, Schreiter ER, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proc. Natl. Acad. Sci. USA. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott EK, Raabe T, Luo L. Structure of the vertical and horizontal system neurons of the lobula plate in Drosophila. J. Comp. Neurol. 2002;454:470–481. doi: 10.1002/cne.10467. [DOI] [PubMed] [Google Scholar]
- 18.Dürr V, Egelhaaf M. In vivo calcium accumulation in presynaptic and postsynaptic dendrites of visual interneurons. J. Neurophysiol. 1999;82:3327–3338. doi: 10.1152/jn.1999.82.6.3327. [DOI] [PubMed] [Google Scholar]
- 19.Longden KD, Krapp HG. State-dependent performance of optic-flow processing interneurons. J. Neurophysiol. 2009;102:3606–3618. doi: 10.1152/jn.00395.2009. [DOI] [PubMed] [Google Scholar]
- 20.Niven JE, Laughlin SB. Energy limitation as a selective pressure on the evolution of sensory systems. J. Exp. Biol. 2008;211:1792–1804. doi: 10.1242/jeb.017574. [DOI] [PubMed] [Google Scholar]
- 21.Haag J, Borst A. Spatial distribution and characteristics of voltage-gated calcium signals within visual interneurons. J. Neurophysiol. 2000;83:1039–1051. doi: 10.1152/jn.2000.83.2.1039. [DOI] [PubMed] [Google Scholar]
- 22.Rosner R, Egelhaaf M, Warzecha AK. Behavioural state affects motion-sensitive neurones in the fly visual system. J. Exp. Biol. 2010;213:331–338. doi: 10.1242/jeb.035386. [DOI] [PubMed] [Google Scholar]
- 23.Shu Y, Hasenstaub A, Badoual M, Bal T, McCormick DA. Barrages of synaptic activity control the gain and sensitivity of cortical neurons. J. Neurosci. 2003;23:10388–10401. doi: 10.1523/JNEUROSCI.23-32-10388.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothman JS, Cathala L, Steuber V, Silver RA. Synaptic depression enables neuronal gain control. Nature. 2009;457:1015–1018. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichardt W. Autocorrelation, a principle for the evaluation of sensory information by the central nervous system. In: Rosenblith WA, editor. Sensory communication. Cambridge, MA: MIT Press; 1961. pp. 303–317. [Google Scholar]
- 26.Srinivasan MV, Poteser M, Kral K. Motion detection in insect orientation and navigation. Vision Res. 1999;39:2749–2766. doi: 10.1016/s0042-6989(99)00002-4. [DOI] [PubMed] [Google Scholar]
- 27.Gotz KG, Wenking H. Visual control of locomotion in the walking fruitfly Drosophila. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1973;85:235–266. [Google Scholar]
- 28.Buchner E. Behavioural analysis of spatial vision in insects. In: Ali MA, editor. Photoreception and vision in invertebrates. New York: Plenum Press; 1984. pp. 561–621. [Google Scholar]
- 29.Duistermars BJ, Chow DM, Condro M, Frye MA. The spatial, temporal and contrast properties of expansion and rotation flight optomotor responses in Drosophila. J. Exp. Biol. 2007;219:3218–3227. doi: 10.1242/jeb.007807. [DOI] [PubMed] [Google Scholar]
- 30.Fry SN, Rohrseitz N, Straw AD, Dickinson MH. Visual control of flight speed in Drosophila melanogaster. J. Exp. Biol. 2009;212:1120–1130. doi: 10.1242/jeb.020768. [DOI] [PubMed] [Google Scholar]
- 31.Götz KG. Optomotorische untersuchung des visuellen systems einiger augenmutanten der fruchtfliege Drosophila. Biol. Cybern. 1964;2:77–92. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- 32.Joesch M, Plett J, Borst A, Reiff DF. Response properties of motion-sensitive visual interneurons in the lobula plate of Drosophila melanogaster. Curr. Biol. 2008;18:368–374. doi: 10.1016/j.cub.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Hietanen MA, Crowder NA, Price NS, Ibbotson MR. Influence of adapting speed on speed and contrast coding in the primary visual cortex of the cat. J. Physiol. 2007;584:451–462. doi: 10.1113/jphysiol.2007.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat. Neurosci. 2004;7:764–772. doi: 10.1038/nn1267. [DOI] [PubMed] [Google Scholar]
- 35.Clifford CW, Ibbotson MR, Langley K. An adaptive Reichardt detector model of motion adaptation in insects and mammals. Vis. Neurosci. 1997;14:741–749. doi: 10.1017/s0952523800012694. [DOI] [PubMed] [Google Scholar]
- 36.Maddess T, Laughlin SB. Adaptation of the Motion-Sensitive Neuron H-1 Is Generated Locally and Governed by Contrast Frequency. P Roy Soc Lond B Biol. 1985;225:251–275. [Google Scholar]
- 37.Van Steveninck RRD, Zaagman WH, Mastebroek HAK. Adaptation of Transient Responses of a Movement-Sensitive Neuron in the Visual-System of the Blowfly Calliphora-Erythrocephala. Biol. Cybern. 1986;54:223–236. [Google Scholar]
- 38.Harris RA, O’Carroll DC, Laughlin SB. Adaptation and the temporal delay filter of fly motion detectors. Vision Res. 1999;39:2603–2613. doi: 10.1016/s0042-6989(98)00297-1. [DOI] [PubMed] [Google Scholar]
- 39.Tatler B, O’Carroll DC, Laughlin SB. Temperature and the temporal resolving power of fly photoreceptors. J. Comp. Physiol. [A] 2000;186:399–407. doi: 10.1007/s003590050439. [DOI] [PubMed] [Google Scholar]
- 40.Warzecha A, Horstmann W, Egelhaaf M. Temperature-dependence of neuronal performance in the motion pathway of the blowfly calliphora erythrocephala. J. Exp. Biol. 1999;202:3161–3170. doi: 10.1242/jeb.202.22.3161. [DOI] [PubMed] [Google Scholar]
- 41.Warzecha AK, Egelhaaf M. Intrinsic properties of biological motion detectors prevent the optomotor control system from getting unstable. Philos T Roy Soc B. 1996;351:1579–1591. [Google Scholar]
- 42.Reiser MB, Dickinson MH. A modular display system for insect behavioral neuroscience. J. Neurosci. Methods. 2008;167:127–139. doi: 10.1016/j.jneumeth.2007.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.