ABSTRACT
Chromosomal DNA is a constant source of information, essential for any given cell to respond and adapt to changing conditions. Here, we investigated the fate of exponentially growing bacterial cells experiencing a sudden and rapid loss of their entire chromosome. Utilizing Bacillus subtilis cells harboring an inducible copy of the endogenous toxin yqcG, which encodes an endonuclease, we induced the formation of a population of cells that lost their genetic information simultaneously. Surprisingly, these DNA-less cells, termed DLCs, did not lyse immediately and exhibited normal cellular morphology for a period of at least 5 h after DNA loss. This cellular integrity was manifested by their capacity to maintain an intact membrane and membrane potential and cell wall architecture similar to those of wild-type cells. Unlike growing cells that exhibit a dynamic profile of macromolecules, DLCs displayed steady protein and RNA reservoirs. Remarkably, following DLCs by time lapse microscopy revealed that they succeeded in synthesizing proteins, elongating, and dividing, apparently forming de novo Z rings at the midcell position. Taken together, the persistence of key cellular events in DLCs indicates that the information to carry out lengthy processes is harbored within the remaining molecular components.
IMPORTANCE
Perturbing bacterial growth by the use of antibiotics targeting replication, transcription, or translation has been a subject of study for many years; however, the consequences of a more dramatic event, in which the entire bacterial chromosome is lost, have not been described. Here, we followed the fate of bacterial cells encountering an abrupt loss of their entire genome. Surprisingly, the cells preserved an intact envelope and functioning macromolecules. Furthermore, cells lacking their genome could still elongate and divide hours after the loss of DNA. Our data suggest that the information stored in the transient reservoir of macromolecules is sufficient to carry out complex and lengthy processes even in the absence of the chromosome. Based on our study, the formation of DNA-less bacteria could serve as a novel vaccination strategy, enabling an efficient induction of the immune system without the risk of bacterial propagation within the host.
INTRODUCTION
DNA provides a living cell with the information required to grow, communicate, differentiate, survive, and die. The bacterial cell contains a centrally located large mass of chromosomal DNA (nucleoid), occupying the majority of its cytoplasmic space, and lacks a membrane-enclosed nucleus. The DNA is typically spread throughout the cell, such that the stored information is accessible for transcription into mRNA molecules that, in turn, are rapidly translated by ribosomes into proteins (1). Electron microscopy studies of bacterial chromosome structure provide evidence that transcription occurs on DNA loops. Based on these observations, it has been proposed that the transcription machinery and ribosomes are located in proximity within these loops, allowing coupled transcription and translation (2–7). Thus, the nucleoid seems to play a major role in facilitating orchestrated transcription and translation, serving not only as a template but also as a physical scaffold for these processes to occur simultaneously. The presence and the architecture of the chromosome as a scaffold is also underlined by its ability to constrain the subcellular distribution of macromolecules, such as ribosomes, and the division machinery components (8–10). Spatial and temporal coordination of DNA accessibility, transcription, and translation is considered a requisite for optimal cellular functionality. This premise is supported by the cellular disarray observed upon disruption of replication, transcription, or translation by antibiotics (11). Nevertheless, protein and RNA synthesis have been shown to occur from plasmids in anucleated minicells, which are produced by misplaced septa in rod-shaped bacteria. Minicells, lacking subcellular organization, are unable to elongate and divide but still harbor some metabolic activities (12–17). However, the consequence of a more extreme circumstance, the abrupt loss of the entire bacterial chromosome in a population of exponentially growing cells, has not been described. Such a catastrophic event would prevent the cell from activating a regulated stress response, due to the absence of any genetic information, leaving the cell mainly with a transient reservoir of RNA and protein molecules. Additionally, such an event would perturb the global cellular architecture by causing the removal of a major cellular scaffold component.
To explore the fate of cells lacking DNA, we induced the degradation of the chromosome of growing Bacillus subtilis cells by activating an endonuclease. Remarkably, we found that, at least for a few hours, cells without DNA remain intact and are able to sustain homeostasis, as well as essential cellular activities. These findings reveal that complex cellular processes can progress in the absence of the nucleoid.
RESULTS
YqcG displays DNase activity.
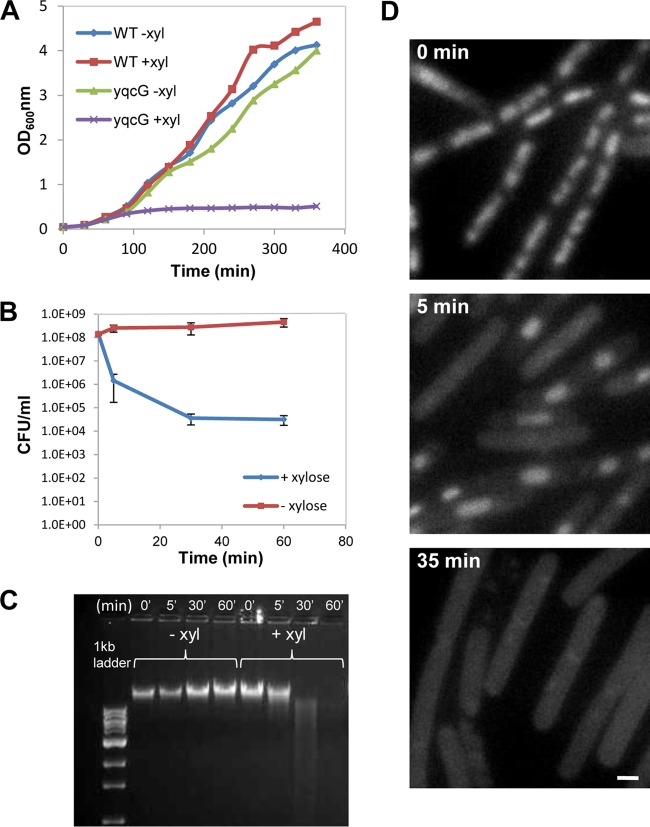
As part of our investigations into components that affect chromosome integrity and dynamics, we characterized the yqcG-yqcF toxin-antitoxin (TA) module (18). Our analysis revealed that the toxin component YqcG harbors robust endonuclease activity, as indicated by the following observations. In the absence of the antitoxin component, inserting yqcG under a xylose-inducible promoter (Pxyl-yqcG) into B. subtilis cells resulted in rapid growth arrest and in a sharp decrease in CFU upon induction (Fig. 1A and B; see Fig. S1A in the supplemental material). The toxicity of yqcG was manifested by a massive decrease in CFU (99.98%), observed as early as 5 min postinduction (Fig. 1B). Furthermore, yqcG-induced cells exhibited rapid disappearance of the chromosome (Fig. 1C and D; see Fig. S1B): at approximately 30 min postinduction, the signal from 4′,6-diamidino-2-phenylindole (DAPI) staining was hardly detectable (out of ~2,000 cells that were visualized, none was found to exhibit chromosomal staining), and in accordance with this, DNA extracted from the cells was largely degraded. Consistent with these findings, when the chromosome was labeled with the DNA binding protein HBsu fused to green fluorescent protein (GFP), mislocalization of the fusion protein was evident subsequent to YqcG production (see Fig. S1C). A recent study reported that the B. subtilis YqcG C-terminal region acts as an RNase when expressed in Escherichia coli (18). However, we found no evidence for such activity when the full-length protein was expressed in B. subtilis, as the RNA remained intact for several hours after yqcG induction (see below). Taken together, our results indicate that YqcG possesses DNase activity when expressed in B. subtilis. This activity could therefore be utilized as a strategy to impose rapid chromosomal degradation. Henceforth, we term cells experiencing a sudden chromosome loss due to YqcG induction “DLCs,” for DNA-less cells.
FIG 1 .
YqcG degrades the DNA of growing B. subtilis cells. (A) Growth curves of wild-type (WT) (PY79) and Pxyl-yqcG ΔyqcG (ME187) strains grown at 37°C in LB medium with or without xylose as indicated. (B) CFU of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with or without xylose were measured at the indicated time points. The time zero sample was plated before xylose addition. Shown are the averages of the normalized values from two independent biological repeats. Standard errors are indicated. (C) Pxyl-yqcG ΔyqcG (ME187) cells were grown at 37°C in LB medium with or without xylose, and genomic DNA was extracted at the indicated time points. The time zero sample was taken before xylose addition. The DNA concentration was determined using NanoDrop, and relatively similar amounts (approximately 50 µg DNA) were loaded onto 1% agarose gels. (D) Fluorescence images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose and stained with DAPI were taken at the indicated time points. The time zero sample was visualized before xylose addition. Scale bar corresponds to 1 µm. Of note, sometimes residual DNA staining is observed by DAPI, usually in the vicinity of the membrane. This could be due to DNA fragments being protected from digestion by the membrane.
Following the integrity of DLCs.
The capability to efficiently produce a synchronized DLC population prompted us to monitor the duration for which overall cellular integrity, activity, and biological functionalities could be preserved after such a deleterious event. As a first step in our investigation, we followed DLC morphology over time, using light microscopy. Surprisingly, DLCs clearly maintained their rod shape and membrane architecture, characteristics typical of wild-type cells, for at least 5 h after YqcG induction (Fig. 2A). Analyzing DLCs at later time points was complicated by the accumulation of suppressor cells, which harbored chromosomal DNA and dominated the culture. These suppressors arose from mutations within the C-terminal region of the yqcG open reading frame (see Fig. S1A in the supplemental material). To overcome this obstacle, we treated the DLC culture with the antibiotic nalidixic acid, which inhibits DNA gyrase and, therefore, should not affect DLCs, while eliminating the growth of suppressors. Remarkably, DLCs were found to be largely capable of maintaining their overall cellular morphology, even as long as 24 h after chromosome loss, though their membrane staining showed a nonhomogenous pattern, indicating some membrane perturbation (Fig. 2A).
FIG 2 .
DLCs maintain cellular integrity. (A) Phase contrast, membrane staining, and DAPI images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose at the indicated time points. The time zero sample was visualized before xylose addition. Nalidixic acid (Nal; 500 µg/ml) was added 5 h postinduction. Scale bar corresponds to 2 µm. Of note, the DAPI signal in DLCs is likely to emanate from leakage of the membrane dye. (B) Pxyl-yqcG ΔyqcG (ME187) cells were grown at 37°C in LB medium with or without xylose, as indicated. After 1 h of incubation, samples were treated with CCCP as indicated. Samples were stained with DiOC6(3) and DAPI and visualized by fluorescence microscopy. Scale bar corresponds to 2 µm. (C) Pxyl-yqcG ΔyqcG (ME187) cells were grown at 37°C in LB medium, and samples taken before (t = 0) and after (t = 1, 3, or 5 h) xylose addition were treated with CCCP, as indicated. Samples were stained with DiOC6(3) and visualized by fluorescence microscopy. Shown are the results for quantification of the fluorescence signal from DiOC6(3)-treated samples. For each time point, 200 cells were analyzed. Standard deviations (SD) are indicated. (D) Pxyl-yqcG ΔyqcG (ME187) cells were grown at 37°C in LB medium, and samples taken before (t = 0) and after (t = 1 to 5 h) xylose addition were incubated with MTT reagent (0.4 mg/ml) for 2 h. The resulting purple formazan was solubilized by SDS and quantified by spectrophotometry at 570 nm. The values presented, measured from 6 × 107 cells, are the average results from triplicates. SD are indicated.
To further examine the destiny of DLCs, we conducted a more detailed examination, focusing on the first 5 h after YqcG induction, when the population was composed solely of DLCs. We first asked whether DLCs that appear intact do indeed harbor a functional membrane. To address this question, DLCs were stained with propidium iodide (PI), which is incapable of penetrating intact membranes of living cells. Consistent with their morphological preservation, DLCs were impermeable to PI (see Fig. S2A in the supplemental material). As a control, when DLC membranes were artificially damaged by SDS treatment PI staining was visible (see Fig. S2B). In accordance with the sustained integrity of the membrane, membrane potential was preserved in DLCs, as indicated by their staining with the lipophilic fluorescent dye 3,3′-dihexyloxacarbocyanine iodide [DiOC6(3)], which reports membrane functionality (Fig. 2B) (19, 20). Furthermore, as would be expected of wild-type cell membranes, DiOC6(3) staining was significantly reduced upon treating DLCs with the proton ionophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP), which uncouples electron transport from ATP synthesis (Fig. 2B and C) (21). Assessment of metabolic activity using the MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay, which monitors the reduction of MTT by NAD(P)H-dependent cellular oxidoreductases, showed that DLCs maintained metabolic activity at approximately half of the level exhibited by the cells before YqcG induction (Fig. 2D).
We have previously shown an intimate connection between cell wall integrity and chromosome organization and segregation, demonstrating that direct perturbation of the cell wall leads to polyploid cells with atypical nucleoid morphologies (22). DLCs offered the opportunity to ask if the link between chromosome structure and cell wall integrity is reciprocal. Since DLCs maintain the rod shape structure, we attempted to elucidate whether their cell wall is intact. To examine the DLC peptidoglycan (PG) structure, cells were labeled with fluorescent wheat germ agglutinin (WGA), which demarcates the cell wall (23, 24). Consistent with preservation of their membrane integrity, DLCs displayed typical bright midcell bands and helical sidewall staining similar to those of normal cells (see Fig. S3 in the supplemental material). Thus, it appears that the presence of the chromosome does not dictate the PG structure. Moreover, once the cell wall is formed, genetic information is not essential for its maintenance for at least 5 h after chromosome loss.
Taken together, our data imply that DLCs remain intact for several hours after experiencing chromosome loss, as manifested by a proper overall cell shape, a membrane that operates as a selective barrier, and a normal PG pattern. These findings suggest that DLCs are not halted in an inactive mode but, instead, seem capable of maintaining basic cellular activities. Thus, although DLCs are no longer alive according to classical definitions, their viability measurements are similar to those of wild-type cells.
Macromolecules are preserved and remain functional in DLCs.
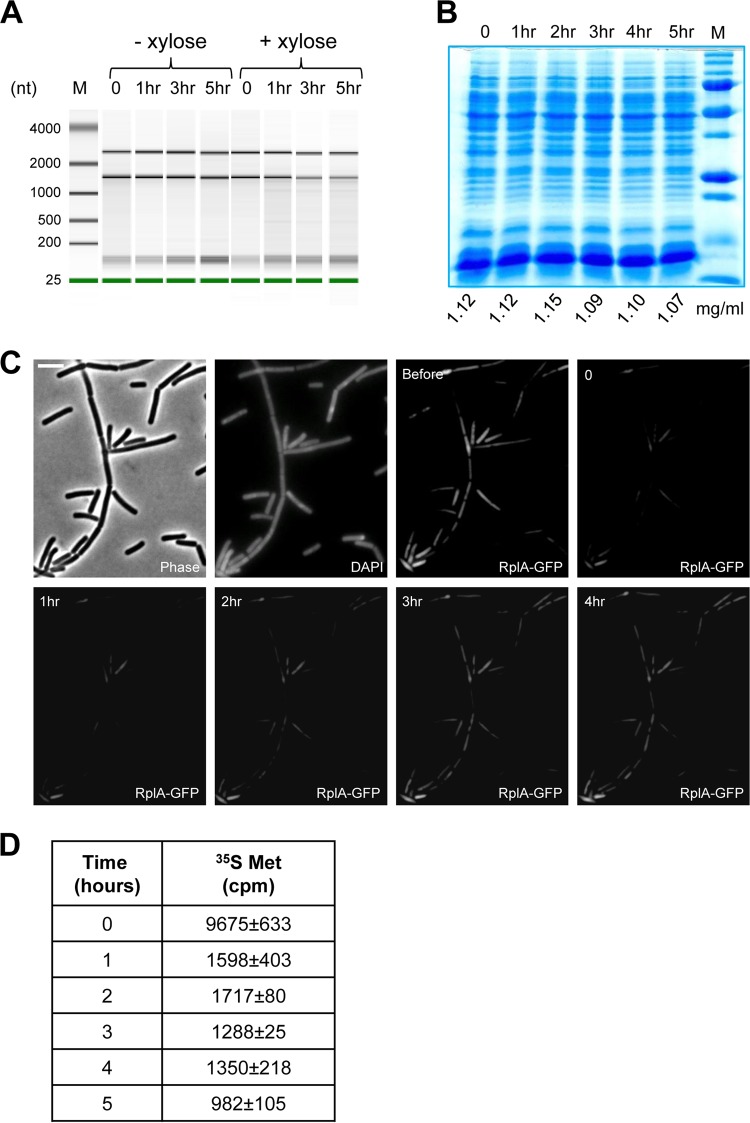
A living cell exhibits dynamic coordination between the key cellular processes of replication, transcription, and translation. DLCs afford tracking of RNA and protein molecules when this synchrony is perturbed by chromosome loss. Intriguingly, RNA extracted from DLCs possessed a profile similar to that of normal cells, showing only slight degradation in the course of 5 h after genome loss (Fig. 3A). In line with the RNA stability observed in DLCs, Bradford and SDS-PAGE analyses of total protein content showed no apparent differences in the overall protein concentration, nor did they show obvious changes in the pattern of protein bands over time (Fig. 3B). Following both the DNA and protein concentrations extracted from the same cultures over time substantiated that the protein concentration remained stable, while the DNA level was reduced (see Fig. S4A in the supplemental material). We next examined in detail the stability of specific proteins with diverse cellular functions: RplA, a ribosomal subunit (25, 26), Spo0J, a DNA binding protein involved in chromosome segregation (27–29), ManP, a membrane transporter of mannose (30), and FtsZ, the major cell division protein (31, 32). To this end, DLCs harboring GFP fusions to each of the representative proteins were subjected to Western blot analysis. In parallel, the level of the housekeeping protein sigma factor A (33, 34) was determined for each of the examined strains as a control. A relatively constant protein level was observed over time for all of the proteins inspected (see Fig. S4B), corroborating the overall lack of significant change in total protein concentration. Accordingly, the intensity of the fluorescent signal deriving from DLCs harboring RplA-GFP or ManP-GFP was relatively steady (see Fig. S4C and D). Based on these observations, we conclude that cells that have lost their genome in a sudden and rapid manner preserve macromolecules in a stable state.
FIG 3 .
Macromolecules remain steady and functional in DLCs. (A) Bioanalyzer pseudogel of RNA extracted from Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with or without xylose at the indicated time points. The time zero sample was taken before xylose addition. All lanes in the pseudogel are scaled to the same intensity range. M, molecular size ladder. (B) SDS-PAGE of proteins extracted from Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium before (t = 0) and after (t = 1 to 5 h) xylose addition. Protein concentrations were determined by Bradford assay and are indicated below the lanes. (C) Xylose was added to growing Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260). After 1 h, cells were incubated at 37°C on an LB agarose pad containing xylose and subjected to FRAP analysis. Shown are phase contrast, DAPI, and RplA-GFP fluorescence images. Time points before (Before) and after (0 to 4 h) photobleaching are indicated. Scale bar corresponds to 4 µm. (D) Pxyl-yqcG ΔyqcG (ME187) cells were grown at 37°C in LB medium, and samples taken before (t = 0) and after (t = 1 to 5 h) xylose addition were incubated with [35S]methionine (35S Met) for 30 min at 37°C. The radioactivity values presented (counts per minute [cpm]), measured from 1 × 107 cells, are the average results of triplicates. SD are indicated. Notably, the experiment was carried out in LB medium, since YqcG degradation activity is less efficient when cells are grown in minimal medium. Therefore, the values obtained for [35S]methionine incorporation are probably an underestimation, as unlabeled methionine was present in the medium.
The observed protein stability could reflect steady-state dynamics or, alternatively, a pause during which the cell is lacking in protein synthesis and degradation. To address this issue, we conducted a fluorescence recovery after photobleaching (FRAP) assay, monitoring DLCs expressing RplA-GFP. Following photobleached DLCs by time lapse microscopy revealed recovery of the signal, though with slow kinetics (26), as evidenced by the increase in fluorescence 1 to 2 h post-bleaching (Fig. 3C; see Fig. S5A in the supplemental material). Importantly, this increase was inhibited when FRAP was conducted in the presence of chloramphenicol (see Fig. S5B and C). Evaluation of protein synthesis by measuring [35S]methionine incorporation indicated that, indeed, DLCs continue to produce proteins with slower kinetics than cells prior to DNA degradation (Fig. 3D).
Thus, DLCs contain RNA molecules that are accessible for translation, as well as functional ribosomes that are capable of protein production. These features suggest that DLCs are most likely kept in a steady-state dynamic mode.
Protein localization within DLCs.
Having established that proteins are preserved within DLCs, we examined the localization of the representative GFP fusion proteins before and after chromosome loss. Notably, a shift in RplA-GFP localization from the typical condensed polar foci to a cytoplasmic diffusible pattern was observed following DNA degradation (Fig. 4A). This change in localization could be driven by the liberation of the ribosomes from their confined cellular zone, which is normally dictated by the chromosomal mass (9). Spo0J-GFP, which binds to the oriC region (27, 29, 35, 36), became more diffusely localized concomitantly with the process of DNA degradation (see Fig. S6 in the supplemental material), as might be expected. Unlike RplA and Spo0J, the localization of the mannose transporter ManP was largely preserved for at least 5 h after YqcG induction (Fig. 4B), reinforcing the view that the cytoplasmic membrane of DLCs remains functional throughout this time. In line with the premise of sustained functionality, approximately 40% of the DLCs contained FtsZ rings (Z rings), in comparison to ~60% of the cells before YqcG induction (see Fig. S7A and B). Notably, around 5% of the DLC Z rings appeared as extended helical structures, while only 1% of the Z rings displayed such pattern prior to YqcG induction. Measuring the Z ring position within DLCs revealed that the majority were properly localized to sites of division in the vicinity of the midcell; however, a slight shift from their customary central position was detected (see Fig. S7C). This typical localization pattern of FtsZ was largely maintained throughout the course of the experiment, raising the possibility that Z ring positioning is independent of the physical presence of the nucleoid. Furthermore, the correct localization of Z rings in DLCs suggests that the division machinery could still operate despite the absence of the chromosome.
FIG 4 .
Expression and localization patterns of RplA and ManP in DLCs. (A and B) Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260) (A) and Pxyl-yqcG ΔyqcG cells harboring manP-gfp (ME249) (B); cells were grown at 37°C in LB medium with xylose and stained with DAPI at the indicated time points. The time zero sample was visualized before xylose addition. Scale bars correspond to 2 µm.
DLCs can elongate and divide.
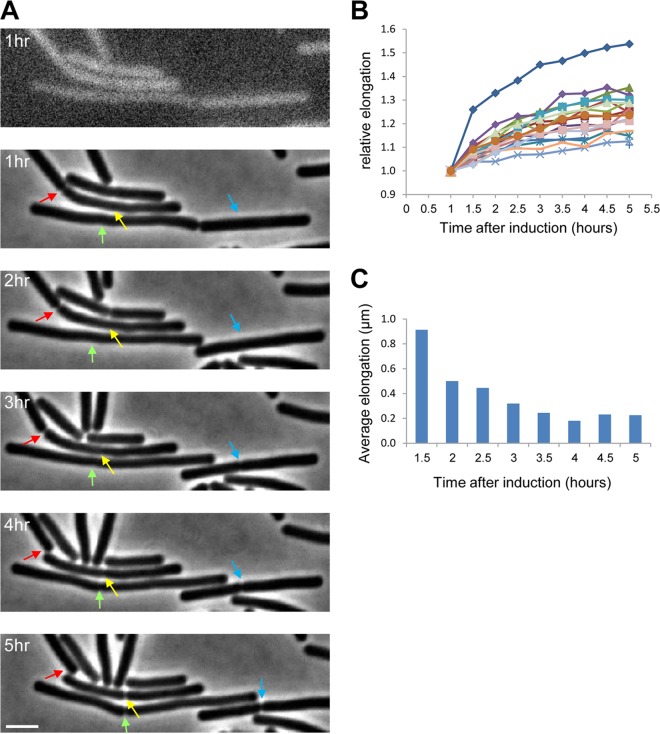
So far, we have shown that DLCs preserve their cellular morphology, contain an intact membrane and stable macromolecules, and exhibit proper localization of the cell division initiator protein FtsZ. These observations raise the question of whether FtsZ can provide a platform to carry out the fundamental process of cell division in the absence of DNA or whether Z rings are halted in a nondynamic state. To address this question, we followed DLCs by time lapse microscopy. Remarkably, we could clearly observe DLCs elongating and eventually separating into individual cells, implying that their Z rings are functional and can facilitate cell division (Fig. 5A). Following the elongation of individual DLCs revealed the elongation rate to be the highest during the initial 2.5 h after DNA loss and to subsequently decrease (Fig. 5B and C). We then attempted to explore whether the observed Z rings are only formed prior to chromosome loss or could also be established de novo. Tracking FtsZ-GFP within DLCs by time lapse microscopy showed that even after DNA loss, FtsZ forms dynamic rings that can undergo assembly and constriction (Fig. 6A and B). Following the Z ring dynamics using FRAP assay revealed that existing rings can successfully recruit new FtsZ molecules (Fig. 6C). Furthermore, the FRAP analysis evidenced that DLCs are capable of forming de novo Z rings (Fig. 6D). The appearance of de novo Z rings in DLCs was approximately 8.5% of the total Z rings and persisted even 5 h after YqcG induction (Fig. 6E). Collectively, these findings suggest that cell division in DLCs is a regulated process rather than spontaneous polymerization and constriction of Z rings.
FIG 5 .
DLCs elongate and divide. (A) Pxyl-yqcG ΔyqcG cells harboring hbsu-gfp (ME233) were grown at 37°C on an LB agarose pad containing xylose and were followed by time lapse microscopy. The top panel shows the signal from HBsu-GFP, indicating DNA loss (see Fig. S1C). The subsequent panels show phase contrast images taken at the indicated time points. Arrows highlight positions of division sites. Each color signifies a given site over time. Scale bar corresponds to 2 µm. (B) Elongation of individual ME233 DLCs was followed by time lapse microscopy. Shown are the relative lengths over time of 20 individual cells for which the length at the 1-h time point was defined as 1. The average elongation of ME233 cells grown without xylose (harboring intact chromosomes) under the same conditions was ×1.5 per 30 min, as calculated from 50 individual cells. (C) The average results for ME233 cell elongation over time were extracted from the data shown in panel B.
FIG 6 .
Z ring dynamics in DLCs. (A) Pxyl-yqcG ΔyqcG cells harboring ftsZ-gfp (ME244) were grown at 30°C on an LB agarose pad containing xylose and were followed by time lapse microscopy. Phase contrast and FtsZ-GFP fluorescence images were taken at the indicated time points. Arrows highlight positions of de novo Z rings, and stars indicate a constricting Z ring leading to cell division. Each color arrow signifies a given Z ring over time. (B) As a complement to the experiment whose results are shown in panel A, DAPI staining, which is toxic to cells, was added at the latest time point (t = 5 h); the results indicate the absence of chromosomal DNA. Of note, the position of some of the cells within the image changed following DAPI addition. (C and D) Xylose was added to growing Pxyl-yqcG ΔyqcG cells harboring ftsZ-gfp (ME244). After 2.5 h, the cells were incubated at 30°C on an LB agarose pad containing xylose and subjected to FRAP analysis. Phase contrast, FtsZ-GFP fluorescence, and DAPI images show recruitment of FtsZ molecules to an existing ring (arrow) (C) or de novo Z ring formation (arrow) (D). Time points before (before) and after (1 h or 2 h) photobleaching are indicated. Scale bars correspond to 2 µm. (E) ME244 cells were followed by time lapse microscopy as described for panel A, and the percentages of de novo Z rings out of the total Z rings for each time point after YqcG induction were calculated. At least 215 Z rings were scored for each time point. Shown are results from two independent biological experiments (Exp.).
DISCUSSION
Chromosome integrity is considered fundamental for the maintenance of any functional cell, with even point mutations being sufficient to trigger cell death and lysis. Here, we investigated the fate of bacterial cells which have lost their entire nucleoid in a sudden and rapid fashion. Surprisingly, DLCs maintained an intact membrane, as well as the characteristic architecture of the cell wall. In addition, DLCs successfully preserved their macromolecules, as indicated by steady RNA and protein levels for a period of at least 5 h. Furthermore, DLCs were not arrested in an inactive mode but could synthesize proteins, elongate, accurately position new Z rings, and divide. Thus, the continuity of key cellular events in cells lacking their chromosome indicates that the cues to carry out complex and lengthy processes are harbored within downstream molecular components.
The chromosome constitutes a physical scaffold that enables simultaneous transcription and translation (2–7) and influences protein localization. In accordance with this structural role, ribosomes were found to be excluded from the nucleoid-occupied regions, localizing mainly to the cell poles and the chromosome periphery (9). Consistent with this view, the ribosomal subunit RplA was observed to become diffusible in DLCs. Alternatively, RplA mislocalization could emanate from the lack of transcription, which has been shown to affect ribosome subcellular positioning (37). A profound effect of the physical presence of the nucleoid is to constrain the localization of the division machinery. It has been demonstrated that chromosome-associated proteins act to directly inhibit the assembly of the division apparatus around the nucleoid (8, 10). While Z ring assembly in cells devoid of DNA could potentially occur all over the cell, Z rings seem to be localized mainly to the midcell in DLCs, though some increase in Z rings displaying helical structures was monitored. This observation is consistent with previous studies showing that Z rings are positioned at the midcell in nucleoid-free E. coli cells formed in response to UV irradiation or derived from parC and mukB mutants and in cells lacking the nucleoid occlusion system (38–41). Although the FtsZ protein was shown to harbor intrinsic properties to assemble into multiple rings in liposomes and generate force for constriction (42), in general, a single Z ring per cell was exhibited by DLCs, suggesting that this phenomenon is not due to random polymerization. Furthermore, following cell elongation, DLCs were frequently capable of completing cell division, implying that the division machinery was functioning properly.
Macromolecule turnover is vital in bacteria to facilitate rapid responses to changing environments and for developmental processes to take place (43–46). Unexpectedly, RNA and protein levels were found to be relatively stable within DLCs, showing slow protein synthesis kinetics. These findings suggest that synthesis and degradation are carried out in DLCs, albeit on a longer time scale than in wild-type cells. Possibly, this slower kinetics is due to a lack of the energy required to facilitate molecular synthesis and degradation, along with the physical lack of a template for new RNA synthesis. Consistent with these possibilities, DLC elongation and division capabilities are energy-consuming processes occurring more slowly than normal. It is conceivable that DLCs are committed to perform particular cellular tasks and harbor energy reservoirs to fuel their occurrence. In light of this notion, normal cells might reserve energy pools to ensure the progression and completion of ongoing pathways.
Although an active apoptotic death pathway has mainly been attributed to eukaryotic cells, accumulating evidence indicates that similar destruction events exist in bacteria (47). Indeed, bacterial cells were shown to activate a regulated stress response, such as SOS or programmed cell death (PCD), following toxin activation, DNA damage, or exposure to antibiotics (48–51). Under such circumstances, morphological and biochemical hallmarks of apoptosis, including DNA fragmentation and membrane depolarization, have been observed in bacterial cells. Since cells lacking a chromosome cannot respond to stress conditions by activating the corresponding set of genes, the maintenance of DLC integrity hours after DNA degradation reinforces the view that active cell death is an attribute of bacteria. Consistent with this model, DNA topoisomerase-targeting antibiotics require protein synthesis to induce death (11, 52). Taken together, bacterial cell death could be an intrinsic apoptotic regulated pathway, demanding gene activation for its accomplishment. DLCs, being unable to induce a stress response, sustain cellular processes without being able to execute active cell death.
Cellular genetic information is generally considered a prerequisite for the performance of complex and prolonged processes. However, evidence for the occurrence of fundamental cellular processes, such as protein synthesis, division, and PCD, was observed in anucleated platelets in mammalians (53). Nevertheless, in contrast to DLCs, chromosome loss in platelets is programmed, taking place in cells destined for such an event. Platelets and DLCs both exemplify the notion that, once the information flows from the DNA to downstream components, the latter become independent, capable of carrying out fundamental processes to sustain cellular existence. Interestingly, studies of minicells, conducted mainly in the 1970s, indicate that they are capable of performing respiration, incorporating amino acids into their cell wall, and synthesizing proteins and RNA from phages and plasmids (12–17).
Despite considerable attempts, we failed to reveal the actual physiological role of the yqcGF TA module. Conducting an array of experiments, such as competition assays between wild-type and ΔyqcGF mutant cells and testing the effects of ΔyqcGF mutation on competence, sporulation, DNA repair, and phage infection, did not expose any detectable phenotype. However, the DLC characterization provided here can serve as a platform for future investigation of specific molecular processes, such as PCD. In this regard, DLCs can also be utilized as recipients for transplantation of synthetic DNA (54). The ability of DLCs to perform sophisticated cellular activities brings about the possibility of utilizing a similar approach for pathogenic bacteria as a potential vaccination strategy. DLCs are likely to be capable of efficiently inducing the immune system without propagating within the host.
MATERIALS AND METHODS
Strains and general methods.
B. subtilis strains are listed in Table S1 in the supplemental material. The plasmid and primers used for this study are described in Text S1 and Table S2. All general methods were carried out as described previously (55). Cultures were inoculated at an optical density at 600 nm (OD600) of 0.05 from an overnight culture, and growth was carried out at 30°C or 37°C in LB medium. During logarithmic phase (OD600 of 0.4 to 0.6), 0.5% xylose was added to induce yqcG expression, as indicated. Of note, induction of YqcG during stationary phase or in minimal medium does not lead to efficient DNA degradation, suggesting that under these conditions, the chromosome is protected from YqcG endonuclease activity.
Fluorescence microscopy.
Fluorescence microscopy was carried out as described previously (22). Briefly, samples (0.5 ml) were taken during logarithmic phase, centrifuged, and resuspended in 10 µl of 1× phosphate-buffered saline (PBS) supplemented with the fluorescent membrane stain FM1-43 (Molecular Probes; Invitrogen) at 1 µg/ml and/or with the DNA stain 4,6-diamidino-2-phenylindole (DAPI) (Sigma) at 2 µg/ml. For cell wall labeling, cells were harvested, gently centrifuged, resuspended in 100 µl 1× T-Base supplemented with wheat germ agglutinin (WGA)-fluorescein isothiocyanate (FITC) (5 µg/ml; Sigma), incubated for 15 min at room temperature, and washed twice with 1× T-Base before imaging. To visualize stained cell walls and GFP-fused proteins, the cells were placed on thin 1× T-Base–1% agarose pads. For SDS treatment, cells (0.5 ml) were incubated with 0.06% SDS for 10 min at room temperature, washed twice with 1× PBS, and stained with DAPI and propidium iodide (PI) (5 µg/ml; Sigma) before imaging. Cells were visualized and photographed using an Axioplan2 microscope (Zeiss) equipped with a CoolSnap HQ camera (Photometrics, Roper Scientific) or an Axio Observer Z1 microscope (Zeiss) equipped with a CoolSnap HQII camera (Photometrics; Roper Scientific). System control and image processing were performed using MetaMorph software (Molecular Devices).
Assessing membrane potential.
To assess membrane potential, cells were grown to logarithmic phase and samples (1 ml) were harvested. Cells were washed once with 1× PBS and incubated for 1 min with 10 nM DiOC6(3) (Invitrogen) diluted in 1× PBS. As a control, before DiOC6(3) staining, cells were incubated with 100 µM CCCP for 5 min. Cells were visualized and photographed by fluorescence microscopy, and signal intensities were evaluated using MetaMorph software (Molecular Devices).
MTT assay.
For estimating cell metabolic activity, the MTT assay was conducted as previously described (56). In brief, cells were grown to logarithmic phase and samples were diluted (1:16, within the linear range) in 1 ml LB medium. MTT reagent was added to a final concentration of 0.4 mg/ml, and samples were incubated at 37°C for 2 h. For dissolving formazan, 100 µl of 10% SDS was added. The level of formazan was measured by spectrophotometer (OD570). A sample of LB medium supplemented with MTT reagent and SDS served as a control.
Pulse and chase [35S]methionine labeling.
For monitoring protein synthesis, cells were grown to logarithmic phase, and samples (400 µl) were incubated with [35S]methionine (24 µCi) at 37°C for 30 min. Ten microliters of a given sample was then spotted in triplicates onto 3MM Whatman paper. The dried filters were incubated in cold 10% 2,4,6-trichloroanisole (TCA) for 30 min, washed with cold 10% TCA for 15 min, and finally left in fresh 10% TCA overnight at 4°C. Next, samples were washed with cold 95% ethanol and left to dry. Radioactivity was determined by scintillation counter (Tri-Carb 2900 TR; Packard). Samples of LB medium supplemented with [35S]methionine and samples of cells incubated in the absence of [35S]methionine served as controls.
For additional methods, see Text S1 in the supplemental material.
SUPPLEMENTAL MATERIAL
Description of additional methods, including DNA and RNA extraction, SDS-PAGE, and Western blot analysis. Download
Induction of YqcG leads to chromosome degradation. (A) Wild-type (PY79), Pxyl-yqcG ΔyqcG (ME187), and Pxyl-yqcGF ΔyqcG (ME198) strains were plated on LB agar without (1, 3) or with (2, 4) xylose. Of note, a few colonies succeeded in forming on plates containing xylose. Sequence analysis of these suppressor colonies revealed that the cells harbored the same stop codon within the yqcG open reading frame. The stop codon was situated within the region encoding the C-terminal part of the protein, suggesting that the toxic activity is located within this region. (B) DNA was extracted from ME187 cells grown at 37°C in LB medium before (t = 0) and after (t = 0.25 to 5 h) xylose addition. Ten microliters from each sample was loaded onto an agarose gel, and DNA concentrations were determined by comparison to a 1-kb DNA ladder. Shown are results from 3 independent biological repeats. Standard deviations (SD) are indicated. (C) Pxyl-yqcG ΔyqcG cells harboring hbsu-gfp (ME233) were grown at 37°C on an LB agarose pad containing xylose and were followed by time lapse microscopy. Shown is fluorescence from HBsu-GFP, signifying the DNA. Scale bar corresponds to 2 µm. Download
DLCs have an intact membrane. (A and B) Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose and stained with PI (5 µg/ml) without (A) or with (B) SDS (0.06%) treatment at the indicated time points postinduction. The time zero sample was visualized before xylose addition. Cells were stained with PI when membrane integrity was artificially damaged by SDS, probably demarcating RNA and residual DNA fragments. Scale bars correspond to 2 µm. Download
DLCs exhibit characteristic cell wall architecture. Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose, labeled with WGA-FITC, and stained with DAPI were taken at the indicated time points. The time zero sample was visualized before xylose addition. Scale bar corresponds to 2 µm. Download
DLCs exhibit protein steady-state dynamics. (A) DNA and proteins from Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium were extracted before (t = 0) and after (t = 0.25 to 5 h) xylose addition. DNA concentrations were determined as described in the legend to Fig. S1B, and protein concentrations were determined by Bradford assay. The ratios between DNA and protein concentrations over time were calculated. Shown are results from 3 independent biological repeats. SD are indicated. (B) Immunoblot analysis of GFP and sigma A (SigA) proteins extracted from the following strains: ME260 (Pxyl-yqcG ΔyqcG rplA-gfp), ME249 (Pxyl-yqcG ΔyqcG manP-gfp), ME244 (Pxyl-yqcG ΔyqcG ftsZ-gfp), ME189 (Pxyl-yqcG ΔyqcG spo0J-gfp), and ME187 (Pxyl-yqcG ΔyqcG) (negative control [nc]). Cells were grown at 37°C (ME260, ME249, ME189, and ME187) or at 30°C (ME244) in LB medium. Samples were taken before (t = 0) and after (t = 1 to 5 h) xylose addition. Each extract was incubated with polyclonal anti-GFP (α GFP, left) and anti-SigA (α SigA, right) antibodies. Bands were quantified, and the ratios between the GFP-labeled proteins and SigA were calculated (bottom graphs). (C) Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260) were grown at 37°C in LB medium with xylose. Samples were visualized by fluorescence microscopy. The time zero sample was visualized before xylose addition. Shown are the results for quantification of the fluorescence signal of RplA-GFP protein at the indicated time points. For each time point, approximately 200 cells were analyzed and SD was calculated (bars). Notably, according to the fluorescence quantification, the level of RplA-GFP seemed to increase during the first hour postinduction. This could be due to the mislocalization of RplA-GFP upon DNA degradation rather than to protein synthesis. It is possible that, normally, fluorescence quenching reduces the signal from RplA-GFP molecules that are clustered together. Consistent with this idea, Western blot analysis revealed the protein levels to be relatively constant throughout the experiment (see panel B). (D) Pxyl-yqcG ΔyqcG cells harboring manP-gfp (ME249) were grown at 37°C in LB medium with xylose. Samples were visualized by fluorescence microscopy. The time zero sample was visualized before xylose addition. Shown are the results for quantification of the fluorescence signal of ManP-GFP protein at the indicated time points. For each time point, approximately 200 cells were analyzed and SD was calculated (bars). Download
DLCs produce RplA-GFP. The results shown are complementary to those shown in Fig. 3C. (A) Xylose was added to growing Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260). After 5 h, cells were incubated at 37°C on an LB agarose pad containing xylose and subjected to FRAP analysis. Shown are phase contrast, RplA-GFP fluorescence, and DAPI images. Time points before (before) and after (0 to 2 h) photobleaching are indicated. Scale bar corresponds to 5 µm. (B) Xylose was added to growing ME260 cells. After 1 h, cells were incubated at 37°C on an LB agarose pad containing xylose and chloramphenicol (5 µg/ml) and subjected to FRAP analysis. Shown are phase contrast, DAPI, and RplA-GFP fluorescence images. Time points before (Before) and after (0 to 4 h) photobleaching are indicated. Scale bar corresponds to 2 µm. (C) Shown are the results for quantification of the fluorescence signal of RplA-GFP protein at the indicated time points for ME260 cells grown in the presence or the absence of chloramphenicol (cm) as described for panel B and in the legend to Fig. 3C, respectively. For each time point, approximately 60 cells were analyzed. Shown are the results of a representative experiment out of two independent biological repeats. Download
Spo0J-GFP localization following DNA degradation. Fluorescence images of Pxyl-yqcG ΔyqcG cells harboring spo0J-gfp (ME189) grown at 37°C in LB medium with xylose and stained with DAPI were taken at the indicated time points (min). The time zero sample was visualized before xylose addition. Scale bar corresponds to 1 µm. Download
Abundance and localization of Z rings in DLCs. (A) Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG cells harboring ftsZ-gfp (ME244) grown at 30°C in LB medium with xylose and stained with DAPI were taken at the indicated time points. The time zero sample was visualized before xylose addition. Scale bar corresponds to 2 µm. (B) Shown are the percentages of ME244 cells exhibiting FtsZ-GFP rings. Cells were grown at 30°C in LB with xylose. The time zero sample was visualized before xylose addition. For each time point, approximately 200 cells were analyzed in each culture. (C) Shown are the percentages of ME244 cells exhibiting FtsZ-GFP rings at the indicated cellular positions. The distance of the Z ring from the pole was calculated relative to the cell length; 0.5 represents the midcell position. Cells were grown at 30°C in LB with xylose. The time zero sample was visualized before xylose addition. For each time point, approximately 100 Z ring-containing cells were analyzed. Download
B. subtilis strains.
Primer list.
ACKNOWLEDGMENTS
This work is supported by a European Research Council (ERC) Advance grant (339984) awarded to S.B.-Y.
YqcG is the subject of a U.S. patent application (Sigal Ben-Yehuda, U.S. patent application 62/109,150).
We thank A. Taraboulos (Hebrew University of Jerusalem, Jerusalem, Israel), A. Grossman (MIT, Cambridge, MA), M. Fujita (Houston University, Houston, TX), and D. Rudner (Harvard, Cambridge, MA) for strains and reagents. We thank I. Rosenshine (Hebrew University of Jerusalem, Jerusalem, Israel) A. Rouvinski (Pasteur Institute, Paris, France), and members of the Ben-Yehuda laboratory for valuable discussions and comments on the manuscript. We are grateful to B. Kalderon and O. Pines and his laboratory members (Hebrew University of Jerusalem, Jerusalem, Israel) for help in performing the radioactive labeling experiments.
Footnotes
Citation Elbaz M, Ben-Yehuda S. 2015. Following the fate of bacterial cells experiencing sudden chromosome loss. mBio 6(3):e00092-15. doi:10.1128/mBio.00092-15.
Contributor Information
Petra Anne Levin, Washington University.
R. John Collier, Harvard Medical School.
REFERENCES
- 1.Lodish HF, Berk A, Krieger M, Kaiser CA, Scott MP, Bretscher A, Polegh H, Matsudaria P. 2008. Molecular cell biology, 6th ed. W. H. Freeman, Basingstoke, United Kingdom. [Google Scholar]
- 2.Hobot JA, Villiger W, Escaig J, Maeder M, Ryter A, Kellenberger E. 1985. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol 162:960–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller OL Jr., Hamkalo BA, Thomas CA Jr.. 1970. Visualization of bacterial genes in action. Science 169:392–395. doi: 10.1126/science.169.3943.392. [DOI] [PubMed] [Google Scholar]
- 4.Robinow C, Kellenberger E. 1994. The bacterial nucleoid revisited. Microbiol Rev 58:211–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryter A, Chang A. 1975. Localization of transcribing genes in the bacterial cell by means of high resolution autoradiography. J Mol Biol 98:797–810. doi: 10.1016/S0022-2836(75)80011-8. [DOI] [PubMed] [Google Scholar]
- 6.Valkenburg JA, Woldringh CL, Brakenhoff GJ, van der Voort HT, Nanninga N. 1985. Confocal scanning light microscopy of the Escherichia coli nucleoid: comparison with phase-contrast and electron microscope images. J Bacteriol 161:478–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woldringh CL, Jensen PR, Westerhoff HV. 1995. Structure and partitioning of bacterial DNA: determined by a balance of compaction and expansion forces? FEMS Microbiol Lett 131:235–242. doi: 10.1111/j.1574-6968.1995.tb07782.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernhardt TG, de Boer PA. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell 18:555–564. doi: 10.1016/j.molcel.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis PJ, Thaker SD, Errington J. 2000. Compartmentalization of transcription and translation in Bacillus subtilis. EMBO J 19:710–718. doi: 10.1093/emboj/19.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu LJ, Errington J. 2004. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Kohanski MA, Dwyer DJ, Collins JJ. 2010. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol 8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adler HI, Fisher WD, Cohen A, Hardigree AA. 1967. Miniature Escherichia coli cells deficient in DNA. Proc Natl Acad Sci U S A 57:321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendelson NH, Reeve JN, Cole RM. 1974. Physiological studies of Bacillus subtilis minicells. J Bacteriol 117:1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mertens G, Reeve JN. 1977. Synthesis of cell envelope components by anucleate cells (minicells) of Bacillus subtilis. J Bacteriol 129:1198–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reeve JN, Cornett JB. 1975. Bacteriophage SPO1-induced macromolecular synthesis in minicells of Bacillus subtilis. J Virol 15:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reeve JN, Mendelson NH, Coyne SI, Hallock LL, Cole RM. 1973. Minicells of Bacillus subtilis. J Bacteriol 114:860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roozen KJ, Fenwick RG Jr, Curtiss R III. 1971. Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol 107:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holberger LE, Garza-Sánchez F, Lamoureux J, Low DA, Hayes CS. 2012. A novel family of toxin/antitoxin proteins in Bacillus species. FEBS Lett 586:132–136. doi: 10.1016/j.febslet.2011.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laflamme C, Ho J, Veillette M, de Latrémoille MC, Verreault D, Mériaux A, Duchaine C. 2005. Flow cytometry analysis of germinating Bacillus spores, using membrane potential dye. Arch Microbiol 183:107–112. doi: 10.1007/s00203-004-0750-9. [DOI] [PubMed] [Google Scholar]
- 20.Sträuber H, Müller S. 2010. Viability states of bacteria-specific mechanisms of selected probes. Cytometry A 77:623–634. doi: 10.1002/cyto.a.20920. [DOI] [PubMed] [Google Scholar]
- 21.Ghoul M, Pommepuy M, Moillo-Batt A, Cormier M. 1989. Effect of carbonyl cyanide m-chlorophenylhydrazone on Escherichia coli halotolerance. Appl Environ Microbiol 55:1040–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elbaz M, Ben-Yehuda S. 2010. The metabolic enzyme ManA reveals a link between cell wall integrity and chromosome morphology. PLoS Genet 6:e1001119. doi: 10.1371/journal.pgen.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayhurst EJ, Kailas L, Hobbs JK, Foster SJ. 2008. Cell wall peptidoglycan architecture in Bacillus subtilis. Proc Natl Acad Sci U S A 105:14603–14608. doi: 10.1073/pnas.0804138105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weart RB, Levin PA. 2003. Growth rate-dependent regulation of medial FtsZ ring formation. J Bacteriol 185:2826–2834. doi: 10.1128/JB.185.9.2826-2834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaltschmidt E, Wittmann HG. 1970. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A 67:1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg A, Sinai L, Smith Y, Ben-Yehuda S. 2012. Dynamic expression of the translational machinery during Bacillus subtilis life cycle at a single cell level. PLoS One 7:e41921. doi: 10.1371/journal.pone.0041921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glaser P, Sharpe ME, Raether B, Perego M, Ohlsen K, Errington J. 1997. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev 11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- 28.Ireton K, Gunther NW, Grossman AD. 1994. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol 176:5320–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin DC, Grossman AD. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92:675–685. doi: 10.1016/S0092-8674(00)81135-6. [DOI] [PubMed] [Google Scholar]
- 30.Reizer J, Bachem S, Reizer A, Arnaud M, Saier MH Jr, Stülke J. 1999. Novel phosphotransferase system genes revealed by genome analysis—the complete complement of PTS proteins encoded within the genome of Bacillus subtilis. Microbiology 145:3419–3429. [DOI] [PubMed] [Google Scholar]
- 31.Bi EF, Lutkenhaus J. 1991. FtsZ ring structure associated with division in Escherichia coli. Nature 354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 32.Lutkenhaus J, Addinall SG. 1997. Bacterial cell division and the Z ring. Annu Rev Biochem 66:93–116. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 33.Lonetto M, Gribskov M, Gross CA. 1992. The sigma 70 family: sequence conservation and evolutionary relationships. J Bacteriol 174:3843–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wösten MM. 1998. Eubacterial sigma-factors. FEMS Microbiol Rev 22:127–150. doi: 10.1016/S0168-6445(98)00011-4. [DOI] [PubMed] [Google Scholar]
- 35.Lewis PJ, Errington J. 1997. Direct evidence for active segregation of oriC regions of the Bacillus subtilis chromosome and co-localization with the Spo0J partitioning protein. Mol Microbiol 25:945–954. doi: 10.1111/j.1365-2958.1997.mmi530.x. [DOI] [PubMed] [Google Scholar]
- 36.Lin DC, Levin PA, Grossman AD. 1997. Bipolar localization of a chromosome partition protein in Bacillus subtilis. Proc Natl Acad Sci U S A 94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mascarenhas J, Weber MH, Graumann PL. 2001. Specific polar localization of ribosomes in Bacillus subtilis depends on active transcription. EMBO Rep 2:685–689. doi: 10.1093/embo-reports/kve160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moriya S, Rashid RA, Rodrigues CD, Harry EJ. 2010. Influence of the nucleoid and the early stages of DNA replication on positioning the division site in Bacillus subtilis. Mol Microbiol 76:634–647. doi: 10.1111/j.1365-2958.2010.07102.x. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues CD, Harry EJ. 2012. The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet 8:e1002561. doi: 10.1371/journal.pgen.1002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pazos M, Casanova M, Palacios P, Margolin W, Natale P, Vicente M. 2014. FtsZ placement in nucleoid-free bacteria. PLoS One 9:e91984. doi: 10.1371/journal.pone.0091984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Q, Yu XC, Margolin W. 1998. Assembly of the FtsZ ring at the central division site in the absence of the chromosome. Mol Microbiol 29:491–503. doi: 10.1046/j.1365-2958.1998.00942.x. [DOI] [PubMed] [Google Scholar]
- 42.Osawa M, Anderson DE, Erickson HP. 2008. Reconstitution of contractile FtsZ rings in liposomes. Science 320:792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker TA, Sauer RT. 2012. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim Biophys Acta 1823:15–28. doi: 10.1016/j.bbamcr.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gottesman S. 1999. Regulation by proteolysis: developmental switches. Curr Opin Microbiol 2:142–147. doi: 10.1016/S1369-5274(99)80025-3. [DOI] [PubMed] [Google Scholar]
- 45.Jenal U, Hengge-Aronis R. 2003. Regulation by proteolysis in bacterial cells. Curr Opin Microbiol 6:163–172. doi: 10.1016/S1369-5274(03)00029-8. [DOI] [PubMed] [Google Scholar]
- 46.Lehnik-Habrink M, Lewis RJ, Mäder U, Stülke J. 2012. RNA degradation in Bacillus subtilis: an interplay of essential endo- and exoribonucleases. Mol Microbiol 84:1005–1017. doi: 10.1111/j.1365-2958.2012.08072.x. [DOI] [PubMed] [Google Scholar]
- 47.Bayles KW. 2014. Bacterial programmed cell death: making sense of a paradox. Nat Rev Microbiol 12:63–69. doi: 10.1038/nrmicro3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aizenman E, Engelberg-Kulka H, Glaser G. 1996. An Escherichia coli chromosomal “addiction module” regulated by guanosine [corrected] 3′,5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A 93:6059–6063. doi: 10.1073/pnas.93.12.6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bos J, Yakhnina AA, Gitai Z. 2012. BapE DNA endonuclease induces an apoptotic-like response to DNA damage in Caulobacter. Proc Natl Acad Sci U S A 109:18096–18101. doi: 10.1073/pnas.1213332109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ. 2012. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol Cell 46:561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erental A, Sharon I, Engelberg-Kulka H. 2012. Two programmed cell death systems in Escherichia coli: an apoptotic-like death is inhibited by the mazEF-mediated death pathway. PLoS Biol 10:e1001281. doi: 10.1371/journal.pbio.1001281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drlica K, Malik M, Kerns RJ, Zhao X. 2008. Quinolone-mediated bacterial death. Antimicrob Agents Chemother 52:385–392. doi: 10.1128/AAC.01617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leytin V. 2012. Apoptosis in the anucleate platelet. Blood Rev 26:51–63. doi: 10.1016/j.blre.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Gibson DG, Glass JI, Lartigue C, Noskov VN, Chuang RY, Algire MA, Benders GA, Montague MG, Ma L, Moodie MM, Merryman C, Vashee S, Krishnakumar R, Assad-Garcia N, Andrews-Pfannkoch C, Denisova EA, Young L, Qi ZQ, Segall-Shapiro TH, Calvey CH, Parmar PP, Hutchison CA III, Smith HO, Venter JC. 2010. Creation of a bacterial cell controlled by a chemically synthesized genome. Science 329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- 55.Harwood C, Cutting SM. 1990. Molecular biological methods for Bacillus. Wiley, Chichester, United Kingdom. [Google Scholar]
- 56.Schaller A, Sun Z, Yang Y, Somoskovi A, Zhang Y. 2002. Salicylate reduces susceptibility of Mycobacterium tuberculosis to multiple antituberculosis drugs. Antimicrob Agents Chemother 46:2636–2639. doi: 10.1128/AAC.46.8.2636-2639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of additional methods, including DNA and RNA extraction, SDS-PAGE, and Western blot analysis. Download
Induction of YqcG leads to chromosome degradation. (A) Wild-type (PY79), Pxyl-yqcG ΔyqcG (ME187), and Pxyl-yqcGF ΔyqcG (ME198) strains were plated on LB agar without (1, 3) or with (2, 4) xylose. Of note, a few colonies succeeded in forming on plates containing xylose. Sequence analysis of these suppressor colonies revealed that the cells harbored the same stop codon within the yqcG open reading frame. The stop codon was situated within the region encoding the C-terminal part of the protein, suggesting that the toxic activity is located within this region. (B) DNA was extracted from ME187 cells grown at 37°C in LB medium before (t = 0) and after (t = 0.25 to 5 h) xylose addition. Ten microliters from each sample was loaded onto an agarose gel, and DNA concentrations were determined by comparison to a 1-kb DNA ladder. Shown are results from 3 independent biological repeats. Standard deviations (SD) are indicated. (C) Pxyl-yqcG ΔyqcG cells harboring hbsu-gfp (ME233) were grown at 37°C on an LB agarose pad containing xylose and were followed by time lapse microscopy. Shown is fluorescence from HBsu-GFP, signifying the DNA. Scale bar corresponds to 2 µm. Download
DLCs have an intact membrane. (A and B) Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose and stained with PI (5 µg/ml) without (A) or with (B) SDS (0.06%) treatment at the indicated time points postinduction. The time zero sample was visualized before xylose addition. Cells were stained with PI when membrane integrity was artificially damaged by SDS, probably demarcating RNA and residual DNA fragments. Scale bars correspond to 2 µm. Download
DLCs exhibit characteristic cell wall architecture. Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium with xylose, labeled with WGA-FITC, and stained with DAPI were taken at the indicated time points. The time zero sample was visualized before xylose addition. Scale bar corresponds to 2 µm. Download
DLCs exhibit protein steady-state dynamics. (A) DNA and proteins from Pxyl-yqcG ΔyqcG (ME187) cells grown at 37°C in LB medium were extracted before (t = 0) and after (t = 0.25 to 5 h) xylose addition. DNA concentrations were determined as described in the legend to Fig. S1B, and protein concentrations were determined by Bradford assay. The ratios between DNA and protein concentrations over time were calculated. Shown are results from 3 independent biological repeats. SD are indicated. (B) Immunoblot analysis of GFP and sigma A (SigA) proteins extracted from the following strains: ME260 (Pxyl-yqcG ΔyqcG rplA-gfp), ME249 (Pxyl-yqcG ΔyqcG manP-gfp), ME244 (Pxyl-yqcG ΔyqcG ftsZ-gfp), ME189 (Pxyl-yqcG ΔyqcG spo0J-gfp), and ME187 (Pxyl-yqcG ΔyqcG) (negative control [nc]). Cells were grown at 37°C (ME260, ME249, ME189, and ME187) or at 30°C (ME244) in LB medium. Samples were taken before (t = 0) and after (t = 1 to 5 h) xylose addition. Each extract was incubated with polyclonal anti-GFP (α GFP, left) and anti-SigA (α SigA, right) antibodies. Bands were quantified, and the ratios between the GFP-labeled proteins and SigA were calculated (bottom graphs). (C) Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260) were grown at 37°C in LB medium with xylose. Samples were visualized by fluorescence microscopy. The time zero sample was visualized before xylose addition. Shown are the results for quantification of the fluorescence signal of RplA-GFP protein at the indicated time points. For each time point, approximately 200 cells were analyzed and SD was calculated (bars). Notably, according to the fluorescence quantification, the level of RplA-GFP seemed to increase during the first hour postinduction. This could be due to the mislocalization of RplA-GFP upon DNA degradation rather than to protein synthesis. It is possible that, normally, fluorescence quenching reduces the signal from RplA-GFP molecules that are clustered together. Consistent with this idea, Western blot analysis revealed the protein levels to be relatively constant throughout the experiment (see panel B). (D) Pxyl-yqcG ΔyqcG cells harboring manP-gfp (ME249) were grown at 37°C in LB medium with xylose. Samples were visualized by fluorescence microscopy. The time zero sample was visualized before xylose addition. Shown are the results for quantification of the fluorescence signal of ManP-GFP protein at the indicated time points. For each time point, approximately 200 cells were analyzed and SD was calculated (bars). Download
DLCs produce RplA-GFP. The results shown are complementary to those shown in Fig. 3C. (A) Xylose was added to growing Pxyl-yqcG ΔyqcG cells harboring rplA-gfp (ME260). After 5 h, cells were incubated at 37°C on an LB agarose pad containing xylose and subjected to FRAP analysis. Shown are phase contrast, RplA-GFP fluorescence, and DAPI images. Time points before (before) and after (0 to 2 h) photobleaching are indicated. Scale bar corresponds to 5 µm. (B) Xylose was added to growing ME260 cells. After 1 h, cells were incubated at 37°C on an LB agarose pad containing xylose and chloramphenicol (5 µg/ml) and subjected to FRAP analysis. Shown are phase contrast, DAPI, and RplA-GFP fluorescence images. Time points before (Before) and after (0 to 4 h) photobleaching are indicated. Scale bar corresponds to 2 µm. (C) Shown are the results for quantification of the fluorescence signal of RplA-GFP protein at the indicated time points for ME260 cells grown in the presence or the absence of chloramphenicol (cm) as described for panel B and in the legend to Fig. 3C, respectively. For each time point, approximately 60 cells were analyzed. Shown are the results of a representative experiment out of two independent biological repeats. Download
Spo0J-GFP localization following DNA degradation. Fluorescence images of Pxyl-yqcG ΔyqcG cells harboring spo0J-gfp (ME189) grown at 37°C in LB medium with xylose and stained with DAPI were taken at the indicated time points (min). The time zero sample was visualized before xylose addition. Scale bar corresponds to 1 µm. Download
Abundance and localization of Z rings in DLCs. (A) Phase contrast and fluorescence images of Pxyl-yqcG ΔyqcG cells harboring ftsZ-gfp (ME244) grown at 30°C in LB medium with xylose and stained with DAPI were taken at the indicated time points. The time zero sample was visualized before xylose addition. Scale bar corresponds to 2 µm. (B) Shown are the percentages of ME244 cells exhibiting FtsZ-GFP rings. Cells were grown at 30°C in LB with xylose. The time zero sample was visualized before xylose addition. For each time point, approximately 200 cells were analyzed in each culture. (C) Shown are the percentages of ME244 cells exhibiting FtsZ-GFP rings at the indicated cellular positions. The distance of the Z ring from the pole was calculated relative to the cell length; 0.5 represents the midcell position. Cells were grown at 30°C in LB with xylose. The time zero sample was visualized before xylose addition. For each time point, approximately 100 Z ring-containing cells were analyzed. Download
B. subtilis strains.
Primer list.