Abstract
The SCID-hu Thy/Liv mouse and human fetal thymic organ culture (HF-TOC) models have been used to explore the pathophysiologic mechanisms of HIV-1 infection in the thymus. We report here that HIV-1 infection of the SCID-hu Thy/Liv mouse leads to the induction of MHC class I (MHCI) expression on CD4+CD8+ (DP) thymocytes, which normally express low levels of MHCI. Induction of MHCI on DP thymocytes in HIV-1-infected Thy/Liv organs precedes their depletion and correlates with the pathogenic activity of the HIV-1 isolates. Both MHCI protein and mRNA are induced in thymocytes from HIV-1-infected Thy/Liv organs, indicating induction of MHCI gene expression. Indirect mechanisms are involved, because only a fraction (<10%) of the DP thymocytes were directly infected by HIV-1, although the majority of DP thymocytes are induced to express high levels of MHCI. We further demonstrate that IL-10 is induced in HIV-1-infected thymus organs. Similar HIV-1-mediated induction of MHCI expression was observed in HF-TOC assays. Exogenous IL-10 in HF-TOC induces MHCI expression on DP thymocytes. Therefore, HIV-1 infection of the thymus organ leads to induction of MHCI expression on immature thymocytes via indirect mechanisms involving IL-10. Overexpression of MHCI on DP thymocytes can interfere with thymocyte maturation and may contribute to HIV-1-induced thymocyte depletion.
Although not well studied during HIV-1 infection, the thymus has been implicated as a site of early viral replication (1–3), and thymic organs from HIV-1-infected fetuses and pediatric patients show profound parenchymal damage and involution (4–6). More significantly, a strong correlation of HIV-1-induced thymus dysfunction to faster AIDS progression has been established in pediatric patients (7). Since the thymus organ is difficult to study in human subjects, a small animal model for the analysis of human thymopoiesis (SCID-hu Thy/Liv mouse) has been constructed by engrafting fragments of human fetal liver and thymus into the immunodeficient C.B-17 scid/scid (SCID) mouse (8, 9). The Thy/Liv organ promotes normal, long term differentiation of human T cells (9, 10). Thymocyte subpopulations are normally represented, a normal TCR V repertoire is displayed (11, 12), and tolerance is induced toward both self MHC Ags and exogenously provided superantigens (13, 14).
After inoculation of the SCID-hu Thy/Liv mouse with HIV-1, replication of pathogenic HIV-1 isolates reaches high levels at 2 wk postinfection (wpi),5 followed by depletion of CD4+ thymocytes with an inversion of the CD4/CD8 ratio between 3–4 wpi (15–18). CD4+CD8+ (DP) thymocytes, comprising 80–85% of the total thymocytes, are significantly depleted. In addition, a higher rate of replication and thymocyte depletion is observed with rapidly replicating, syncytium-inducing virus isolated from AIDS patients than with slowly replicating, nonsyncytium-inducing virus isolated from the same patients before AIDS development or from long term nonprogressor patients (19). As observed in the SIV-infected rhesus macaque (20), replication of HIV-1 in the SCID-hu mouse is dependent upon an intact nef open reading frame (21). Analysis of the other HIV-1 accessory genes, such as vpr, vpu, and vif, has demonstrated that, unlike in tissue cultures, mutations in these genes significantly slow down the replication and cytopathic effects of HIV-1 (22, 23). Thus, the SCID-hu Thy/Liv mouse provides a relevant in vivo model to evaluate primary HIV-1 replication and pathogenicity.
Both direct and indirect mechanisms of thymocyte depletion have been implicated in HIV-1-infected thymus organs (16, 18, 24, 25). Target cell depletion may be achieved by a number of HIV-1-encoded factors with cytotoxic or cytostatic activities, as demonstrated in T cells in vitro. For example, vpr has been shown to lead to G2/S phase cell cycle arrest in infected target cells (26, 27). Other HIV-1 proteins, such as Tat, Nef, and gpl20/gp41, have also demonstrated cytotoxic activity in various cell culture systems (28–31). Apoptosis has been associated with HIV-1-induced T cell death both in vitro and in vivo (32–36). In the Thy/Liv organ, some thymocytes with condensed nuclei are detected in HIV-1-infected Thy/Liv organs by thin section light microscopy and electron microscopy (16). Biochemically, partial chromosomal loss (detected by propidium iodide staining) (16) and DNA strand breaks (detected by terminal deoxynucleotide transferase labeling) (24) are associated with HIV-1-induced thymocyte depletion. DNA stand breaks are detected both in HIV-1-infected and in uninfected cells. However, data from a recent report suggest that necrosis by cytolytic infection may be a major mechanism of HIV-1-induced thymocyte depletion, and significant levels of apoptosis are only detected at late times postinfection (25). In addition, the intrathymic T progenitor cells can be directly infected and depleted to lead to thymocyte depletion by blocking T cell development (24).
The thymus microenvironment is essential for T cell development. Destruction of thymic epithelial cells has been reported in the human thymus and in the SCID-hu Thy/Liv organ after HIV-1 infection (4, 17). This may block T cell development and result in thymocyte depletion. Indeed, two recent reports suggest that hemopoietic progenitor cells in HIV-1-infected Thy/Liv organs are preferentially depleted by indirect mechanisms (37, 38). It is not clear, however, whether the HIV-1-infected thymic stromal cells are still functional in supporting de novo human T cell development. Only transient T cell development is reported after efficient inhibition of HIV-1 replication (39).
During T cell maturation in the thymus, MHC class I (MHCI) is differentially expressed on different subpopulations of thymocytes. Of note, the expression of MHCI on CD4+CD8+ DP thymocytes is lower than that on more mature CD4+ or CD8+ SP thymocytes (40). Thus, lowered expression of MHCI on DP thymocytes is associated with and may be important for normal thymocyte maturation. It has been reported that intrathymic transfer of semiallogeneic thymocytes can induce transient allotolerance in host-derived T cells (41). Supporting the idea that proper levels of interaction between MHCI and CD8/TCR on developing thymocytes are important for normal thymocyte maturation, ectopic expression of MHCI on thymocytes leads to thymocyte depletion in a transgenic mouse model (42). Similar results are reported in transgenic mice expressing high levels of CD8 on thymocytes (43). Interference with intrathymic selection signals of immature thymocytes is probably involved. We report here that HIV-1 infection of the thymus organ leads to enhanced expression of MHCI on DP thymocytes by indirect mechanisms in the SCID-hu Thy/Liv mouse and in human fetal thymus organ cultures (HF-TOC).
Materials and Methods
Reagents
mAbs reactive with CD3, CD4, and CD8 were purchased from Becton Dickinson (San Jose, CA). Anti-MHCI mAb W6/32 was obtained from Biodesign (Kennebunk, ME). HC-10 mAb and a cDNA encoding the MHCI gene (44) were provided by Ed Collins (University of North Carolina, Chapel Hill, NC). Human recombinant IL-10, IL-7, and IL-2 were purchased from Endogen (Woburn, MA). HIV-1 isolates used in this study have been described previously: NL4-3 (45); JD and EW (24); A6/87, A7/88, B5/85, B11/88, and D9/90 (46); 89.6 (47); and JR-CSF (15).
Infection of SCID-hu Thv/Liv mice
Animal transplantation procedures for SCID-hu Thy/Liv construction have been previously described (9). Infection of SCID-hu Thy/Liv mice was performed as previously described (24). Briefly, SCID-hu Thy/Liv mice (4–6 mo after transplantation) were infected with supernatant collected from PHA-activated PBMC containing no HIV-1 (mock) or 4 × 103 to 104 TCID50/ml of HIV-1. Fifty microliters (200–2000 TCID50) were injected into each thymus graft. The Thy/Liv organs were harvested at the indicated times, thymocyte suspension was prepared, and thymocyte subpopulations were analyzed by FACS (CD4-PE, CD8-TC, MHCI-FITC).
The study was approved by the institutional review boards, and animal experimentation guidelines were followed.
HF-TOC assays
The HF-TOC procedures are essentially as previously described (48). Briefly, human fetal thymi (19–24 gestational wk) were dissected into approximately 2-mm3 fragments containing about two to four intact thymic lobules and transferred into either mock supernatant or virus-containing supernatant. The vials were gently rocked at room temperature for 2 h, and the fragments were transferred to 0.45-μm pore size Nucleopore filters (Millipore, Bedford, MA) atop gelfoam boats (Upjohn, Kalamazoo, MI) saturated in HF-TOC medium (RPMI, 10% FCS, 50 μg/ml streptomycin, 50 U/ml penicillin G, 1 × MEM vitamin solution (Life Technologies, Gaithersburg, MD), and 1 × insulin/transferrin/sodium selenite medium supplement (Sigma, St. Louis, MO)) in 6-well tissue culture plates. The fragments were then cultured at 37°C with 5% CO2 for 4–8 days with daily changes of culture medium.
HIV-1 replication and cytokine assays
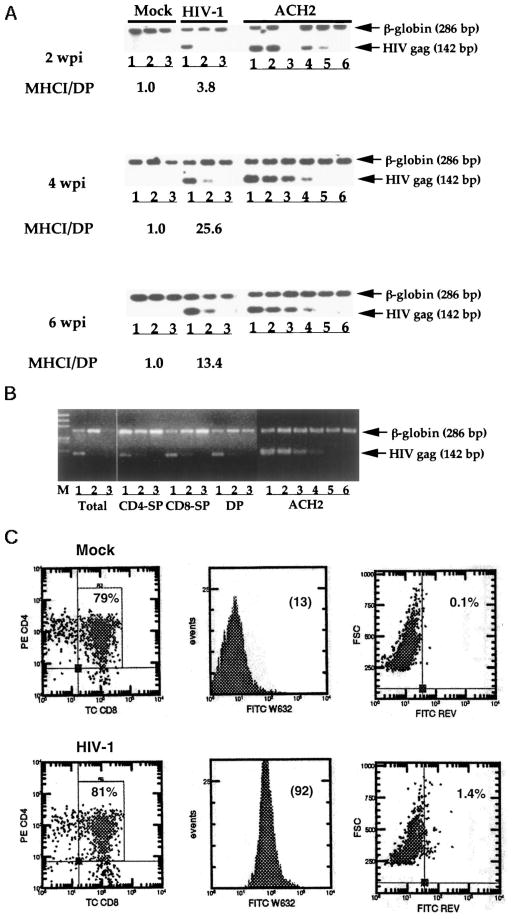
Measurement of cell-associated p24 production (picograms per 106 thymocytes) was performed using a p24 ELISA kit (DuPont, Wilmington, DE). Semiquantitative DNA PCR analysis was performed essentially as previously described (24). Briefly, 10,000 human thymocytes from Thy/ Liv grafts or HF-TOC fragments were assayed by 10-fold dilution of infected cells into uninfected human cells. Genomic DNA was prepared from the mixed cells (Fig. 4, lane 1, 10,000 sample cells; Fig. 4, lane 2, 1,000 sample cells plus 9,000 normal human cells; Fig. 4, lane 3, 100 sample cells plus 9,900 normal human cells). ACH2 cells (1 HIV-1 genome/cell) were used as a standard control. A standard titration represents samples with 100, 10, 1, 0.1, 0.01, and 0%, respectively, ACH2 cells. For detecting HIV-1 Rev+ thymocytes, cells were stained with CD4-PE and CD8-TC, fixed and permeabilized, and followed by intracellular staining of Rev (anti-Rev-FITC) as previously described (49). CEM parental cells or CEM stably expressing an HIV-1 rev transgene was used as the control.
FIGURE 4.
Indirect induction of MHCI expression by HIV-1 infection. A, Semiquantitative PCR assays (24) were used to analyze HIV-1 proviral DNA loads in total thymocytes at various times postinfection (2–6 wpi). A representative mock- or HIV-1-infected sample is shown to demonstrate the relative proviral DNA loads. The fold induction of MHCT on DP thymocytes by HIV-1 infection is indicated. Lane 3 of ACH2 control cells at 2 wpi was from PCR run in the absence of human cell DNA. Lanes 1–3 represent 100, 10, and 1% sample cells mixed with normal thymocytes, respectively. For ACH2 cells, lanes 1– 6 represent 100, 10, 1, 0.1, 0.01, and 0% ACH2 cells mixed with normal human thymocytes. A total of 1O4 cells were used in each PCR reaction. Similar results were observed in three independent experiments. B, Purified thymocyte subpopulation from a SCID-hu Thy/Liv mouse infected at 14 dpi (NL4-3). No significant thymocyte depletion was detected at this time point, and MHCI levels on DP thymocytes were induced by 7-fold in this Thy/Liv organ. Thymocyte sub-populations were purified to >90%, and PCR analysis was performed as described above. CD4−SP, CD4+CD8− thymocytes; CD8−SP, CD4−CD8+ thymocytes; DP, CD4+CD8+ thymocytes. C, The majority of DP thymocytes with enhanced MHCI expression are not productively infected by HIV-1. Mock or HIV-1 (JD)-infected SCID-hu Ty/Liv mice were analyzed at 2 wpi. Total live cells were gated based on light scatter profiles. The percentage of the DP subpopulation is shown (left panel). MHCI expression of DP thymocytes is shown as the mean channel fluorescence (MCF) in the histograms (middle panel). Intracellular HIV-1 Rev expression was detected by FACS, and the percentage of Rev+ cells is shown (right panel). At least three independent experiments with duplicate samples were performed to confirm the results.
Cell-associated IL-10 was measured with an IL-10 ELISA kit (Bio-Source International, Camarillo, CA). Briefly, thymocytes were prepared from SCID-hu Thy/Liv organs or from HF-TOC fragments as described above. Equal numbers of thymocytes were lysed, and standard ELISA was performed as described in the kit instructions. IL-10 levels from mock-infected HF-TOC fragments were used as baselines (1×) in each experiment.
Western blot and RNA blot analysis
Western blot analysis was performed with total thymocyte cell extracts prepared from mock- or HIV-1-infected Thy/Liv organs between 10–15 days postinfection (dpi). Thymocytes were meshed out of the thymic stromal cells, and protein extracts from equal number of thymocytes (3 × 106) were run on SDS-PAGE. The anti-MHCI mAb (HC-10) and the chemiluminescent detection system (Amersham, Arlington Heights, IL) were used for Western detection. Total RNA (7.5 μg) isolated from thymocytes prepared as described above were used to analyze MHCI gene expression by standard Northern blot with an MHCI cDNA as probe 44). RNA samples were confirmed by ribosomal RNA bands and by blotting with a human β-actin cDNA probe.
Results
Induction of MHCI expression on DP thymocytes in HIV-infected Thy/Liv organs
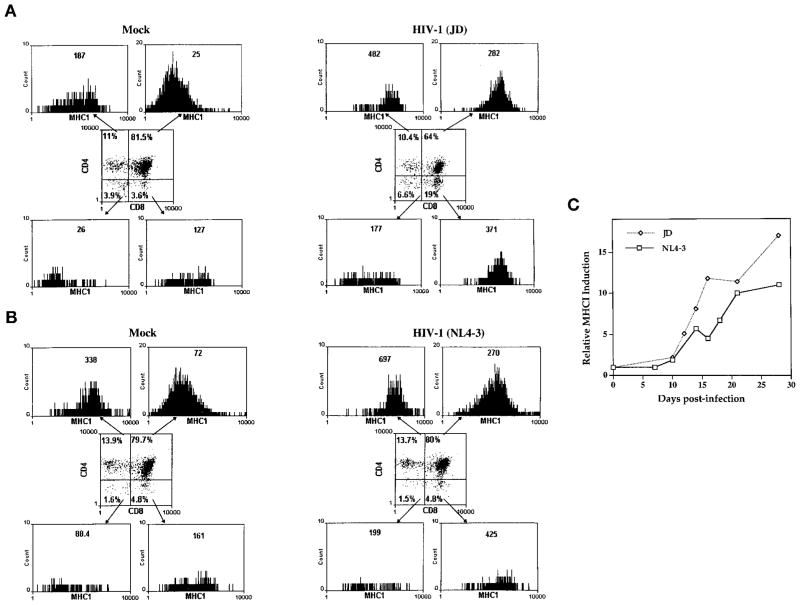
We analyzed phenotypic changes in thymocytes in HIV-1-infected Thy/Liv organs. During human thymocyte maturation, MHCI expression was low in immature CD4+CD8+ DP thymocytes and high in CD4+ or CD8+ SP thymocytes (Fig. 1). As shown in two representative Thy/Liv organs at 14 dpi with mock or HIV-1, all DP thymocytes were induced by HIV-1 infection to express 11-fold (JD infected; Fig. 1A) or 4-fold (NL4-3 infected; Fig. 1B) higher levels of MHCI. The differences in basal level MCF of MHCI in the two experiments were due to the setting differences of the FACScan cytometer in different experiments. Multiple mock samples in the same experiment always showed similar basal MHCI levels (data not shown). After HIV-1 infection, surface MHCI expression on CD4−CD8− (DN) and CD4+ or CD8+ SP thymocytes was also enhanced by 2- to 6-fold and 2- to 3-fold, respectively. This suggests that direct infection of thymocytes is not necessary for MHCI induction.
FIGURE 1.
HIV-1 infection-induced MHCI expression on immature thymocytes in the SCID-hu Thy/Liv mouse. A, Mock- or HIV-1 (JD)-infected Thy/Liv organs were harvested at 14 dpi and analyzed for surface MHCI expression (W6/32) on each thymocyte subpopulation. Total live cells were gated based on light scatter profiles. The percentage of each subpopulation is shown. MHCI expression of each subpopulation is shown as the mean channel fluorescence (MCF) in the histograms. B, As in A, NL4-3-infected Thy/Liv organs (14 dpi) showed similar induction of MHCI on immature thymocytes. The difference in basal MHCI levels was due to different flow cytometer settings in different experiments. At least five SCID-hu Thy/Liv mice mock infected or infected with each HIV-1 isolate were analyzed in more than three independent experiments, and similar results were observed. C, The kinetics of MHCI induction on DP thymocytes after infection with two HIV-1 isolates from multiple experiments are summarized. JD (diamond) and NL4-3 (square) are pathogenic viruses that replicate to peak levels at 2 wpi and deplete thymocytes after 3–4 wpi (24). Shown is the average fold induction of MHCI on DP thymocytes (MCF from HIV-1-infected/mock samples) derived from 5–10 SCID-hu Thy/Liv mice at each time point.
Aggregate data from multiple experiments using two different HIV-1 isolates are summarized in Fig. 1C and Table I. The induction of MHCI expression on DP thymocytes appeared to precede HIV-1-induced thymocyte depletion (18, 24). At later stages postinfection, even higher levels of MHCI on DP thymocytes were induced (Fig. 1C and Table I). In agreement with their activity in replication and pathogenicity in the thymus, HIV-1 isolates from patients before AIDS (A6/87 and B5/85) or from long term non-progressors (D9/90) showed significantly lower levels of MHCI induction than isolates from the same patients after AIDS progression (A7/88 and B11/88) or other pathogenic isolates, such as NL4-3, JD, and EW (19, 24). All DP cells were affected, and the level of induction correlated with the level of HIV-1 replication, as measured by p24 (Table I and data not shown).
Table I.
Correlation of HIV-1 replication/pathogenicity with MHCI induction on DP thymocytes
| HIV-1 (N)a | HIV-1 Replicationb | Pathogenicityc | Fold Induction (SE)d |
|---|---|---|---|
| NL4-3 (25) | +++ | +++ | 10 (1.6) |
| JD (10) | +++ | +++ | 17 (2.7) |
| EW (9) | +++ | +++ | 22 (2.6) |
| A6/87 (5) | + | +/− | 3.7 (0.2) |
| A7/88 (4) | +++ | +++ | 22 (2.4) |
| B5/85 (3) | + | +/− | 2.1 (0.5) |
| B11/88 (3) | +++ | +++ | 6.8 (2.5) |
| D9/90 (6) | + | +/− | 3.3 (0.4) |
HIV-1 clones/isolates with different replication (p24 production) and pathogenicity (thymocyte depletion) were compared in MHCI induction. N is the number of SCID-hu Thy/Liv mice (3–4 wk postinfection) analyzed.
Thymocyte-associated p24 levels (pg/106 thymocytes) and proviral DNA loads by PCR are summarized. +++, significant levels of p24 (>200 pg/106 thymocytes) at 2–4 wpi; +, <100 pg/106 thymocytes at 4–6 wpi.
Significant depletion of DP thymocytes and inversion of CD4+/CD8+ ratios at 3–4 wpi indicate high pathogenicity (+++). Failure to show significant thymocyte depletion at 6–8 wpi is marked as +/−.
Fold induction indicates mean channel fluorescence (MCF) of MHCI (W6/32) staining on CD4+ CD8+ DP thymocytes from HIV-1 infected Thy/Liv organs divided by MCF of DP thymocytes from mock-infected Thy/Liv organs.
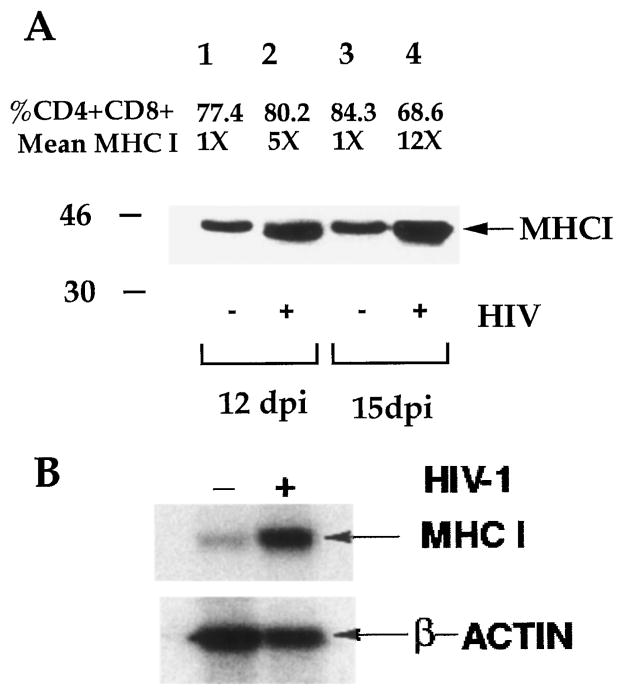
To confirm the induced expression of MHCI in HIV-1-infected thymus organs, total thymocytes from mock- or HIV-1-infected (12 or 15 dpi) Thy/Liv organs were harvested, and MHCI protein was measured by Western blot analysis with the HC-10 mAb, which reacts with the unfolded MHCI protein of HLA-A, -B, and -C alleles. Mock- and HIV-1-infected Thy/Liv organs showed similar percentages of SP and DP thymocytes at 12 dpi and a slight reduction of DP thymocytes at 15 dpi. In agreement with FACS detection of surface MHCI expression on thymocytes, total MHCI proteins in thymocytes were induced significantly in HIV-1-infected samples (Fig. 2A). Thus, HIV-1 infection of the SCID-hu Thy/Liv mouse led to induction of MHCI protein expression in thymocytes.
FIGURE 2.
Induction of MHCI gene expression in HIV-1-infected Thy/ Liv organs. A, SCID-hu Thy/Liv mice infected with mock supernatant (−) or with JD (+) were harvested at 12 and 15 dpi, and total cell proteins from an equal number of thymocytes were analyzed by Western blot with the anti-MHCI mAb, HC-10. Relative MHCI levels on DP thymocytes and percentage of DP thymocytes from mock- and HIV-1-infected Thy/Liv organs are presented. B, Total RNA samples from a mock- or HIV-1-infected SCID-hu Thy/Liv mouse at 2 wpi were analyzed by Northern blot. Relative expression levels of MHCI mRNA was quantified by phosphorimager. The fold HIV-1-induced MHCI RNA expression (7.8) is shown. The β-actin mRNA bands were used to show the relative amounts of total RNA from mock- or HIV-1-infected samples. The experiment was repeated three times with similar results.
To test whether HIV-1 induced MHCI gene transcription in thymocytes, MHCI mRNA levels were analyzed using an MHCI cDNA probe (44). Similar levels of induction of MHCI RNA was observed in HIV-1-infected Thy/Liv organs at 14 dpi (7- to 8-fold; Fig. 2B). Therefore, HIV-1 infection of the SCID-hu Thy/Liv mouse induced MHCI mRNA expression in immature thymocytes, probably via increased transcription.
Induction of MHCI on DP thymocytes in HIV-1-infected HF-TOC
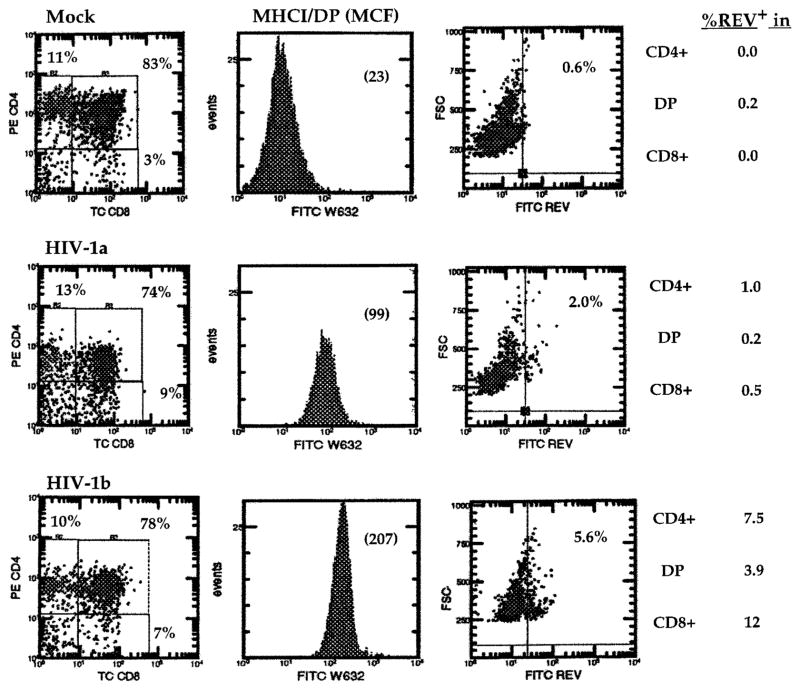
To rule out the possibility that murine host factors of SCID-hu Thy/Liv mice may contribute to the phenotypic changes, we performed similar experiments in the HF-TOC model (48). As in the SCID-hu Thy/Liv mouse, HIV-1 infection of HF-TOC also led to induction of MHCI expression on DP cells (Fig. 3 and Table II), before any significant thymocyte depletion. Thus, HIV-1 infection of the human thymus induced MHCI expression on DP thymocytes. In addition, HIV-1 isolates with different tropism determinants were all able to induce MHCI expression. CXCR4-tropic isolates (NL4-3 and EW), CCR5-tropic virus (JR-CSF), and dual tropic isolate (89.6 and JD) were able to efficiently induce MHCI expression on DP thymocytes. HXB2 failed to replicate efficiently in SCID-hu Thy/Liv mice or in HF-TOC (23, 48) and showed no induction of MHCI.
FIGURE 3.
Induction of MHCI expression in HIV-1-infected HF-TOC assays. HF-TOC were infected with mock or HIV-1 (NL4-3) supernatant and analyzed at 7 dpi. Two representative HIV-1-infected HF-TOC samples were shown (HIV-1a and HIV-1b). MHCI expression on DP thymocytes is shown as the mean channel fluorescence (MCF) in parentheses. The histogram on the right shows cells expressing intracellular HIV-1 Rev detected by a separate FACS analysis (Rev-FITC/CD4-PE/CD8-TC). HIV-1 infection (percentage of Rev+) in each thymocyte subpopulation is presented. CEM cell expression of HIV-1 Rev proteins (49) was used as a positive control (not shown). HIV-1a and HIV-1b indicate two independent samples infected with NL4-3. Three independent experiments were performed with similar results
Table II.
HIV-1 replication and MCHI induction on DP thymocytes in HF-TOC
| HIV-1 (N)a | Replicationb | Coreceptor Usagec | Fold Induction (SE)d |
|---|---|---|---|
| NL4-3 (11) | + | CXCR4 | 9 (2.6) |
| JD (5) | + | CXCR4/CCR5 | 19 (5.7) |
| EW (4) | + | CXCR4 | 24 (4.6) |
| HXB2 (11) | − | CXCR4 | 1.2 (0.6) |
| 89.6 | + | CXCR4/CCR5 | 7 (1.9) |
| JRCSF | + | CCR5 | 5 (1.7) |
HIV-1 clones/isolates with different replication (p24 production) were compared in MHCI induction. N is the number of HF-TOC assays analyzed. HXB2 is unable to replicate in HF-TOC or SCID-hu Thy/Liv mice (23).
Peak p24 levels of >5000 pg/106 thymocytes are indicated as +; levels of <50 pg/106 thymocytes are indicated as −.
HIV-1 coreceptor usage was determined by CXCR4- and CCR5-dependent fusion or replication assays. JD and EW were tested for this study (K.D. and L.S., unpublished results).
Fold induction indicates mean channel fluorescence (MCF) of MHCI (W6/32) staining on DP thymocytes from HIV-infected HF-TOC divided by MCF on DP thymocytes from mock-infected HF-TOC.
Indirect mechanisms are involved in MHCI induction on DP thymocytes
Using FACS-based detection of HIV-1 Rev proteins (49) in HIV-1-infected HF-TOC samples, we showed that <5% of the DP thymocytes were productively infected, whereas all or most DP thymocytes were induced to express 5- to 9-fold higher levels of MHCI (Fig. 3). Therefore, indirect mechanisms were involved in the induction of MHCI expression on DP thymocyte cells.
As previously reported in HIV-1-infected SCID-hu Thy/Liv mice (15, 17, 21–24), analysis of HIV-1 proviral DNA loads at various times postinfection (2–6 wpi) estimated that <10% of total thymocytes were directly infected (Fig. 4A). High levels of MHCI induction on the majority of DP thymocytes were observed in the HIV-1-infected Thy/Liv organs (4- to 25-fold). Thus, most DP thymocytes with high MHCI expression were not directly infected, especially at early times postinfection.
When DP thymocytes from HIV-1-infected Thy/Liv organs at 2 wpi were purified, and proviral DNA was measured, <10% (1–10%, assuming one proviral genome per cell) were directly infected (Fig. 4B). No significant thymocyte depletion was detected at this time point, and MHCI levels on all DP thymocytes were induced by 7-fold in this Thy/Liv organ infected with NL4-3.
When productively infected thymocytes were measured by intracellular staining of HIV-1 Rev, a very low level (1–5%) of total thymocytes was detected (Fig. 4C) at 2 wpi. As shown in a representative Thy/Liv organ at 2 wpi, DP thymocytes (81%) were induced to express 7-fold higher MHCI. Therefore, the majority of the DP cells, although induced to express high levels of MHCI, were not directly infected by HIV-1.
Induction of IL-10 production in HIV-1-infected thymus organs and IL-10-induced MHCI expression on DP thymocytes
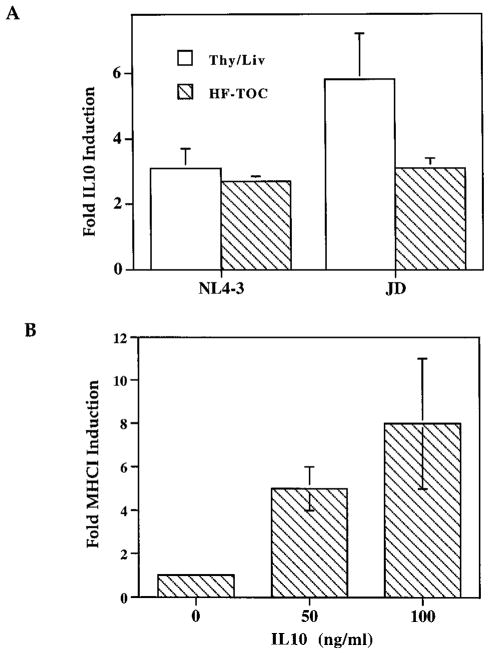
To identify possible cytokines induced by HIV-1 infection, we analyzed the production of a number of cytokines in HIV-1-infected Thy/Liv organs or HF-TOC (50). IL-10 production was induced in HIV-1-infected Thy/Liv organs or HF-TOC by a number of HIV-1 isolates (Fig. 5A and data not shown). IL-10 has been reported to be induced in HIV-1-infected patients (51) and in lymphoid organs of feline immunodeficiency virus-infected cats (52). In addition, IL-10 has been reported to, depending on cell type, inhibit or induce MHC class II expression (53). Both NL4-3- and JD-infected Thy/Liv organs showed significant induction (2- to 5-fold) of IL-10 expression at 2 wpi, before significant thymocyte depletion (Fig. 5A). Similar induction was observed in the HF-TOC model. As induction of IL-10 is not directly correlated with levels of MHIC induction (Fig. 5A), we further tested the effect of exogenous IL-10 on MHCI expression in the HF-TOC model. When provided exogenously in HF-TOC assays, IL-10 induced expression of MHCI on DP thymocytes in a dose-dependent fashion (Fig. 5B). Therefore, HIV-1 infection of the thymus organ led to up-regulation of IL-10 production. Increased levels of IL-10 in the thymus can partly contribute to the induction of MHCI expression. Other cytokines are also likely to contribute to the induction of MHCI expression in HIV-infected thymus organs.
FIGURE 5.
IL-10 is involved in the induction of MHCI expression on DP thymocytes. A, IL-10 is induced in HIV-1-infected thymus organs. IL-10 was measured by ELISA with thymocyte cell lysates from SCID-hu Thy/Liv organs or HF-TOC fragments. SCID-hu Thy/Liv mice infected with NL4-3 (n = 5) or JD (n = 4) at 2 wpi were analyzed. For HF-TOC assays, NL4-3 (n = 8) or JD (n = 10) infection was analyzed at 4–8 dpi. Fold IL-10 induction indicates IL-10 levels in HIV-1-infected samples divided by IL-10 levels from mock-infected samples. SEs are shown as error bars. B, Induction of MHCI expression by IL-10 in HF-TOC. HF-TOC was analyzed at 4 days postculture in the absence or the presence of IL-10. Fold MHCI induction indicates relative MHCI expression on DP thymocytes from IL-10-treated samples over that from mock-treated samples. Two independent experiments with duplicate samples were performed with similar results. SEs are shown.
Discussion
HIV-1 pathogenesis in the thymus plays an important role in AIDS progression in pediatric patients (7). We report here that HIV-1 infection of the human thymus leads to enhanced expression of MHCI on immature thymocytes in SCID-hu Thy/Liv mice and in HF-TOC assays via paracrine mechanisms.
In PBMC or T cell lines, it has been reported that the MHCI level is down-regulated by HIV-1 infection. HIV-1 Tat, Nef, and Vpu have all been reported to reduce surface expression of MHCI in HIV-1-infected cells (54–56). In our thymus models, the majority of the thymocytes affected were not directly infected by HIV-1, suggesting that indirect (viral or host) mediators were induced by HIV-1 infection. Therefore, the mechanisms of HIV-1-induced MHCI expression in immature thymocytes are different from those of MHCI suppression in PBL or T cell lines directly infected by HIV-1.
In addition to production of viral proteins, HIV-1 infection in the Thy/Liv organ leads to increased production of cytokines such as IL-4, IL-6, IL-10, and TGF-β (50). IFN-γ, which stimulates MHCI gene expression, was not significantly induced by HIV-1 in the Thy/Liv organ at 2 wpi (50) (data not shown). Among the induced cytokines, IL-4 showed no significant activity on MHCI induction in HF-TOC (data not shown). IL-10, which was shown to inhibit MHC class II expression in macrophages (53), appeared to induce MHCI expression on thymocytes in HF-TOC (Fig. 5B). However, the level of IL-10 induction is not directly correlated to that of MHCI induction (Fig. 5A and data not shown). Other viral or host factors or a combination of factors may also be involved in the MHCI induction. IL-10-neutralizing Ab may be employed in the HF-TOC model to confirm that IL-10 is involved in HIV-1-induced MHCI expression on thymocytes, although efficient Ab penetration of the HF-TOC fragments has not been demonstrated.
An important question related to HIV-1-induced thymocyte depletion is whether direct infection is required. The replication level of HIV-1 in the Thy/Liv organ is relatively low, especially at early time points. Less than 10% (maximal estimation) of thymocytes are infected at 2 wpi as measured by PCR detection of proviral DNA (15, 17, 18, 21–24, 57). This is consistent with the lack of significant mutations during HIV-1 infection in the SCID-hu Thy/ Liv mouse (57). Our data show that HIV-1 infection leads to the induction of MHCI expression on all DP thymocytes, yet only a small fraction of them are directly infected by HIV-1 (Figs. 1, 3, 4, and 5). Therefore, indirect mechanisms are clearly involved, at least at early (10–15 dpi) times postinfection. A recent report suggests that higher viral loads may be detected at later times postinfection in some biopsies of HIV-1-infected Thy/Liv organs (25, 39). In the SCID-hu Thy/Liv organs we analyzed, HIV-1 infection appeared to be comparably lower by PCR even at later time points. Experimental differences, such as viral infection and/or PCR assays, may contribute to the discrepancy. The biopsy analysis of HIV-1-infected Thy/Liv organs, in contrast to whole organs, may also have biased sampling due to uneven distribution of HIV-1-infected cells in the Thy/Liv organ.
MHCI expression is usually low on DP thymocytes during thymopoiesis (Fig 1A), suggesting that low levels of MHCI on DP cells are associated with and may be required for proper thymocyte selection and maturation. It has been reported that thymocytes expressing allo-MHC can induce allospecific tolerance in host T cells (41). Indeed, in transgenic mice overexpressing MHCI (42) or CD8 (43) on thymocytes, severe thymocyte depletion was observed. This may be due to increased affinity/avidity among TCR, CD8, and MHCI on DP thymocytes to interfere with proper intrathymic selection (58).
The exact mechanisms and the significance of HIV-1-induced MHCI expression on immature thymocytes are not clear. As in the transgenic mouse models, induction of MHCI expression on DP thymocytes by HIV-1 may contribute to HIV-1-induced thymocyte depletion. MHCI on DP thymocytes may interact with CD8/TCR to interfere with the proper selection signals required for thymocyte maturation and survival. In addition, MHCI itself or in combination with other receptors may transduce signals affecting cell survival (59, 60). Further studies of HIV-1 pathogenesis in the SCID-hu Thy/Liv mouse and in the HF-TOC model will not only help understand the mechanisms of HIV-1-induced thymus destruction, but will also shed light on the mechanisms of thymocyte selection and maturation.
Acknowledgments
We thank Drs. Jenny Ting, Roland Tisch, Jeff Frelinger, and Ed Collins for helpful discussions and reagents; Jennifer Auten and Suzan Salimi for technical assistance; Larry Arnold for assistance with flow cytometry; and the tissue culture, oligonucleotide, and the Division of Laboratory Animal Science facilities of the Lineberger Comprehensive Cancer Center at University of North Carolina-Chapel Hill for support. We thank the National Institutes of Health AIDS Research and Reference Reagent Program for providing the 89.6 virus.
Footnotes
This work was supported in part by a Basil O’Connor Scholar award from March of Dimes (to L.S.), National Institutes of Health Grant AI41356 (to L.S.), a National Institutes of Health training grant (to K.D.), and a fellowship from the Irvington Institute for Immunological Research (to K.D.).
Abbreviations used in this paper: wpi, weeks postinfection; DP, double positive; HF-TOC, human fetal thymus organ culture; MHCI, MHC class I; SP, single positive; dpi, days postinfection.
References
- 1.Courgnaud V, Laure F, Brossard A, Bignozzi C, Goudeau A, Barin F, Brechot C. Frequent and early in utero HIV-1 infection. AIDS Res Hum Retroviruses. 1991;7:337. doi: 10.1089/aid.1991.7.337. [DOI] [PubMed] [Google Scholar]
- 2.Harris PJ, Candeloro PD, Bunn JE. HIV infection of the adult thymus: an even more conventional theory explaining CD cell decrease and CD8 cell increase in AIDS. Med Hypotheses. 1991;36:379. doi: 10.1016/0306-9877(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 3.Tremblay M, Numazaki K, Goldman H, Wainberg MA. Infection of human thymic lymphocytes by HIV-1. J Acquired Immune Defic Syndr. 1990;3:356. [PubMed] [Google Scholar]
- 4.Joshi V, Oleske JM. Pathologic appraisal of the thymus gland in acquired immunodeficiency syndrome in children. Arch Pathol Lab Med. 1985;109: 142. [PubMed] [Google Scholar]
- 5.Rosenzweig M, Clark DP, Gaulton GN. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Seemayer TA, Laroche AC, Russo P, Malebranche R, Arnoux E, Guerin J-M, Pierre G, Dupuy J-M, Gartner JG, Lapp WS, et al. Precocious thymic involution manifested by epithelial injury in the acquired immune deficiency syndrome. Hum Pathol. 1984;15:469. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- 7.Kourtis AP, Ibegbu C, Nahmias AJ, Lee FK, Clark WS, Sawyer MK, Nesheim S. Early progression of disease in HIV-infected infants with thymus dysfunction. N Engl J Med. 1996;335:1431. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 8.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: a model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 9.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krowka JF, Sarin S, Namikawa R, McCune JM, Kaneshima H. Human T cells in the SCID-hu mouse are phenotypically normal and functionally competent. J Immunol. 1991;146:3751. [PubMed] [Google Scholar]
- 11.Vandekerckhove BA, Krowka JF, McCune JM, de Vries J, Spits H, Roncarolo MG. Clonal analysis of the peripheral T cell compartment of the SCID-hu mouse. J Immunol. 1991;146:4173. [PubMed] [Google Scholar]
- 12.Vandekerckhove BA, Baccala R, Jones D, Kono DH, Theofilopoulos AN, Roncarolo MG. Thymic selection of the human T cell receptor Vβ repertoire in SCID-hu mice. J Exp Med. 1992;176:1619. doi: 10.1084/jem.176.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandekerckhove BA, Namikawa R, Bacchetta R, Roncarolo MG. Human hematopoietic cells and thymic epithelial cells induce tolerance via different mechanisms in the SCID-hu mouse thymus. J Exp Med. 1992;175:1033. doi: 10.1084/jem.175.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waller EK, Sen MA, Kamel OW, Hansteen GA, Schick MR, Weissman IL. Human T-cell development in SCID-hu mice: staphylococcal enterotoxins induce specific clonal deletions, proliferation, and anergy. Blood. 1992;80:3144. [PubMed] [Google Scholar]
- 15.Aldrovandi GM, Feuer G, Gao L, Jamieson B, Kristeva M, Chen IS, Zack JA. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 16.Bonyhadi ML, Rabin L, Salimi S, Brown DA, Kosek J, McCune JM, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 17.Stanley SK, McCune JM, Kaneshima H, Justement JS, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su L. HIV-1 pathogenesis and therapeutic intervention in the SCID-hu Thy/Liv mouse: a model for primary HIV-1 infection in the human thymus. Rev Med Virol. 1997;7:157. doi: 10.1002/(sici)1099-1654(199709)7:3<157::aid-rmv197>3.0.co;2-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneshima H, Su L, Bonyhadi ML, Connor RI, Ho DD, McCune JM. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kestler Hd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson BD, Aldrovandi GM, Planelles V, Jowett JBM, Gao L, Bloch LM, Chen ISY, Zack JA. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J Virol. 1994;68:3478. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aldrovandi GM, Zack JA. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J Virol. 1996;70:1505. doi: 10.1128/jvi.70.3.1505-1511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su L, Kaneshirna H, Bonyhadi ML, Lee R, Auten J, Wolf A, Du B, Rabin L, Hahn BH, Terwilliger E, et al. Identification of HIV-1 determinants for replication in vivo. Virology. 1997;227:45. doi: 10.1006/viro.1996.8338. [DOI] [PubMed] [Google Scholar]
- 24.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune JM. HIV-1 induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 25.Jamieson BD, Uittenbogaart CH, Schmid I, Zack JA. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J Virol. 1997;71:8245. doi: 10.1128/jvi.71.11.8245-8253.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jowett J, Planelles V, Poon B, Shah N, Chen M, Chen I. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogel M, Wu L, Emerman M. The human immunodeficiency virus type 1 vpr gene prevents cell proliferation during chronic infection. J Virol. 1995;69:882. doi: 10.1128/jvi.69.2.882-888.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 29.Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Park I, Cooper A, Sodroski J. Molecular determinants of acute single-cell lysis by HIV-1. J Virol. 1996;70:1340. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 32.Laurent CA, Krust B, Muller S, Riviere Y, Rey CM, Bechet JM, Montagnier L, Hovanessian AG. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 33.Terai C, Kornbluth RS, Pauza CD, Richman DD, Carson DA. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991;87:1710. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ameisen JC, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol Today. 1991;12:102. doi: 10.1016/0167-5699(91)90092-8. [DOI] [PubMed] [Google Scholar]
- 35.Finkel TH, Tudor-Williams G, Banda NK, Cotton MF, Curiel T, Monks C, Baba TW, Ruprecht RM, Kupfer A. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV-and SIV-infected lymph nodes. Nat Med. 1995;1:129. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 36.Finkel TH, Banda NK. Indirect mechanism of HIV pathogenesis: how does HIV kill T cells? Curr. Opin Immunol. 1994;6:605. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 37.Jenkins M, Hanley MB, Moreno MB, Wieder E, McCune JM. Human immunodeficiency virus-1 infection interrupts thymopoiesis and multi-lineage hematopoiesis in vivo. Blood. 1998;91:2672. [PubMed] [Google Scholar]
- 38.Koka PS, Fraser JK, Bryson Y, Bristol GC, Aldrovandi GM, Daar ES, Zack JA. Human immunodeficiency virus inhibits multilineage hematopoiesis in vivo. J Virol. 1998;72:5121. doi: 10.1128/jvi.72.6.5121-5127.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Withers-Ward ES, Amado RG, Koka PS, Jamieson BD, Kaplan AH, Chen IS, Zack JA. Transient renewal of thymopoiesis in HIV-infected human thymic implants following antiviral therapy. Nat Med. 1997;3:1102. doi: 10.1038/nm1097-1102. [DOI] [PubMed] [Google Scholar]
- 40.Lawlor DA, Zemmour J, Ennis PD, Parham P. Evolution of class-I MHC genes and proteins: from natural selection to thymic selection. Annu Rev Immunol. 1990;8:23. doi: 10.1146/annurev.iy.08.040190.000323. [DOI] [PubMed] [Google Scholar]
- 41.Shimonkevitz RP, Bevan MJ. Split tolerance induced by the intrathymic adoptive transfer of thymocyte stem cells. J Exp Med. 1988;168:143. doi: 10.1084/jem.168.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz R, Mellor AL. Self major histocompatibility complex class I antigens expressed solely in lymphoid cells do not induce tolerance in the CD4+ T cell compartment. J Exp Med. 1996;184:1573. doi: 10.1084/jem.184.4.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robey E, Kioussis RFD, Sha W, Loh D, Axel R, Fawlkes B. The level of CD8 expression can determine the outcome of thymic selection. Cell. 1992;69:1089. doi: 10.1016/0092-8674(92)90631-l. [DOI] [PubMed] [Google Scholar]
- 44.Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci USA. 1992;89: 3429. doi: 10.1073/pnas.89.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adachi A, Gendelman HE, Koenig S, Folks T, Willey R, Rabson A, Martin MA. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connor RI, Mohri H, Cao Y, Ho DD. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J Virol. 1993;67:1772. doi: 10.1128/jvi.67.4.1772-1777.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strizki JM, Turner JD, Collman RG, Hoxie J, Gonzalez-Scarano F. A monoclonal antibody (12G5) directed against CXCR-4 inhibits infection with the dual-tropic human immunodeficiency virus type 1 isolate HIV-1(89.6) but not the T-tropic isolate HIV-1(HxB) J Virol. 1997;71:5678. doi: 10.1128/jvi.71.7.5678-5683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonyhadi ML, Su L, Auten J, McCune JM, Kaneshima H. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res Hum Retroviruses. 1995;11:1073. doi: 10.1089/aid.1995.11.1073. [DOI] [PubMed] [Google Scholar]
- 49.Riggs R, Dando J, Escaich S, Plavec I, Bohnlein E. Detection of intracellular HIV-1 Rev protein by flow cytometry. J Immunol Methods. 1995;188: 187. doi: 10.1016/0022-1759(95)00237-5. [DOI] [PubMed] [Google Scholar]
- 50.Bonyhadi M, Su L, Auten J, McCune JM, Kaneshima H. Cytokine dysregulation in human fetal thymus organ culture and in SCID-hu Thy/Liv mice following infection with HIV-1. Keystone Symposium: Control and Manipulation of the Immune Response; Keystone, CO. 1995. [Google Scholar]
- 51.Clerici M, Wynn TA, Berzofsky JA, Blatt SP, Hendrix CW, Sher A, Coffman RL, Shearer GM. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J Clin Invest. 1994;93:768. doi: 10.1172/JCI117031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy JK, Ritchey JW, Rottman JB, Davidson MG, Liang YH, Jordan HL, Tompkins WA, Tompkins MB. Elevated interleukin-10-to-interleukin-12 ratio in feline immunodeficiency virus-infected cats predicts loss of type 1 immunity to Toxoplasma gondii. J Infect Dis. 1998;178:503. doi: 10.1086/515632. [DOI] [PubMed] [Google Scholar]
- 53.Chadban SJ, Tesch GH, Foti R, Lan HY, Atkins RC, Nikolic-Paterson DJ. Interleukin-10 differentially modulates MHC class II expression by mesangial cells and macrophages in vitro and in vivo. Immunology. 1998;94:72. doi: 10.1046/j.1365-2567.1998.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Howcroft T, Strebel K, Martin M, Singer D. Repression of MHC class I gene promoter activity by two-exon Tat of HIV. Science. 1993;260:1320. doi: 10.1126/science.8493575. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard J. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 56.Kerkau T. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jamieson BD, Pang S, Aldrovandi GM, Zha J, Zack JA. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J Virol. 1995;69:6259. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alam S, Travers P, Wung J, Nasholds W, Redpath S, Jameson S, Gascoigne N. T-cell-receptor affinity and thymocyte positive selection. Nature. 1996;381:616. doi: 10.1038/381616a0. [DOI] [PubMed] [Google Scholar]
- 59.Genestier L, Paillot R, Bonnefoy-Berard N, Meffre G, Flacher M, Fevre D, Liu Y, Le Bouteiller P, Waldmann H, Engelhard V, et al. Fas-independent apoptosis of activated T cells induced by antibodies to the HLA class I al domain. Blood. 1997;90:3629. [PubMed] [Google Scholar]
- 60.Pettersen R, Gaudernack G, Olafsen M, Lie S, Hestdal K. The TCR-binding region of the HLA class I a2 domain signals rapid Fas-independent cell death: a direct pathway for T cell-mediated killing of target cells? J Immunol. 1998;160:4343. [PubMed] [Google Scholar]