Abstract
Objective
Population-based studies suggest that Black women with localized endometrial cancer have shorter survival compared to White patients because of inequalities in treatment. The purpose of this investigation was to determine if there is a racial disparity in outcome between Black and White patients with early stage endometrial cancer treated similarly in a clinical trial setting.
Methods
A retrospective review of 110 Black and 1049 White patients with stage I and II endometrial cancer was performed using data from a randomized, placebo controlled trial performed by the Gynecologic Oncology Group (GOG) that evaluated postoperative estrogen replacement therapy (ERT) and the risk of cancer recurrence. Demographic, pathologic, treatment and outcome related data were collected and analyzed using regression and survival analysis.
Results
Estimates of recurrence-free survival (RFS) suggested that Black patients may be more likely to have disease recurrence, particularly those on ERT. Within a median follow-up of three years, 5 of 56 Black endometrial cancer patients in the ERT group were identified with recurrent disease compared to only 8 of 521 White patients. Adjusted for age, BMI and tumor grade, the relative risk of recurrence among Blacks in the ERT group was 11.2 (95% CI: 2.86-43.59, p=0.0005).
Conclusions
Our findings suggest that RFS may be shorter among Black women with stage I endometrial cancer, even in a clinical trials setting in which patients receive similar treatment and followup. This increased risk of recurrence appears to be most evident in Black women with endometrial cancer who maintain ERT following primary treatment.
Keywords: race, gynecologic, malignancies, outcome
Introduction
In the United States, Black women have higher mortality rates for cancer compared to other racial groups, particularly among malignancies that affect the uterus [1]. Although only 7% of the 40,000 newly diagnosed cases of endometrial cancer in 2005 occurred in Black women, almost 14% of the women that died of their disease were Black [2]. According to Surveillance Epidemiology and End Results (SEER) data published by the American Cancer Society, approximately 80% more Blacks with endometrial cancer die of disease each year compared with Whites [3]. The reasons for racial disparity in cancer outcome are multifactorial and may be associated with barriers to access of care, inequalities in treatment, higher rates of co-morbidities among minorities that affect therapy, and differences in the types of tumors that are observed among Black women with endometrial cancer [4-9].
Previous work by our group evaluated racial disparities in endometrial cancer outcome while controlling for potential confounding prognostic factors. In a retrospective review of advanced stage endometrial cancer patients participating in treatment trials conducted by the GOG, we found that Black women had a 20% higher mortality rate compared to White women even when receiving similar care in a clinical trial setting. This disparity persisted despite controlling for demographic and pathologic confounding variables [10].
Management of localized endometrial cancer typically consists of hysterectomy and possible surgical staging. Adjuvant therapy is given depending on pathologic features that would predict a higher likelihood of recurrence (i.e. poor differentiation, deep myometrial invasion, capillary lymphatic space involvement, or non-endometrioid histology). Although variations in management of early stage endometrial cancer would appear to be less likely to be a source of observed racial disparities in outcome, population-based studies have revealed that Black women with stage I endometrial cancer have a worse overall survival compared to White women [6]. The objective of our study was to determine whether RFS or overall survival (OS) is different for stage I endometrial cancer patients in a clinical trial setting in which patients received similar care, and in which data was collected in a manner that facilitated analysis that controlled for confounding influences.
Methods
We reviewed data from participants in GOG 137, a randomized double-blind trial of ERT versus placebo in stage I or II endometrial cancer patients [11]. All patients were diagnosed with primary, histologically confirmed, endometrial adenocarcinoma. Surgical management consisted of a hysterectomy and surgical staging and other procedures were left to the discretion of the surgeon. Patients were subsequently initiated on therapy versus placebo within 20 weeks of their operative procedure. The primary endpoint was RFS, defined as the time from randomization to recurrence and the secondary endpoint was OS, defined as the time from randomization to death from all causes. Further details of the eligibility criteria and results of the study have been previously published [11].
In the current analysis, we evaluated demographic data, as well as tumor characteristics in the evaluation of outcome among Black and White women with stage I and II endometrial cancer. Racial designation as Black or White reflected a non-uniform collection of both self-described and investigator-reported methods. Patients from other racial groups were excluded from this analysis. RFS and OS distributions were estimated by Kaplan-Meier method and differences by race or treatment group were compared using the logrank test. The relative risk adjusted for age, BMI and tumor grade was also estimated using a Cox proportion hazards model. These three variables were chosen for adjustment because they showed differences in the distributions between white and black patients. The type of hysterectomy was not included for adjustment due to relative small number of black patients having LAVH (n=6), although it also showed somewhat difference in distribution between white and black patients. All statistical analyses were performed using Statistical Analysis System (SAS) version 9.1 (Cary, NC).
Results
One thousand forty-nine (1049) White patients and 110 Black patients participated in GOG 137 trial. Patient characteristics by race are summarized in Table 1. Black women with endometrial cancer were more likely to be older and have a larger body habitus in comparison to White women enrolled in the trial. Although the depth of myometrial invasion and the proportion of non-endometrioid histologic subtypes was similar in the endometrial cancers from the two racial groups, Black women more often presented with tumors that were poorly differentiated. A comparison of the clinical and pathologic features revealed that the distribution of stage, histologic type, depth of myometrial invasion and grade were similar among Blacks and Whites with endometrial cancer.
Table 1.
Patient Characteristics of White and Black Participants of GOG 137
| White | Black | ||||
|---|---|---|---|---|---|
|
|
|||||
| No. patients
(n=1049) |
(%) | No. patients
(n=110) |
(%) | P value | |
| Age Group (years) | |||||
| <50 | 249 | (23.7) | 17 | (15.5) | |
| 50-59 | 344 | (32.8) | 34 | (30.9) | |
| 60-69 | 284 | (27.1) | 46 | (41.8) | 0.007 |
| ≥ 70 | 172 | (16.4) | 13 | (11.8) | |
| BMI Group (Kg/m2) 1 | |||||
| < 25.0 | 216 | (21.8) | 11 | (10.8) | |
| 25.0-29.9 | 180 | (18.2) | 11 | (10.8) | |
| 30.0-39.9 | 335 | (33.8) | 56 | (54.9) | 0.0002 |
| ≥ 40.0 | 259 | (26.2) | 24 | (23.5) | |
| Stage | |||||
| IA | 399 | (38.0) | 43 | (39.1) | |
| IB | 513 | (48.9) | 51 | (46.4) | |
| IC | 80 | (7.6) | 6 | (5.5) | 0.377 |
| II | 57 | (5.4) | 10 | (9.1) | |
| Histology | |||||
| Endometrioid | 740 | (70.5) | 75 | (68.2) | |
| Other | 309 | (29.5) | 35 | (32.8) | 0.606 |
| Tumor Grade | |||||
| 1 | 620 | (59.1) | 62 | (56.4) | |
| 2 | 345 | (32.9) | 29 | (26.4) | 0.004 |
| 3 | 84 | (8.0) | 19 | (17.3) | |
| Hysterectomy | |||||
| TAH | 918 | (87.5) | 104 | (94.5) | |
| LAVH | 131 | (12.5) | 6 | (5.5) | 0.030 |
| PELN Assessed | |||||
| Yes | 814 | (77.6) | 93 | (84.6) | |
| No | 235 | (22.4) | 17 | (15.5) | 0.093 |
| PALN Assessed | |||||
| Yes | 620 | (59.1) | 71 | (64.6) | |
| No | 429 | (40.9) | 39 | (35.5) | 0.267 |
| PE Washing | |||||
| Yes | 944 | (90.0) | 104 | (94.6) | |
| No | 105 | (10.0) | 6 | (5.5) | 0.123 |
| CLS Invasion | |||||
| Positive | 47 | (4.5) | 6 | (5.5) | |
| Negative | 1002 | (95.5) | 104 | (94.6) | 0.642 |
| Myometrial Invasion 2 | |||||
| None | 904 | (86.2) | 94 | (85.5) | |
| < 50% | 106 | (10.1) | 12 | (10.9) | 0.965 |
| ≥ 50% | 39 | (3.7) | 4 | (3.6) | |
| Adjuvant XRT | |||||
| Yes | 109 | (10.4) | 10 | (9.1) | |
| No | 940 | (89.6) | 100 | (90.9) | 0.669 |
| ERT | |||||
| Yes | 521 | (49.7) | 56 | (50.9) | |
| No | 528 | (50.9) | 54 | (49.1) | 0.804 |
67 patients had missing data on weight or height
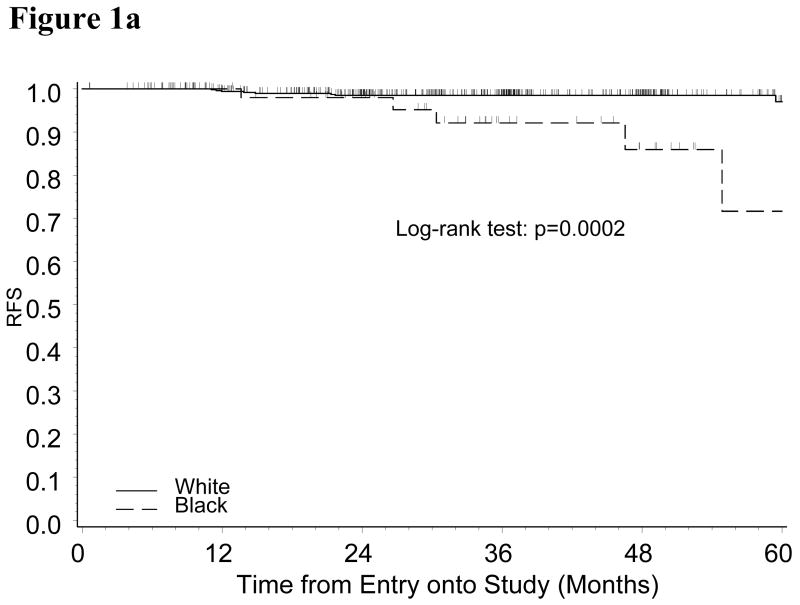
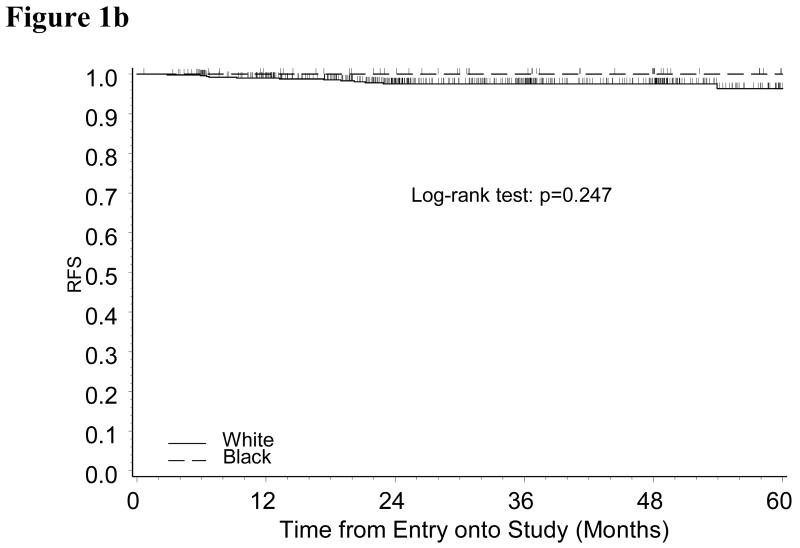
With a median follow-up period of 35 months, only 25 recurrences (20 Whites and 5 Blacks) (Table 2) and 43 deaths (34 Whites and 9 Blacks) were identified. The estimate of RFS suggested that Black patients may be more likely to have disease recurrence (Logrank test: p=0.085). Multivariate analysis adjusted for age, BMI and tumor grade showed that the relative risk of disease recurrence for black patients versus white patients was 2.55 (95% CI: 0.91-7.11, p=0.074). Analysis by treatment group indicated that the increased risk for Black patients was observed among ERT group, but not in the placebo group (Figure 1A and 1B). Five out of 56 Black endometrial cancer patients in ERT group were diagnosed with recurrent disease within three years of diagnosis compared to only eight out of 521 White patients. In the ERT group, the relative risk for Black versus White patients adjusted for age, BMI and tumor grade was 11.2 (95% CI: 2.86-43.59, p=0.0005).
Table 2.
Clinical and pathologic characteristics of the recurrent endometrial cancer cases
| Patient No. | Race | Age | Stage | Histology | Grade | CLS |
|---|---|---|---|---|---|---|
| Patients with ERT treatment who experienced disease recurrence | ||||||
| Adenocarcinoma, | ||||||
| 1 | B | 57 | IIA | unspecified | 1 | Negative |
| 2 | B | 63 | IIA | Endometrioid | 2 | Negative |
| 3 | B | 65 | IB | Endometrioid | 3 | Negative |
| 4 | B | 73 | IIA | Serous | 2 | Negative |
| 5 | B | 66 | IB | Endometrioid
Adenocarcinoma, |
1 | Negative |
| 6 | W | 52 | IB | unspecified | 2 | Negative |
| 7 | W | 66 | IIB | Endometrioid | 2 | Positive |
| 8 | W | 69 | IC | Endometrioid | 3 | Negative |
| 9 | W | 78 | IB | Endometrioid | 3 | Negative |
| 10 | W | 73 | IC | Endometrioid
Adenocarcinoma, |
3 | Positive |
| 11 | W | 76 | IB | unspecified | 2 | Negative |
| 12 | W | 54 | IB | Endometrioid | 2 | Negative |
| 13 | W | 79 | IC | Endometrioid | 2 | Negative |
| Patients from placebo treatment who experienced disease recurrence | ||||||
| 14 | W | 68 | IIA | Endometrioid | 2 | Positive |
| 15 | W | 65 | IB | Clear cell | 3 | Negative |
| 16 | W | 35 | IC | Endometrioid | 2 | Negative |
| 17 | W | 65 | IA | Serous | 3 | Negative |
| 18 | W | 59 | IC | Endometrioid | 2 | Positive |
| 19 | W | 55 | IB | Endometrioid | 1 | Negative |
| 20 | W | 56 | IA | Endometrioid
Adenocarcinoma, |
2 | Negative |
| 21 | W | 71 | IA | unspecified
Adenocarcinoma, |
1 | Negative |
| 22 | W | 59 | IA | unspecified | 1 | Negative |
| 23 | W | 77 | IB | Endometrioid | 2 | Negative |
| 24 | W | 53 | IB | Endometrioid
Adenocarcinoma, |
2 | Negative |
| 25 | W | 79 | IA | unspecified | 1 | Negative |
Note: B: Black; White, W; CLS, capillary space involvement
Figure 1.
Figure 1a: Kaplan-Meier Estimate of Recurrence-free Survival (RFS) for Patients Randomized to ERT Group by Race
Figure 1b: Kaplan-Meier Estimate of Recurrence-free Survival (RFS) for Patients Randomized to Placebo Group by Race
A similar hormone-dependent association between race and OS was observed. A comparison of OS estimates showed that Black patients with stage I/II endometrial cancer had increased mortality compared to White patients with disease (logrank test: p=0.015). The relative risk between Black patients and White patients adjusted for age, BMI and tumor grade was 2.24 (95% CI: 1.01-4.94, p=0.047). Subgroup analysis suggested that the racial disparity in survival was only seen in the patients on ERT. In the ERT group, the adjusted relative risk for Black versus White patients was 3.00 (95% CI: 1.06-8.50, p=0.038).
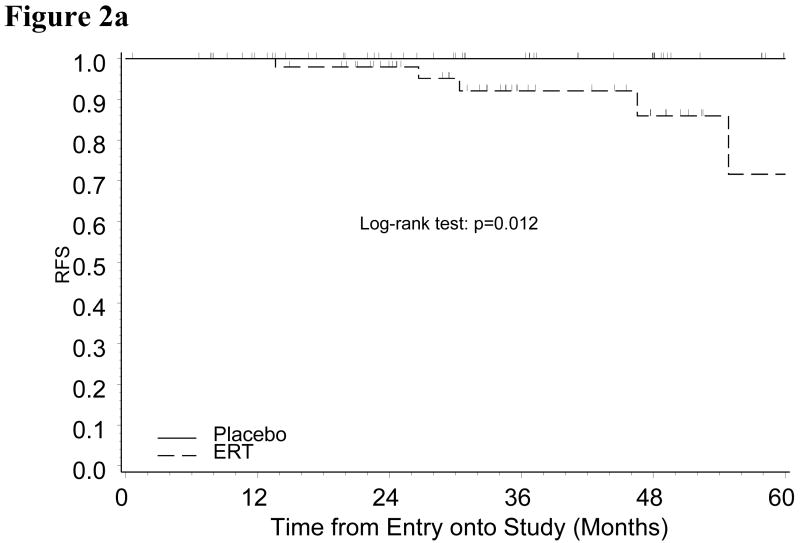
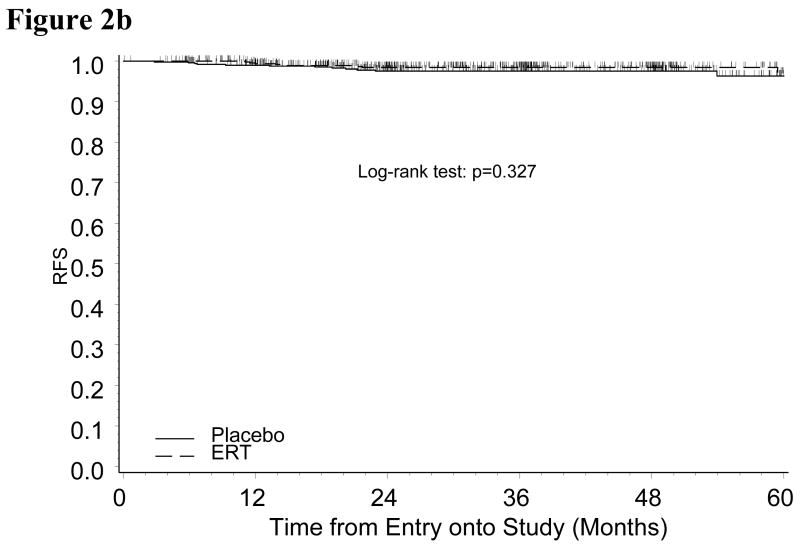
In an effort to further evaluate the relationship of race and ERT on outcome, we restricted the study population to either Black or White patients to compare the ERT and placebo groups (patient characteristics well balanced between ERT and placebo in either subgroup of Black or White patients, data not shown). Black patients in the ERT group had a significantly increased risk of disease recurrence compared to those in placebo group (p=0.012) (Figure 2A). This relationship was not true for White women with endometrial cancer (p=0.327) (Figure 2B).
Figure 2.
Figure 2a: Kaplan-Meier Estimate of Recurrence-free Survival (RFS) for Black Patients by Treatment
Figure 2b: Kaplan-Meier Estimate of Recurrence-free Survival (RFS) for White Patients by Treatment
Discussion
Multiple studies report that RFS and OS in endometrial cancer patients is shorter among Blacks compared to Whites and that inequalities in treatment may be important in the etiology of this racial disparity. The National Cancer Data Base Report on Endometrial Cancer revealed that survival was poorer for Black women compared to Caucasian women, even among patients with stage I disease [6]. In an analysis of almost 36,000 women with stage I endometrial cancer (1600 of which were Black) the authors found that five-year survival was 70% for Blacks and almost 95% for Whites. Among patients with stage I disease, 7.7% of Black women did not undergo surgery, whereas 2.2% of White women did not undergo surgery. Although Black women with endometrial cancer underwent lymph node dissection more often than White women, approximately the same proportions of both groups received postoperative adjuvant therapy [6]. Similar findings have been reported by Maddison et al in an analysis of data from the Detroit area SEER [12]. Although these studies suggest that a proportion of Blacks with endometrial cancer may not receive appropriate surgical therapy, they do not take into account co-morbidities that might influence whether a patient is offered surgery as part of their treatment. A Pattern of Care analysis of the SEER database evaluated 320 White and 99 Black women with stage I endometrial cancer and found that over 90% of women received guideline therapy and there were no differences in primary surgery or adjuvant radiotherapy between Blacks and Whites [13]. In our current study, all patients underwent hysterectomy for treatment of primary disease. The frequency of postoperative adjuvant therapy was also similar suggesting that differences in treatment did not account for the racial disparities in RFS and OS observed for a subset of Blacks receiving ERT.
Comparison of the demographic and clinical characteristics of Black and White patients revealed some differences between the groups. Black women enrolled in this GOG trial were more often older than the White participants, which can influence overall survival and may even shorten RFS [14,15]. Although Black patients with endometrial cancer were more likely to be obese or morbidly obese, recent data from the GOG has indicated that BMI does not appear to correlate with RFS in patients with endometrial cancer [16].
Population based differences in survival among Black patients with endometrial cancer also have been attributed to higher proportions of patients presenting with undifferentiated tumors and cancers with non-endometrioid histology [5,6,8,9]. Sherman and Devesa showed that 53% of the total mortality among Blacks was associated with non-endometrioid tumors compared to only 36% of Whites [9]. In our current study, a relatively equal distribution in the proportion of non-endometrioid tumors and the degree of myometrial invasion was noted between Black and White patients with early stage (94% were stage I) disease that were enrolled in the study. However, Black women more often had poorly differentiated tumors, which can be associated with a higher risk of recurrence. In an assessment of the clinical and pathologic characteristics of the patients with recurrent disease, we did not observe a significant differential proportion of undifferentiated tumors between Blacks and Whites that ultimately recurred. Only one of the five recurrences among Black women had a tumor that was poorly differentiated (Table 2).
Many previous studies have reported that rates of recurrence among endometrial cancer patients appear to be unaffected by the use of postoperative ERT [17-20]. Because in vivo growth of Ishikawa endometrial cancer cells are stimulated by exogenous estrogen, there is a theoretical risk of estrogen stimulating residual cancer cells to develop into a recurrent tumor [21,22]. Results from the WHI trial prompted closure of the GOG trial evaluating postoperative estrogen therapy in endometrial cancer patients after the accrual rate slowed substantially [23]. Our current findings have suggested that Black women with endometrial cancer who maintain ERT postoperatively may be at an increased risk of cancer recurrence. Although our findings reached statistical significance, limited numbers of outcome events should prompt the reader to interpret these findings with caution. We purposely chose to refrain from performing definitive multivariate analysis on the data because of the potential error that can be associated with a limited sample size. Although the clinicopathologic similarities between the Black and White patients suggest that the observed association between race and outcome among patients taking ERT may be a “true” observation, this could not be confirmed in the absence of definitive multivariate modeling. In addition, the majority of patients in this study had stage IA and IB disease with tumors that primarily consisted of grade 1 and grade 2 disease. Therefore, there may be even greater uncertainty regarding the association of hormone replacement therapy and recurrence of disease among African Americans with more advanced stage or less differentiated tumors.
Previous studies have observed differences in the rates of molecular alterations in the tumors from Black and White women with endometrial cancer suggesting that there may be a biologic etiology for the racial disparities in recurrence and poor survival. Although the majority of these studies have evaluated patients with advanced stage disease, Clifford et al showed that mutant p53 was overexpressed three times more often in Blacks compared to Whites with stage I endometrial cancer [23-27]. Recurrence of cancer was observed more than twice as frequent in cases with p53 overexpression as in cases with normal p53 expression. In addition, recurrence was seen in 14% of Blacks compared to only 8% of Whites, suggesting that the frequency of p53 mutation may contribute to the racial disparity in endometrial cancer survival [27]. In our current study, racial disparities in RFS and OS were observed despite patients receiving similar followup in a clinical trial setting following comparable surgery and adjuvant radiotherapy. These finding suggest a potential biologic etiology, particularly since the racial disparity was observed only among those patients receiving ERT. Variation in the metabolism of estrogen may be one possible explanation for differences in recurrence noted among Blacks and Whites with endometrial cancer. Although specific polymorphisms in genes involved in estrogen metabolism have been associated with an increased risk of endometrial cancer in patients on long-term hormone replacement therapy, there have not been any studies reporting a racial disparity in the frequency of these genetic alterations and an associated risk of recurrence among patients with endometrial cancer receiving estrogen following surgical treatment [28].
Recurrence of stage I endometrial cancer is more common among primary tumors with grade 3 histology and Blacks more often have poorly differentiated tumors. However, only 1 of the 5 recurrent endometrial cancers among Blacks in this study was undifferentiated and therefore we can't explain their worse RFS on the basis of a preponderance of grade 3 tumors. Since Estrogen Receptor (ER) is more commonly overexpressed among grade 1 and 2 tumors compared to grade 3, these findings suggest that estrogen has some influence on recurrence in Blacks with stage I disease, particularly since the recurrences among Black women were only noted among those patients receiving ERT. Previous reports have suggested that hypersensitivity may occur in some breast cancers through hyperacetylation of the ERα promoter or truncation of ER [29]. Investigation of racial differences in ER expression among endometrial cancers has not been reported to date.
In conclusion, our findings suggest that racial disparities in RFS and OS in endometrial cancer may be observed even among patients with early stage endometrial cancer who receive similar followup. These data suggest that recurrent disease is more frequent among Black women taking ERT, a finding that has not been previously reported. Because accrual of patients onto a randomized trial involving estrogen is likely to be a challenge given the adverse results associated with hormone replacement therapy, a definitive study to resolve these findings is not likely to be forthcoming. Clinicians will continue to rely on limited evidence-based information to support practice guidelines in the administration of estrogen replacement therapy to endometrial cancer patients.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical and Data Center (CA 37517).
The following Gynecologic Oncology Group member institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University Medical Center, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian – St. Luke's Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Southwest Oncology Group, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Cooper Hospital/University Medical Center, Columbus Cancer Council, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia, University of Chicago, Tacoma General Hospital, Eastern Collaborative Oncology Group, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Brookview Research Inc., Ellis Fischel Cancer Center, M.D. Anderson CCOP.
References
- 1.http://www.cancer.org/downloads/STT/CAFF2005AACorrPWSecured.pdf
- 2.Ghafoor A, Jemal A, Cokkinides V, et al. Cancer statistics for African Americans. Cancer Clin J. 2000;52:326–41. doi: 10.3322/canjclin.52.6.326. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Liu JR, Conaway M, Soper JT, et al. Relationship between race and interval to treatment in endometrial cancer. Obstet Gynecol. 1995;86:486–90. doi: 10.1016/0029-7844(95)00238-m. [DOI] [PubMed] [Google Scholar]
- 5.Randall TC, Armstrong K. Differences in treatment and outcome between African-American and white women with endometrial cancer. J Clin Oncol. 2003;21:4200–6. doi: 10.1200/JCO.2003.01.218. [DOI] [PubMed] [Google Scholar]
- 6.Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base Report on endometrial carcinoma in African-American women. Cancer. 1998;83:2629–37. doi: 10.1002/(SICI)1097-0142(19981215)83:12<2629::AID-CNCR30>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 7.Cossrow N, Falkner B. Race/ethnic issues in obesity and obesity-related comorbidities. J Clin Endocrinol Metab. 2004;89:2590–4. doi: 10.1210/jc.2004-0339. [DOI] [PubMed] [Google Scholar]
- 8.Plaxe SC, Saltzstein SL. Impact of ethnicity on the incidence of high-risk endometrial carcinoma. Gynecol Oncol. 1997;65:8–12. doi: 10.1006/gyno.1996.4594. [DOI] [PubMed] [Google Scholar]
- 9.Sherman ME, Devesa SS. Analysis of racial differences in incidence, survival and mortality for malignant tumors of the uterine corpus. Cancer. 2003;98:176–86. doi: 10.1002/cncr.11484. [DOI] [PubMed] [Google Scholar]
- 10.Maxwell GL, Brown M, Tian C, et al. Racial disparity in advanced endometrial cancer: a Gynecologic Oncology Group study. Cancer. 2006;107:2197–205. doi: 10.1002/cncr.22232. [DOI] [PubMed] [Google Scholar]
- 11.Barakat RR, Bundy BN, Spirtos NM, et al. Randomized double-blind trial of estrogen replacement therapy versus placebo in stage I or II endometrial cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2006;24:587–92. doi: 10.1200/JCO.2005.02.8464. [DOI] [PubMed] [Google Scholar]
- 12.Madison T, Schottenfeld D, James SA, et al. Endometrial cancer: Socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am J Public Health. 2004;94:2104–11. doi: 10.2105/ajph.94.12.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trimble EL, Harlan LC, Clegg LX, et al. Pre-operative imaging, surgery and adjuvant therapy for women diagnosed with cancer of the corpus uteri in community practice in the United States. Gynecol Oncol. 2005;96:741–8. doi: 10.1016/j.ygyno.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 14.Modesitt SC, Huang B, Shelton BJ, et al. Endometrial cancer in Kentucky: The impact of age, smoking status, and rural residence. Gynecol Oncol. 2006;103:300–6. doi: 10.1016/j.ygyno.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Jolly S, Vargas CE, Kumar T, et al. The impact of age on long-term outcome in patients with endometrial cancer treated with postoperative radiation. Gynecol Oncol. 2006;103:87–93. doi: 10.1016/j.ygyno.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 16.von Gruenigen VE, Tian C, Frasure H, et al. Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: A Gynecologic Oncology Group Study. Cancer. 2006;107:2786–91. doi: 10.1002/cncr.22351. [DOI] [PubMed] [Google Scholar]
- 17.Creasman WT, Henderson D, Hinshaw W, et al. Estrogen replacement therapy in the patient treated for endometrial cancer. Obstet Gynecol. 1986;67(3):326–30. [PubMed] [Google Scholar]
- 18.Lee RB. Estrogen replacement therapy following treatment for stage I endometrial carcinoma. Gyn Oncol. 1990;67:189–91. doi: 10.1016/0090-8258(90)90171-g. [DOI] [PubMed] [Google Scholar]
- 19.Suriano KA, McHale M, McLaren CE, et al. Estrogen replacement therapy in endometrial cancer patients: a matched control study. Obstet Gynecol. 2001;97(4):555–60. doi: 10.1016/s0029-7844(00)01221-7. [DOI] [PubMed] [Google Scholar]
- 20.Chapman JA. Estrogen replacement in surgical stage I and II endometrial cancer survivors. Obstet Gynecol. 1996;175:1195–2000. doi: 10.1016/s0002-9378(96)70027-3. [DOI] [PubMed] [Google Scholar]
- 21.Holinka CF, Hata H, Kuramoto H, et al. Responses to estradiol in a human endometrial adenocarcinoma cell line (Ishikawa) J Setroid Biochem. 1986;24(1):85–9. doi: 10.1016/0022-4731(86)90036-1. [DOI] [PubMed] [Google Scholar]
- 22.Farnell YZ, Ing NH. The effects of estradiol and selective estrogen receptor modulators on gene expression and messenger RNA stability in immortalized sheep endometrial stromal cells and human endometrial adenocarcinoma cells. J Steroid Biochem Mol Biol. 2003;84:453–61. doi: 10.1016/s0960-0760(03)00066-9. [DOI] [PubMed] [Google Scholar]
- 23.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women Health Initiative randomized controlled trial. JAMA. 2002;288:321–3. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Kohler MF, Carney P, Dodge R, et al. p53 overexpression in advanced-stage endometrial adenocarcinoma. Am J Obstet Gynecol. 1996;175(5):1246–52. doi: 10.1016/s0002-9378(96)70036-4. [DOI] [PubMed] [Google Scholar]
- 25.Santin AD, Bellone S, Siegel ER, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (Her-2/neu): A major prognostic indicator in uterine serous papillary cancer. Obstet Gynecol. 2005;192:813–8. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 26.Maxwell GL, Risinger JI, Hayes KA, et al. Racial disparity in the frequency of PTEN mutations, but not microsatellite instability, in advanced endometrial cancers. Clin Cancer Res. 2000;6:2999–3005. [PubMed] [Google Scholar]
- 27.Clifford SL, Kaminetsky CP, Cirisano FD, et al. Racial disparity in overexpression of the p53 tumor suppressor gene in stage I endometrial cancer. Am J Obstet Gynecol. 1997;176:S229–S232. doi: 10.1016/s0002-9378(97)70380-6. [DOI] [PubMed] [Google Scholar]
- 28.Rebbeck TR, Troxel AB, Wang Y, et al. Estrogen sulfation genes, hormone replacement therapy, and endometrial cancer risk. J Natl Cancer Inst. 2006;98:1311–20. doi: 10.1093/jnci/djj360. [DOI] [PubMed] [Google Scholar]
- 29.Jones DA, Cho J, Salamon E, Stefano GB. Risk factors for breast cancer and the prognosis of African American women: estrogen's role. Med Sci Monit. 2003;9:RA131–9. [PubMed] [Google Scholar]