Abstract
More than 90% of individuals who use cocaine also report concurrent ethanol use, but only a few studies, all conducted with vertebrates, have investigated pharmacodynamic interactions between ethanol and cocaine. Planaria, a type of flatworm often considered to have the simplest ‘brain’, is an invertebrate species especially amenable to the quantification of drug-induced behavioral responses and identification of conserved responses. Here, we investigated stereotypical and environmental place conditioning (EPC) effects of ethanol administered alone and in combination with cocaine. Planarians displayed concentration-related increases in C-shape movements following exposure to ethanol (0.01 – 1%) (maximal effect: 9.9 ± 1.1 C-shapes/5 min at 0.5%) or cocaine (0.1 – 5 mM) (maximal effect: 42.8 ± 4.1 C-shapes/5 min at 5 mM). For combined administration, cocaine (0.1 – 5 mM) were tested with submaximal ethanol concentrations (0.01, 0,1%), the observed effect for the combination was enhanced compared to its predicted effect, indicating synergism for the interaction. The synergy with ethanol was specific for cocaine, as related experiments revealed that combinations of ethanol and nicotine did not result in synergy. For EPC experiments, ethanol (0.0001 – 1%) concentration-dependently increased EPC, with significant environmental shifts detected at 0.01 and 1%. Cocaine (0.001 – 1 μM) produced an inverted U-shaped concentration-effect curve, with a significant environmental shift observed at 0.01 μM. For combined exposure, variable cocaine concentrations (0.001 – 1 μM) were administered with a statistically ineffective concentration of ethanol (0.0001%). For each concentration of cocaine, the environmental shift was enhanced by ethanol, with significance detected at 1 μM. Cocaethylene, a metabolite of cocaine and ethanol, also produced C-shapes and EPC. Lidocaine (0.001 – 10 μM), an anesthetic and analog of cocaine, did not produce EPC or C-shape movements. Evidence from planarians that ethanol produces place-conditioning effects, motor dysfunction, and interacts synergistically with cocaine suggests that aspects of ethanol neuropharmacology are conserved across species.
Keywords: cocaine, ethanol, cocaethylene, planaria, nicotine, synergy, place preference
Introduction
For cocaine-dependent patients, up to 90% receiving inpatient treatment and 50% receiving outpatient treatment are also dependent on ethanol (Lacoste et al., 2010). Ethanol counters anxiety precipitated by cocaine withdrawal but also facilitates cocaine craving that increases relapse rates (Lacoste et al., 2010). Further, promising anti-cocaine medications, notably modafinil, are often less effective in patients who simultaneously abuse cocaine and ethanol (Anderson et al., 2009). Studies conducted in rats and mice indicate that the presence of ethanol can alter the pharmacological profile of cocaine, and vice versa (Busse et al., 2004, 2005; Sobel and Riley, 1997; Masur et al., 1989; Aston-Jones et al., 1984).
Standard vertebrate assays (e.g. self-administration, drug discrimination, conditioned place preference (CPP)) provide invaluable information about relative abuse liability and mechanism but are less amenable to the rapid, mathematical, reproducible, and cost-effective quantification of the myriad of drug-drug interactions available to polydrug abusers. Assays developed in planarians provide an alternative (Raffa and Rawls, 2010). Planarians have a centralized nervous system (cephalic ganglia and nerve cord processes) and multiple neurotransmitter systems, including glutamate, dopamine, serotonin, acetylcholine, and GABA (Eriksson and Panula, 1994; Buttarelli et al., 2008; Nishimura et al., 2010). Planarians display mammalian-like behavioral responses, including stereotyped movements, spontaneous withdrawal, behavioral sensitization, cross-sensitization, and environmental place conditioning (EPC), during exposure to several classes of abused substances (Kusayama and Watanabe, 2002; Pagan et al., 2008, 2009; Palladini et al., 1996; Passarelli et al., 1999; Rowlands and Pagan, 2008; Rawls et al., 2010a, 2011; Raffa and Valdez, 2001). Cocaine administration produces motor dysfunction, behavioral sensitization, and environmental place conditioning (EPC) in planarians (Pagan et al., 2013; Ramoz et al., 2012; Rawls et al., 2010b), but less is known about impacts of ethanol on planarians. Here, we investigated stereotypical and place-conditioning effects of ethanol administered alone and in combination with cocaine in planarians through application of dose-addition analysis, a mathematical approach that examines interactions between agonist drugs in combination by comparing the experimentally determined effect for a combination (e.g. ethanol + cocaine) with its predicted additive effect (Tallarida, 2011, 2012).
Methods
Animals and Chemicals
Planarians (Dugesia Dorotocephala) were purchased from Carolina Biological (Burlington, NC, USA) and used within three days of arrival. Ethanol and nicotine hydrogen tartrate salt were purchased from Sigma-Aldrich (St. Louis, MO, USA). Cocaine hydrochloride and cocaethylene fumarate was generously provided by the National Institute on Drug Abuse (NIDA) (Bethesda, MD, USA). Stock and treatment solutions were prepared daily in tap water containing AmQuel® water conditioner (1 ml Amquel per 1 gallon of water).
Activity experiments
Individual planarians were removed from their home jars and placed for 5 min into a petri dish (5.5 cm diameter) containing water, cocaine (0.1, 1, 3, 5 mM), ethanol (0.01, 0.1, 0.5, 1 %), or nicotine (0.1, 1, 3, 5 mM). C-shape movements, previously defined as stereotypical movements, were quantified over the 5-min interval by a trained observer with a stopwatch who was blinded to drug treatment (Rawls et al., 2010a, 2011). The response was quantified each time the planarian made a C-shaped behavior and then relaxed, and this sequence translated to “1” individual C-shape. The duration of individual C-shapes was not quantified. Quantifying the frequency of a specific in vivo response is also common practice in rodent models, such as the quantification of withdrawal signs in physically-dependent animals (e.g. wet-dog shakes, escape behavior, teeth chattering, eye blinking). Prior work has demonstrated that C-shape movements displayed by planarians are not caused by changes in the pH or osmolarity of the solution (Raffa et al., 2010). Drug concentrations were based on prior planarian studies (Rawls et al., 2010a, b; Pagan et al., 2013). Concentration-response data were analyzed using dose-addition analysis, which is best described in terms of two agonist drugs with overtly similar effects, e.g., each capable of producing stereotypical activity (Tallarida and Raffa, 2010). The expected effect of the combination (cocaine, ethanol) was calculated from individual concentration-response data and compared with the observed effect of the combination (Tallarida, 2012). The difference between the observed and expected effect is the basis for assessing synergism. For comparative purposes, ethanol was tested in combination with nicotine.
EPC experiments
Based on the fact that planarians display a natural preference for a dark environment, or aversion from the light, we used a biased, counterbalanced conditioning design to assess ethanol or cocaine conditioning effects (Ramoz et al., 2012). Dark and “ambient” light environments were created by covering half (top and bottom) of a petri dish containing water with black paper. Individual planarians were placed at the midline of the dish and given free access to both the light and dark sides of the dish. The time spent in the non-preferred, or more aversive, setting (light) over a 5-min interval was determined (pretest). Planarians were conditioned with either ethanol (0, 0.0001, 0.001, 0.01 and 1%) or cocaine (0, 0.001, 0.01 0.01 and 1 μM) for 30 min in the non-preferred environment. Immediately following conditioning, planarians were placed at the midline of a petri dish containing water and allowed free access to the light and dark sides of the dish for 5 min. The time spent in the non-preferred environment was again determined (posttest), and the difference in time spent in the drug-paired environment (posttest minus pretest times) was determined to assess effects of drug conditioning. For combination experiments, each concentration of cocaine (0, 0.001, 0.01 0.01 and 1 μM) was tested in the presence of a statistically ineffective concentration of ethanol (0.0001%). Separate experiments tested the effects of cocaethylene, a metabolite of cocaine and alcohol, on EPC (0, 1, 10, 100, 1000, and 100,000 μM) and C-shapes (0, 100, 250, 750, 1000, and 3000 μM), and the effects of lidocaine (0, 0.01, 0.1, 1, 10 μM), an anesthetic and structural analog of cocaine, on EPC.
Statistical analysis
Comparisons of group means (± S.E.M.) for experimental sets involving individual drugs were evaluated by one-way ANOVA, and in cases of significance, followed by Dunnett’s test to identify differences between individual groups. Combination data were analyzed by either dose-addition analysis (Tallarida, 2012) that incorporates a Student’s t-test to compare expected and observed responses or a two-way ANOVA followed by Bonferroni’s test to identify individual group differences. Values of P < 0.05 were considered statistically significant.
Results
Effects of cocaine and ethanol on planarian C-shape movements
Consistent with normal behavior, planarians tested in water did not display paroxysms or C-shape movements. In contrast, planarians exposed to cocaine or ethanol displayed recurrent C-shapes (representative photographs shown in Fig. 1 for exposure to 5 mM cocaine). The onset of C-shapes during cocaine exposure was rapid, beginning within 10 s following exposure. The duration of each individual C-shape movement was approximately 1 s. For quantification, cocaine produced a concentration-dependent increase in C-shapes [F(3, 28) = 43.00, P < 0.0001] (one-way ANOVA), with the 5 mM producing the greatest effect (42.75 ± 4.11 movements) (Fig. 2A). Ethanol also produced C-shapes [F(3, 28) = 12.47, P < 0.0001] (one-way ANOVA), with a concentration of 1% producing the maximal effect (8.75 ± 0.98 movements) (Fig. 2A).
Fig 1.
Representative photographs of C-shape movements displayed when planarians were tested in 5 mM cocaine.
Fig 2.
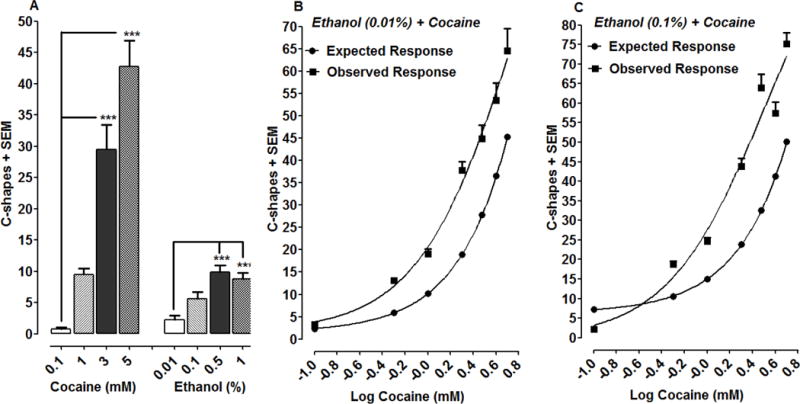
Ethanol interacts synergistically with cocaine to produce C-shape movements in planarians. 2A) Effects of cocaine or ethanol on C-shape movements over a 5-min exposure interval. ***P < 0.001 compared to lowest concentration of cocaine (0.1 mM) or ethanol (0.01%). N=8 planarians/group. 2B–C) Fixed concentrations of ETOH (0.01, 1%) were administered with increasing concentrations of cocaine (0,1, 0.5, 1, 2, 3, 4, 5 mM). C-shape movements were quantified over a 5-min exposure interval and data were presented as mean C-shapes + SEM versus log cocaine dose. N=8 planarians/group. A Student’s t-test comparing the expected and observed responses reveal synergy for the interaction (2B, P = 0.003 and 2C, P = 0.017).
Results with the individual agents suggested that an appropriate testing paradigm for combination studies was one in which fixed concentrations of ethanol were paired with variable concentrations of cocaine (Tallarida, 2012). Thus, from the individual concentration-effect curves, each ethanol concentration was converted to its effective equivalent of cocaine concentration, thereby enabling the total concentration of each combination to be expressed as cocaine concentration + effective equivalent (Tallarida, 2012, Tallarida and Raffa, 2010). In that regard, two fixed concentrations of ethanol (0.01, 0.1%) were tested with increasing concentrations of cocaine (0,1, 0.5, 1, 2, 3, 4, 5 mM) and the results are presented in Figs. 2B and 2C. The expected effect of each combination was calculated mathematically prior to experimental testing. For illustrative purposes an ethanol concentration of 0.01% is equi-effective to a cocaine concentration of 0.106 (e.g., the identical C-shape response is produced by either 0.01% alcohol or 0.106 mM cocaine). Thus, a combination of 0.01% alcohol and 1 mM cocaine is actually equivalent to a cocaine concentration of 0.106 + 1 = 1.106, yielding an expected effect of 10.24 C-shapes as determined from the cocaine-concentration response curve (Fig. 2B). The observed effect for the combination (0.01% ethanol, 1 mM cocaine) was 19.125 ± 1.56 C-shapes, a value well above the expected effect of 10.24 (Fig. 2B). In fact, for all seven combinations involving 0.01% ethanol, the observed effect was greater than the expected effect (Fig. 2B). Analysis of the expected and observed responses with a Student’s t-test yielded a P value of 0.003, which is highly indicative of synergism. Application of this approach to combinations involving 0.1% ethanol (Fig. 2C) also revealed differences between observed and expected effects (P = 0.017), confirming synergy for the ethanol-cocaine interaction.
To determine if the synergy shown for ethanol was specific for cocaine or extended to another psychoactive substance, a combination of ethanol and nicotine was analyzed (Fig. 3). Nicotine produced a concentration-dependent increase in C-shape movements [F(3, 28) = 227.6, P < 0.0001] (one-way ANOVA), with 5 mM nicotine eliciting the greatest effect (37.70 ± 0.99 counts) (Fig. 3A). In this case, the ethanol concentration (0.8%) was fixed and tested in combination with increasing concentrations of nicotine (1, 2, 4 mM). Analysis of the expected and observed responses, shown in Fig. 3B, with a Student’s paired t-test revealed no significant difference, a finding that is indicative of simple additivity (Fig. 3B).
Fig 3.
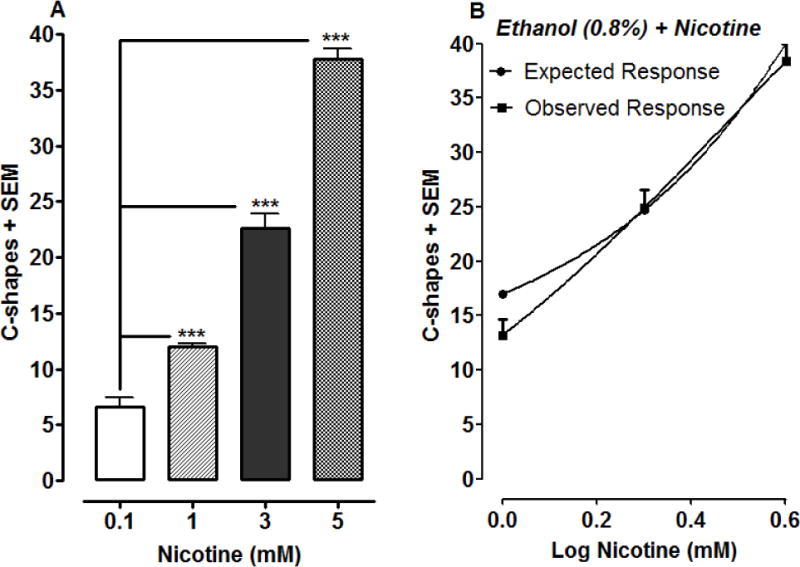
A combination of ethanol and nicotine displays simple additivity against C-shape movements. 3A) Effects of nicotine on C-shape movements over a 5-min exposure interval. ***P < 0.001 compared to lowest concentration (0.1 mM) of nicotine. N=8 planarians/group. 3B) A fixed concentration (0.8%) of ethanol was administered with increasing concentrations of nicotine (1, 2, 4 mM). C-shape movements were quantified over a 5-min exposure interval and data were presented as mean C-shapes + SEM versus log nicotine dose. N=8 planarians/group. A Student’s t-test comparing the expected and observed responses revealed simple additivity for the interaction (P > 0.05).
Effects of cocaine and ethanol on planarian EPC
The majority of drug-naïve planarians spent a greater amount of time in the dark than in the ambient light environment during the pre-test (see Table 1). Effects of ethanol and cocaine, administered alone and in combination, on EPC are presented in Fig. 4. For ethanol (Fig. 4A), one-way ANOVA indicated a significant main effect in experiments testing the effects of different concentrations of ethanol on EPC [F(4, 35) = 3.78, P = 0.0117]. Two concentrations of ethanol, 0.01 and 1%, produced significant environmental shifts relative to the water control (P < 0.05 for 0.01% and P < 0.01 for 1%). A maximal environmental shift of 139 ± 21 s was produced by a concentration of 1% ethanol. For the effects of cocaine on EPC (Fig. 4B), one-way ANOVA indicated a significant main effect [F(4, 30) = 3.21, P < 0.05]. A concentration of 0.01 μM cocaine produced the maximal environmental shift (95 ± 12 s), which was significantly greater than the environmental shift induced by water (34 ± 11 s) (P < 0.05). Overall, cocaine produced an inverted U-shaped concentration-effect curve across the concentrations tested here (Fig. 4B), with weaker and statistically insignificant environmental shifts detected for concentrations that were both lower and higher than 0.01 μM cocaine. For combinations, a fixed concentration of ethanol (0.0001%) that was statistically ineffective when given by itself was administered concurrently with graded concentrations of cocaine (Fig. 4C). For experiments (Fig. 4C), two-way ANOVA revealed a significant effect of ethanol [F(1, 70) = 9.39, P < 0.01]. Bonferroni analysis indicated that the effect produced by the highest concentration of cocaine (1 μM) was significantly enhanced in the presence of 0.0001% ethanol (P < 0.05) (Fig. 4C). Although effects of each of the other concentrations of cocaine were enhanced in the presence of 0.0001% ethanol, none of the effects reached statistical significance (P > 0.05).
Table 1. Drug-naïve planarians spend a greater percentage of time in a dark environment.
For each set of CPP experiments, data regarding the initial time spent in the dark versus light environments during the pre-test is presented below. The environment in which planarians spent the least amount of time was designated as the non-preferred environment. Since a biased CPP design was used, drug conditioning always occurred in the non-preferred environment.
Fig 4.
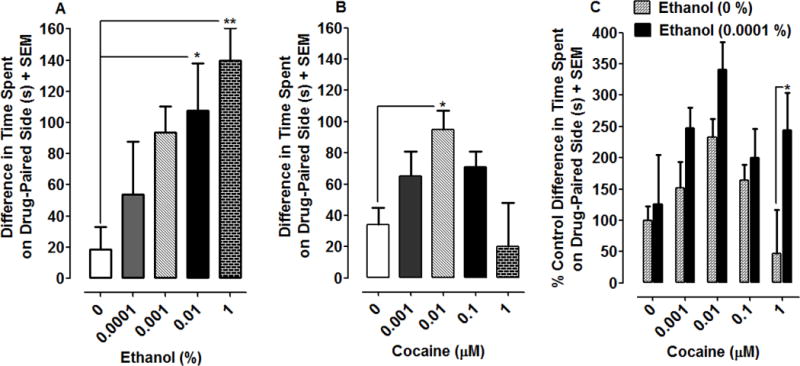
Ethanol and cocaine both produce EPC in planarians, and a combination of ethanol and cocaine produces enhanced EPC. Data are presented as the mean difference in time spent on the drug-paired side (s) + SEM for ethanol (4A) and cocaine (4B). N = 7–8 planarians per group. *P < 0.05 or **P < 0.01 compared to respective water controls. 4C) Data are presented as percentage of water control environmental shift (i.e., this environmental shift is the difference in time spent on the drug-paired side) + SEM. N = 7–8 planarians per group except for the water control (N=13). *P < 0.05 compared to group treated with cocaine by itself.
Effects of cocaethylene on planarian C-shape movements and EPC
Effects of cocaethylene on C-shape movements and EPC are presented in Fig. 5. Cocaethylene produced a concentration-dependent increase in C-shapes [F(5, 42) = 173, P < 0.0001] (one-way ANOVA) (Fig. 5A). Post-hoc analysis revealed that the highest concentrations of cocaethylene produced significant C-shapes compared to the lowest tested concentration (i.e., 2.12 ± 0.35 C-shapes), with 750 μM (7.37 ± 0.80 movements, P < 0.01), 1000 μM (12.5 ± 1.83 movements, P < 0.001), and 3000 μM (38.49 ± 1.45 movements, P < 0.001) all producing significant enhancement of C-shape movements. For EPC experiments (Fig. 5B), one-way ANOVA indicated a significant main effect [F(4, 34) = 3.09, P < 0.05]. Two concentrations of cocaethylene, 0.01 and 1 μM, produced significant environmental shifts relative to water control (P < 0.05). A maximal environmental shift of 106 ± 19 s was produced by a concentration of 1 μM cocaethylene.
Fig 5.
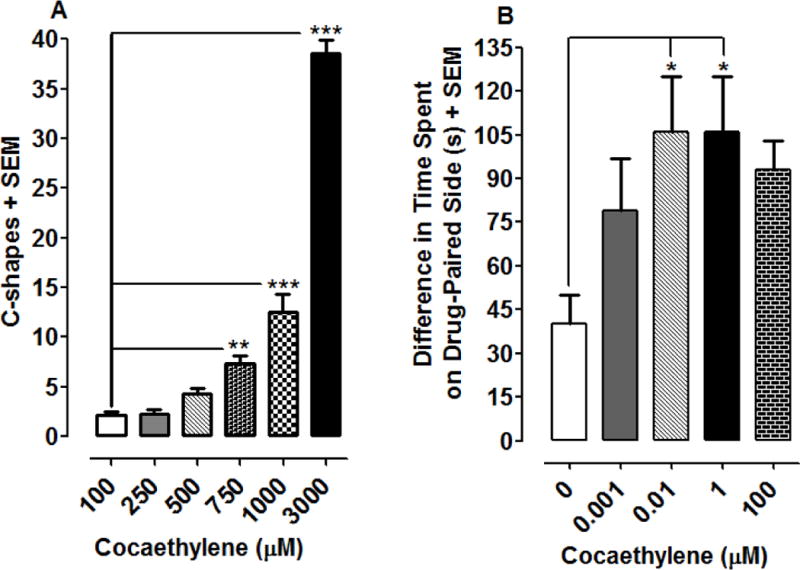
Cocaethylene produces C-shape movements and EPC in planarians. 5A) Effects of cocaethylene on C-shape movements over a 5-min exposure interval. **P < 0.01 or ***P < 0.001 compared to lowest concentration of cocaethylene (0.1 μM). N=8 planarians/group. 5B) Effects of cocaethylene on EPC. Data are presented as the mean difference in time spent on the drug-paired side (s) + SEM. N = 7–8 planarians per group. *P < 0.05 compared to the water control.
Effects of lidocaine on planarian C-shape movements and EPC
Effects of lidocaine on EPC are presented in Fig. 6. One-way ANOVA conducted on the data set did not reveal a significant main effect [F(4, 35) = 0.08369, P > 0.05], thus indicating that lidocaine, at the concentrations tested here, did not produce significant effects in the planarian EPC assay.
Fig 6.
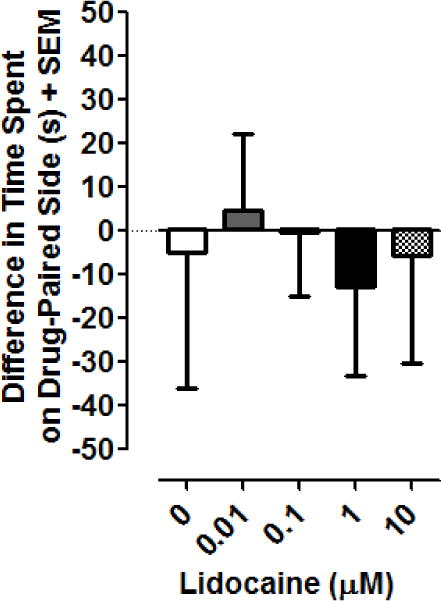
Lidocaine does not produce EPC in planarians. Data are presented as the mean difference in time spent on the drug-paired side (s) + SEM. N = 8 planarians per group.
Discussion
We tested the hypothesis that planarians, the simplest animal to possess a body plan common to all vertebrates and most invertebrates, would display motor dysfunction and place-conditioning effects when exposed to ethanol alone and in combination with cocaine. That is, indeed, what we found. Planarians exposed to ethanol by itself displayed concentration-dependent increases in C-shape movements and EPC. For combination experiments that quantified C-shape movements, ethanol interacted synergistically with cocaine (Tallarida, 2012). The synergism was specific to cocaine as similar experiments testing combinations of ethanol and nicotine revealed only a simply additive interaction. Synergy between ethanol and cocaine in planarians, or other invertebrates for that matter, has not been previously reported to our knowledge, but it should be noted that Lmo genes regulate behavioral responses to ethanol and cocaine in Drosophila melanogaster and the mouse (Lasek et al., 2011). Thus, in planarians, it is possible that Lmo genes contributed to the stereotypical and rewarding effects produced by ethanol and cocaine as well as the synergy that was demonstrated for the combination. Interactions between ethanol and cocaine have been studied more extensively in rats and mice. For rat experiments synergy has been demonstrated for ethanol and cocaine interactions in a taste-aversion learning assay whereas additivity was demonstrated on schedule-controlled responding (Sobel and Riley, 1997). Rotarod performance in both rats and mice was investigated using a design in which active doses of ethanol were administered with inactive doses of cocaine (Rech et al., 1978). The combinations disrupted rotarod performance of rats above levels observed with ethanol alone, whereas the combination did not enhance or suppress effects of ethanol alone in mice. Another study measured activity levels of mice following administration of a stimulant dose of ethanol by itself and in combination with different doses of cocaine (Masur et al., 1989). Both compounds increased activity when administered alone, and the combination resulted in additive responses.
Ethanol and cocaine both produced C-shape movements in the present experiments, a finding consistent with prior evidence that acute exposure to psychoactive drugs such as cocaine, nicotine, methamphetamine, and the ‘bath salt’ constituent mephedrone (i.e., 4-methylmethcathinone) also elicit C-shapes in planarians (Pagan et al., 2013; Ramoz et al., 2012; Rawls et al., 2010a, 2011). Ethanol was less efficacious than cocaine as a maximum of about 9 and 43 C-shapes were detected during exposure to ethanol and cocaine, respectively. The disparity in efficacies is unclear, but one possibility is that ethanol displays mixed stimulant and depressant effects, with the latter action serving to dampen the number of C-shape movements. Although rats also display increased stereotypical activity following exposure to cocaine, ethanol and other psychoactive drugs (Devenport et al., 1983; Koob, 1992), the relationship between C-shape movements in planarians and stereotyped movements in rodents is unclear despite manifestation of both behavioral phenomena after presentation of a common drug-induced stimulus. C-shape movements have been described as muscle contractions that present following exposure to cholinergic agonists (Nishimura et al., 2010) and as “seizure-like activity” based on evidence that proconvulsants produce stereotyped movements that are suppressed by administration of antiepileptic drugs (Raffa et al., 2010; Ramakrishnan and Desaer, 2011). Given that cocaine and ethanol can produce toxicity in humans and rats, particularly at high doses (Zagnoni and Albano, 2002), it cannot be discounted that proconvulsant or toxic actions of cocaine, ethanol, or both, contributed to the production of C-shape movements. For cocaine there was also significant disparity between concentrations that produced EPC and C-shapes. EPC was elicited by nanomolar concentrations of cocaine, with the environmental shift peaking at 0.01 μM, whereas millimolar concentrations were necessary to elicit C-shapes. No such disparity was evident for ethanol, as EPC and C-shapes were produced by overlapping concentrations, an outcome perhaps related to ethanol’s only modest ability to induce C-shapes. Motility was not tested in the present study, although it could be predicted that concentrations of cocaine and ethanol that produce the most robust stereotyped movements would cause the most pronounced reduction in motility, and vice versa (Baker et al., 2011; Rawls et al., 2011).
Ethanol and cocaine both produced EPC in planarians, a finding consistent with evidence that drugs of abuse, such as nicotine and mephedrone, and natural rewards, such as common table sugar, also produce EPC in planarians (Rawls et al., 2011; Ramoz et al., 2012; Zhang et al., 2013). The efficacies of ethanol and cocaine, and the shapes of their concentration-response curves, were different. Ethanol was more efficacious, producing a maximal environmental shift of nearly 150 s compared to approximately a 100 s shift for cocaine. Ethanol was also more efficacious than sucrose, which was previously shown to produce a maximal environmental shift of about 110 s (Zhang et al., 2013). The shapes of the concentration curves for ethanol and cocaine also differed. Cocaine produced an inverted-U shape concentration-response curve in which a concentration of 0.01 μM produced the maximal environmental shift with lower and higher concentrations producing weaker shifts. Comparable inverted-U shape dose-response curves for cocaine have been observed with rats (e.g. 10 mg/kg producing maximal EPC with doses of 3 and 30 mg/kg producing weaker responses) (Zakharova et al., 2009). Cocaine-induced EPC in rats can be both reduced and strengthened by ethanol in a manner that is dependent on cocaine dose and thought to be related to whether or not the dose of cocaine is rewarding or aversive (Busse et al., 2004; Busse and Riley, 2002). Rat studies indicate that a fixed, ineffective dose of ethanol reduces EPC produced by higher doses of cocaine (e.g. 30 and 40 mg/kg); however, when the same dose of ethanol is administered in combination with lower, ineffective doses of cocaine (2.5 and 5 mg/kg), rats display EPC (Busse et al., 2004). In planarians a somewhat different profile was observed when a fixed concentration of ethanol that did not produce significant EPC by itself was administered with variable concentrations of cocaine. Ethanol did not reduce environmental shifts induced by any of the cocaine concentrations but when administered with the highest concentration of cocaine, which did not produce an environmental shift by itself, planarians did display significant EPC. Thus, while modest overall effects of cocaine and ethanol combinations on planarian EPC were observed, our results in this species are suggestive of a slight enhancement rather than inhibition of EPC.
A liability of the planarian assay is that it is not well-suited for identifying pharmacokinetic mechanisms underlying synergistic interactions, including the one identified here for cocaine and ethanol. It is conceivable that planarians contain enzymes that convert cocaine and ethanol into cocaethylene, a behaviorally-active metabolite that targets mammalian neural reward pathways and contributes to the locomotor, reinforcing and addictive properties of the combination (Katz et al., 1992; Sobel and Riley, 1999; Schechter, 1995). Fruit flies (Drosophila melanogaster) express a cellular mechanism through which cocaine can be converted to cocaethylene, possibly through ethanol-sensitive enzymes (Torres and Horowitz, 1999). Along these lines, we investigated behavioral effects of cocaethylene in planarians, and found that the metabolite, similar to cocaine and ethanol, produced C-shapes and EPC following it exogenous application. Similar to cocaine, cocaethylene produced rewarding effects and motor dysfunction that were discernible by concentration, with lower concentrations producing EPC and higher concentrations producing C-shapes. As more information emerges about the genome of planarians, future studies are planned to better link behavioral, neurochemical and cellular data to determine if endogenous cocaethylene contributes to the pharmacological effects of cocaine and ethanol in planarians.
The relationship between EPC responses in planarians and mammals is not yet clear. It is evident that drugs of abuse from different classes, as well as natural reinforcers such as table sugar, can produce EPC in planarians and mammals. From the conventional point of view, the EPC assay is typically used to assess the rewarding effects of a substance, especially in the context in which the rewarding effect is associated with the environment (Napier et al., 2013). For planarians, it is unclear whether the rewarding effects of addictive substances such as ethanol and cocaine are responsible for the EPC. Our experiments revealed that lidocaine, an analog of cocaine that produces anesthetic effects but not rewarding effects, did not produce EPC. While the effects of cocaine and lidocaine on EPC in planarians were separable, a caveat is that lidocaine may not be biologically activity in this organism since there is no existing evidence that lidocaine acts on Na+ channels in planarians. Nonetheless, prior pharmacological evidence does suggest that planarians express functional Na+ channels. For example, carbamazepine, a clinically approved antiepileptic drug, inhibits convulsant-like effects in planarians (Ramakrishnan and Desaer, 2011), and it is widely accepted that the anti-seizure properties of carbamazepine are largely due to its inhibition of voltage-gated sodium channels (McLean and Macdonald, 1986).
It is generally accepted that enhanced dopamine transmission underlies the rewarding effects of addictive substances, and it has been shown that sucrose produces EPC in planarians that is inhibited by antagonists of dopamine receptors (Zhang et al., 2013). However, in regard to ethanol and table sugar, it is possible that taste contributes to EPC. The digestive system of planarians is comprised of a mouth and gastrovacular cavity connected by a pharynx, and their natural diet consists of segmented worms and dead fish. It is known that chemoreceptors concentrated in the auricles at the side of the planarian head respond to gustatory and olfactory stimuli and that olfactory/taste signals received in the head region are conveyed in the main lobes of the brain (Okamoto et al., 2005). Future studies will be aimed at linking reward and appetite pathways in planarians to the pharmacological effects of ethanol. It should also be mentioned that anxiolytic effects of rewarding substances could contribute to EPC displayed by planarians.
In summary, ethanol produced place-conditioning and motor effects in planarians and interacted synergistically with cocaine. Our results suggest that certain elements of ethanol’s neuropharmacological profile are conserved across species (Raffa and Rawls, 2010) and demonstrate the applicability of planarian assays in the quantification of polydrug interactions, particularly when used with dose-addition analysis (Tallarida, 2012).
References
- Anderson AL, Reid MS, Li SH, Holmes T, Shemanski L, Slee A, Smith EV, Kahn R, Chiang N, Vocci F, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104:133–139. doi: 10.1016/j.drugalcdep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones S, Aston-Jones G, Koob GF. Cocaine antagonizes anxiolytic effects of ethanol. Psychopharmacology (Berl) 1984;84:28–31. doi: 10.1007/BF00432019. [DOI] [PubMed] [Google Scholar]
- Baker D, Deats S, Boor P, Pruitt J, Pagán OR. Minimal structural requirements of alkyl γ-lactones capable of antagonizing the cocaine-induced motility decrease in planarians. Pharmacol Biochem Behav. 2011;100(1):174–9. doi: 10.1016/j.pbb.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse GD, Lawrence ET, Riley AL. The modulation of cocaine-induced conditioned place preferences by alcohol: effects of cocaine dose. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:149–155. doi: 10.1016/j.pnpbp.2003.09.031. [DOI] [PubMed] [Google Scholar]
- Busse GD, Lawrence ET, Riley AL. The effects of alcohol preexposure on cocaine, alcohol and cocaine/alcohol place conditioning. Pharmacol Biochem Behav. 2005;81:459–465. doi: 10.1016/j.pbb.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Busse GD, Riley AL. Modulation of cocaine-induced place preferences by alcohol. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1373–1381. doi: 10.1016/s0278-5846(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pellicano C, Pontieri FE. Neuropharmacology and behavior in planarians: translations to mammals. Comp Biochem Physiol C Toxicol Pharmacol. 2008;147:399–408. doi: 10.1016/j.cbpc.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Buttarelli FR, Pontieri FE, Margotta V, Palladini G. Acetylcholine/dopamine interaction in planaria. Comp Biochem Physiol C Toxicol Pharmacol. 2000;125:225–231. doi: 10.1016/s0742-8413(99)00111-5. [DOI] [PubMed] [Google Scholar]
- Devenport LD, Merriman VJ, Devenport JA. Effects of ethanol on enforced spatial variability in the 8-arm radial maze. Pharmacol Biochem Behav. 1983;1:55–9. doi: 10.1016/0091-3057(83)90251-4. [DOI] [PubMed] [Google Scholar]
- Dawn. Substance Abuse and Mental Health Services Administration. Center for Behavioral Health Statistics and Quality; Rockville, MD: 2010. [Google Scholar]
- Eriksson KS, Panula P. Gamma-Aminobutyric acid in the nervous system of a planarian. J Comp Neurol. 1994;345:528–536. doi: 10.1002/cne.903450405. [DOI] [PubMed] [Google Scholar]
- Katz JL, Terry P, Witkin JM. Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene) Life Sci. 1992;50:1351–1361. doi: 10.1016/0024-3205(92)90286-x. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kusayama T, Watanabe S. Reinforcing effects of methamphetamine in planarians. Neuroreport. 2002;11:2511–2513. doi: 10.1097/00001756-200008030-00033. [DOI] [PubMed] [Google Scholar]
- Lasek AW, Giorgetti F, Berger KH, Tayor S, Heberlein U. Lmo genes regulate behavioral responses to ethanol in Drosophila melanogaster and the mouse. Alcohol Clin Exp Res. 2011;35:1600–1606. doi: 10.1111/j.1530-0277.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste J, Pedrera-Melgire M, Charles-Nicolas A, Ballon N. [Cocaine and alcohol: a risky association] Presse Med. 2010;39:291–302. doi: 10.1016/j.lpm.2009.05.011. [DOI] [PubMed] [Google Scholar]
- McLean MJ, Macdonald RL. Carbamazepine and 10,11-epoxycarbamazepine produce use- and voltage-dependent limitation of rapidly firing action potentials of mouse central neurons in cell culture. J Pharmacol Exp Ther. 1986;238:727–738. 1986. [PubMed] [Google Scholar]
- Masur J, Souza-Formigoni ML, Pires ML. Increased stimulatory effect by the combined administration of cocaine and alcohol in mice. Alcohol. 1989;6:181–182. doi: 10.1016/0741-8329(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Napier TC, Herrold AA, de Wit H. Using conditioned place preference to identify relapse prevention medications. Neurosci Biobehav Rev. 2013;37(9 Pt A):2081–2086. doi: 10.1016/j.neubiorev.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Kitamura Y, Taniguchi T, Agata K. Analysis of motor function modulated by cholinergic neurons in planarian Dugesia japonica. Neuroscience. 2010;168:18–30. doi: 10.1016/j.neuroscience.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Takeuchi K, Agata K. Neural projections in planarian brain revealed by fluorescent dye tracing. Zoolog Sci. 2005;22:535–546. doi: 10.2108/zsj.22.535. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Deats S, Baker D, Montgomery E, Wilk G, Tenaglia M, Semon J. Planarians require an intact brain to behaviorally react to cocaine, but not to react to nicotine. Neuroscience. 2013;246:265–70. doi: 10.1016/j.neuroscience.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Azam M, Urban KR, Bidja AH, Roy DM, Feeney RB, Afshari LK. Reversal of cocaine-induced planarian behavior by parthenolide and related sesquiterpene lactones. Pharmacol Biochem Behav. 2008;89:160–170. doi: 10.1016/j.pbb.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Pagán OR, Rowlands AL, Fattore AL, Coudron T, Urban KR, Bidja AH, Eterović VA. A cembranoid from tobacco prevents the expression of nicotine-induced withdrawal behavior in planarian worms. Eur J Pharmacol. 2009;615:118–124. doi: 10.1016/j.ejphar.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladini G, Ruggeri S, Stocchi F, De Pandis MF, Venturini G, Margotta V. A pharmacological study of cocaine activity in planaria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:41–45. doi: 10.1016/s0742-8413(96)00053-9. [DOI] [PubMed] [Google Scholar]
- Passarelli F, Merante A, Pontieri FE, Margotta V, Venturini G, Palladini G. Opioid-dopamine interaction in planaria: a behavioral study. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1999;124:51–55. doi: 10.1016/s0742-8413(99)00048-1. [DOI] [PubMed] [Google Scholar]
- Phillips K, Luk A, Soor GS, Abraham JR, Leong S, Butany J. Cocaine cardiotoxicity: a review of the pathophysiology, pathology, and treatment options. Am J Cardiovasc Drugs. 2009;9:177–196. doi: 10.2165/00129784-200909030-00005. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Finno KE, Tallarida CS, Rawls SM. Topiramate-antagonism of L-glutamate-induced paroxysms in planarians. Eur J Pharmacol. 2010;649:1–3. 150–3. doi: 10.1016/j.ejphar.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffa RB, Rawls SM. A Model for Drug Action and Abuse. Landes Bioscience; Austin, TX: 2010. [Google Scholar]
- Raffa RB, Valdez JM. Cocaine withdrawal in Planaria. Eur J Pharmacol. 2001;430:143–145. doi: 10.1016/s0014-2999(01)01358-9. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan L, Desaer C. Carbamazepine inhibits distinct chemoconvulsant-induced seizure-like activity in Dugesia tigrina. Pharmacol Biochem Behav. 2011;99:665–670. doi: 10.1016/j.pbb.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Ramoz L, Lodi S, Bhatt P, Reitz AB, Tallarida C, Tallarida RJ, Raffa RB, Rawls SM. Mephedrone (“bath salt”) pharmacology: insights from invertebrates. Neuroscience. 2012;208:79–84. doi: 10.1016/j.neuroscience.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Yuvasheva E, Raffa RB. First evidence that drugs of abuse produce behavioral sensitization and cross sensitization in planarians. Behav Pharmacol. 2010b;4:301–13. doi: 10.1097/FBP.0b013e32833b0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Karaca F, Madhani I, Bhojani V, Martinez RL, Abou-Gharbia M, Raffa RB. β-lactamase inhibitors display anti-seizure properties in an invertebrate assay. Neuroscience. 2010a;169:1800–1804. doi: 10.1016/j.neuroscience.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Patil T, Tallarida CS, Baron S, Kim M, Song K, Ward S, Raffa RB. Nicotine behavioral pharmacology: clues from planarians. Drug Alcohol Depend. 2011;118:274–279. doi: 10.1016/j.drugalcdep.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rech RH, Vomachka MK, Rickert DE. Interactions between depressants (alcohol-type) and stimulants (amphetamine-type) Pharmacol Biochem Behav. 1978;8:143–151. doi: 10.1016/0091-3057(78)90331-3. [DOI] [PubMed] [Google Scholar]
- Rowlands AL, Pagán OR. Parthenolide prevents the expression of cocaine-induced withdrawal behavior in planarians. Eur J Pharmacol. 2008;583:170–172. doi: 10.1016/j.ejphar.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Cocaethylene produces conditioned place preference in rats. Pharmacol Biochem Behav. 1995;51(2–3):549–52. doi: 10.1016/0091-3057(95)00053-y. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Riley AL. The interaction of cocaine and alcohol on schedule-controlled responding. Psychopharmacology (Berl) 1997;129:128–134. doi: 10.1007/s002130050172. [DOI] [PubMed] [Google Scholar]
- Sobel BF, Riley AL. The interaction of cocaethylene and cocaine and of cocaethylene and alcohol on schedule-controlled responding in rats. Psychopharmacology (Berl) 1999;145:153–161. doi: 10.1007/s002130051044. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer. 2011;2:1003–1008. doi: 10.1177/1947601912440575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Revisiting the isobole and related quantitative methods for assessing drug synergism. J Pharmacol Exp Ther. 2012;342:2–8. doi: 10.1124/jpet.112.193474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Raffa RB. The application of drug dose equivalence in the quantitative analysis of receptor occupation and drug combinations. Pharmacol Ther. 2010;127:165–174. doi: 10.1016/j.pharmthera.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres G, Horowitz JM. Cocaethylene synthesis in Drosophila. Neurosci Lett. 1999;263:201–204. doi: 10.1016/s0304-3940(99)00156-1. [DOI] [PubMed] [Google Scholar]
- Zagnoni PG, Albano C. Psychostimulants and epilepsy. Epilepsia. 2002;2:28–31. doi: 10.1046/j.1528-1157.2002.043s2028.x. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Miller J, Unterwald E, Wade D, Izenwasser S. Social and physical environment alter cocaine conditioned place preference and dopaminergic markers in adolescent male rats. Neuroscience. 2009;163(3):890–7. doi: 10.1016/j.neuroscience.2009.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tallarida CS, Raffa RB, Rawls SM. Sucrose produces withdrawal and dopamine-sensitive reinforcing effects in planarians. Physiol Behav. 2013;15:112–113:8–13. doi: 10.1016/j.physbeh.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


