Abstract
Background
Adherence to oral naltrexone has been poor and can be improved somewhat with behavioral therapy. We compared Behavioral Naltrexone Therapy (BNT) to Compliance Enhancement (CE) and tested efficacy of single-dose injection naltrexone (XR-NTX; 384 mg) with behavioral therapies at further improving aherence to oral naltrexone.
Methods
A 24-week, randomized, placebo-controlled trial (N=125) compared four treatment conditions following inpatient detoxification and oral naltrexone induction: (1) BNT+XR-NTX; (2) BNT+ placebo injection; (3) CE+ XR-NTX; and (4) CE+placebo injection. All participants were maintained on oral naltrexone throughout the trial. Primary outcome was retention in treatment.
Results
Of 89 randomized participants, 78.7% (70/89) completed 4 weeks, 58.2% (54/89) completed 8 weeks, 47.2% (42/89) completed 12 weeks, and 25.8% (23/89) completed 24 weeks. A Cox proportional hazards regression modeled time to dropout as a function of treatment condition, baseline opioid dependence severity (bags per day of heroin use), and their interaction. Interaction of conditions by baseline severity was significant (X23 = 9.19, p = .027). For low-severity patients (<6 bags/day), retention was highest in the BNT-XRNTX group (60% at 6 months), as hypothesized. For high-severity (> 6 bags/day) patients, BNT-XR-NTX did not perform as well, due to high early attrition.
Conclusion
For low-severity heroin users, single-dose XR-NTX improved long-term treatment retention when combined with behavioral therapy. In higher-severity opioid-dependent patients, XR-NTX was less helpful, perhaps because, combined with oral naltrexone, it produced higher blood levels and more withdrawal discomfort. When cost considerations recommend oral naltrexone following XR-NTX, the latter should be phased in slowly.
Keywords: Opiate dependence treatment, Pharmacotherapy trials, Injection naltrexone, oral naltrexone, Opioid antagonist
1. INTRODUCTION
Opioid dependence represents a serious public health problem affecting a growing number of individuals in the United States. It is estimated that there are 1 million heroin addicts in need of treatment and nearly 2 million untreated prescription opioid addicts in the U.S. (NSDUH, 2011). Agonist maintenance with methadone or buprenorphine is not available or acceptable to many patients, and not all patients respond well to agonists. Naltrexone, a mu-opioid antagonist, acts by a different mechanism and offers an alternative approach to agonist treatment. Naltrexone blocks the effects of opioids, while producing no agonist effects itself, and thus may be helpful to patients who are not suitable for agonist maintenance or have already failed trials of agonist treatment. However, the effectiveness of naltrexone in pill form had been limited by poor adherence and was rarely utilized in practice (Johannson et al., 2006). Prior studies suggested the effectiveness of contingency management, and involvement of significant others at improving adherence to oral naltrexone (Preston et al., 1999; Carroll et al., 2001). Long-acting injectable or implantable formulations of natlrexone, by circumventing the need for daily pill adherence, also improved effectiveness (Comer et al., 2006; Hulse et al., 2005; Krupitsky et al., 2011).
In prior Stage I trials conducted to improve adherence with oral naltrexone for opioid dependence, we developed and tested Behavioral Naltrexone Therapy (BNT), a manual-based therapy integrating elements of Network Therapy, Community Reinforcement Approach, Relapse Prevention Therapy and Motivational Interviewing (Rothenberg et al., 2002; Nunes et al., 2006; Sullivan et al., 2006). BNT was developed to address four potential limitations of naltrexone maintenance: 1) Difficulty transitioning from opiates to naltrexone; 2) Poor adherence; 3) Possible dysphoric effects; and 4) Inadequate psychotherapeutic context. The aims in this early Stage II trial were (1) to test the efficacy of BNT compared to a standard therapy (Compliance Enhancement, a control condition simulating outpatient pharmacotherapy management) for the treatment of opioid dependence; and (2) to test the efficacy of a single dose of a long-acting injectable formulation of naltrexone (XR-NTX; Depotrex, BIOTEK) in reducing early attrition on oral naltrexone and improving long-term outcome of Behavioral Naltrexone Therapy (BNT). In previous trials, we had observed high rates of attrition in the first 4 weeks after inpatient detoxification (Nunes et al., 2006; Sullivan et al., 2006; Rothenberg et al., 2002). By including a single administration of XR-NTX as a condition in the present trial, we hoped to provide a treatment condition in which patients could remain abstinent long enough to engage in therapy and benefit from the elements of BNT. We hypothesized that the combination of BNT and XR-NTX would perform best, resulting in the highest rates of retention among the four treatment groups.
2. METHODS
2.1 Participants
Individuals seeking treatment for opioid dependence at the Substance Treatment and Research Service (STARS) outpatient clinic of Columbia University, in New York City were recruited for this study. Clinical screening included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) (SCID Axis I/P version; First et al., 1995) and a clinical interview assessing substance abuse severity. Medical assessment included history, laboratory tests, electrocardiogram (ECG), a physical examination, and a psychiatric evaluation. Included were men and women 18-60 years old, who met DSM-IV criteria for current opioid dependence and used opioids daily. Participants were required to identify a significant other who was able to attend sessions and monitor compliance. Individuals with major severe affective or psychotic disorder were excluded. Other exclusion criteria included: 1) regular use of methadone (> 30 mg per week); 2) history of accidental opioid overdose in the past 3 years (since a prior overdose event likely raises the risk for subsequent overdoses, and a loss of opioid tolerance can increase risk of overdose if oral naltrexone is abruptly discontinued), 3) ongoing treatment with prescription opioids; 4) physiological dependence on alcohol or sedative-hypnotics, and 5) unstable medical disorders which might make participation hazardous.
2.2 Study Procedures
2.2.1 General Procedures
Following study consent, participants were admitted to an inpatient unit at the New York State Psychiatric Institute for the purpose of detoxification and naltrexone induction. We employed a modification of a buprenorphine-assisted, rapid opioid detoxification and naltrexone induction procedure (Collins et al., 2005). Briefly, participants were stabilized on buprenorphine for 1 day, followed by a washout period of 1-2 days, then received increasing daily doses of naltrexone (12.5 mg, 25 mg, 50 mg, 100 mg) while precipitated withdrawal symptoms were treated with clonidine, clonazepam, and other adjuvant medications. After receiving the 50-mg dose of naltrexone participants were stratified by two levels of baseline heroin use (< 6 bags per day vs. 7 or more bags per day), and by two levels of dysphoria (none or minimal as indicated by Ham-D total score less than 12, versus mild or greater dysphoria with Ham-D>12). During the pilot trial, these variables were found to be independent predictors of dropout (Sullivan et al., 2006). The stratification of low-severity vs. high-severity patients reflects a binary construct of baseline opioid use, in which physiological severity is defined based on the amount of heroin or other opioids that the patient reports taking, on a daily basis, prior to seeking treatment. Heroin amount is quantified as “bags per day,” which is the common unit used in illicit sales in the region where our treatment studies are located. Approximate equivalents with respect to prescription opioids the patient was taking (e.g., oxycodone) were calculated. The cut-off point of high severity based on the opioid equivalent of 6 bags of heroin or more per day has been shown to predict worse outcome in prior studies of naltrexone treatment of opioid dependence, and to interact with treatment (Sullivan et al., 2006, Nunes et al., 2006, Carpenter et al., 2009, Brooks et al., 2010).
Participants were then randomized by a research pharmacy to one of four conditions in a two-by-two factorial design: (1) Behavioral Naltrexone Therapy (BNT) plus one dose (384 mg) of XR-NTX; Depotrex, BIOTEK) prior to hospital discharge; (2) BNT plus placebo injection; (3) Compliance Enhancement, simulating standard treatment with oral naltrexone plus XR-NTX injection; and (4) CE plus placebo injection.
Both participants and study personnel were blind to medication assignment. The BIOTEK product used in this trial was a prototype of injectable naltrexone that did not achieve marketability. However, this injectable naltrexone (Depotrex) demonstrated plasma levels of naltrexone above 1 ng/ml for approximately 4 weeks after administration of 384 mg naltrexone (Comer et al. 2002), which is comparable bioavailability to the current commercial XR-NTX product (Vivitrol, Alkermes; Bigelow et al., 2012). Participants were discharged on Day 8 with small amounts of adjuvant medications that they had been receiving in the hospital (clonidine, trazodone, and zolpidem), and these were tapered off during the first two weeks of outpatient treatment.
Following discharge, all participants received oral naltrexone during the 24-week study. Naltrexone tablets were encapsulated with 25 mg of riboflavin, added by the research pharmacy as a urine marker to assess compliance. All BNT participants received oral naltrexone in the research clinic, under observed ingestion conditions, for the first two weeks in doses of 100 mg on Mondays and Wednesdays and 150 mg on Fridays before transitioning to home-based administration monitored by their significant other or family member. This dosing schedule for oral naltrexone was selected in order to ensure that patients received naltrexone under conditions of observed ingestion during each study visit in Weeks 1-2, and that they received a dose sufficient to provide a 48- to 72-hour blockade, lasting until the next study visit. During these initial two weeks of the study, monitored ingestion of oral naltrexone was considerd clinically necessary, in order to reduce the risk of relapse and overdose during this perioid of heightened vulnerability. Liver function tests were obtained every week for 4 weeks and then monthly, and there were no patients demonstrating elevated liver enzymes considered to be naltrexone-related. From Week 1, CE participants received medication to be self-administered at home. Participants were given an emergency supply of naltrexone to take at home in case of a missed visit (Carroll et al., 2001).
Participants were required to attend the clinic three times per week. During each visit, participants gave an observed urine specimen and completed self-report measures of drug use, craving, and mood. Both groups attended twice per week therapy visits, and for the BNT group, the second session was a network session with their monitor.
Participants met with a research psychiatrist once per week to monitor their progress in treatment, and review medication safety and adherence. All urine specimens were tested on-site for opiates and two samples per week were sent to the laboratory for a full toxicology panel. Each urine sample submitted during the trial was observed under UV light for riboflavin fluorescence indicating adherence to oral naltrexone. Participants who did not come for a visit to submit a urine sample for 14 consecutive days during the 24-week medication trial were classified as study drop-outs. Participants were reimbursed $10 per week during the treatment period and $25 for follow-up visits, for their time taken to complete the research assessments.
2.2.2 Behavioral Naltrexone Therapy
Participants in the Behavioral Naltrexone Therapy (BNT) condition received a manual-guided therapy based on the integration of emprically validated treatments for subtance use incorporating motivational and cognitive-behavioral techniques (Rothenberg et al., 2002; Nunes et al., 2006). BNT incorporates elements from network therapy (Galanter, 1993), in that a significant other monitors ingestion of oral naltrexone at home and attends one join therapy session weekly with the patients. In BNT sessions, therapists also delivered elements of relapse prevention therapy (Carroll et al., 1991) for drug avoidance and Motivational Interviewing (Miller and Rollnick, 2002) to address ambivalence and secure a commitment to abstinence.
In addition, BNT adapted aspects from the Community Reinforcement Approach focused on the identification of alternate reinforcers (e.g., relationship enhancement; social skills; seeking employment; social skills) to support longer-term lifestyle changes.
In BNT, voucher-based contingency management was offered to increase attendance and adherence to oral naltrexone (Higgins et al., 1994). Starting with 50 cents, each patient could earn 80 cents worth of additional voucher credits in an escalating schedule when demonstrating compliance with naltrexone in the first two weeks and opioid-free urines thereafter throughout the 6-month outpatient program, for a potential total of $1320 in incentives.
Therapy was provided by clinical psychologists trained in BNT and Relapse Prevention. All treatment sessions were provided within an individual therapy framework. Therapy sessions were audio-taped for supervisory and adherence purposes. Therapists participated in weekly supervision sessions to assure adherence to the intervention procedures and to prevent therapeutic drift.
2.2.3 Compliance Enhancement
Participants in the Compliance Enhancement (CE) condition received a manual-guided intervention delivered by a trained research psychiatrist. CE was intended to model basic medication management, and to control for professional attention. CE includes basic psychoeducation, problem-solving supportive psychotherapy, and 12-step principles. Patients assigned to the CE condition, similarly to those in BNT, attended three visits per week in the first two weeks. During this time, the medical staff monitored adherence to nalrexone. Beyond the first two weeks, patients in CE, similarly to BNT, had two clinic visits per week, one visit with the psychiatrist and one visit with the clinic nurse and research assistant. In contrast to BNT, there were approximately two sessions involving significant others, significant others did not monitor naltrexone administration, and there were no vouchers based on compliance or abstinence. CE did not involve the more elaborate skills training, relationship therapy, and fostering of competing reinforcers adapted from CRA into the maintenance phase of BNT.
2.3 Assessments and Data analysis
Primary outcome was retention in treatment since dropout from opioid antagonist treatment is most commonly associated with relapse to opiate use, even if participants cannot be located and thus relapse is not directly measured (Nunes et al., 2006; Sullivan et al., 2006, 2013). Cox proportional hazards regression was used to model time to dropout as a function of treatment group (BNT plus XR-NTX; BNT plus placebo injection; CE plus XR-NTX; and CE plus placebo injection), with the group that received placebo injection plus the control behavioral therapy (Compliance Enhancement) as the reference group, with cases retained throughout the trial censored at end of study (Month). Baseline severity of opioid dependence, operationalized as self-reported bags per day of heroin, was entered as a continuous covariate, and the interactions between baseline and treatment was tested. The stratification of low-severity vs. high-severity patients reflects a binary construct of baseline opioid use, in which physiological severity is defined based on the amount of heroin or other opioids that the patient reports taking, on a daily basis, prior to seeking treatment. Heroin amount is quantified as “bags per day,” which is the common unit used in illicit sales in the region where our treatment studies are located. Approximate equivalents with respect to prescription opioids the patient was taking (e.g. oxycodone) were calculated. The cut-off point of high severity based on the opioid equivalent of 6 bags of heroin or more per day has been shown to predict worse outcome in prior studies of naltrexone treatment of opioid dependence, and to interact with treatment (Sullivan et al., 2006, Nunes et al., 2006, Carpenter et al., 2009, Brooks et al., 2010).
3. RESULTS
3.1 Sample description
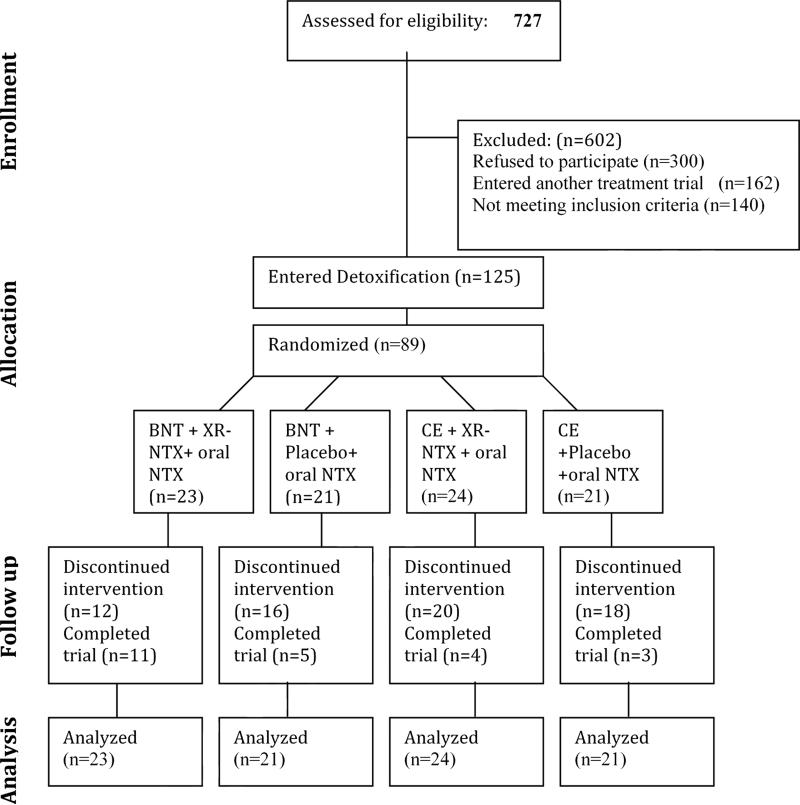
We screened in person 727 individuals for eligibility (See Figure 1 for the CONSORT Flow Diagram). Of those who were screened, 300 individuals declined to participate (223 failed to complete the evaluation and were lost to follow-up, 42 requested immediate detoxification, 35 were not interested in inpatient detoxification or treatment medications) and 140 individuals were not eligible to participate (47 had significant medical problems, 32 had significant psychiatric co-morbidities, 21 were using methadone regularly, and 40 were not eligible for other reasons). In addition, 162 participants entered other treatment studies conducted concurrently at our clinic.
Figure 1.
CONSORT Diagram for Study Participant Flow
A total of 125 individuals consented to the study and entered the inpatient detoxification protocol, and 89 participants were randomized. Participants were on average 38.1 years of age (SD=9.2), mostly male (75.2%) and White (42.4%), Hispanic (36.8%), or African-American (16.8%). The majority of participants were daily heroin users, using on average 6.6 (SD= 4.0) bags of heroin per day (range 1-25), with 52.8% reporting primarily intranasal use. Twenty-nine of those participants (23%) decided to withdraw from study participation during the first 4-5 days of detox. After stratification and completion of detoxification and oral naltrexone induction, participants were randomized equally to four study arms; BNT + oral naltrexone + XR-NTX injections (n=23), BNT + oral NTX + Placebo injections (n=21), CE + oral NTX + XR-NTX injections (n=24) and CE + oral NTX + Placebo injections (n=21). Demographic characteristics of the 89 randomized participants are presented in Table 1. None was found to be significantly different across the three groups.
Table 1.
Demographics of the sample (N=89) randomized to depot naltrexone vs. placebo and BNT vs. CE for 24-week trial of oral naltrexone maintenance.
| BNT + XR-NTX (n=23) | BNT+Placebo (n=21) | CE+XR-NTX (n=24) | CE+Placebo (n=21) | Test Statistic; P-value | Total (n=89) | |
|---|---|---|---|---|---|---|
| Age (mean) | 38.1 ± 9.6 | 40.1 ± 9.8 | 37.3 ± 7.8 | 36.7 ± 8.5 | F3, 85 = 0.60; p=.62 | 38.0 ± 8.9 |
| Gender (female) | 21.7% | 19.0% | 12.5% | 33.3% | Fisher's exact test; p = .42 | 21.3% |
| Ethnicity | ||||||
| -Caucasian | 43.5% | 57.1% | 33.3% | 38.1% | Fisher's exact p = .52 | 42.7% |
| -Hispanic | 34.8% | 28.6% | 54.2% | 33.3% | 38.2% | |
| -African American | 17.4% | 14.3% | 12.5% | 28.6% | 18.0% | |
| -Asian | 4.4% | 0.0% | 0.0% | 0.0% | 1.1% | |
| Severity of Use (mean bags of heroin/day) | 6.6 ± 4.6 | 5.9 ± 3.8 | 6.1 ± 2.3 | 6.4 ± 3.1 | F3, 85 = 0.17; p= .92 | 6.2 ± 3.5 |
| Percentage of Heavy Use (>6 bags of heroin/day) | 34.8% | 28.6% | 37.5% | 33.3% | X23 = 0.42; p= .94 | 33.7% |
3.2 Retention in Treatment
Of the 89 randomized participants, 78.7% (n=70) completed at least 4 weeks of treatment, 58.4% (n=52) completed Week 8, 47.2% (n=42) completed Week 12, and 25.8% (n=23) completed all 24 weeks of the trial. Of the 66 patients who did not complete the trial, 48 were non-compliant with attendance (i.e., dropped out/relapsed) and 18 relapsed to opiate use while still attending sessions but were unable to restart naltrexone following an episode of drug use.
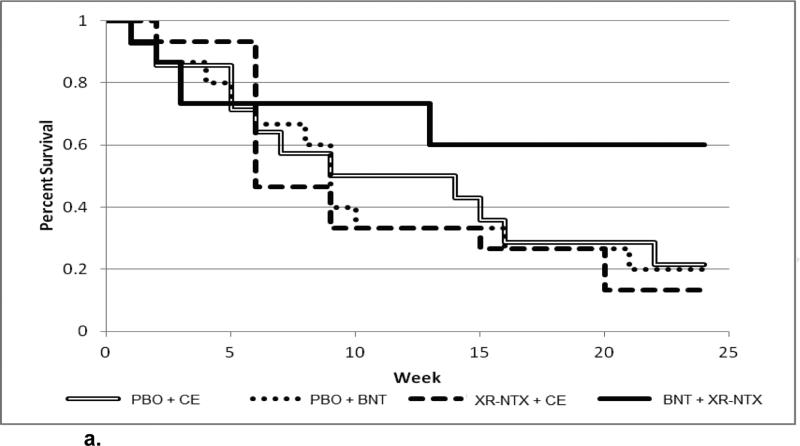
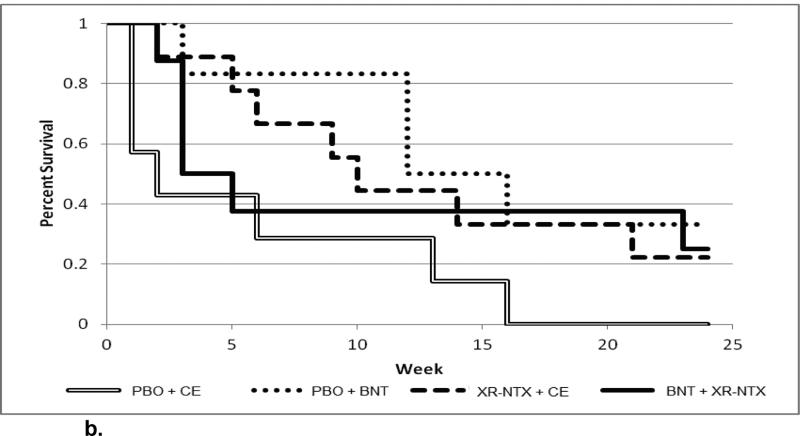
When the Cox proportion hazards regression was fit modeling time to dropout from treatment as a function of treatment conditions (categorical variable), baseline opioid severity (bags per day of heroin; continuous variable), and the interaction between condition and baseline opioid severity, the interaction of treatment condition by baseline opioid severity was significant (X23 = 9.19, p = .027). Only to illustrate the continuous nature of the interaction, the continuous baseline opioid severity was dichotomized: The sample was split into lower baseline opioid severity (≤ 6 bags per day) (n = 59), and higher baseline opioid severity (> 6 bags per day) (n = 30)..The dichotomization was based on median severity that had emerged from our previous studies and it well illustrates the behavior of the continuous interaction term that was part of the statistical model. Figure 2a shows the observed survival curves for subjects with low baseline opioid severity, depicting time to dropout from treatment for each of the four treatment conditions. Figure 2b shows the observed survival curves for subjectes with high baseline opioid severity. For subjects with low baseline opioid severity (Figure 2a), the group that received an active injection of naltrexone prior to discharge from hospital followed by Behavioral Naltrexone Therapy (BNT) appears to have the best retention (60% retained at month 6), while the other three groups have similar retention, ranging from 14% to 24% retained at 6 months. In contrast, for subjects with high baseline opioid severity (Figure 2b), the group that received placebo injection plus control therapy (Compliance Enhancement) has low retention (0% retained by month 6); the other three groups appear to diverge over the first 12 weeks, with placebo + BNT having the best retention, and active naltrexone injection + BNT having the worst retention, with all three groups then converging to end up in the range of 25% to 35% retained at 6 months.
Figure 2.
Kaplan-Meier curve for low-severity opioid dependence, stratified by treatment group
Figure 2.
Kaplan-Meier curve for high-severity opioid dependence, stratified by treatment group
Note, that in the active naltrexone injection + BNT condition, there was very high dropout during the first 4 weeks (only 65.2% retained by 4 weeks) with very little dropout thereafter (47.8% retained overall at 6 months).
3.3 Opioid Positive Urines
Across all treatment groups, opioid use was very low for all participants retained in the study. This is consistent with prior findings that patients who repeatedly “test the blockade” tend to drop out of treatment. By contrast, while patients were engaged in treatment and taking naltrexone, opioid use virtually ceases, except for occasional episodes of use to confirm the blockade (Sullivan et al., 2006, 2013).
3.4 Naltrexone Blood Levels
Table 2 shows the serum levels for naltrexone and 6-beta-naltrexol across Weeks 1-4. The data was right skewed, so medians and interquartile ranges are displayed instead of means and standard deviations. The 2 serum level outcomes were analyzed separately with longitudinal mixed effects models over the 4 weeks using a lognormal distribution as a function of treatment, time, and time by treatment interaction. A random intercept and autoregressive (AR1) covariance structure were used to account for the within-subject correlation. The interaction term was not significant for either outcome and was dropped from the model. There was a significant main effect of treatment on serum level for naltrexone (F1,97 = 33.62, p <.0001 ) and on 6-beta-naltrexol (F1,97 = 26.04, p <.0001). Patients who received the active injection naltrexone in combination with oral naltrexone had significantly higher levels of naltrexone and its active metabolite across Weeks 1-4, compared to patients who received the placebo injection and were maintained on oral naltrexone alone.
Table 2.
Serum Naltrexone Levels and 6-beta-Naltrexol Levels in Patients who Received Oral Naltrexone with Active vs. Placebo Injection Naltrexone
| Serum Naltrexone Levels | 6-beta-Naltrexol Levels | |||
|---|---|---|---|---|
| Active median (IQR) | Placebo median (IQR) | Active median (IQR) | Placebo median (IQR) | |
| Week 1 | 16.5 (12.9, 32.1) | 6.0 (1.2, 12.7) | 13.4 (9.2, 26.7) | 6.0 (1.2, 12.3) |
| Week 2 | 20.1 (11.5, 29.7) | 5.1 (3.3, 8.2) | 13.2 (8.1, 24.2) | 5.1 (3.3, 8.2) |
| Week 3 | 23.3 (12.8, 35.2) | 6.0 (0.4, 15.3) | 19.2 (10.6, 32.4) | 5.1 (0.3, 13.9) |
| Week 4 | 23.7 (9.0, 66.7) | 14.7 (3.5, 24.3) | 20.3 (7.9, 57.6) | 14 (3.5, 24.0) |
3.5 Adverse Effects
Two serious adverse events occurred during this trial: (1) one male participant randomized to BNT + placebo admitted to disguising non-adherence to his medication with his monitor and subsequently experienced a non-fatal overdose requiring an emergency room visit; he was removed from the study and referred for buprenorphine maintenance; and (2) a female participant, following negative urine pregnancy tests during screening, was found to have a positive urine pregnancy test after admission to detoxification and was not randomized. The patient was informed of these results, administratively removed, and referred to a local methadone program in her community. One patient who developed elevated transaminases was found to have recent-onset hepatitis C, and a second patient developed transaminases deemed related to a gallstone; in both instances, the transaminases resolved, and in the former case the patient continued daily naltrexone at 25 mg per day. The adverse effects most frequently reported by participants were: insomnia (49.4%), muscle aches (33.7%), fatigue (28.1%), GI upset (28.1%), and headache (21.3%) . These symptoms are consistent with the subacute opioid withdrawal syndrome observed during the first few weeks of naltrexone maintenance (Mariani et al., 2009).
4. DISCUSSION
This study sought to improve the outcome of oral naltrexone treatment for opioid dependence by testing a combination of a behavioral therapy aimed at increasing adherence (BNT) with a single injection of long-acting naltrexone (XR-NTX) at the outset of treatment. Our original hypothesis, that BNT-XR-NTX would be associated with the highest treatment retention, was not confirmed as a general finding. Rather, the analysis yielded a significant interaction between severity of opioid dependence at baseline and treatment group assignment, which supports our hypothesis partly. Among patients with lower severity (fewer bags per day of heroin), retention in treatment was highest in the BNT-XR-NTX group, as hypothesized: 60% retained at 6 months after treatment initiation. This is comparable to retention rates seen with buprenorphine maintenance (Gryczynski et al., 2014, Hser et al., 2014, Mattick et al., 2014). However, among patients with higher severity of opioid dependence, the BNT-XR-NTX combination did not perform as well, apparently due to high dropout in the first month of treatment. This finding was contrary to the expectation that XR-NTX, by ensuring adequate naltrexone blood levels would reduce dropout during the first month.
Of note is the safety advantage of XR-NTX in that blood levels of naltrexone fall slowly during Weeks 4-5. In addition to providing a window of time in which to gradually transition to oral naltrexone, if indicated by cost or other clinical considerations, this feature also reduces the risk of overdose by ensuring that the patient does not suddenly lose the opioid blockade. While opioid detoxification reduces tolerance and increases the risk of overdose death, naltrexone protects against overdose during the period of adherence. There is no evidence that naltrexone increases overdose death risk to any greater extent than does detoxification.
We previously reviewed studies of behavioral therapy to improve outcome of oral naltrexone (Preston et al., 1999, Carroll et al., 2001, Sullivan et al., 2006), and observed what appeared to be a ceiling on the effectiveness oral naltrexone with no better than 20% to 30% retained by 6 months after treatment initiation (Nunes et al., 2006). The addition of a single injection of long-acting naltrexone at the outset of treatment was an effort to raise that ceiling and improve long-term retention on oral naltrexone. Since this study was conducted, injection naltrexone (Vivitrol) was approved by the FDA, having very similar pharmacokinetics to the Depotrex used in this study, including blood levels and opioid blockade (Comer et al., 2006, Bigelow et al., 2012). Vivitrol is an expensive medication, and the present results do suggest that transition to oral naltrexone may be a viable option for some patients. Thus, if the high cost of XR-NTX represents a barrier to treatment, these findings underscore the value of administering XR-NTX post-detoxification prior to transitioning to oral naltrexone, as a pragmatic strategy for supporting abstinence and treatment retention.
However, while the pharmacy cost of XR-NTX may be prohibitive for some individuals, it is important to note that healthcare utilization studies have found that XRNTX for the treatment of opioid dependence is associated with fewer hospitalizations; total healthcare costs for XR-NTX were found to be similar to those for oral naltrexone or buprenorphine and 49% lower than those for methadone (Hartung et al., 2014, Baser et al., 2011). Likewise, XR-NTX was more cost-effective than psychosocial treatments for alcohol dependence and resulted in fewer inpatient hospitalization days (Bryson et al., 2011, Mark et al., 2010), compared to oral naltrexone or other oral medications.
In high-severity patients, BNT-XR-NTX did not perform as well; high dropout occurred in the first 4 weeks. The most likely explanation for this early attrition is that BNT-XR-NTX patients experienced more subacute withdrawal during the first few weeks of abstinence, because their system was exposed to the combination of naltrexone from both the injection and from the oral naltrexone; compliance with the latter was actively encouraged by the BNT therapy. This clinical phenomenon of malaise, fatigue, and low-grade opioid withdrawal symptoms commonly seen during the first few weeks of antagonist maintenance has been termed the “naltrexone flu” (Mariani et al., 2009). Patients who received the active injection naltrexone in combination with oral naltrexone had significantly higher levels of naltrexone and its active metabolite across Weeks 1-4, compared to patients who received the placebo injection and were maintained on oral naltrexone alone.
Our finding in the present analysis of a baseline severity-by-treatment interaction is consistent with earlier reported results of an interaction effect, where Behavioral Naltrexone Therapy (BNT) was found to be superior to standard Compliance Enhancement therapy (CE) especially among patients with higher levels of opioid use at baseline (Nunes et al., 2006, Carpenter et al., 2009, Brooks et al., 2010). Compared to standard psychopharmacological support, BNT is more complicated and labor-intensive to implement, but behavioral therapy of this type appears to be a worthwhile treatment to support naltrexone adherence, particularly for more severely ill opioid-dependent patients.
4.1 Limitations
Study limitations include a relatively small per group sample size and the treatment setting of a research clinic, which may limit the generalizability of findings to community-based treatment programs. The primary reason for the relatively small sample size was the complexity and labor-intensive nature of the study procedures, which included: (1) a 7-day inpatient detoxification (not acceptable to many patients) and induction onto oral/injection naltrexone (less acceptable than opioid agonist therapy to some patients), (2) thrice-weekly observed ingestion of oral naltrexone, supervised by a research nurse or physician three times per week, in the first two study weeks (necessitating frequent trips to the research clinic); (3) participation of a significant other (not acceptable to all patients) and (4) attendance at 50 therapy and medical visits (thrice-weekly in Weeks 1-2, then twice weekly in Weeks 3-24).
Behavioral Naltrexone Therapy (BNT), the manualized therapy delivered in this trial, may be too intensive and complex a treatment platform for adoption in some community-based treatment settings. BNT involves twice weekly individual sessions with a trained therapist, which is not consistent with the resources available in most community-based treatment. However, the principles of BNT (motivation enhancement, significant other to help monitor adherence, incentives, and relapse prevention skills) could be delivered in a more streamlined manner. Future research should seek to simplify this intervention to adapt it to community settings.The BIOTEK product used in this trial was a prototype of injectable naltrexone that did not achieve marketability, although it yielded plasma naltrexone levels similar to the current commercial product (Vivitrol; Alkermes). Thus, our study findings should be generalizable to treatment carried out with Vivitrol.
There are few data available on relapse rates after discontinuing naltrexone treatment for opioid dependence. Future studies should examine the question of how long a course of naltrexone should continue, as well as whether there is a particular duration of treatment after which naltrexone could be safely discontinued. This is a particularly salient question, given the expense of the currently available injection product (Vivitrol). The present findings provide a possible solution to this clinical dilemma, as they offer partial support for a strategy of substituting oral naltrexone as a lower-cost maintenance treatment after a successful course of injection naltrexone. These results also suggest the benefits of a gradual transition; oral naltrexone should be phased in slowly in Weeks 4-5 post-injection, as the blood levels from the XR-NTX are declining.
4.2. Conclusions
In summary, antagonist therapy remains an important tool in the armamentarium of treatments for opioid dependence, and deserves serious clinical consideration, especially for individuals during the early phase of their opioid dependence, and for those appropriately seeking abstinence from opioid agonists as a treatment goal. Our findings replicate prior work from this group (Rothenberg et al., 2002, Nunes et al., 2006) suggesting the efficacy of behavioral therapy in improving outcome with oral naltrexone treatment for opioid dependence. While we had hypothesized that the combination of BNT and XR-NTX would result in the highest treatment retention, we found a significant interaction between baseline severity of use and treatment group. For low-severity patients, the BNT-XR-NTX treatment proved superior to the other treatment conditions. For such patients, administration of a single injection of XR-NTX, even if maintenance on the injection is cost-prohibitive, can be expected to considerably increase abstinence rates and treatment retention on oral naltrexone up to six months. But for high-severity heroin users, BNT with oral naltrexone (without active naltrexone injection) worked best , as such individuals were likely spared the increased opioid withdrawal symptoms and dysphoria that accompanied the combined use of oral and depot naltrexone, as confirmed by higher naltrexone and 6-beta-naltrexol levels during Weeks 1 and 2. A dose of XR-NTX without oral naltrexone should provide an equivalent or better outcome, as it would minimize the effect of non-compliance. These results suggest the value of even a single injection of XR-NTX in enhancing the effectiveness of antagonist treatment, and the importance of slowly phasing in oral naltrexone in post-injection Weeks 4 and 5 following a course of XR-NTX maintenance.
Further analyses are warranted to examine predictors of early attrition from naltrexone treatment, and their moderation of treatment effects. Clinical trials are needed testing maintenance on long-acting injectable naltrexone as an alternative to oral naltrexone for the maintenance treatment of opioid dependence, and testing the role of behavioral therapy in supporting injectable naltrexone treatment.
Highlights.
We conducted a 24-week placebo-controlled trial comparing 4 treatment conditions in combination with oral naltrexone maintenance for opioid dependence.
For low-severity opioid users, retention was highest (60% at 6 months) in Behavioral Naltrexone Therapy with a single administration of injection naltrexone (XR-NTX) post-detoxification.
For high-severity opioid users, BNT-XR-NTX + oral naltrexone did not perform as well, due to high early attrition and likely increased withdrawal comfort from oral naltrexone combined with XR-NTX.
When patients are transitioned from XR-NTX, oral naltrexone should be phased in slowly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Maria A. Sullivan, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032.
Adam Bisaga, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 amb107@columbia.edu.
Andrew Glass, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 glassan@nyspi.columbia.edu.
Kaitlyn Mishlen, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 mishlen@nyspi.columbia.edu.
Martina Pavlicova, Columbia University 722 W. 168th Street, MSPH Box 12 New York, NY 10032 mp2370@columbia.edu.
Kenneth M. Carpenter, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 carpent@nyspi.columbia.edu
John J Mariani, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 mariani@nyspi.columbia.edu.
Frances R. Levin, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 Frl2@columbia.edu
Edward V. Nunes, Columbia University and the New York State Psychiatric Institute 1051 Riverside Drive, Unit 120 New York, NY 10032 nunesed@nyspi.columbia.edu
REFERENCES
- Baser O, Chalk M, Fiellin DA, Gastfriend DR. Cost and utilization outcomes of opioid-dependence treatments. Am. J. Manag. Care. 2011;17(Suppl. 8):S235–248. [PubMed] [Google Scholar]
- Bigelow GE, Preston KL, Schmittner J, Dong Q, Gastfriend DR. Opioid challenge evaluation of blockade by extended-release naltrexone in opioid-abusing adults: dose-effects and time-course. Drug Alcohol Depend.1. 2012;123:57–65. doi: 10.1016/j.drugalcdep.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks AC, Comer SD, Sullivan MA, Bisaga A, Carpenter KM, Raby WM, Yu E, O'Brien CP, Nunes EV. Long-acting injectable naltrexone versus oral naltrexone maintenance therapy with psychosocial intervention for heroin dependence: a quasi-experiment. J. Clin. Psychiatry. 2010;71:1371–1378. doi: 10.4088/JCP.09m05080ecr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson WC, McConnell J, Korthuis PT, McCarty D. Extended-release naltrexone for alcohol dependence: persistence and health care costs and utilization. Am. J. Manag. Care. 2011;17(Suppl. 8):S222–234. [PMC free article] [PubMed] [Google Scholar]
- Carpenter KM, Jiang H, Sullivan MA, Bisaga A, Comer SD, Raby WN, Brooks AC, Nunes EV. Betting on change: modeling transitional probabilities to guide therapy development for opioid dependence. Psychol. Addict. Behav. 2009;23:47–55. doi: 10.1037/a0013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Ball SA, Nich C, O'Connor PG, Eagan DA, Frankforter TL, Triffleman EG, Shi J, Rounsaville BJ. Targeting behavioral therapies to enhance naltrexone treatment of opioid dependence: efficacy of contingency management and significant other involvement. Arch. Gen. Psychiatry. 2001;58:755–761. doi: 10.1001/archpsyc.58.8.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Libby B, Sheehan J, Hyland N. Motivational interviewing to enhance treatment initiation in substance abusers: an effectiveness study. Am. J. Addict. 2001;10:335–339. doi: 10.1080/aja.10.4.335.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Keller DS. Relapse prevention strategies for the treatment of cocaine abuse. Am. J. Drug Alcohol Abuse. 1991;17:249–265. doi: 10.3109/00952999109027550. [DOI] [PubMed] [Google Scholar]
- Collins ED, Kleber HD, Whittington RA, Heitler NE. Anesthesia-assisted vs. buprenorphine- or clonidine-assisted heroin detoxification and induction: a randomized trial. JAMA. 2005;294:903–13. doi: 10.1001/jama.294.8.903. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, Kleber HD, Nuwayser ES, Kerrigan JH, Fischman MW. Depot naltrexone: long-lasting antagonism of the effects of heroin in humans. Psychopharmacology. 2002;159:351–360. doi: 10.1007/s002130100909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comer SD, Sullivan MA, Yu E, Rothenberg JL, Kleber HD, Kampman K, Dackis C, O'Brien CP. Injectable, sustained-release naltrexone for the treatment of opioid dependence: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2006;63:210–218. doi: 10.1001/archpsyc.63.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research. New York State Psychiatric Institute; New York: 2002. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr., Oslin D, O'Brien CP, Imms P, Riggs DS. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Galanter M. Network therapy for addiction: a model for office practice. Am. J. Psychiatry. 1993;150:28–36. doi: 10.1176/ajp.150.1.28. [DOI] [PubMed] [Google Scholar]
- Gryczynski J, Mitchell SG, Jaffe JH, O'Grady KE, Olsen YK, Schwartz RP. Leaving buprenorphine treatment: patients’ reasons for cessation of care. J. Subst. Abuse Treat. 2014;46:356–361. doi: 10.1016/j.jsat.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung DM, McCarty D, Fu R, Wiest K, Chalk M, Gastfriend DR. Extended-release naltrexone for alcohol and opioid dependence: a meta-analysis of healthcare utilization studies. J. Subst. Abuse Treat. 2014;47:113–121. doi: 10.1016/j.jsat.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger CJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch. Gen. Psychiatry. 1994;51:568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- Hser YI, Saxon AJ, Huang D, Hasson A, Thomas C, Hillhouse M, Jacobs P, Teruya C, McLaughlin P, Wiest K, Cohen A, Ling W. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109:79–87. doi: 10.1111/add.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulse GK, Tait RJ, Comer SD, Sullivan MA, Jacobs IG, Arnold-Reed D. Reducing hospital presentations for opioid overdose in patients treated with sustained release naltrexone implants. Drug Alcohol Depend. 2005;79:351–357. doi: 10.1016/j.drugalcdep.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BA, Berglund M, Lindgren A. Efficacy of maintenance treatment with naltrexone for opioid dependence: a meta-analytical review. Addiction. 2006;101:491–503. doi: 10.1111/j.1360-0443.2006.01369.x. [DOI] [PubMed] [Google Scholar]
- Mariani JJ, Sullivan MA, Bisaga K, Carpenter KA, Murray, Levin FR, Nunes EV. College on Problems of Drug Dependence 71st Annual Scientific Meeting. Reno, Nevada: 2009. Naltrexone-Induced Protracted Opioid Withdrawal Symptoms. [Google Scholar]
- Mark TL, Montejano LB, Kranzler HR, Chalk M, Gastfriend DR. Comparison of healthcare utilization among patients treated with alcoholism medications. Am. J. Manag. Care. 2010;16:879–888. [PMC free article] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst. Rev. 2014;2:CD002207. doi: 10.1002/14651858.CD002207.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2nd ed. The Guilford Press; New York: 2002. [Google Scholar]
- National Survey on Drug Use and Health (NSDUH) U.S. Department of Health and Human Services. Substance Abuse and Mental Health Services Administration. Office of Applied Studies. [04/03/13];Results from the 2011 National Survey on Drug Use and Health: Volume I. Summary of National Findings. 2011 http://www.samhsa.gov/data/NSDUH/2k11State/NSDUHsaeTables2011.pdf.
- Nunes EV, Rothenberg JL, Sullivan MA, Carpenter KM, Kleber HD. Behavioral therapy to augment oral naltrexone for opioid dependence: a ceiling on effectiveness? Am. J. Drug Alcohol Abuse. 2006;32:504–517. doi: 10.1080/00952990600918973. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Oslin DW, Kampman KM, Dundon WD, Xie H, Gallis TL, Dackis CA, O'Brien CP. A double-blind, placebo-controlled trial combining setraline and naltrexone for treating co-occurring depression and alcohol dependence. Am. J. Psychiatry. 2010;167:668–675. doi: 10.1176/appi.ajp.2009.08060852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Umbricht A, DeJesus A, Montoya ID, Schuster CR. Improvement in naltrexone treatment compliance with contingency management. Drug Alcohol Depend. 1999;54:127–135. doi: 10.1016/s0376-8716(98)00152-5. [DOI] [PubMed] [Google Scholar]
- Rothenberg JL, Sullivan MA, Church SH, Seracini A, Collins E, Kleber HD, Nunes EV. Behavioral naltrexone therapy: an integrated treatment for opiate dependence. J. Subst. Abuse Treat. 2002;23:351–360. doi: 10.1016/s0740-5472(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Rothenberg JL, Vosburg SK, Church SH, Feldman SJ, Epstein EM, Kleber HD, Nunes EV. Predictors of retention in naltrexone maintenance for opioid dependence: analysis of a stage I trial. Am. J. Addict. 2006;15:150–159. doi: 10.1080/10550490500528464. [DOI] [PubMed] [Google Scholar]
- Sullivan MA, Bisaga A, Mariani JJ, Glass A, Levin FR, Comer SD, Nunes EV. Naltrexone treatment for opioid dependence: does its effectiveness depend on testing the blockade? Drug Alcohol Depend. 2013;133:80–85. doi: 10.1016/j.drugalcdep.2013.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]