Abstract
In addition to mean blood pressure, blood pressure variability is hypothesized to have important prognostic value in evaluating cardiovascular risk. We aimed to assess the prognostic value of blood pressure variability within 24 hours. Using MEDLINE, EMBASE and Cochrane Library to April 2013, we conducted a systematic review of prospective studies of adults, with at least one year follow-up and any day, night or 24-hour blood pressure variability measure as a predictor of one or more of the following outcomes: all-cause mortality, cardiovascular mortality, all cardiovascular events, stroke and coronary heart disease. We examined how blood pressure variability is defined and how its prognostic use is reported. We analysed relative risks adjusted for covariates including the appropriate mean blood pressure and considered the potential for meta-analysis. Our analysis of methods included 24 studies and analysis of predictions included 16 studies. There were 36 different measures of blood pressure variability and 13 definitions of night- and day-time periods. Median follow-up was 5.5 years (interquartile range 4.2–7.0). Comparing measures of dispersion, coefficient of variation was less well researched than standard deviation. Night dipping based on percentage change was the most researched measure and the only measure for which data could be meaningfully pooled. Night dipping or lower night-time blood pressure was associated with lower risk of cardiovascular events. The interpretation and use in clinical practice of 24-hour blood pressure variability, as an important prognostic indicator of cardiovascular events, is hampered by insufficient evidence and divergent methodologies. We recommend greater standardisation of methods.
Introduction
Hypertension typically accounts for up to 50% of an individual’s cardiovascular (CV) risk.[1] Traditionally, this risk has been attributed to mean blood pressure (BP) load. However, it is now thought this explanation is unable to fully account for the effect of rising BP level on CV risk and the inherent variability of an individual’s BP may also be a significant contributing factor.[2]
The variability of BP can be evaluated over a variety of different timescales, over a lifetime,[3] years,[4] different seasons,[5] month-to-month (visit-to-visit),[6] day-to-day,[7] within 24 hours,[8] and by different methods: beat-to-beat, measured intra-arterially[9] or non-invasively.[10] The aetiology, pathogenesis and prognosis, of BP variability over these different timescales and methods are likely to vary considerably.[11]
Recent attention has focused on the predictive value of visit-to-visit and day-to-day BP variability[11] and the subsequent risk of stroke[12] and mortality.[13] The treatment of normotensives with antihypertensive agents, which reduce BP variability, can reduce CV morbidity, further supporting the hypothesis that variability is a potentially modifiable risk factor, regardless of baseline BP.[14] In addition, recent economic analyses and National Institute for Clinical Excellence (NICE) recommendations for the systematic use of ambulatory BP monitoring, could potentially increase the emphasis on the predictive ability of BP variability within 24 hours.[15–16] In this paper, we use the term ‘24-hour BP variability’ to refer to BP variability within 24 hours based on ambulatory BP measurement during the day, night or over 24 hours.
The prognostic value of 24-hour BP variability has been reviewed previously by a systematic review that included night dipping measures [17] and a mini-review of other 24-hour measures of BP variability including standard deviation.[18] Our aim was to revisit, update and expand on these reviews, by systematic review, in investigating to what extent the existing literature establishes the value of 24-hour BP variability as a prognostic index.
Methods
Data Sources and Search Strategy
We searched MEDLINE (1946-April 2013), EMBASE (1980-April 2013) and the Cochrane Library (Issue 3, 2013) using a sensitive search strategy for prognostic studies.[19,20] Our search terms included combinations of terms relating to BP variability (e.g. “blood pressure variation” and “dipping”), time of day (e.g. “day” “nocturnal” and “diurnal”) and cardiovascular risk (e.g. “hypertension”, “risk” and “mortality”). Our full search strategy is given in S1 Appendix. One author carried out the initial screen of titles and abstracts for relevance. In updating this initial search, papers were screened independently by two authors who first considered abstracts and titles, and then full texts and reference lists. Disagreements were resolved by discussion and review with a third author. Several authors were contacted for further information.
All extracted data were verified and checked, discrepancies were discussed and agreements on values reached. The list of extracted variables is given in S2 Appendix.
Study Selection
Our study protocol is given in S3 Appendix. We included papers describing randomized controlled trials and observational cohort prognostic studies of 24-hour BP variability with: an adult population (aged 18 years or over); follow-up of at least one year; systolic, diastolic or both ambulatory BP measurements; BP measured at night, during the day or spanning 24 hours; and, reporting one or more of the following outcomes: all-cause mortality, CV mortality, all fatal and non-fatal CV events, stroke and coronary heart disease. Several groups of papers with duplicate or overlapping patient populations were included because they reported different measures, outcomes or methods of analysis. Each group was counted as a single study.
Quality Assessment
Methodological quality was assessed by individual paper. Two authors independently carried out the assessments and disagreements were resolved by discussion and review with a third author. There is no established quality checklist for prognostic studies,[21] so we based our evaluation of quality on a framework for appraisal of prognostic studies[22] used previously.[23] We considered six criteria: (1) all were recruited in the same setting (from a general population or from a clinic population but not both); (2) clinical and demographic characteristics were described; (3) relative risks were adjusted for covariates including appropriate mean BP; (4) follow-up length was sufficient for the clinical outcomes (mean or median follow-up was at least five years); (5) follow-up was complete (at least 80%); and (6) outcomes assessment was objective or independently adjudicated.
Statistical Analysis
Statistical analyses were by individual study and were carried out using STATA, version 13 (StataCorp, College Station, TX). Data from all studies that satisfied the eligibility criteria for inclusion in the systematic review were included in our analysis of methods to understand how 24-hour BP is described. We classified the measures of variability within a three-component framework: (1) class of measurement (e.g. standard deviation), (2) type of BP measured (e.g. systolic) and (3) timing (e.g. day). We examined the definitions of night and day.
Only studies that provided relative risks adjusted for the appropriate mean BP were included in our analysis of relative risks. Relative risks expressed as continuous variables were scaled to a common basis: 5mmHg increase for all dispersion measures; 10% increase for night-day ratio; 1mmHg increase for night dipping expressed as day-night difference; 10mmHg increase for measures of morning surge. We evaluated the potential for data pooling using meta-analysis and, where appropriate, we investigated further by applying data synthesis based on the DerSimonian and Laird method, inputting beta coefficients and standard errors into a random effects model. From the Mantel-Haenszel model we assessed the I-squared statistic for heterogeneity.[24] I-squared over 50% was defined as high heterogeneity.
In the analysis of relative risks, studies were separated by the hypertensive status of their patient populations. We defined a population as hypertensive if at least 80% were reported as hypertensive. We compared the predictive power of corresponding systolic and diastolic measures based on the same populations where possible. Sensitivity analyses assessed the effect of statistical heterogeneity and, in the diastolic-systolic comparison, the effect of relative risks rescaled to 1 SD increase.
Results
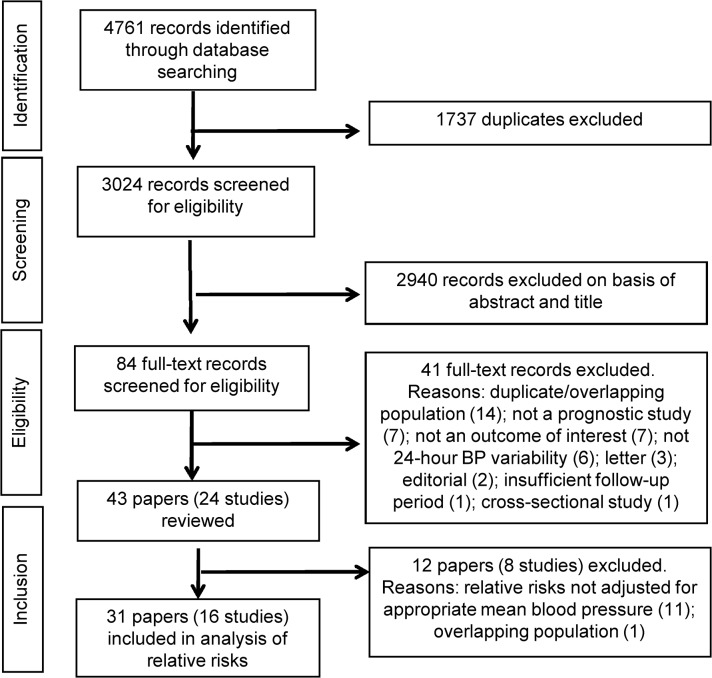
From 4,761 search results we screened 84 full-text records for eligibility (Fig 1) and, of these, 41 were excluded (S1 Table), leaving 43 included papers in our systematic review. These papers are listed in S2 Table where their references are numbered with a prefix “W”. Twenty-four papers were from five studies with overlapping or duplicate data sets: Chieti University (n = 3), International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO, n = 10), which included several meta-analyses; Jichi Medical School (n = 4), Progetto Ipertensione Umbria Montioraggio Ambulatoriale (PIUMA, n = 5) and Hadassah Hebrew University (n = 2). There were 24 studies in total in our review based on the 43 papers (Table 1).
Fig 1. Study flow chart.
Table 1. Studies included in systematic review.
| Ref | Study | Countries | Population | n | Age (yrs) | Follow-up (yrs) |
|---|---|---|---|---|---|---|
| Chieti University | ||||||
| W1a | Pierdomenico 2005 (excl) | Italy | Hypertensive, untreated | 1088 | 49.0 | 4.7 |
| W1b | Pierdomenico 2006 | Italy | Hypertensive, treated | 1472 | 59.0 a | 4.9 |
| W1c | Pierdomenico 2009 | Italy | Hypertensive | 1280 | 58.0 a | 4.8 |
| International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO) | ||||||
| W2a | Boggia 2007* | Europe, Asia, South America | Mixed b | 7458 | 56.8 | 9.6 |
| W2b | Fagard 2008* | International | Hypertensive | 3468 | 60.8 | 6.6 |
| W2c | Fagard 2009* | International | Hypertensive | 3468 | 60.8 | 6.6 |
| W2d | Hansen 2006 | Denmark | Mixed b | 1700 | 55.8 a | 9.5 |
| W2e | Hansen 2010* | Europe, Asia, South America | General | 8938 | 53.0 | 11.3 |
| W2f | Li 2010* | Europe, Asia, South America | General | 5645 | 53.0 | 11.4 |
| W2g | Metoki 2006 | Japan | General | 1430 | 61.1 | 10.4 |
| W2h | Ohkubo 2002 (excl) | Japan | General | 1542 | 61.0 | 9.2 |
| W2i | Ohkubo 1997 (excl) | Japan | General | 1542 | 61.0 | 5.1 |
| W2j | Pringle 2003 | Europe | Hypertensive | 744 | 69.5 | 4.4 |
| Jichi Medical School | ||||||
| W3a | Eguchi 2009 | Japan | Hypertensive, diabetes | 300 | 67.8 | 4.5 |
| W3b | Kabutoya 2010 | Japan | Hypertensive | 811 | 72.3 | 3.4 |
| W3c | Kario 2001 | Japan | Hypertensive | 575 | 72.5 a | 3.4 |
| W3d | Kario 2003 | Japan | Hypertensive | 519 | 72.0 | 3.4 |
| Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) | ||||||
| W4a | Verdecchia1994 | Italy | Hypertensive | 959 | 52.9 a | Not given |
| W4b | Verdecchia 1997 | Italy | Hypertensive | 1522 | 52.0 | 4.2 |
| W4c | Verdecchia 2007 | Italy | Hypertensive | 2649 | 51.2 | 6.0 |
| W4d | Verdecchia 2008 (excl) | Italy | Hypertensive | 2934 | 50.9 | 7.0 |
| W4e | Verdecchia 2012 | Italy | Hypertensive | 3012 | 50.8 | 8.4 |
| Hadassah Hebrew Univeristy | ||||||
| W5a | Ben Dov 2007 | Israel | Mixed | 3957 | 55.0 | 6.5 |
| W5b | Israel 2011 | Israel | Mixed | 2627 | 56.5 a | 6.5 |
| Other studies | ||||||
| W6 | Amici 2008 | Italy | Mixed | 42 | 65.9 | 5.0 |
| W7 | Astrup 2007 (excl) | Denmark | Type II diabetes mellitus | 104 | 61.0 a | 9.2 |
| W8 | Bouhanick 2008 (excl) | France | Hypertensive, diabetes mellitus | 97 | 60.8 a | 5.5 |
| W9 | Brotman 2008 | US | Mixed | 621 | 61.3 | 6.3 a |
| W10 | de la Sierra 2012 | Spain | Hypertensive | 2115 | 66.1 | 4.0 |
| W11 | Eto 2005 | Japan | Hypertensive | 106 | 73.9 | 2.8 |
| W12 | Gosse 2004 | France | Hypertensive | 507 | 49.0 | 7.0 |
| W13 | Iqbal 2011 (excl) | UK | Hypertensive | 297 | 60.1 | 5.5 |
| W14 | Liu 2003 (excl) | Japan | End stage renal failure on haemodialysis | 80 | 60.2 a | 2.8 |
| W15 | Mancia 2007 | Italy | General | 2012 | 50.9 a | 12.3 |
| W16 | Mena 2005 | Venezuala | Mixed | 312 | 66.9 a | 1.9 |
| W17 | Minutolo 2011 | Italy, US | Hypertensive, non-dialysis chronic kidney disease | 436 | 65.1 | 4.2 |
| W18 | Muxfeldt 2009 | Brazil | Hypertensive | 556 | 65.8 | 4.8 |
| W19 | Nakamura 1995 (excl) | Japan | Chronic ischaemic cerebro-vascular disease | 76 | 63.7 | 2.3 |
| W20 | Nakano 2004 | Japan | Type II diabetes mellitus | 364 | 54.0 | 7.2 |
| W21 | Otsuka 1996 (excl) | Japan | Hypertensive | 176 | 51.6 a | 6.0 |
| W22 | Rothwell 2010 | Europe | Hypertensive | 843 | Not given | 5.5 |
| W23 | Sturrock 2000 (excl) | UK | Diabetes mellitus | 75 | 52.1 a | 3.5 |
| W24 | Zweiker 1994 (excl) | Austria | Hypertensive | 116 | 59.0 | 2.6 |
Abbreviations: (excl)—Excluded from analysis of relative risks; ARV—average real variability; CoV—coefficient or variation; CV—cardiovascular; MS—morning surge; SD—standard deviation.
*Relative risks/hazard ratios based on meta-analysis
a Given for subgroups of patients so mean for all patients was estimated by calculating a weighted average
b Provided separate relative risks for hypertensive and normotensive patients for night-day ratio (Boggia 2007, n = 3436 hypertensive, n = 4022 normtensive, CV events and all-cause mortality, systolic BP; Hansen 2006, n = 682 hypertensive, n = 1018 normotensive, CV events, systolic and diastolic BP)
Of these 24 studies, 13 had populations from within Europe, nine had non-European populations and two studies were based on international databases. Thirteen studies involved hypertensive populations, of which two studies involved patients with diabetes and one study involved patients with chronic kidney disease. One study reported hypertensive and mixed populations. Ten studies involved populations with mixed hypertensive status. Of these, five were reported as general or mixed populations, one had a population with chronic ischemic cerebrovascular disease, three had a population with diabetes and another study had a population with end-stage renal failure. Numbers of patients enrolled in the studies varied between 42 and 8938 (median 843, interquartile range 300–2115). Average age of the patient population varied between 49.0 and 73.9 years (median 60.2, interquartile range 53.0–65.1 years).
Methodological Quality Assessments
We assessed the methodological quality of 42 of the 43 papers as insufficient information was provided to evaluate the quality of one paper.(W22)
No papers met all six criteria, 19 papers met five criteria, 12 met four criteria, 10 met three criteria, and one paper met two criteria (S3 Table). The number of papers satisfying each criterion ranged from 12 to 40 papers. Length of follow-up varied between 1.9 years and 12.3 years (median 5.5, interquartile range 4.2 to 7.0 years), was missing in one paper (W4a) and was less than five years in 17 papers, with follow-up ranging from 1.9 to 4.9 years. The criterion most often met was providing a description of clinical and demographic characteristics (40 papers, 95.2%) and the criterion least often met was outcomes assessment being objective or independently adjudicated (12 papers, 28.6%).
Describing 24-Hour BP Variability
Reviewing the literature, we found that 24-hour BP variability was referred to, in general terms, as “short-term variability” [25] or “24-hour ambulatory blood pressure variability” (W13), or more specifically as “diurnal” variation or variability (W9), “circadian” blood pressure profile, pattern, variation or variability [26] (W4b, W4d), or with reference to a particular type, or measure, of 24-hour BP variability.
Measuring 24-Hour BP Variability
Among the 24 included studies, we found 36 different measures of 24-hour BP variability across the three different components of variation: class, type and timing (Table 2).
Table 2. Components of 24-hour BP variability measures.
| Class | BP type | Timing | No of studies (references) | No of measures |
|---|---|---|---|---|
| Dispersion measures | ||||
| Standard deviation | Systolic or diastolic | Day, night or 24hrs | 8 (W1a, W1b, W1c, W2e, W2j, W3a, W4c, W11, W15, W16, W21) | 6 |
| Coefficient of var. a | Systolic | Day or 24hrs | 2 (W11,W22) | 2 |
| Average real var. b | Systolic or diastolic | 24hrs | 3 (W1c, W2e, W16) | 2 |
| SDday-night c | Systolic or diastolic | 24hrs | 1 (W2e) | 2 |
| Circadian amplitude | Systolic or diastolic | 24hrs | 1 (W21) | 2 |
| Night dipping measures | ||||
| Night dipping 1 (night-day ratio) d | Systolic, diastolic or both | 24hrs | 14 (W2a, W2b, W2c, W2d, W2g, W2h, W2i, W3a, W3b, W3c, W4a, W4b, W4d, W4e, W5a, W7, W8, W9, W10, W13, W14, W17, W18, W23, W24) | 3 |
| Night dipping 2 e | Systolic, diastolic or both | 24hrs | 3 (W15, W19, W20) | 3 |
| Night dipping 3 f | Systolic or diastolic | 24hrs | 1 (W21) | 2 |
| Morning pressure surge measures | ||||
| Preawakening 1 g | Systolic | 24hrs | 4 (W2f, W2g, W3d, W4e, W5b) | 1 |
| Preawakening 2 h | Systolic | 24hrs | 1 (W12) | 1 |
| Preawakening 3 i | Systolic | 24hrs | 1 (W5b) | 1 |
| Preawakening 4 j | Systolic | 24hrs | 1 (W5b) | 1 |
| Sleep trough 1 k | Systolic or diastolic | 24hrs | 2 (W3a, W4e) | 2 |
| Sleep trough 2 l | Systolic | Both | 4 (W2f, W2g, W3d, W5b, W6) | 1 |
| Sleep trough 3 m | Systolic | Both | 1 (W5b) | 1 |
| Sleep trough 4 n | Systolic | Both | 1 (W13) | 1 |
Based on 24 studies included in the review.
astandard deviation / mean BP
b average of absolute value of successive pairs of BP measurements
c weighted average of day standard deviation and night standard deviation
dpercentage nocturnal fall
e difference of mean day BP and mean night BP
f adjusted day-night difference, (mean day BP-mean night BP)/mean 24-hour BP
gmean BP in 2 hours after awakening—mean BP in 2 hours preawakening
hdifference between BP on rising and BP in 30 minutes before rising
idifference between mean BP in first hours after awakening and mean BP in last hour preawakening
jdifference between mean BP in 3 hours after awakening and mean BP in 3 hours preawakening
kdifference between mean BP in 2 hours after awakening and mean of the 3 BP readings centred on the lowest BP readings during sleep
ldifference between mean BP in 2 hours after awakening and mean of all the BP readings during sleep
mdifference between mean BP in 2 hours after awakening and lowest mean of 3 BP consecutive BP readings during the night
n≥20/15mmHg rise in first two BP readings from 7am compared to average night BP
Class of measurement
We identified 3 measures of night dipping, 8 measures of morning pressure surge and 5 measures of dispersion. Among nine studies reporting dispersion measures, standard deviation (SD) was the dispersion measure most often reported (n = 8). Eighteen studies reported measures of night dipping which differed in their unit of measurement (Table 2). Most (n = 14) reported dipping measures defined by percentage fall or an equivalent definition based on night-day ratio. Seven studies reported morning surge measures. The morning surge classes differed in the timing and number of BP measurements used in the definition of the BP surge. There were nine different definitions of morning surge.
Type of BP measured
Of 36 measures, 21 (58.3%) measures were based on readings of systolic BP only, 12 (33.3%) on diastolic BP only, and three (0.8%) were based on both BPs, either monitored together (W2d, W2h, W2i, W4a) or combined in a weighted average (W7, W19).
Timing of measurement
Of 36 BP variability measures, seven were based on day-time readings only or night-time readings only (Table 2). Of 24 studies, definitions of day-time and night-time were given by 21 studies, producing 13 different definitions (Table 3). The most common definition was based on the time spent in and out of bed (n = 8).
Table 3. Variation of definitions of night and day.
| Daytime | Day-time hours | Night-time | Night-time hours | No of studies | References |
|---|---|---|---|---|---|
| Diary entries | |||||
| Time awake or time between rising and lying down | variable | Time asleep or time between lying down and rising | variable | 8 | W1a, W1b, W1c, W3a, W3b, W3c, W3d, W4b, W4d, W4e, W5a, W5b, W10, W12, W17, W18 |
| Time when > 8 hours awake | variable | Time when > 4 hours asleep | variable | 1 | W2g, W2h, W2i |
| Short fixed clock-time intervals | |||||
| 10am to 8pm | 10 | Midnight to 6am | 6 | 3 | W2a, W2b, W2c, W2e, W2f, W2j, W4c, W4d, W21 |
| 9am to 9pm | 12 | 1am to 6am | 5 | 1 | W22 |
| 10am to 10.59pm | 13 | 1am to 6.59pm | 6 | 1 | W23 |
| Long fixed clock-time intervals | |||||
| 6am to 10pm | 16 | 10pm to 6am | 8 | 3 | W4a, W4d, W19, W20 |
| 7am to 11pm | 16 | 11pm to 7am | 8 | 1 | W15 |
| 6am to midnight | 18 | Midnight to 6am | 6 | 2 | W2d, W18 |
| 7am to 10pm | 15 | 10pm to 7am | 9 | 1 | W8 |
| 9am to 9pm | 12 | 9pm to 9am | 12 | 1 | W24 |
| 8am to 8pm | 12 | 8pm to 8am | 12 | 1 | W14 |
| 6am to 9pm | 15 | 9.30pm to 5.30am | 8 | 1 | W11 |
| 6am to 11pm | 17 | 11pm to 6am | 7 | 2 | W9, W16 |
In studies with multinational patient populations, adjustments were made for time differences. Three studies did not provide definitions.(W6, W7, W13)
Defining Cardiovascular Outcomes
The most commonly studied outcomes were all fatal and non-fatal CV events were the most common (18 studies of each, 75.0%) and coronary heart disease was the outcome least often reported (2 studies). Authors used 26 different terms to refer to cardiovascular events. These definitions involved between two and 11 conditions (median 5 conditions, inter-quartile range 4–7). Myocardial infarction and stroke were the conditions most often included in definitions. Twelve studies defined stroke of which four included transient ischemic attack in the definition. Two studies defined coronary heart disease, each providing a different definition.
Predicting Cardiovascular Outcomes
Fourteen studies (27 papers) and 25 of the 31 measures of BP variability were included in our analysis of relative risks (Fig 1).
Expressing relative risk
Associations of BP variability with cardiovascular outcomes were expressed as categorical or continuous expressions of relative risks or hazard ratios. Categorisations included: for dispersion measures, ‘high’ and ‘low’, defined by above vs. below the mean (W11); for night dipping measures, with varying thresholds, two, three of four categories, risers (also known as reverted or inverted dippers), non-dippers (reduced or decreased dippers), dippers (normal dippers) and extreme dippers; and, for morning surge measures, two or more categories with thresholds based on the top decile,(W2f, W3d) top tertile,(W6) quintiles(W2g, W18) or quartiles(W4e) of the morning pressure surge. Of 24 papers reporting relative risks expressed for categories of night dipping, four (16.7%) also reported relative risks expressed as a continuous variable in the same paper.(W2a, W2d, W10, W18)
Of 43 papers, 32 (74.4%) adjusted for covariates including the appropriate mean BP, 5 (11.6%) adjusted for covariates excluding the appropriate mean BP and 6 papers (14.0%) provided unadjusted analyses.
Extent of study of measures
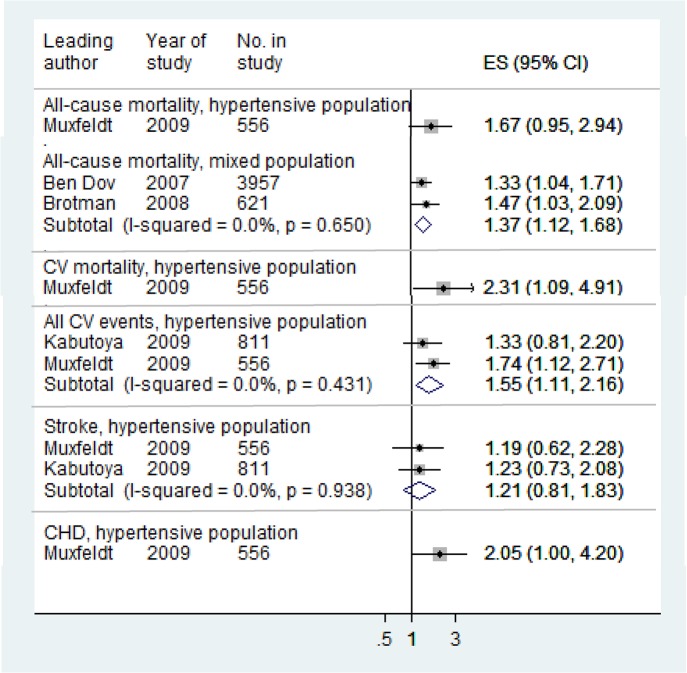
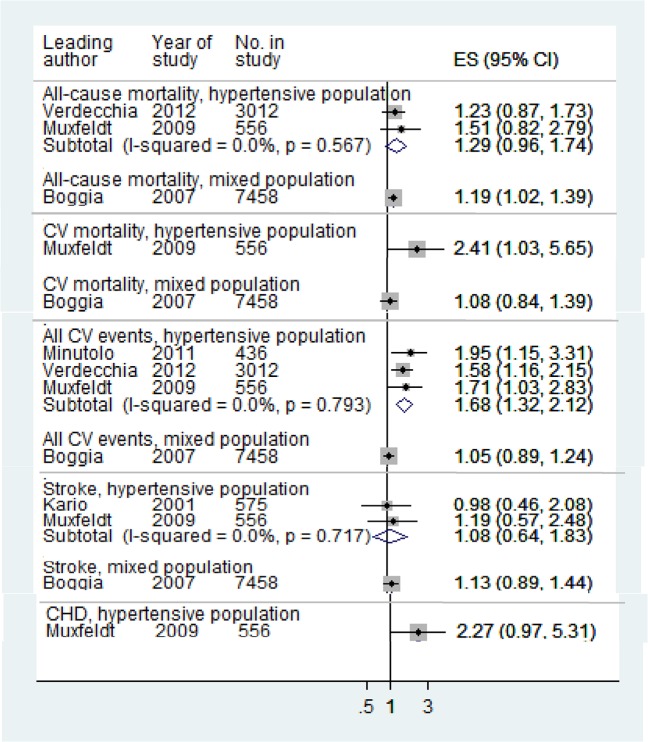
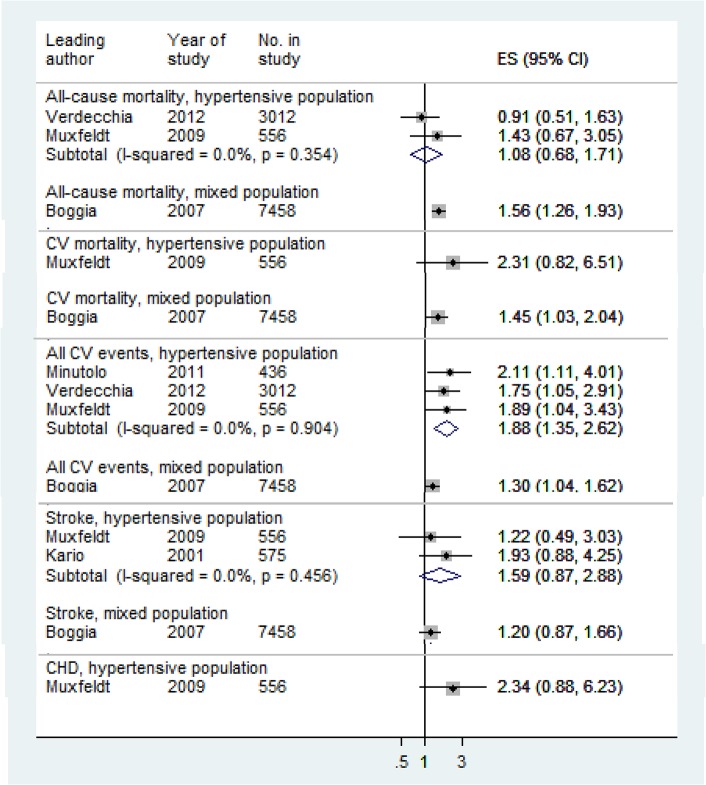
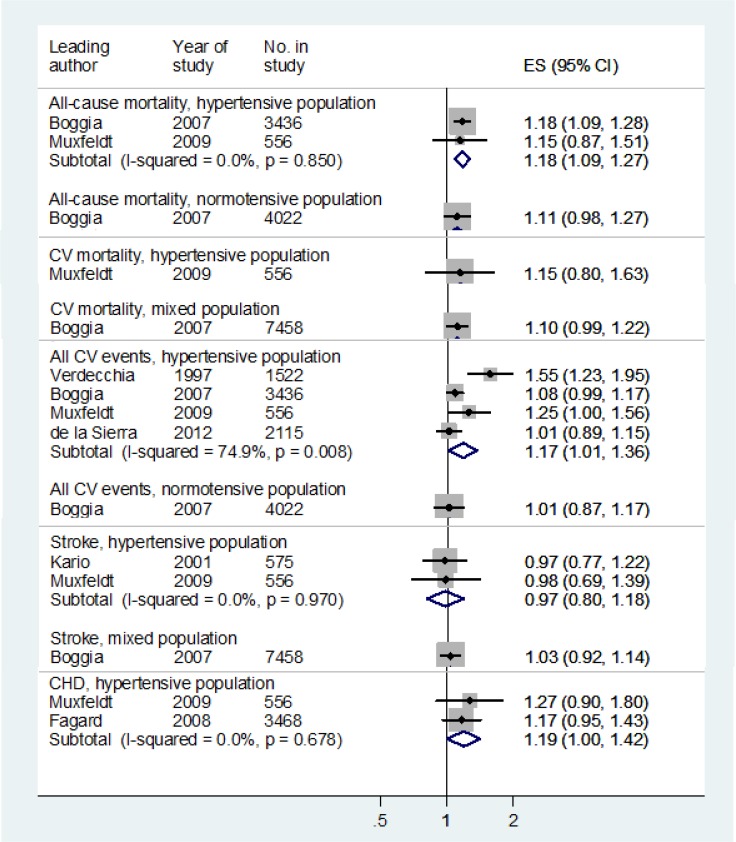
Night dipping, based on percentage change (Night dipping 1) was the only measure for which relative risks were reported by more than two studies, and therefore the only measure for which we pooled relative risks (Table 4 and S4 Table). For both hypertensive and mixed populations, night dipping was associated with lower risk of cardiovascular events (Figs 2 and 3) while rising blood pressure at night compared to day was associated with increased risk (Fig 4). Night-day ratio (Fig 5) also provided evidence of predictive power in hypertensive populations, as did dispersion measures in general populations (Table 5).
Table 4. Numbers of studies reporting 24-hour blood pressure variability measures as a prognostic index of cardiovascular events.
| Class | BP | Timing | All-cause mortality | CV mortality | All CV events | Stroke | CHD |
|---|---|---|---|---|---|---|---|
| Hypertensive populations | |||||||
| SD | Systolic | Day | 0 | 1 | 2 | 1 | 0 |
| SD | Systolic | Night | 0 | 1 | 2 | 1 | 0 |
| SD | Diastolic | Day | 0 | 0 | 1 | 0 | 0 |
| SD | Diastolic | Night | 0 | 0 | 1 | 0 | 0 |
| CoV | Systolic | Day | 0 | 0 | 1 | 0 | 0 |
| Night dipping 1 | Systolic | 24 hrs | 2* | 1 | 4* | 2 | 2* |
| Night dipping 1 | Diastolic | 24 hrs | 1* | 1 | 1* | 1 | 2* |
| Pre-awakening 1 | Systolic | 24 hrs | 0 | 0 | 1 | 1 | 0 |
| Pre-awakening 2 | Systolic | 24 hrs | 0 | 0 | 1 | 0 | 0 |
| Sleep trough 1 | Systolic | 24 hrs | 0 | 0 | 2 | 0 | 0 |
| Sleep trough 1 | Diastolic | 24 hrs | 0 | 0 | 1 | 0 | 0 |
| Sleep trough 2 | Systolic | 24 hrs | 0 | 0 | 0 | 1 | 0 |
| Mixed populations | |||||||
| SD | Systolic | Day | 1 | 1 | 0 | 0 | 1 |
| SD | Systolic | Night | 1 | 1 | 0 | 0 | 0 |
| SD | Systolic | 24 hrs | 2* | 2* | 1* † | 1* | 0 |
| SD | Diastolic | Day | 1 | 1 | 0 | 0 | 0 |
| SD | Diastolic | Night | 1 | 1 | 0 | 0 | 0 |
| SD | Diastolic | 24 hrs | 2* | 2* | 1* | 1* | 0 |
| ARV | Systolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| ARV | Diastolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| SDday-night | Systolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| SDday-night | Diastolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| Night dipping 1‡ | Systolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| Night dipping 1‡ | Diastolic | 24 hrs | 1* | 1* | 1* | 1* | 0 |
| Night dipping 2 | Systolic | 24 hrs | 1 | 1 | 0 | 0 | 0 |
| Night dipping 2 | Diastolic | 24 hrs | 1 | 1 | 0 | 0 | 0 |
| Pre-awakening 1 | Systolic | 24 hrs | 1 | 0 | 0 | 1 | 0 |
| Pre-awakening 3 | Systolic | 24 hrs | 1 | 0 | 0 | 0 | 0 |
| Pre-awakening 4 | Systolic | 24 hrs | 1 | 0 | 0 | 0 | 0 |
| Sleep trough 2 | Systolic | 24 hrs | 1 | 0 | 0 | 0 | 0 |
| Sleep though 3 | Systolic | 24 hrs | 1 | 0 | 0 | 0 | 0 |
Providing continuous expressions of relative risk.
* Includes studies reporting relative risks/hazard ratios based on pooled data from other studies.
†Further data from an extra study could not be included as there was insufficient information to extract the data
‡Night-day ratio; SD—standard deviation, ARV—average real variability, CoV—coefficient of variation
Fig 2. Associations of systolic night dipping 1 with cardiovascular outcomes: non-dippers verses dippers for all patients.
Relative risks:>1 increased risk;< 1 reduced risk.
Fig 3. Associations of systolic night dipping 1 with cardiovascular outcomes: non-dippers verses dippers, excluding risers and dippers.
Relative risks:>1 increased risk;< 1 reduced risk. Pooled relative risk for all CV events, hypertensive populations, excluding Minutolo 2009 (non-dialysis chronic kidney disease): 1.61 (1.24, 2.10)
Fig 4. Associations of systolic night dipping 1 with cardiovascular outcomes: risers verses dippers.
Relative risks:>1 increased risk;< 1 reduced risk. Pooled relative risk for all CV events, hypertensive populations, excluding Minutolo 2009 (non-dialysis chronic kidney disease): 1.81 (1.23, 2.66)
Fig 5. Associations of night-day ratio with cardiovascular outcomes.
Relative risks:>1 increased risk;< 1 reduced risk. Pooled relative risk for all CV events, hypertensive population excluding de la Sierra 2012 only (did not control for treatment): 1.25 (1.00, 1.56), I-squared = 79.0%; excluding Verdecchia 1997 only:1.07 (0.99, 1.17), I-squared = 23.7%; excluding de la Sierra 2012 and Verdecchia 1997: 1.12 (0.98, 1.27), I-squared 33.6%.
Table 5. Comparing predictive power of corresponding systolic and diastolic measures.
| Systolic | Diastolic | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Author | n | Population | BPV measure | BP | Outcome | RR | 95% CI | RR | 95% CI | ||
| 1 | Hansen 2010 | 8938 | G | SD, 24hrs | Systolic | All cause mortality | 1.00 | 0.98 | 1.02 | 1.04 | 1.01 | 1.06 |
| 2 | Mancia 2007 | 2012 | G | SD, 24hrs | Systolic | All cause mortality | 1.00 | 0.84 | 1.20 | 1.19 | 0.94 | 1.51 |
| 3 | Mancia 2007 | 2012 | G | SD, day | Systolic | All cause mortality | 1.17 | 0.99 | 1.38 | 1.41 | 1.14 | 1.75 |
| 4 | Mancia 2007 | 2012 | G | SD, night | Systolic | All cause mortality | 1.09 | 0.91 | 1.32 | 1.26 | 1.01 | 1.58 |
| 5 | Hansen 2010 | 8938 | G | SDdn | Systolic | All cause mortality | 1.03 | 1.00 | 1.06 | 1.08 | 1.05 | 1.12 |
| 6 | Hansen 2010 | 8938 | G | ARV | Systolic | All cause mortality | 1.05 | 1.02 | 1.08 | 1.07 | 1.04 | 1.11 |
| 7 | Boggia 2007 | 7458 | M | Night dipping 1 | Systolic | All cause mortality | 1.17 | 1.09 | 1.25 | 1.13 | 1.06 | 1.21 |
| 8 | Muxfeldt 2009 | 556 | H | Night dipping 1 | Systolic | All cause mortality | 1.15 | 0.87 | 1.51 | 0.98 | 0.77 | 1.25 |
| 9 | Mancia 2007 | 2012 | G | Night dipping 2 | Systolic | All cause mortality | 0.99 | 0.97 | 1.00 | 0.98 | 0.96 | 1.00 |
| 10 | Hansen 2010 | 8938 | G | SD, 24hrs | Systolic | CV mortality | 1.01 | 0.98 | 1.04 | 1.06 | 1.02 | 1.10 |
| 11 | Mancia 2007 | 2012 | G | SD, 24hrs | Systolic | CV mortality | 0.91 | 0.66 | 1.26 | 1.15 | 0.76 | 1.76 |
| 12 | Mancia 2007 | 2012 | G | SD, day | Systolic | CV mortality | 1.21 | 0.89 | 1.66 | 1.67 | 1.13 | 2.48 |
| 13 | Mancia 2007 | 2012 | G | SD, night | Systolic | CV mortality | 1.05 | 0.76 | 1.45 | 1.29 | 0.86 | 1.93 |
| 14 | Hansen 2010 | 8938 | G | SDdn | Systolic | CV mortality | 1.02 | 0.98 | 1.06 | 1.10 | 1.04 | 1.15 |
| 15 | Hansen 2010 | 8938 | G | ARV | Systolic | CV mortality | 1.07 | 1.03 | 1.12 | 1.12 | 1.07 | 1.17 |
| 16 | Boggia 2007 | 7458 | M | Night dipping 1 | Systolic | CV mortality | 1.10 | 0.99 | 1.22 | 1.11 | 1.00 | 1.24 |
| 17 | Muxfeldt 2009 | 556 | H | Night dipping 1 | Systolic | CV mortality | 1.15 | 0.80 | 1.63 | 1.02 | 0.76 | 1.38 |
| 18 | Mancia 2007 | 2012 | G | Night dipping 2 | Systolic | CV mortality | 0.98 | 0.95 | 1.00 | 0.96 | 0.93 | 0.99 |
| 19 | Hansen 2010 | 8938 | G | SD, 24hrs | Systolic | CV events | 1.01 | 0.99 | 1.03 | 1.02 | 0.99 | 1.05 |
| 20 | Eguchi 2009 | 300 | HD | SD, day | Systolic | CV events | 1.15 | 0.82 | 1.58 | 2.03 | 0.88 | 1.35 |
| 21 | Eguchi 2009 | 300 | HD | SD, night | Systolic | CV events | 1.38 | 1.00 | 1.92 | 1.85 | 1.19 | 2.89 |
| 22 | Hansen 2010 | 8938 | G | SDdn | Systolic | CV events | 1.02 | 0.99 | 1.04 | 1.04 | 1.00 | 1.07 |
| 23 | Hansen 2010 | 8938 | G | ARV | Systolic | CV events | 1.03 | 1.00 | 1.06 | 1.04 | 1.01 | 1.08 |
| 24 | Pierdomenico 2009 | 1280 | H | ARV | Systolic | CV events | 2.07 | 1.31 | 3.28 | 1.36 | 0.92 | 2.02 |
| 25 | Boggia 2007 | 7458 | M | Night dipping 1 | Systolic | CV events | 1.06 | 0.98 | 1.15 | 1.08 | 1.01 | 1.15 |
| 26 | Muxfeldt 2009 | 556 | H | Night dipping 1 | Systolic | CV events | 1.25 | 1.00 | 1.56 | 1.13 | 0.94 | 1.37 |
| 27 | Eguchi 2009 | 300 | HD | Sleep trough 1 | Systolic | CV events | 1.02 | 0.86 | 1.24 | 1.05 | 0.83 | 1.36 |
| 28 | Hansen 2010 | 8938 | G | SD, 24hrs | Systolic | Stroke | 0.99 | 0.96 | 1.03 | 1.03 | 0.89 | 1.20 |
| 29 | Hansen 2010 | 8938 | G | SDdn | Systolic | Stroke | 1.01 | 0.97 | 1.06 | 1.05 | 0.99 | 1.11 |
| 30 | Hansen 2010 | 8938 | G | ARV | Systolic | Stroke | 1.04 | 1.00 | 1.09 | 1.08 | 1.03 | 1.13 |
| 31 | Boggia 2007 | 7458 | M | Night dipping 1 | Systolic | Stroke | 1.03 | 0.92 | 1.14 | 1.04 | 0.94 | 1.16 |
| 32 | Muxfeldt 2009 | 556 | H | Night dipping 1 | Systolic | Stroke | 0.98 | 0.69 | 1.39 | 0.96 | 0.72 | 1.28 |
| 33 | Muxfeldt 2009 | 556 | H | Night dipping 1 | Systolic | CHD | 1.27 | 0.90 | 1.80 | 1.11 | 0.83 | 1.48 |
G—general; M—mixed; H—hypertensive; HD—hypertensive, diabetes; SD—standard deviation; ARV—average real variability; RR—relative risk. Relative risks were scaled to the same increase except Pierdomenico 2009 (categorical expressions of relative risks). Sensitivity analysis (relative risks rescaled to 1 SD increase) are shown in S5 Table.
Other variability measures for which the predictive value for different cardiovascular outcomes has been assessed by more than one study are the diastolic and systolic measures of standard deviation (Table 4), again largely based on studies of patients with mixed hypertensive status. Further measures which have been researched by single studies across different cardiovascular outcomes are the systolic measures of pre-awakening 1 and sleep trough 2, which were evaluated over mixed populations (S4 Table). Measures, which have been evaluated less well include coefficient of variation, most of the morning surge measures and diastolic measures in general (Table 4).
Comparing the predictive power of systolic and diastolic measures
Comparisons could be made using data from five papers (four studies). When relative risks were scaled to the same increase, involving 33 comparisons (Table 5), the diastolic measure had stronger predictive power than the systolic measure in 15 cases (45.5%), weaker power in 3 cases (9.1%) and neither had predictive power in 15 cases (45.5%). When relative risks were scaled to 1 SD increase, involving 30 comparisons (S5 Table), the diastolic measure had stronger predictive power in 14 cases (46.7%), weaker power in 7 cases (10.0%) and neither had predictive power in 13 cases (43.3%). The majority of these comparisons involved general populations.
Discussion
In our review, we found variation of terms used to refer to blood pressure variability within 24 hours and a diverse set of measures of 24-hour BP variability with variation in the definitions and terminology of individual measures. Several measures of BP variability depended on differences between day-time BP and night-time BP but there were no universally consistent definitions of the hours that constitute day and night. Further inconsistencies exist in the definitions of CV outcomes and in the expression of relative risks.
The power of prediction varied across the variability measures well researched and other measures less well-researched. The basis of analysis varied widely, between one and five studies and involving between less than 100 patients and several thousand patients. We have reported the smaller studies to highlight the diversity of methods.
Night dipping, or lower night-time blood pressure, based on percentage change was the most researched measure, and having relative risks reported by more than two studies, across cardiovascular outcomes. Night dipping was associated with lower risk of cardiovascular events. In many cases the predictive power of measures had only been assessed by a single study
The majority of 24-hour BP variability measures involved systolic BP measurements. Where we could compare the prognostic value of systolic and diastolic measures, we found measures of diastolic BP variability had higher predictive power more often than not compared to the corresponding systolic measures.
Our findings are consistent with those of other studies where variations in methods and definitions were reported[17, 27–29] The superiority of diastolic BP variability measures over systolic measures has also been reported. (W2e, W15) It has been suggested that the differences are accounted for by arterial stiffness(W2e) and the greater dependence of overall BP variability on diastolic BP as it covers a greater proportion of the cardiac cycle.(W15)
We have presented a comprehensive, systematic review of the methods of analysis and prognostic value of 24-hour BP variability. We expand on previous reviews[17,18] by exploring methods of analysis further, by considering other measures of BP variability and examining relative risks of more cardiovascular outcomes, and via systematic review. Our study is also strengthened by narrowing our inclusion criteria to prospective cohort studies and populations from randomised controlled trials. By reviewing the literature systematically, we avoided selection bias. Restricting our analysis to fully-adjusted relative risks reduced the risk of confounding.
There were twice as many studies of hypertensive populations than general populations in our review which suggests possible selection bias. We could not account for possible bias. Nor could we account for physical activity during ambulatory monitoring or address the methodological quality limitations, nor the significance of the variation of definitions of cardiovascular events and day-time and night-time definitions of the studies in our review. Our analysis of the predictive value of measures is tentative, given the heterogeneity of studies.
It is understood that the prognostic significance of diastolic BP declines with age while that of systolic BP increases with age[30] but, given the limitations of our data, we were unable to investigate this issue further. A more comprehensive assessment would compare the predictions of systolic and diastolic BP of more measures, across different populations, involve more studies, and stratify by age, across different cardiovascular outcomes.
Whilst new measures of 24-hour BP variability have been proposed,[31–34] there is clearly a need to focus on understanding better the predictive value of fewer measures and a better standardisation of methods. Concerns have been expressed about categorizations of patients by night dipping status being mainly based on arbitrary criteria and the inability to reproduce as patients can change dipping status between readings.[17,35,36] There are several other concerns about categorising continuous variables including loss of statistical power, obscuring any non-linearity in the relation between variable and outcome, and the problem with viewing those with similar values but on opposite sides of the threshold very differently.[37] It has been recommended that, for BP variability measures of nocturnal fall, categorical expressions of relative risks are accompanied by continuous analyses and that relative risks, based on ambulatory BP data, are adjusted for the appropriate mean BP.[17](W2a)
There are no guidelines on how day-time and night-time should be measured. [38] The most common definitions of night-time and day-time were based on time asleep and awake but measuring these accurately is challenging, as highlighted by others [17]. Patient-filled diary cards are simple and popular [39] but can be inaccurate due to recall bias. Accelerometry-based monitors could provide more accurate estimations of periods asleep and awake,[40] but these devices are not widely available. The use of narrow, fixed clock-time intervals have defined day-time and night-time BPs that are approximately within 1–2mmHg of the BPs when awake and asleep.[41] This is achieved by avoiding the transition periods in the morning and evening when rapid changes in BP can occur[41] and involve the periods when a variable proportion of patients may be in or out of bed.(W4d) Consistency in the prognostic value of diurnal BP variability across these different definitions has been reported, (W4d) which may suggest that variations in definitions of night and day are less important than other sources of variation in methods.
The existence of methodological and clinical heterogeneity[42] leads to substantial difficulties in interpreting prognostic data. The sheer number of measures limits the utility of 24-hour BP variability and its interpretation and use in clinical practice, particularly as an important prognostic indicator of CV events. If variability is going to inform clinical practice, there is an urgent need to harmonize methodology sufficiently to draw conclusions across the research field with emphasis of future research directed towards variability measures with evidence of prognostic power.
Drawing together insights from this study and consolidating guidance issued by others, we conclude that there are several areas in which methodological change in future prognostic studies of BP variability is needed if the field is to advance:
To improve the methodological quality of prognostic studies, all studies should attempt to follow-up all patients and carry out objective or independent outcome assessment. To facilitate the pooling of data, there is a need to standardise: (a) definitions of outcomes, particularly composite outcomes; (b) definitions of variability measures, particularly thresholds of night dipping, the dispersion measure which should be used, and what is meant by the term “morning surge”; and, (c) definitions of night and day. Standardisation would produce greater consistency across studies and this could enhance the opportunity for meta-analysis. To reduce confounding, relative risks should be adjusted for the appropriate mean BP (the mean must be defined across the same time period as the variability measure) and adjusted for treatment in studies of hypertensive patients. Clarity in reporting is also important, for example, by presenting patient characteristics, and by stating when variability was measured in studies of hypertensive patients i.e. whether under treatment or not. To reduce bias and avoid problems with categorisation, categorical expressions of relative risks would benefit from being accompanied by continuous analyses. Finally, to increase the evidence base of the prognostic value of BP variability, it would be helpful if individual patient data were made available and if research efforts probed further, for example, by stratifying relative risks by age group, and evaluating the risk of other cardiovascular outcomes beyond the primary outcomes of interest.
Supporting Information
(DOC)
(DOCX)
(DOCX)
(DOC)
Only one reason is listed.
(DOCX)
References are grouped by study and are listed alphabetically.
(DOCX)
Evaluated by individual paper.
(DOCX)
Includes studies reporting relative risks/hazard ratios based on pooled data from other studies.
(DOCX)
Categorical expressions of relative risks. G—general; M—mixed; H—hypertensive; HD—hypertensive, diabetes; SD—standard deviation; ARV—average real variability; RR—relative risk. Relative risks:>1 increased risk;< 1 reduced risk.
(DOCX)
Acknowledgments
We thank Nia Roberts (University of Oxford) for carrying out the search strategy and David Judge (University of Oxford) for his assistance in formatting the files for journal submission.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This project presents independent research commissioned by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research funding scheme (RP-PG-0407-10347). The views expressed in this paper are those of the authors and not necessarily of the NHS, the NIHR or the Department of Health.
References
- 1. Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008; 371: 1513–8. 10.1016/S0140-6736(08)60655-8 [DOI] [PubMed] [Google Scholar]
- 2. Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010; 375: 938–948. 10.1016/S0140-6736(10)60309-1 [DOI] [PubMed] [Google Scholar]
- 3. Kannel WB, Sorlie P, Gordon T. Labile hypertension: a faulty concept? The Framingham study. Circulation. 1980; 61: 1183–87. [DOI] [PubMed] [Google Scholar]
- 4. Grove JS, Reed DM, Yano K, Hwang L. Variability in systolic blood pressure—a risk factor for coronary heart disease? Am J Epidemiol. 1997; 145: 771–6. [DOI] [PubMed] [Google Scholar]
- 5. Sega R, Cesana G, Bombelli M, Grassi G, Stella ML, Zanchetti A, et al. Seasonal variations in home and ambulatory blood pressure in the PAMELA population. J Hypertens. 1998; 16: 1585–92. [DOI] [PubMed] [Google Scholar]
- 6. Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010; 375: 895–905. 10.1016/S0140-6736(10)60308-X [DOI] [PubMed] [Google Scholar]
- 7. Kikuya M, Ohkubo T, Metoki H, Asayama K, Hara A, Obara T, et al. Day-by-Day Variability of Blood Pressure and Heart Rate at Home as a Novel Predictor of Prognosis. Hypertension. 2008; 52: 1045–1050. 10.1161/HYPERTENSIONAHA.107.104620 [DOI] [PubMed] [Google Scholar]
- 8. Mancia G, Bombelli M, Facchetti R, Madotto F, Corrao G, Trevano FQ, et al. Long-term prognostic value of blood pressure variability in the general population: Results of the Pressioni Arteriose Monitorate e Loro Associazioni Study. Hypertension. 2007; 49: 1265–1270. [DOI] [PubMed] [Google Scholar]
- 9. Mena L, Pintos S, Queipo NV, Aizpúrua JA, Maestre G, Sulbarán T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005; 23: 505–11. [DOI] [PubMed] [Google Scholar]
- 10. Parati G, Ongaro G, Bilo G, Glavina F, Castiglioni P, Di Rienzo M, et al. Non-invasive beat-to-beat blood pressure monitoring: new developments. Blood Press Monit. 2003; 8: 31–6. [DOI] [PubMed] [Google Scholar]
- 11. Stergiou GS, Parati G. How to best assess blood pressure?: The ongoing debate on the clinical value of blood pressure average and variability. Hypertension. 2011; 57: 1041–1042. 10.1161/HYPERTENSIONAHA.111.172924 [DOI] [PubMed] [Google Scholar]
- 12. Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010; 9: 469–80. 10.1016/S1474-4422(10)70066-1 [DOI] [PubMed] [Google Scholar]
- 13. Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population. Hypertension. 2010; 57: 160–166. [DOI] [PubMed] [Google Scholar]
- 14. Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, et al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA. 2004; 292: 2217–25. [DOI] [PubMed] [Google Scholar]
- 15.National Institute for Clinical Excellence. Hypertension: clinical management of primary hypertension in adults. NICE guideline. August 2011. Available: http://www.nice.org.uk/guidance/cg127.
- 16. Krakoff LR. Home blood pressure monitoring: international recognition. Hypertension. 2014; 63: 670–671. 10.1161/HYPERTENSIONAHA.113.02886 [DOI] [PubMed] [Google Scholar]
- 17. Hansen TW, Li Y, Boggia J, Thijs L, Richart T, Staessen JA. Predictive role of nighttime blood pressure. Hypertension. 2011; 57: 3–10. 10.1161/HYPERTENSIONAHA.109.133900 [DOI] [PubMed] [Google Scholar]
- 18. Asayama K, Schutte R, Li Y, Hansen TW, Staessen JA. Blood pressure variability in risk stratification: what does it add? Clin Exp Pharmacol Physiol. 2014; 41: 1–8. 10.1111/1440-1681.12091 [DOI] [PubMed] [Google Scholar]
- 19. Haynes RB, Wilczynski N, McGibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound prognostic studies in MEDLINE: an analytic survey. J Am Med Inform Assoc. 1994; 1: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong S-L, Wilczynski NL, Haynes RB. Optimal search strategies for detecting clinically sound prognostic studies in EMBASE: an analytic survey. J Med Libr Assoc. 2006; 91: 41–47. [PMC free article] [PubMed] [Google Scholar]
- 21. Altman DG. Systematic reviews of evaluations of prognostic variables. BMJ. 2001; 323: 224–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heneghan C, Badenock D. Evidence-based medicine toolkit, 2nd ed. Oxford Blackwell Publishing; 2006. [Google Scholar]
- 23. Ward AM, Takahashi O, Stevens R, Heneghan C. Home measurement of blood pressure and cardiovascular disease: systematic review and meta-analysis of prospective studies. J Hypertens. 2012; 30: 449–56. 10.1097/HJH.0b013e32834e4aed [DOI] [PubMed] [Google Scholar]
- 24.The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0, [updated March 2011]. Available: www.cochrane.org.
- 25. Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012; 60: 512–517. 10.1161/HYPERTENSIONAHA.112.194340 [DOI] [PubMed] [Google Scholar]
- 26. Kukla C, Sander D, Schwarze J, Wittich I, Klingelhöfer J. Changes of circadian blood pressure patterns are associated with the occurrence of lucunar infarction. Arch Neurol. 1998; 55: 683–8. [DOI] [PubMed] [Google Scholar]
- 27. Fagard R, Staessen JA, Thijs L. The relationships between left ventricular mass and daytime and night-time blood pressures: a meta-analysis of comparative studies. J Hypertens. 1995; 13: 823–9. [DOI] [PubMed] [Google Scholar]
- 28. van Ittersum FJ, Ijzerman RG, Stehouwer CD, Donker AJ. Analysis of twenty-four-hour ambulatory blood pressure monitoring: what time period to assess blood pressures during waking and sleeping? J Hypertens. 1995; 13:1053–8. [DOI] [PubMed] [Google Scholar]
- 29. Peixoto Filho AJ, Mansoor GA, White WB. Effects of actual versus arbitrary awake and sleep times on analyses of 24-h blood pressure. Am J Hypertens. 1995; 8: 676–80. [DOI] [PubMed] [Google Scholar]
- 30. Franklin SS, Gustin W 4th, Wong ND, Larson MG, Weber MA, Kannel WB, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997; 96: 308–315. [DOI] [PubMed] [Google Scholar]
- 31. Parati G, Vrijens B, Vincze G. Analysis and interpretation of 24-h blood pressure profiles: appropriate mathematical models may yield deeper understanding. Amer J of Hypertens. 2008; 21: 123–125. [DOI] [PubMed] [Google Scholar]
- 32. Manios E, Stamatelopoulos K, Tsivgoulis G, Barlas G, Koroboki E, Tsagalis G, et al. Time rate of blood pressure variation: a new factor associated with coronary atherosclerosis. J Hypertens. 2011; 29: 1109–14. 10.1097/HJH.0b013e3283454ff4 [DOI] [PubMed] [Google Scholar]
- 33. Stergiou GS, Mastorantonakis SE, Roussias LG. Morning blood pressure surge: the reliability of different definitions. Hypertens Res. 2008; 31: 1589–94. 10.1291/hypres.31.1589 [DOI] [PubMed] [Google Scholar]
- 34. Kario K. Morning surge in blood pressure and cardiovascular risk: evidence and perspectives. Hypertension. 2010; 56: 765–773. 10.1161/HYPERTENSIONAHA.110.157149 [DOI] [PubMed] [Google Scholar]
- 35. Omboni S, Parati G, Palatini P, Vanasia A, Muiesan ML, Cuspidi C, et al. Reproducibility and clinical value of nocturnal hypotension: prospective evidence from the SAMPLE study. Study on Ambulatory Monitoring of Pressure and Lisinopril Evaluation. J Hypertens. 1998; 16: 733–8. [DOI] [PubMed] [Google Scholar]
- 36. Mochizuki Y, Okutani M, Donfeng Y, Iwasaki H, Takusagawa M, Kohno I, et al. Limited reproducibility of circadian variation in blood pressure dippers and nondippers. Am J Hypertens. 1998; 4: 403–9. [DOI] [PubMed] [Google Scholar]
- 37. Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ 2006; 332:1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. ; Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005; 45: 142–61. [DOI] [PubMed] [Google Scholar]
- 39. O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, et al. , on behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003; 21:821–848. [DOI] [PubMed] [Google Scholar]
- 40. Wetzler ML, Borderies JR, Bigaignon O, Guillo P, Gosse P. Validation of a two-axis accelerometer for monitoring patient activity during blood pressure or ECG holter monitoring. Blood Press Monit. 2003; 8: 229–235. [DOI] [PubMed] [Google Scholar]
- 41. Fagard R, Brguljan J, Thijs L, Staessen J. Prediction of the actual awake and asleep blood pressures by various methods of 24 h pressure analysis. J Hypertens. 1996; 14: 557–563. [DOI] [PubMed] [Google Scholar]
- 42.Bandolier. Available: http://www.medicine.ox.ac.uk/bandolier/booth/glossary/hetero.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(DOCX)
(DOC)
Only one reason is listed.
(DOCX)
References are grouped by study and are listed alphabetically.
(DOCX)
Evaluated by individual paper.
(DOCX)
Includes studies reporting relative risks/hazard ratios based on pooled data from other studies.
(DOCX)
Categorical expressions of relative risks. G—general; M—mixed; H—hypertensive; HD—hypertensive, diabetes; SD—standard deviation; ARV—average real variability; RR—relative risk. Relative risks:>1 increased risk;< 1 reduced risk.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.