SUMMARY
The SCID-hu Thy/Liv mouse is a model for the analysis of human thymopoiesis. It has been constructed by engrafting fragments of human fetal liver and thymus into the immunodeficient C.B-17 scid/scid (SCID) mouse. The resulting ‘Thy/Liv’ organ promotes long-term differentiation of human T cells. Given the apparently normal physiology of the SCID-hu Thy/Liv organ, it has been used to explore the pathophysiologic mechanisms of HIV-1 infection in vivo, and to test therapeutic modalities such as anti-HIV-1 drugs and haematopoietic stem cell (HSC)-based gene therapy. In this review, I will summarise what we have learned from the SCID-hu Thy/Liv model, with a focus on recent findings in HIV-1 replication and therapy. Unique HIV-1 determinants have been identified which are required for replication in the Thy/Liv organ but not for replication in PBMC or in T cell lines in vitro. The mechanism of HIV-1 induced thymus depletion is not clear. It is correlated with high levels of HIV-1 replication. Both direct and indirect mechanisms may be involved. In addition to preclinical evaluation of anti-HIV-1 drugs, the SCID-hu Thy/Liv mouse has also been successfully used to test the feasibility of HSC-based gene therapy.
A number of improved SCID-hu models have been constructed to meet different requirements. Using these SCID-hu Thy/Liv models, current/future efforts will provide insightful information for understanding pathogenesis and designing therapeutic interventions against HIV-1 infection in humans, especially in paediatric patients.
INTRODUCTION
It has become increasingly clear that the pathophysiologic correlates leading to T cell depletion during HIV-1 disease may be clarified by direct evaluation of interactions between the virus and defined haematolymphoid organs.1,2 Though not well studied during HIV-1 infection, the thymus has been implicated as a site of early viral replication3–8 and thymic organs from HIV-1 infected fetuses and paediatric patients show profound parenchymal damage and involution.5,6,9,10 More significantly, a strong correlation of HIV induced thymus dysfunction has been established with faster AIDS progression in paediatric patients.11 In addition, early thymus destruction appears to be a common feature of other lentivirus diseases in FIV-infected cats12 and SIV-infected monkeys.13
In the thymus, where most T cells are derived, CD4 is present not only on mature (CD3+CD4+CD8−) T cells and macrophages, but also on less mature thymocytes (CD3−/low CD4+CD8+) and intrathymic progenitor (CD3−CD4+CD8−) cells.14,15 Since the thymus organ is difficult to study in human subjects, a small animal model (SCID-hu Thy/Liv mouse) for the analysis of human thymopoiesis has been constructed by engrafting fragments of human fetal liver and thymus into the immunodeficient C.B-17 scid/scid (SCID) mouse.16 The resulting ‘Thy/Liv’ organ promotes longterm differentiation of human T cells in a manner which appears physiologically normal.17,18 Thymocyte subpopulations are represented within the organ in expected proportions, a normal T cell receptor Vfl repertoire is displayed,19,20 and tolerance is induced towards both ‘self’ major histocompatibility antigens and exogenously provided superantigens.21,22
Given the apparently normal physiology of the SCID-hu Thy/Liv organ, we and others23–26 have used this model to explore the pathophysiologic mechanisms of HIV-1 infection in vivo. In this review, I will summarise what we have learned from the SCID-hu Thy/Liv model, with a focus on recent findings in HIV-1 replication and therapy. A number of reviews of earlier reports have been published.27,28 A different humanised SCID model, hu-PBL-SCID,29 which is transplanted with mature human PBMC, will not be covered here.
THE SCID-HU THY/LIV MOUSE AS A MODEL FOR PRIMARY HIV-1 INFECTION AND PATHOGENESIS IN THE HUMAN THYMUS
In contrast to in vitro models of HIV-1 infection, the SCID-hu Thy/Liv mouse provides an intact human lymphoid organ for HIV-1 infection. Multiple cell types are present and, most importantly, most target cells are in their physiological resting stage. In addition, normal T cell development and maturation occur in the Thy/Liv organ over 12 months after transplantation.17,18
The organ system is permissive for infection with primary HIV-1 isolates.30 Both macrophages and T cells are infected in the Thy/Liv organ. Most T cell line-adapted HIV-1 strains, however, failed to replicate efficiently.31 The infection proceeds in a dose- and time-dependent manner and is suppressed by in vivo administration of nucleoside analogues such as zidovudine (AZT).32
In an attempt to analyse AZT resistant mutants arising during in vivo selection, multiple rounds of infection of the Thy/Liv organ in the presence of increasing concentrations of AZT have failed to generate AZT-resistant HIV-1 mutants (J. McCune and H. Kaneshima., personal communication). This is consistent with the finding that very few mutations have accumulated during the infection process.33 Thus HIV-1 replication in this model reflects a low level, primary infection in the absence of immune selection.
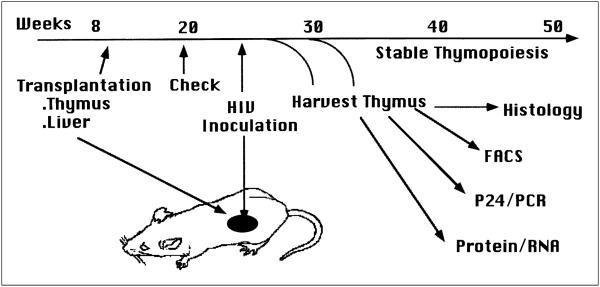
After intra-organ inoculation of the SCID-hu Thy/Liv with HIV-1 (Figure. 1), HIV-1 replication reaches high levels followed by depletion of CD4+ thymocytes with an inversion of the CD4/CD8 ratio.23,24,34 CD4+CD8+ thymocytes, which comprise 80–85% of total thymocytes, are significantly depleted about 1 week after HIV-1 reaches peak infection (Figure 2).24,35 In addition, a more accelerated pace of replication and thymocyte depletion is observed with rapidly replicating, syncytium-inducing (SI) virus isolated from AIDS patients than with slowly replicating, non-syncytium-inducing (NSI) virus isolated from the same patients before AIDS development, or from long term non-progressor patients.36,37 Thus, the Thy/Liv organ provides a relevant in vivo model to evaluate primary HIV-1 replication and pathogenicity.
Figure 1.
HIV-1 infection of the SCID-hu Thy/Liv mouse. Human fetal thymus and liver fragments transplanted under renal capsules in SCID mice support stable human thymopoiesis for over a year. HIV-1 inoculation and endpoint (1–6 wpi) analyses are illustrated.
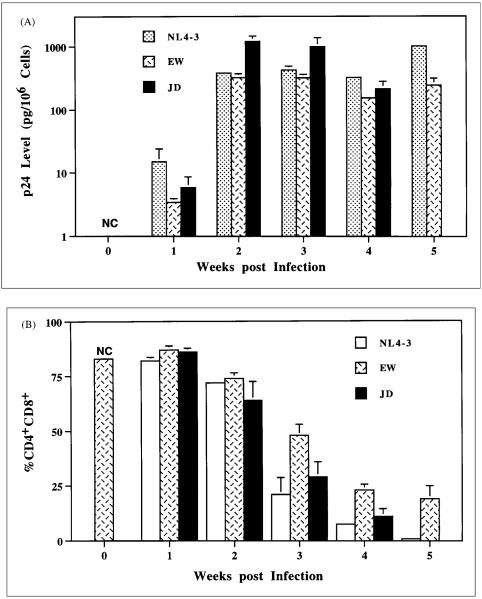
Figure 2.
Kinetics of HIV-1 replication and pathogenesis in the Thy/Liv organ. (A) Production of viral p24 antigen after HIV-1 infection. Thymocyte-associated p24 antigen is measured and standardised as pg/106 cells). NL4–3 is a T-tropic HIV-1 clone. EW and JD are two primary patient isolates. No Thy/Liv organs infected by JD are analysed at 5 wpi. NC: Negative controls of mock infected Thy/Liv organs. (B) Depletion of CD4+CD8+ thymocytes after HIV-1 infection. SCID-hu mice are analysed by FACS staining of CD4 and CD8. %CD4+CD8+, which consist of 80%–85% of total thymocytes in normal human thymi and Thy/Liv organs, is a good measure of thymocyte depletion.
ANALYSIS OF HIV-1 FACTORS UNIQUELY REQUIRED FOR REPLICATION IN THE THY/LIV ORGAN
Studies of HIV-1 accessory genes in the SCID-hu Thy/Liv model
As is observed in the SIV-infected rhesus macaque,38 replication and pathologic effects (e.g. thymocyte depletion) of HIV-1 (both NL4–3 and JRCSF) in the SCID-hu mouse are dependent upon an intact nef open reading frame39 Analysis of the other HIV-1 accessory genes such as vpr, vpu and vif has demonstrated that, unlike in tissue cultures, mutations in these genes significantly slowed down the replication and cytopathic effects of HIV-1-NL4-3.40
A close correlation between the levels of HIV-1 replication and thymocyte depletion has been established (Table 1). Thus, NL4-3, JD, EW and primary SI isolates replicated to high levels in about 2–3 weeks post inoculation (wpi) and lead to early thymocyte depletion at about 3–4 wpi. JRCSF, NL4-3 mutants (nef-, vpu- or vif-) and primary NSI isolates replicated to peak levels in about 5–6 weeks and significant thymocyte depletion occurred at about 7–8 weeks post infection. In addition, high input HIV-1 accelerated both HIV-1 replication and its associated thymocyte depletion.40 Therefore, prolonged presence of high viral replication is required to cause thymocyte depletion.
Table 1.
Correlation of HIV-1 replication and thymus depletion
| HIV-1 isolates | PBMCa | Thy/Live replicationb |
Thy/Liv depletionc |
|---|---|---|---|
| Clones | |||
| NL4–3 | +++ | +++ | +++ |
| NL4–vif | − d | +/− | +/− |
| NL4–vpr | +++ | ++ | ++ |
| NL4–vpu | +++ | + | + |
| NL4–nef | +++ | +/− | +/− |
| JRCSF | +++ | ++ | ++ |
| JRCSF-nef | +++ | + | NDSe |
| Lai/IIIB | +++ | − | − |
| HXB2 | +++ | − | − |
| HXB2/LW | +++ | +++ | NDS |
| Primary isolates | |||
| SM | +++ | +++ | +++ |
| TY | +++ | +++ | +++ |
| EW | +++ | +++ | +++ |
| JD | +++ | +++ | +++ |
| A–NSI | + | +/− | +/− |
| A–SI | +++ | +++ | +++ |
| B–NSI | + | +/− | +/− |
| B–SI | +++ | +++ | ++ |
| LTNP–NSI | + | +/− | +/− |
Data from the following reports are summarised. NL4–323,33,35,39,40,43 and its mutant derivatives,39,40 JRCSF23,24,33,34,39 and JRCSF–nef,39 Lai/IIIB31 HXB2 and HXB2/LW.31,43 SM,24,34 TY24 EW24,35 JD35 A–NSI, SI and B–NSI, SI.36 LTNP (long-term non-progressor)–NSI.37
Replication in PHA-activated PBMC as measured by p24 or RT production.
HIV-1 replication in the Thy/Liv organ measured by cell- associated p24 or by semi-quantitative DNA PCR analyses.
Thymocyte depletion after HIV-1 infection as analysed by FACS analysis.
NL4–vif mutant replicates in certain T cell lines.
No data shown.
HIV-1 env determinants required for efficient replication in the Thy/Liv organ
Comparison of ‘attenuated’ HIV-1 isolates with ‘pathogenic’ ones in vitro and in vivo should help to identify important viral determinants for replication and pathogenesis in vivo. The Lai/IIIB isolate and its associated infectious molecular clones (e.g. HXB2) were found to infect T cell lines such as H9.41 When a laboratory worker was accidentally infected by Lai/IIIB, however, HIV-1 was isolated only from inoculation of primary PBMC, but not from T cell lines.42 The SCID-hu Thy/Liv model was used to study the replication of HXB2 and of HXB2 recombinant viruses with HIV-1 fragments isolated from the infected laboratory worker.43 HXB2 showed no or very low levels of replication in the Thy/Liv organ. Replacement of its subgenomic fragment encoding the envelope gene with a corresponding fragment from the LW87-1 isolate generated a recombinant virus (HXB2/LW) which replicated actively in SCID-hu mice.
The specific env determinants have been mapped to the V1–V3 regions of the HIV-1 genome. Six unique mutations in the V3 loop region have been identified which contribute to most of its increased replication in vivo. These changes affected target cell tropism and/or overall infectivity of the virus. However, HXB2/LW showed no enhanced replication activity in PBMC. Thus, altered or expanded host cell range may contribute to its enhanced replication in the Thy/Liv organ.
The unique structural determinants in HIV-1 appear to be necessary for infectivity in vivo, but not in PBMC or in immortalised T cell lines. Interestingly, the relevant changes did not affect the nef gene, previously implicated for pathogenicity of SIV in rhesus macaques,38 or of HIV-1 in SCID-hu mice.39,40 The vpu and vpr genes, which have also been reported to affect HIV-1 replication in SCID-hu Thy/Liv mice,40 of HXB2/LW also remain defective. Thus, unique features of the V3 region of env that are necessary for infection of thymic target cells are revealed by phenotypic and molecular analyses of HIV-1 isolates in the SCID-hu Thy/Liv mouse.
A variation of the SCID-hu Thy/Liv model which transplants more human tissues has been developed.25 In this model, HIV-1 infection can be initiated by intraperitoneal inoculation.44 However, the Lai/IIIB isolate appears to infect the Thy/Liv organ in this model and leads to thymus depletion. The difference may be due to its long duration (6 months) of infection and/or to activation of human thymocytes in this model by some unknown mechanisms.
MECHANISMS OF HIV-1 INDUCED THYMUS DEPLETION
HIV-1 replication in the Thy/Liv organ leads to thymocyte depletion with a preference for CD4+ thymocytes. As mentioned above, a close correlation exists between levels of HIV-1 replication and thymocyte depletion (Table 1). Kinetic analysis indicated that thymocyte depletion occurs about 1–2 weeks after HIV-1 peak replication is achieved (Figure 2). This indicates that high levels of viral replication are required to lead to thymus depletion. Both viral encoded proteins and host factors may be involved. The SCID-hu Thy/Liv mouse has been used to address the following questions regarding HIV-1 induced thymus depletion.
How do thymocytes die in response to HIV-1 infection in the Thy/Liv organ?
Apoptosis has been associated with HIV-1 induced T cell death both in vitro and in vivo.45–49 It appears to be associated, at least partly, with HIV-1 induced thymocyte depletion in the Thy/Liv organ. Morphologically, some thymocytes with condensed nuclei are detected in HIV-1 infected Thy/Liv organs by thin section light microscopy and by electron microscopy.24 Biochemically, partial chromosomal loss (detected by propidium iodide staining)24 and DNA strand breaks (detected by terminal deoxynucleotide transferase labelling)35 are associated with HIV-1 induced thymocyte depletion, although the characteristic chromosomal DNA ladder associated with most forms of apoptosis is not consistently observed (Su, Bonyhadi, Kaneshima and McCune, unpublished observation). Experiments performed by a different research group using the Thy/Liv model have failed to demonstrate evidence of significant levels of apoptosis during HIV-1 induced thymocyte depletion. Necrosis appears to be the major mechanism of HIV-1 induced T cell death (J. Zack, personal communication). Differences in experimental procedures and in animal maintenance conditions may contribute to the discrepancy.
Are all dead or dying thymocytes directly infected with HIV-1?
Regardless of how thymocytes die after HIV-1 infection, a very important question is whether direct infection is required for the thymocyte to die. The replication level of HIV-1 in the Thy/Liv organ is relatively low. At peak times, about 10% thymocytes are infected as measured by PCR detection of proviral DNA.23,33–35,39,40,43 This is consistent with lack of significant mutation during infection33 and failure to generate AZT-resistant mutants (unpublished results), as discussed earlier. Using flow cytometric cell sorting coupled with a semi-quantitative PCR assay for detection of HIV-1 DNA, it was shown that most of the thymocytes within the HIV-1 infected Thy/Liv organ with induced DNA strand breaks in the chromosome were not infected with HIV-1. Thus, both cells with DNA strand breaks and without DNA strand breaks were infected at the same level (about 10% as measured by PCR). Therefore, HIV-1 replication may induce changes in the thymus to cause cell death (or chromosomal breakages) of uninfected, as well as infected, thymocytes.35 Likewise, it has been recently reported that most apoptotic cells are not productively infected in lymph nodes from HIV-1 infected human patients or from SIV infected monkeys.48
What contributes to the thymus depletion following HIV-1 infection?
Both direct and indirect mechanisms of cell death induction may be involved. The plateau and slight reduction of HIV-1 replication during thymus depletion may be interpreted as gradual depletion of direct HIV-1 target cells (Figure 2).24,35 Thus, HIV-1 infects and depletes the target cells and leads to lower levels of HIV-1 replication. Reduced replication may be achieved by a number of HIV-1 encoded factors with cytotoxic or cytostatic activities as demonstrated in T cells cultured in vitro. For example, vpr has been shown to lead to G2/S phase cell cycle arrest in infected target cells by a cytostatic mechanism.50 Other HIV-1 proteins, such as tat, nef and gp120/gp41, have also demonstrated cytotoxic activity in various cell culture systems.51–54
Besides evidence discussed above of DNA strand breaks in uninfected cells, there is also evidence showing that, at least in some infected thymocytes, HIV-1 infection does not lead to their immediate destruction. For example, CD3+CD8+CD4− cells from HIV-1 infected Thy/Liv organs have been shown to carry HIV-1 proviral DNA 23,34 (Su, unpublished results). It has recently been shown that the HIV-1+ CD8 single positive cells are derived from HIV-1 infected CD4+ progenitors,55 possibly CD4+CD8+ and/or CD3−CD4+CD8− cells. Thus, HIV-1 infection of CD4+CD8+, CD3−CD4+CD8− or earlier progenitor cells does not necessarily lead to their immediate cytolysis.
Is thymocyte depletion due in part to destruction of intrathymic T progenitor cells?
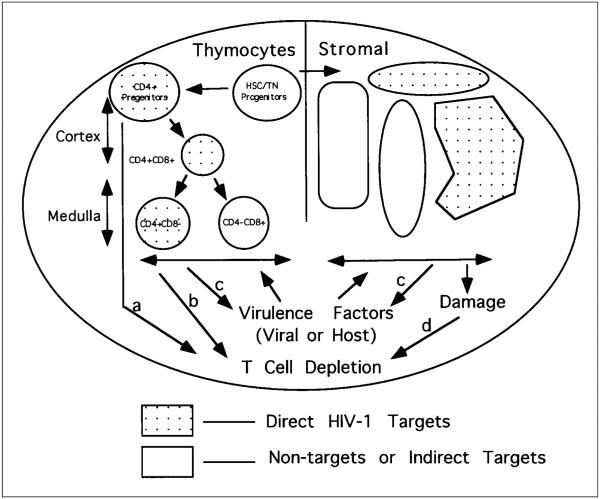
Besides the CD4+CD8+ immature thymocytes, the intrathymic CD3−CD4+CD8− T progenitor cell subpopulation constitutes a target for HIV-1 infection in vivo. Some HIV-1 isolates infect this population and deplete it; others infect it and do not lead to its immediate depletion.35 As illustrated in Figure 3, infection of progenitor cells by HIV-1 may lead to the following: (1) generation of a reservoir to transmit the HIV-1 genome to progeny cells; (2) destruction of a population that could provide progeny cells (reduction of progenitor cell pool); and (3) introduction of a maturational block upon normal thymocyte differentiation processes. Induction of cell death, either by direct or indirect mechanisms, may occur through pathway a or pathway b (Figure 3).
Figure 3.
A model for multiple target cells and pathogenic pathways of HIV-1 pathogenesis in the human thymus. HIV-1 infection may occur in many different types of cells in the thymus (both thymocytes and stromal cells). Infection and destruction of intrathymic T progenitor cells will block the supply of new thymocyte maturation, leading to thymus depletion (pathway a). Direct infection of CD4+ thymocytes may lead to their destruction (pathway b). In addition, virulence factors induced after HIV-1 infection may directly induce thymocyte death or indirectly by enhancing HIV-1 replication in the thymocytes (pathway c). Furthermore, destruction of thymic stromal cells following HIV-1 infection may contribute to thymocyte death induction (pathway d). HSC: haematopoietic stem cell. TN: Triple negative (CD3−CD4−CD8−) thymocytes.
In addition to production of viral proteins, HIV-1 infection also leads to cytokine dysregulation in humans.56 A large number of cytokines are produced in the thymus which play important roles in modulating T cell development. HIV-1 infection in the Thy/Liv organ leads to increased production of cytokines such as IL4, IL6, IL10. In addition, TNFa and TGFfl are also induced.57 The contribution of the increased levels of cytokines to thymocyte depletion is not clear and needs future attention. These viral and host ‘virulence factors’ may cause thymocyte depletion either directly or indirectly by enhancing HIV-1 replication, as shown in pathway c (Figure 3).
The thymus microenvironment is essential for T cell development. Direct infection and destruction of thymic epithelium cells have been reported in the human thymus and in HIV-1 infected Thy/Liv organs.5,34 This may lead to blockage in T cell development and result in thymocyte death. Thus, HIV-1 infection of the Thy/Liv organ may cause destruction of the thymus microenvironment to induce thymocyte depletion (pathway d, Figure 3). It is not clear, however, whether the HIV-1 infected thymic stromal cells are still functional in supporting de novo human T cell development.
SCREENING ANTI-HIV-1 DRUGS IN THE SCID-HU MODEL
As mentioned above, the Thy/Liv model provides an in vivo system to study the human thymus organ in a normal, physiologically relevant state. In addition, the SCID-hu mouse also provides an animal model to test toxicity and bioavailability of therapeutic compounds.58 The SCID-hu Thy/Liv model has been used to evaluate anti-retroviral drugs since the beginning of its construction. AZT, either administered before or post exposure to HIV-1, appeared to inhibit HIV-1 replication in the model.32,59 In recent years, the Thy/Liv model has been used to evaluate and screen various compounds for their efficacy, toxicity and in vivo formulations.60,61 This includes AZT, didanosine, nevirapine and bicyclams (singly or in combinations). This will provide useful preclinical information in drug evaluation and clinical trials.
Novel peptide-based therapeutic agents can also be tested in this model. It has been reported that IL10, but not IL12, can inhibit HIV-1 replication in the SCID-hu Thy/Liv mouse.25
PRECLINICAL STUDIES OF HSC-BASED GENE THERAPY OR CELL THERAPY FOR AIDS
Gene delivery via the haematopoietic stem cell (HSC) offers an attractive means to introduce antiviral genes into both T cells and macrophages for AIDS gene therapy. HSC can be isolated from a number of tissue sources, including bone marrow and peripheral blood, and are used to reconstitute all haematopoietic lineages in transplant recipients.62,63 Recently, haematopoietic progenitor cells have been efficiently transduced with murine leukaemia virus-based vectors.64,65 In addition, a retroviral vector encoding an anti-HIV-1 ribozyme has been shown to inhibit HIV replication in macrophage-like cells derived from the transduced stem/progenitor cells.65 Due to the difficulty of deriving human T cells from HSCs in vitro, it is difficult to demonstrate efficacy in the T lineage. The SCID-hu Thy/Liv model offers an ideal system to evaluate the potential of HSC-based gene therapy for AIDS.
The HIV-1 rev protein is critically required for the transport of unspliced HIV-1 mRNA into the cytoplasm and thus for the expression of HIV structural proteins.66 A trans-dominant mutant of HIV-1 rev, RevM10, has been shown to inhibit HIV-1 replication in PBMCs without affecting the growth and functions of the transduced cells.67,68 A clinical trial using retrovirally modified PBMCs, however, has demonstrated that RevM10 modified PBMC are short-lived in vivo. Thus, HSC-based gene therapy may be necessary to obtain long termanti-HIV-1 PBMCs in AIDS patients. It was unknown, however, whether the transduced gene will express at sufficient levels in T and myeloid cells and what effect (if any) RevM10 expression may have on the ability of transduced HSCs to differentiate into lymphoid or myeloid lineages.
To address these issues, experiments were performed to show that RevM10 could be efficiently transduced into cord blood derived HSC/progenitor cells, which develop into primary T cells69 or myeloid cells expressing the RevM10 gene in the SCID-hu Thy/Liv model or SCID-hu Bone model,70 respectively. After reconstitution of the Thy/Liv implants in SCID mice (SCID-hu Thy/Liv) with the transduced HSC/progenitor cells, normal thymocytes were derived and a significant number of donor derived thymocyte cells were found to express the RevM10 gene69 or a marker gene.71 It was further demonstrated that sufficient levels of RevM10 expression could be achieved to suppress HIV-1 replication in primary T cells derived from retrovirally transduced human HSCs69 Thus, the RevM10 gene did not appear to inhibit the differentiation of HSC/progenitor cells into T cells in the Thy/Liv organ. The level of retrovirus-mediated RevM10 expression in T cells derived from transduced HSCs was sufficient to suppress HIV-1 replication.
CONCLUSIONS
I have briefly summarised the most recent findings in HIV-1 infection and therapy using the SCID-hu Thy/Liv mouse. Its application to understanding HIV-1 infection, pathogenesis and therapy has proven that insighful information can be obtained about the HIV-1 disease process in vivo and about the feasibility of various therapeutic modalities. Further studies for assessing HIV-1 effects on the thymus environment, mechanisms of HIV-1 replication and associated thymocyte depletion, as well as tests of novel therapeutic agents and protocols are being explored by an increasing number of research groups. The preclinical studies of HSC-based gene therapy in the SCID-hu mouse have helped to launch a Phase I/II clinical trial in HIV-1 infected patients (SyStemix, Inc., 1997).
A number of improved SCID-hu models have been constructed to meet different requirements. For example, human fetal liver, thymus, bone and spleen fragments have been co-transplanted into SCID mice to form a human joint organ with T cell, B cells, myeloid cells and red blood cells.72 This model will be useful to test HIV-1 pathogenesis and to study HSC differentiation into all lineages in the same organ. In addition, implanting human lung tissues intraperitoneally in the SCID-hu Thy/Liv mouse has created a model to study HIV-1 infection of the lung (macrophages) and its transmission to the Thy/Liv organ.26 Using current or improved SCID-hu Thy/Liv models, future efforts are directed to addressing the following outstanding questions.
What viral factors (virulence factors) are uniquely required for replication and/or pathogenicity in the Thy/Liv organ?
What host factors are involved in HIV-1 induced thymus depletion?
What are the distributions of co-receptors (CCR5 and fusin) in the Thy/Liv organ?
What target cells are infected in the Thy/Liv organ by different HIV-1 isolates?
How significant is HIV-1 induced indirect cell killing in the Thy/Liv model?
Can HIV-1 infected/depleted Thy/Liv organs still support de novo thymopoiesis from HSC/progenitor cells carrying an antiviral gene or in the presence of antiviral drugs?
Are the findings of studying SCID-hu Thy/Liv mice reflective of HIV-1 infection in humans (paediatric infection in particular)?
Answers to these questions will further prove the usefulness of the model and provide insightful information for understanding pathogenesis and designing therapeutic interventions against HIV-1 infection in humans, especially in paediatric patients.
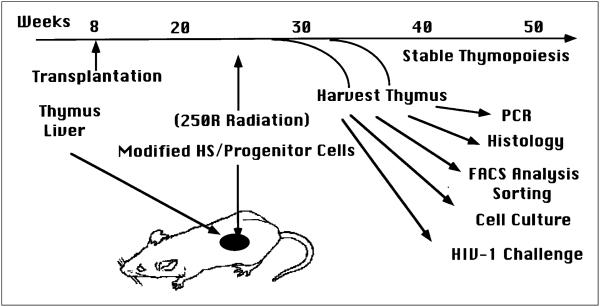
Figure 4.
Preclinical studies of HSC-based gene therapy in the SCID-hu Thy/Liv model. Retroviral-modified HSC/progenitors can be used to reconstitute the Thy/Liv organ which is irradiated to deplete resident thymocytes. Donor cells can be identified by mismatched HLA markers or by retroviral marking. Thymocytes from reconstituted Thy/Liv organs can be isolated and characterised in vitro to study gene expression, T cell function and resistance to HIV-1 infection.
ACKNOWLEDGEMENTS
I would like to thank Drs Mike McCune, Hideto Kaneshima and Mark Bonyhadi for helpful discussions, and SyStemix, Inc. and the Lineberger Comprehensive Cancer Center at UNC-Chapel Hill for previous and current supports.
Contract grant sponsor: SyStemix Inc., Lineberger Comprehensive Cancer Center at UNC-Chapel Hill
Abbreviations used
- HSC
haematopoietic stem cell
- AZT
zidovudine
- wpi
weeks post-inoculation
- SI
syncytium-inducing
- NSI
non-syncytium-inducing
REFERENCES
- 1.Fauci AS. Multifactoral nature of human immunodeficiency virus disease: implications for therapy. Science. 1993;262:1011–1018. doi: 10.1126/science.8235617. [DOI] [PubMed] [Google Scholar]
- 2.Fauci AS. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 3.Courgnaud V, Laure F, Brossard A, et al. Frequent and early in utero HIV-1 infection. AIDS Res. Hum. Retroviruses. 1991;7:337–341. doi: 10.1089/aid.1991.7.337. [DOI] [PubMed] [Google Scholar]
- 4.Harris PJ, Candeloro PD, Bunn JE. HIV infection of the adult thymus: an even more conventional theory explaining CD4 cell decrease and CD8 cell increase in AIDS. Med. Hypotheses. 1991;36:379–380. doi: 10.1016/0306-9877(91)90015-q. [DOI] [PubMed] [Google Scholar]
- 5.Joshi V, Oleske JM. Pathologic appraisal of the thymus gland in acquired immunodeficiency syndrome in children. Arch. Pathol. Lab. Med. 1985;109:142–146. [PubMed] [Google Scholar]
- 6.Schuurman HJ, Krone WJA, Broekhuizen R, et al. The thymus in acquired immune deficiency syndrome: comparison with other types of immunodeficiency disease, and presence of components of human immunodeficiency virus type 1. Am. J. Pathol. 1989;131:1329–1334. [PMC free article] [PubMed] [Google Scholar]
- 7.Seemayer TA, Lapointe N, Michaud J, Russo P, Goldman H, Dupuy JM. The thymus in the acquired immunodeficiency syndrome: pathological and theoretical consideration. In: CaV J, editor. Progress in Immunodeficiency Research and Therapy I. Griscelli Elsevier; Amsterdam: 1984. [Google Scholar]
- 8.Tremblay M, Numazaki K, Goldman H, Wainberg MA. Infection of human thymic lymphocytes by HIV-1. J. Acquir. Immune Defic. Syndr. 1990;3:356–360. [PubMed] [Google Scholar]
- 9.Rosenzweig M, Clark DP, Gaulton GN. Selective thymocyte depletion in neonatal HIV-1 thymic infection. AIDS. 1993;7:1601–1605. doi: 10.1097/00002030-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Seemayer TA, Laroche AC, Russo P, et al. Precocious thymic involution manifested by epithelial injury in the acquired immune deficiency syndrome. Hum. Pathol. 1984;15:469–474. doi: 10.1016/s0046-8177(84)80082-9. [DOI] [PubMed] [Google Scholar]
- 11.Kourtis AP, Ibegbu C, Nahmias AJ, et al. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 1996;335:1431–1436. doi: 10.1056/NEJM199611073351904. [DOI] [PubMed] [Google Scholar]
- 12.Beebe AM, Dua N, Faith TG, Moore PF, Pedersen NC, Dandekar S. Primary stage of feline immunodeficiency virus infection: viral dissemination and cellular targets. J. Virol. 1994;68:3080–3091. doi: 10.1128/jvi.68.5.3080-3091.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baskin GB, Murphy-Corb M, Martin LN, Davison-Fairburn B, Hu F-S, Kuebler D. Thymus in simian immunodeficiency virus-infected rhesus monkey. Lab. Invest. 1991;65:400–407. [PubMed] [Google Scholar]
- 14.Galy A, Verma S, Barcena A, Spits H. Precursors of CD3+CD4+CD8+ cells in the human thymus are defined by expression of CD34. Delineation of early events in human thymus development. J. Exp. Med. 1993;178:391–401. doi: 10.1084/jem.178.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraft DL, Weissman IL, Waller EK. Differentiation of CD3−4−8− human fetal thymocytes in vivo: characterization of a CD3−4+8− intermediate. J. Exp. Med. 1993;178:265–277. doi: 10.1084/jem.178.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: a model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 17.Krowka JF, Sarin S, Namikawa R, McCune JJ, Kaneshima H. Human T cells in the SCID-hu mouse are phenotypically normal and functionally competent. J. Immuno. 1991;146:3751–3756. [PubMed] [Google Scholar]
- 18.Namikawa R, Weilbaecher KN, Kaneshima H, Yee EJ, McCune JM. Long-term human hematopoiesis in the SCID-hu mouse. J. Exp. Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandekerckhove BA, Krowka JF, McCune JM, de VJ, Spits H, Roncarolo MG. Clonal analysis of the peripheal T cell compartment of the SCID-hu mouse. J. Immunol. 1991;146:4173–4179. [PubMed] [Google Scholar]
- 20.Vandekerckhove BA, Baccala R, Jones D, Kono DH, Theofilopoulos AN, Roncarolo MG. Thymic selection of the human T cell receptor V beta repertoire in SCID-hu mice. J. Exp. Med. 1992;176:1619–1624. doi: 10.1084/jem.176.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandekerckhove BA, Namikawa R, Bacchetta R, Roncarolo MD. Human hematopoietic cells and thymic epithelial cells induce tolerance via different mechanisms in the SCID-hu mouse thymus. J. Exp. Med. 1992;175:1033–1043. doi: 10.1084/jem.175.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waller EK, Sen MA, Kamel OW, Hansteen GA, Schick MR, Weissman IL. Human T-cell development in SCID-hu mice: staphylococcal enterotoxins induce specific clonal deletions, proliferation, and anergy. Blood. 1992;80:3144–3156. [PubMed] [Google Scholar]
- 23.Aldrovandi GM, Feuer G, Gao L, et al. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 24.Bonyhadi ML, Rabin L, Salimi S, et al. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 25.Kollmann Tr, Pettoello-Mantovani M, Zhung X, et al. Disseminated human immunodeficiency virus 1 (HIV-1) infection in SCID-hu mice after peripheral inoculation with HIV-1. J. Exp. Med. 1994;179:513–522. doi: 10.1084/jem.179.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grandadam M, Cesbron JY, Candotti D, et al. Dose-dependent systemic human immunodeficiency virus infection of SCID-hu mice after intraperitoneal virus injection. Res. Virol. 1995;146:101–112. doi: 10.1016/0923-2516(96)81079-x. [DOI] [PubMed] [Google Scholar]
- 27.McCune J, Kaneshima H, Krowka J, et al. The SCID-hu mouse: a small animal model for HIV infection and pathogenesis. Annu. Rev. Immunol. 1991;9:399–429. doi: 10.1146/annurev.iy.09.040191.002151. [DOI] [PubMed] [Google Scholar]
- 28.Kaneshima H. HIV-1-associated pathology in hemato-lymphoid organs and the experimental evaluation in the SCID-hu mouse. Int. J. Hematol. 1996;63:253–264. doi: 10.1016/0925-5710(96)00452-5. Review. [DOI] [PubMed] [Google Scholar]
- 29.Mosier DE, Gulizia RJ, MacIsaac PD, Torbett BE, Levy JA. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993;260:689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- 30.Namikawa R, Kaneshima H, Lieberman M, Weissman IL, McCune JM. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 31.Rabin L, Kaneshima H, Shih CC, McCune JM. Direct comparison of the infectivity of HIV isolates in vitro and in vivo in the SCID-hu mouse. Int. Conf. AIDS. 1990;6:113. [Google Scholar]
- 32.McCune JM, Namikawa R, Shih CC, Rabin L, Kaneshima H. Suppression of HIV infection in AZT-treated SCID-hu mice. Science. 1990;247:564–566. doi: 10.1126/science.2300816. [DOI] [PubMed] [Google Scholar]
- 33.Jamieson BD, Pang S, Aldrovandi G, Zack JA. In vivo pathogenic properties of two clonal human immunodeficiency virus type 1 isolates. J. Virol. 1995;69:6259–6264. doi: 10.1128/jvi.69.10.6259-6264.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stanley SK, McCune JM, Kaneshima H, et al. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su L, Kaneshima H, Bonyhadi M, et al. HIV-1 induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 36.Kaneshima H, Su L, Bonyhadi ML, Connor RI, Ho DD, McCune JM. Rapid-high, sycytium-inducing isolates of HIV-1 induce cytopathicity in the human thymus of the SCID-hu mouse. J. Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneshima H, Bonyhadi M, Su L, Connor R, Ho D, McCune JM. HIV infection induces thymocyte depletion in the SCID-hu mouse. In. Conf. AIDS. 1994;10:7–12. [Google Scholar]
- 38.Kestler HD, Ringler DJ, Mori K, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson BD, Aldrovandi GM, Planells V, et al. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 1994;68:3478–3485. doi: 10.1128/jvi.68.6.3478-3485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aldrovandi GM, Zack JA. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J. Virol. 1996;70:1505–1511. doi: 10.1128/jvi.70.3.1505-1511.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn BH, Shaw GM, Arya SK, Popovic M, Gallo RC, Wong SF. Molecular cloning and characterization of the HTVL-III virus associated with AIDS. Nature. 1984;312:166–169. doi: 10.1038/312166a0. [DOI] [PubMed] [Google Scholar]
- 42.Weiss SH, Goedert JJ, Gartner S, et al. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988;239:68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]
- 43.Su L, Kaneshima H, Bonyhadi M, et al. Identification of HIV-1 molecular determinants for replication in vivo. Virology. 1997;227:45–52. doi: 10.1006/viro.1996.8338. [DOI] [PubMed] [Google Scholar]
- 44.Kollman T, Kim A, Pettoello-Mantovani M, et al. Divergent effects of chronic HIV-1 infection on human thymocyte maturation in SCID-hu mice. J Immunol. 1995;154:907–921. [PubMed] [Google Scholar]
- 45.Laurent CA, Krust B, Muller S, et al. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991;185:829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- 46.Terai C, Kornbluth RS, Pauza CD, Richman DD, Carson DA. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Invest. 1991;87:1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ameisen JC, Capron A. Cell dysfunction and depletion in AIDS: the programmed cell death hypothesis. Immunol. Today. 1991;12:102–105. doi: 10.1016/0167-5699(91)90092-8. see comments. [DOI] [PubMed] [Google Scholar]
- 48.Finkel TH, Tudor-Williams G, Banda NK, et al. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nature Med. 1995;1:129–134. doi: 10.1038/nm0295-129. [DOI] [PubMed] [Google Scholar]
- 49.Finkel TH, Banda NK. Indirect mechanism of HIV pathogenesis: how does HIV kill T cells? Curr. Op. Immunol. 1994;6:605–615. doi: 10.1016/0952-7915(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 50.Bartz S, Rogel M, Emerman M. HIV-1 cell cycle control: Vpr is cytostatic and mediates G2 accumulation by a mechanism which differs from DNA damage checkpoint control. J. Virol. 1996;70:2324–2331. doi: 10.1128/jvi.70.4.2324-2331.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li CJ, Friedman DJ, Wang C, Metelev V, Pardee AB. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science. 1995;268:429–431. doi: 10.1126/science.7716549. [DOI] [PubMed] [Google Scholar]
- 52.Cheng-Mayer C, Seto D, Tateno M, Levy JA. Biologic features of HIV-1 that correlate with virulence in the host. Science. 1988;240:80–82. doi: 10.1126/science.2832945. [DOI] [PubMed] [Google Scholar]
- 53.Cao C, Park I, Cooper A, Sodroski J. Moecular determinants of acute single-cell lysis by HIV-1. J. Virol. 1996;70:1340–1354. doi: 10.1128/jvi.70.3.1340-1354.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 55.Kitchen S, Uittenbogaart C, Zack JA. Mechanisms of HIV-1 localization and expression in CD4-negative thymocytes; 4th Conf. Retroviruses and Opport. Inf.; 1997. p. S746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clerici M, Bevilazqua M, Vago T, Villa ML, Shearer GM, Norbiato G. An immunoendocrinological hypothesis of HIV infection. Lancet. 1994;343:1552–1553. doi: 10.1016/s0140-6736(94)92944-0. [DOI] [PubMed] [Google Scholar]
- 57.Bonyhadi M, Su L, Auten J, McCune JM, Kaneshima H. Cytokine dysregulation in human fetal thymus organ culture and in SCID-hu Thy/Liv mice following infection with HIV-1; Keystone Symposium: Control and Manipulation of the Immune Response.1995. [Google Scholar]
- 58.McCune J, Kaneshima H, Rabin L, Shih C, Namikawa R. Preclinical evaluation of anti-viral compounds in the SCID-hu mouse. Annal N. York Scie. 1990;616:281–286. doi: 10.1111/j.1749-6632.1990.tb17848.x. Review. [DOI] [PubMed] [Google Scholar]
- 59.Shih CC, Kaneshima H, Rabin L, et al. Postexposure prophylaxis with zidovudine suppresses human immunodeficiency virus type 1 infection in SCID-hu mice in a time-dependent manner. J. Infect. Dis. 1991;163:625–627. doi: 10.1093/infdis/163.3.625. [DOI] [PubMed] [Google Scholar]
- 60.Datema R, De Clercq E, Henson G, Rabin L, Rosenwirth B. Antiviral properties of bicyclams and non-immunosuppressive cyclosporin analogs; Program Abstr Intersci Conf Antimicrob Agents Chemother.1994. p. 279. [Google Scholar]
- 61.Rabin L, Hincenbergs M, Moreno M, et al. A standardized small animal model for preclinical ef icacy testing of anti-HIV-1 compounds; 3rd Conf Retro and Opportun Infect.1996. p. 120. [Google Scholar]
- 62.Baum CM, Weissman IL, Tsukamoto AS, Buckle AM, Peault B. Isolation of a candidate human hematopoietic stem-cell population. Proc. Natl. Acad. Sci. USA. 1992;89:2804–2808. doi: 10.1073/pnas.89.7.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nienhuis AA. Gene transfer into hematopoietic stem cells. Blood Cells. 1994;20:141–147. [PubMed] [Google Scholar]
- 64.Hanley ME, Nolta JA, Parkman R, Kohn DB. Umbilical cord blood cell transduction by retroviral vectors: preclinical studies to optimize gene transfer. Blood Cells. 1994;20:539–543. [PubMed] [Google Scholar]
- 65.Yu M, Leavitt MC, Maruyama M, et al. Intracellular immunization of human fetal cord blood stem/progenitor cells with a ribozyme against human immunodeficiency virus type 1. Proc. Nat. Acad. Sci. 1995;92:699–703. doi: 10.1073/pnas.92.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Malim MH, Böhnlein S, Hauber J, Cullen BR. Functional dissection of the HIV-1 Rev trans- activator—derivation of trans-dominant repressor of Rev function. Cell. 1989;58:205–214. doi: 10.1016/0092-8674(89)90416-9. [DOI] [PubMed] [Google Scholar]
- 67.Bev D, Dobrovnik M, Hauber J, Böhnlein E. Inhibition of human immunodeficiency virus type 1 replication in human t cells by retroviral-mediated gene transfer of a dominant-negative Rev trans-activator. Proc. Nat. Acad. Sci. 1992;89:9870–9874. doi: 10.1073/pnas.89.20.9870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nabel GJ, Fox BA, Post L, Thompson CB, Woffendin C. A molecular genetic intervention for AIDS—effects of a transdominant negative form of Rev. Human Gene Therapy. 1995;5:79–92. doi: 10.1089/hum.1994.5.1-79. [DOI] [PubMed] [Google Scholar]
- 69.Bonyhadi M, Moss K, Voytoich A, et al. T cells derived in vivo fromRevM10-transduced human hematopoietic stem/progenitor cells inhibit HIV-1 replication. J. Virol. 1997;71:4707–4716. doi: 10.1128/jvi.71.6.4707-4716.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su L, Lee R, Bonyhadi M, Forestell S, Bohnlein E, Kaneshima H. Hematopoietic stem cell-based gene therapy for AIDS: efficient transduction and expression of RevM10 in vivo and in vitro. Blood. 1997;89:2283–2290. [PubMed] [Google Scholar]
- 71.An DS, Koyanagi Y, Zhao J, et al. High-efficiency transduction of human lymphoid progenitor cells and expression in differentiated T cells. J. Virol. 1997;71:1397–1404. doi: 10.1128/jvi.71.2.1397-1404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fraser CC, Kaneshima H, Hansteen G, Kilpatrick M, Hoffman R, Chen BP. Human allogeneic stem cell maintenance and differentiation in a long-term multilineage SCID-hu-graft. Blood. 1995;86:1680–1693. [PubMed] [Google Scholar]