Abstract
Pancreatic islet mass, represented by islet equivalent (IEQ), is the most important parameter in decision making for clinical islet transplantation. To obtain IEQ, the sample of islets is routinely counted manually under a microscope and discarded thereafter. Islet purity, another parameter in islet processing, is routinely acquired by estimation only.
In this study, we validated our digital image analysis (DIA) system developed using the software of Image Pro Plus for islet mass and purity assessment. Application of the DIA allows to better comply with current good manufacturing practice (cGMP) standards. Human islet samples were captured as calibrated digital images for the permanent record. Five trained technicians participated in determination of IEQ and purity by manual counting method and DIA. IEQ count showed statistically significant correlations between the manual method and DIA in all sample comparisons (r >0.819 and p < 0.0001). Statistically significant difference in IEQ between both methods was found only in High purity 100μL sample group (p = 0.029). As far as purity determination, statistically significant differences between manual assessment and DIA measurement was found in High and Low purity 100μL samples (p<0.005), In addition, islet particle number (IPN) and the IEQ/IPN ratio did not differ statistically between manual counting method and DIA.
In conclusion, the DIA used in this study is a reliable technique in determination of IEQ and purity. Islet sample preserved as a digital image and results produced by DIA can be permanently stored for verification, technical training and islet information exchange between different islet centers. Therefore, DIA complies better with cGMP requirements than the manual counting method. We propose DIA as a quality control tool to supplement the established standard manual method for islets counting and purity estimation.
Keywords: digital image analysis, islet quantification, islet purity, and islet transplantation
INTRODUCTION
Human pancreatic islet transplantation is a clinical cell therapy for patients who undergo total pancreatectomy due to benign pancreatic disease or trauma (autologous islet transplantation) or for select patients with type I diabetes (allogeneic islet transplantation) (9,11). As islet product is regarded as a “drug”, it has to be processed in the Clean Room of a current Good Manufacture Practice (cGMP) facility and meet all release criteria required by the FDA in the United States before being released for transplantation to the patient. Among all release criteria, islet mass is the most crucial to assure a positive clinical outcome after transplantation. Since islets size falls in a broad range (50 to 400 μm), islet equivalents (IEQ) was established to measure islet mass based on islet size and number in 1990 (8). The procedures to determine IEQ are described as follows. A sample of islet suspension is stained with Dithizone (DTZ), which chelates the zinc of the insulin granules in beta cells of the pancreatic islets, resulting in a red color. The acinar cells remain unstained and white. The diameter of individual islets is measured using a calibrated grid with 50 μm increments in the eyepiece of a phase contrast microscope. The concept of IEQ is derived from an assumption that islets are spherical and the volume of IEQ is equal to the volume of a 150μm diameter islet. An IEQ calculation table was created by displaying the islet size groups (diameter), the number of islets per group, and conversion factors (mean group volume /volume of one IEQ) used to calculate IEQ per size group (8). The total IEQ is calculated by the multiplication of the sum of IEQ in all size groups in the entire sample and dilution factor. Islet purity is a parameter used during islet processing. At the culture stage, islets are cultured in defined purity ranges, which are high purity (> 70%), middle purity (40 to 69%) and low purity (30 to 39%). When the final islet product is transferred into the infusion bag for clinical transplantation, islet purity is one of the parameters used to determine the number of infusion bags needed. Islet purity is not calculated or measured, but only roughly estimated by technicians (8).
The above method used to assess IEQ has been widely accepted and applied in research, in clinical islet isolation, and in transplantation for the last two decades. However, this method has obvious shortcomings, including technical bias, limited time for verification, and infeasibility of long-term sample preservation. In 2010, we reported preliminary results of islet mass quantification using our DIA protocol (12). Next, we confirmed the advantages of our DIA protocol as a part of an Islet Cell Resource (ICR) study (5). Recently, Friberg et al (2) reported a different DIA system that allows for reduced variability of islet count compared to manual counting methods. Although, many other DIA systems have been tested previously, shortcomings affecting accuracy of the results existed in these studies (1, 2, 3, 4, 6, 7, 10, Hui Jian Zhang, University of Minnesota-personal communication,). Among the issues was that although islet samples stained with DTZ were manually counted immediately, the images might have been taken hours later or even on the following day. Some of the islets might have dissociated in the extended incubation in the DTZ solution. Another concern was that the picture taken was not complete and represented only part of the entire islet sample. A clinically applicable and reproducible DIA protocol has never been well described and validated in well-controlled human islet samples.
The aim of this study was to rigorously validate our DIA protocol in determination of IEQ and purity of human islet preparations. If successful, it would allow introduction of the DIA technique as a clinically applicable tool to supplement the current practice with a verifiable cGMP-compliant record.
MATERIALS AND METHODS
Materials
Software, Microscope and Camera
Image-pro plus version 7.0, is purchased from MediaCybernetics (Bethesda, MD). Zoom stereomicroscope, SMZ1000 and 1.5-x ED Plan WD 45 high magnification lens are made by Nikon (Japan). Microscope camera XC50 is ordered from Olympus America Inc, (Central Valley, PA). Additional lens (0.63 ×) connected to the top of the adaptor for the camera is made by Diagnostic Instruments (Sterling Heights, MI).
Reagents
Dithizone (DTZ), and Dimethyl sulfoxide (DMSO) are from Sigma (St. Louis, MO). Dulbecco’s phosphate buffered saline (DPBS) and CMRL1066, supplemented, are from Mediatech (Manassas, VA). Human albumin 25% USP is manufactured by CSL Behring (King of Prussia, PA). Heparin sodium injection USP is manufactured by Hospira (Lake Forest IL). Insulin-like growth factor-1 (IGF-1) is ordered from Cell Sciences (Canton MA).
Disposable Supplies
Most of disposable items are ordered from Fisher Scientific (Pittsburgh, PA). They are 250 mL conical tube; 50 mL conical tube; culture dish, 60 × 15 mm; large-orifice pipet tip; pipetter-specific tip; 10 mL syringe, syringe filter, 0.22 um; Kimax flask; one-hole rubber stoppers and glass Pasteur pipets. Kimax flask and rubber stopper are used for islet wash before taking picture. T-75 culture flask is ordered from Sarstedt (Nümbrecht, Germany).
Methods
DIA protocol
Our DIA protocol has been prepared to meet the quality control criteria for reproducible data acquisition and analysis. Therefore, any deviations of the protocol will affect accuracy of the data analysis. The methods below describe details of our DIA protocol including: islet sample collection; image capture of hemocytometer for image spatial calibration; image capture of islet sample; digital image analysis; application of HK template for the calculation of sample IEQ, purity and IPN; and manual – image analysis.
Islet sample collection
The islet isolation protocol, as part of the Clinical Pancreatic Islet Transplantation Study was approved by the Institutional Review Board of University of Chicago and the FDA. Human pancreatic islet isolation was performed in the clean room of a cGMP facility. Islet processing was designed based on the Clinical Islet Transplantation (CIT) protocol (http://www.isletstudy.org/). Islet samples were collected from 13 isolations. In one clinical transplant grade pancreas, immediately after purification islets were washed and suspended in CIT Culture Media (CMRL 1066 Supplemented, 0.5% Human Serum Albumin, 10iu/mL Heparin and 0.1 μg/mL IGF-1) and transferred to T-75 culture flasks. High and low purity islet cultures were processed separately. The total volume of islet suspension was 100 mL in the high purity and 75 mL in the low purity flasks. Twenty of 150 μL and twenty of 100 μL samples were collected from the high purity preparation and named group H150 and group H100, respectively. Another twenty samples of 100 μL were collected from the low purity preparation and named group L100. Under a biological safety cabinet, one technician gently mixed the islets with culture media then immediately opened the lid, the second technician inserted an autoclaved wide mouth pipet tip with extension (large-orifice pipet tip and pipetter-specific tip) to the middle level of the islet suspension and aspirated the pre-determined amount of islet sample to the center of a culture dish. Islet samples were stored in a culture incubator with 5% CO2 at 37 °C. Additional 12 pairs of islet high purity samples (100 μ L per sample, n=24) collected at post-purification stage from 12 cases of islet isolation were also analyzed to investigate the reproducibility of the methods in this study.
Image capture of hemacytometer
The grid in hemacytometer is considered as the gold standard for microscopic measurement. Serial pictures with different zoom magnifications must be pre-captured from individual microscope for calibration purpose. The hemacytometer pictures are saved with microscope ID and zoom magnifications.
Imaging of the islet sample
DTZ solution contained 50 mg DTZ, 10 mL DMSO and 20 mL DPBS in a 50 mL conical tube covered with aluminum foil to protect it from light. The DTZ solution was filtered before being used. Islet sample was stained with DTZ and photographed right after collection. For the single isolation with multiple samples, four culture dishes were removed from the CO2 incubator at one time for DTZ staining and image capturing. Islets per culture dish were stained with one to two drops of DTZ solution for at least 2 min before 3 to 5 mL culture media were added to the dish. After swirling islets to the center of dish, any air bubbles were carefully aspirated off the surface of the media by a Pasteur pipet connected to a vacuum flask. It was important for an ideal islet picture to include all islets per sample in one picture, avoid overlapping islets and show good resolution with a black background. In the ideal islet picture, islets are shown red and acinar white or slightly yellow (Figure 1). It was important that the islet pictures are saved with the sample ID, Microscope ID and zoom magnification.
Figure 1.
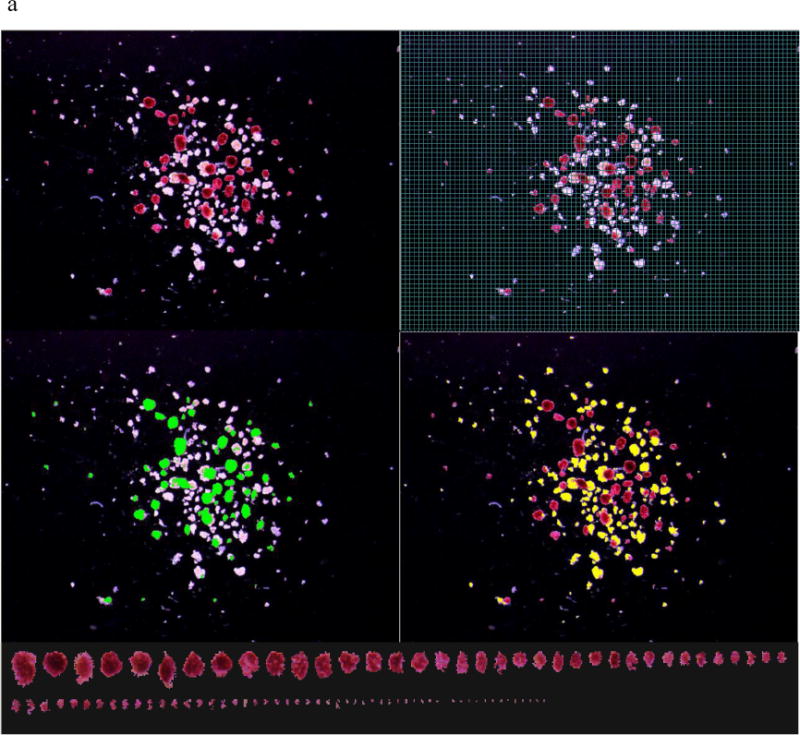
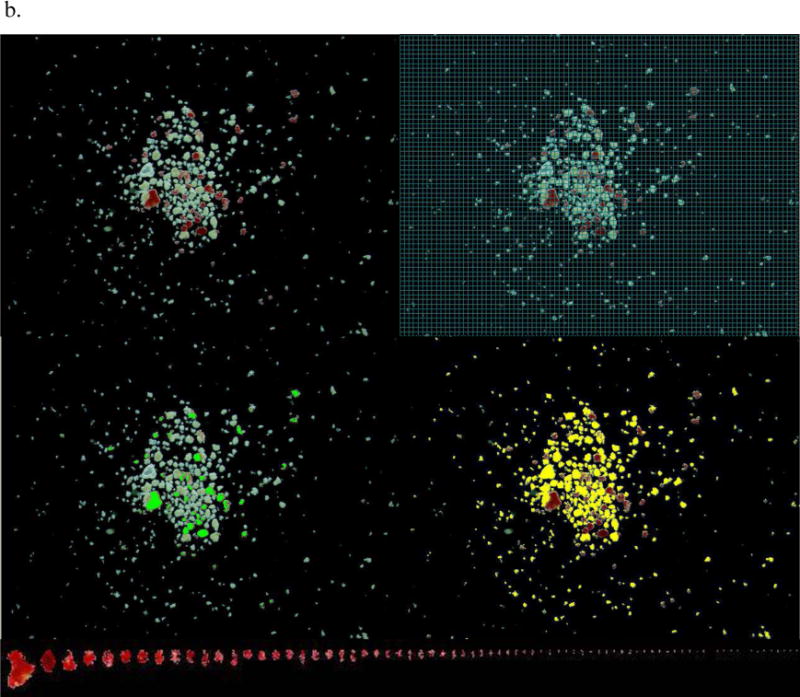
a. An islet sample with middle purity was captured in a calibrated image. The islet information in this sample includes: IEQ 78 by Manual I and 95 by DIA; Purity 54% by Manual I and 53% by DIA. b. An islet sample with low purity was captured in a calibrated image. The islet information in this sample includes: IEQ 24 by Manual I and 18 by DIA; Purity 20 % by Manual I and 12% by DIA.
Note: Each picture was taken with spacious background so that all islets in the sample could be included in one image. Top-left: Islets stained with DTZ (red) and acinar tissue (unstained). Top-right: a grid mask was placed on the image for Manual I counting (A 50 micron grid was too small to be seen in this printed image, a 100 micron grid is displayed instead, as an example). Mid-left: Islets outlined in green in DIA. The pixels in the green marked area were converted to μm2 and used for islet area and volume calculation. Mid-right: Acinar tissue outlined in yellow in DIA. The pixels in the yellow market area were converted to μm2 and used for total acinar area calculation. Bottom: All of the islets in the sample were lined up according to their sizes. Islet size smaller than 50 μm was not included in IEQ calculation according to the principle of IEQ calculation.
Digital image analysis
Software Image-pro Plus 7.0 was used for this study. To calibrate for digital image analysis, a hemacytometer grid was used to determine pixel per μm following manufacturer’s instructions. The software was set to calculate islet and acinar, measurements including area, radius (max), radius (min) and perimeter. The window showing sorted islets was used to identify the islets with improper boundary for correction (Figure 1).
HK template
HK islet template, a custom designed excel template (by Dr. Hermann J Kissler), has been routinely applied in islet processing cGMP setting since 2007. The template is an essential tool in our DIA protocol. It contains the following sections. Section I: general information includes isolation ID, donor ID, date, name of camera, microscope ID, zoom magnification, pixel/μm, calibration date, sample ID, islet isolation stages, purity level, total volume, sample volume and operator initials. Section II: analyzed data are copied and pasted from Image-pro plus. Section III reports: two islet information reports (DIA and DIA-E,) are produced in this section. DIA uses spherical volume formula to calculate the results. In DIA, the IEQ is calculated as the result of the volume of an islet in the image divided by the volume of a 150 μm spherical islet. DIA-E applies ellipsoid volume formula and the IEQ is calculated based on volume of an islet in the image divided by the volume of a 150 μm ellipsoidal islet. DIA reports contain islet size groups; sample IEQ; total IEQ; sample islet particle number (IPN); total IPN; IEQ/IPN ratio and purity.
Manual Image analysis
In the routine manual count method, a technician collects islet sample in a culture dish and counts islets under the microscope with a reticule. We called it Manual Dish method (Manual D), which was not used in this study. Instead islets were counted directly on the digital image with a calibrated grid. We called this method, Manual Image method (Manual I). For this we employed a calibrated 50 μm Grid Mask over the islet image for manual analysis (Figure 1). After counting, the islets are marked by using the annotation function. Thus the missed islets are easily found and the repeated counting is avoided. Islets in different size groups were counted and entered into the IEQ calculation table (8).
Operators
Five well-trained technicians participated in IEQ and Purity analysis using Manual I and DIA.
Statistical analysis
Linear regression was used in the comparison of IEQ. Paired t-test was used in the comparisons of IEQ, Purity, IPN and IEQ/IPN ratio. Statistical significance was determined by the p value < 0.05.
RESULTS
IEQ Results of sixty samples from single islet isolation
Analysis of IEQ
Five technicians using Manual I and DIA analyzed 20 islet samples from each of H150, H100 and L100 group separately. In other words, every technician analyzed a total of 60 samples by Manual I and 60 samples by DIA. Linear regression test showed statistically significant correlation in the IEQ results between DIA and Manual I (Table 1). Paired t-test reported that statistically significant difference in IEQ count was found between Manual I and DIA in H100 group (Table 2. p = 0.029) but not in H150 and L100 groups (Table 2). Significant differences were found when IEQ of DIA E was compared to those of Manual I and DIA (all p< 0.001) (Table 2) and linear regression was significantly correlated (Table 1) among comparisons. Comparative analyses in this study also include inter-sample variation (ISV), inter-technician variation (Inter-TV) and intra-technician variation (Intra-TV) (Table 2 and Figure 2).
Table 1.
Summary of linear regression reports on IEQ counted from islet sample groups
| Manual I vs. DIA | Manual I vs. DIA E | DIA vs. DIA E | |||||
|---|---|---|---|---|---|---|---|
| n | R | P | r | P | r | P | |
| H150 | 20 | 0.881 | <0.0001 | 0.872 | <0.0001 | 0.9892 | <0.0001 |
| H100 | 20 | 0.898 | <0.0001 | 0.837 | <0.0001 | 0.9801 | <0.0001 |
| L100 | 20 | 0.819 | <0.0001 | 0.7496 | <0.0001 | 0.9429 | <0.0001 |
| 12 Cases | 24 | 0.985 | <0.0001 | 0.9862 | <0.0001 | 0.9983 | <0.0001 |
Table 2.
Comparison of IEQ between manual count and digital image analysis.
| Inter-Sample Variation (ISV) | Inter-Technician Variation (Inter-TV) | |||||
|---|---|---|---|---|---|---|
| Manual I | DIA | DIA-E | Manual I | DIA | DIA-E | |
| High 150 | ||||||
| Mean ±SD | 242 ± 65 | 242 ± 62 | 185 ± 47 | 242 ± 22 | 242 ± 20 | 185 ± 16 |
| CV% ± SD | 24 ± 4 | 26 ± 1 | 26 ± 1 | 9 ± 3 | 8 ± 3 | 8 ± 3 |
|
| ||||||
| p value | ||||||
| Manual I vs. | 0.86 | <0.0001 | 0.86 | <0.0001 | ||
| DIA vs. | <0.0001 | <0.0001 | ||||
|
| ||||||
| High 100 | ||||||
| Mean ±SD | 168 ± 36 | 161 ± 42 | 124 ± 33 | 168 ± 16 | 161 ± 13 | 124 ± 10 |
| CV% ± SD | 21 ± 1 | 26 ± 2 | 26 ± 2 | 9 ± 3 | 8 ± 3 | 9 ± 3 |
|
| ||||||
| p value | ||||||
| Manual I vs. | 0.029 | <0.0001 | 0.029 | <0.0001 | ||
| DIA vs. | <0.0001 | <0.0001 | ||||
|
| ||||||
| Low 100 | ||||||
| Mean ±SD | 83 ± 23 | 80 ± 20 | 62 ± 16 | 83 ± 7.2 | 80 ± 7.7 | 62 ± 6.3 |
| CV% ± SD | 28 ± 3 | 25 ± 1 | 26 ± 2 | 9 ± 4 | 10 ± 4 | 10 ± 2 |
|
| ||||||
| p value | ||||||
| Manual I vs. | 0.182 | <0.0001 | 0.182 | <0.0001 | ||
| DIA vs. | <0.0001 | <0.0001 | ||||
Note: Every islet sample group (H150, H100 and L100) contains 20 islet image samples respectively. Five technicians participated IEQ counting. Results came from 100 counted data per islet sample group. ISV and Inter-TV were not significantly different among Manual I, DIA and DIA-E. All p values were determined by paired t-test.
Figure 2.
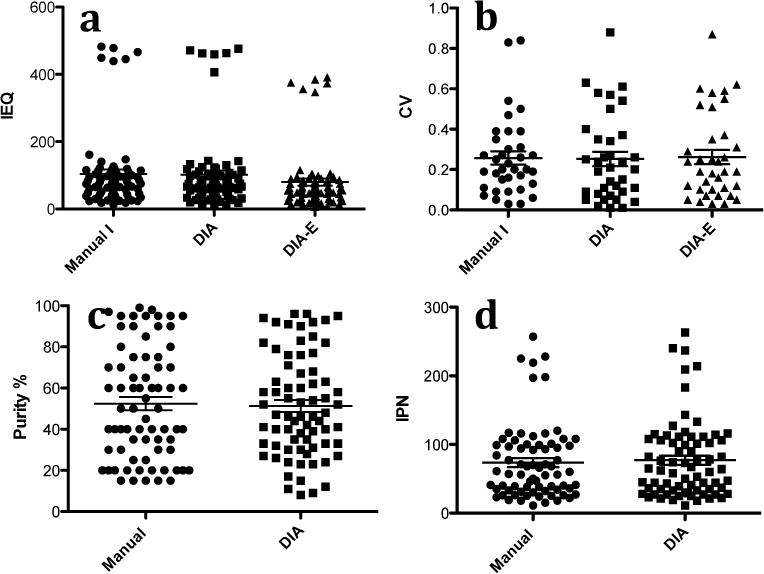
24 islet samples collected from 12 isolations were analyzed by Manual I and DIA. a: IEQ was obtained from 72 individual counts (3 technicians counted 24 sample each, p = 0.0516). b: CV was calculated from paired islet sample per case (n=36, 3 technicians counted 12 pairs each, p = 0.82). c: Purity was obtained from 72 individual counts (p =0.47). d: IPN was obtained from 72 individual counts (p=0.07).
ISV is the variation in IEQ of samples 1 to 20 from the same group (group of H150, or H100 or L100) assessed by one technician. ISV values in Table 2 are means from the individual mean, SD and CV calculated from the results of five technicians by Manual I or DIA.
Inter-TV refers to the variation of IEQ count in a single sample by 5 technicians. The values of Inter-TV in Table 2 show the means of the individual mean, SD and CV calculated from the results the single sample counted by five technicians in the same sample group (group H150, or H100, or L100).
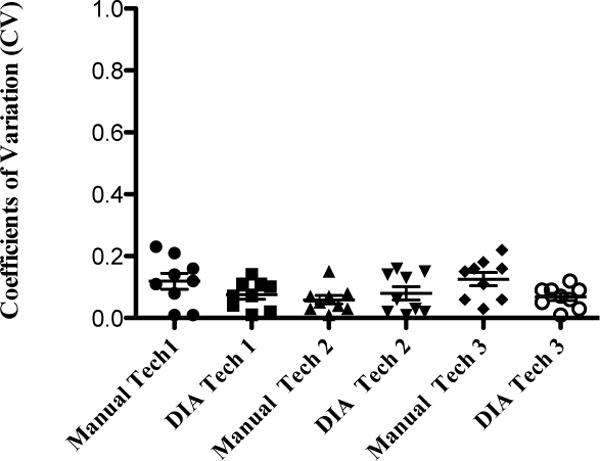
Intra-TV was assessed on the same islet image three times by the same technician at least three days apart by Manual I and DIA. Three technicians participated in this work. Three islet images from each sample group (group H150, or H100, or L100) were used separately for Intra-TV analysis. IEQ counts were consistent for each technician using Manual I and DIA with the mean coefficient variations, (CVs) < 10% (range 1 to 23%, Figure 2).
IEQ Results of twelve pairs of samples from twelve islet isolations
Three technicians using Manual I and DIA analyzed 12 pairs of islet samples. Statistically significant difference was not found in the IEQ between Manual I and DIA (p = 0.0516, Figure. 3a). The wide range of CVs reflected the mean CV per pair of sample (Figure 3b). Linear regression showed the results analyzed by Manual I, DIA and DIA E were statistically significant correlated (Table 1). Statistically significant differences were found in the IEQ between Manual I and DIA E (p < 0.0001), and DIA and DIA E (p < 0.0001).
Figure 3.
Intra-TV was assessed by re-analysis of IEQ on the same islet image three times by the same technician at least three days apart. Individual CV for each sample IEQ count is presented for each technician. The mean CVs for Manual I and DIA was < 10%.
In summary, IEQ results determined by Manual I and DIA were statistically significantly correlated. Statistically significant difference in IEQ quantification by Manual I and DIA was found in H100 group only. Based on different calculation formula, the IEQ counted by DIA-E differed significantly from Manual I and DIA in all sample groups.
Analysis of purity measurement
Technicians assessed islet purity in Manual I based on their experience. On the other hand, the purity calculated by DIA is the result of the measured total islet areas divided by the measured total areas of acinar tissue and islets. In the purity comparison of Manual I to DIA (sixty samples of the single islet isolation, Table 3), statistically significant differences were found in the group H100 (p<0.005) and the group L100 (p<0.001) but not in the group H150. Figure 3c showed the purity results of 12 pairs of samples of 12 islet isolations analyzed by three technicians. Statistically significant difference was not found in the 12 pairs of samples.
Table 3.
Comparison of Purity, IPN and Ratio of IEQ/IPN between Manual I and DIA
| Manual I | DIA | Manual I | DIA | Manual I | DIA | |
|---|---|---|---|---|---|---|
|
|
||||||
| High 150 μL (n=20) | High 100 μL (n=20) | Low 100 μL (n=20) | ||||
| Purity | ||||||
| Mean ±SD | 76 ± 6 | 75 ± 6 | 78 ± 6 | 76 ± 5 | 31 ± 5 | 37 ± 7 |
| CV% | 7 | 5 | 6 | 4 | 5 | 5 |
|
| ||||||
| p | 0.08 | 0.005 | 0.001 | |||
|
| ||||||
| IPN | ||||||
| Mean ±SD | 128 ± 21 | 127 ± 24 | 95 ± 19 | 91 ± 19 | 67 ± 14 | 67 ± 18 |
| CV% | 12 | 16 | 6 | 8 | 10 | 10 |
|
| ||||||
| p | 0.99 | 0.6 | 0.99 | |||
|
| ||||||
| IEQ/IPN Ratio | ||||||
| Mean ±SD | 1.9 ± 0.2 | 2.0 ± 0.2 | 1.8 ± 0.2 | 1.8 ± 0.2 | 1.4 ± 0.3 | 1.3 ± 0.2 |
| CV% | 11 | 11 | 12 | 9 | 20 | 12 |
|
| ||||||
| p | 0.65 | 0.75 | 0.13 | |||
Note: Every islet sample group (H150, H100 and L100) contains 20 islet image samples respectively. Five technicians participated IEQ counting. Results came from 100 counted date per islet sample group. Manual I Purity represents the results of the subjective purity estimation by the technicians based on the image. There were statistically significant differences in the purity determination between Manual I and DIA in the group H100 and the group L100 but not in the group H150. All comparisons in IPN and IQE/IPN ratio between Manual I and DIA were not statistically significant.
Analysis of islet particle number (IPN)
We found no statistically significant difference in IPN between Manual I and DIA in this study (Table 3 and Figure 3d). Islet size distribution showed similar patterns in both Manual I and DIA (Figure 4). Additionally, IPN assessed by DIA-E showed that mean ± SD and CV were 121 ± 22 and 18% in the group High 150; 86 ± 17 and 20% in the group High 100, and 60 ± 15 and 25% in the group L100, respectively. Comparing IPN by DIA-E to IPN by Manual I and DIA in the samples of the single islet isolation, statistically significant differences between Manual I and DIA-E were only found in the group High 100 (p=0.005).
Figure 4.
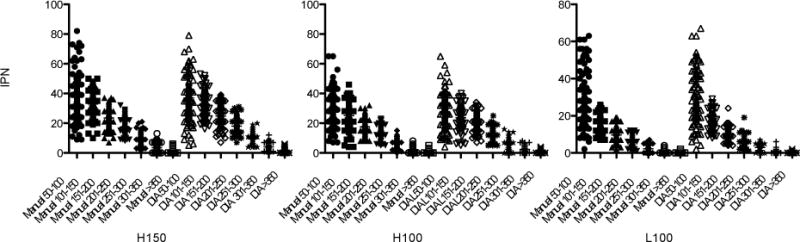
Islet distribution in size group was shown by Manual I and DIA. There was no statistically significant difference found in IPN between Manuals I and DIA.
Analysis IEQ/IPN ratio
We found no statistically significant difference of the IEQ/IPN ratio between Manual I and DIA in the same groups (Table 3). However, statistically significant differences in IEQ/IPN ratio were found between high and low purity samples (group H150 vs group L100 and group H100 vs group L100, all p< 0.001) regardless, if Manual I or DIA was used (Figure 5).
Figure 5.
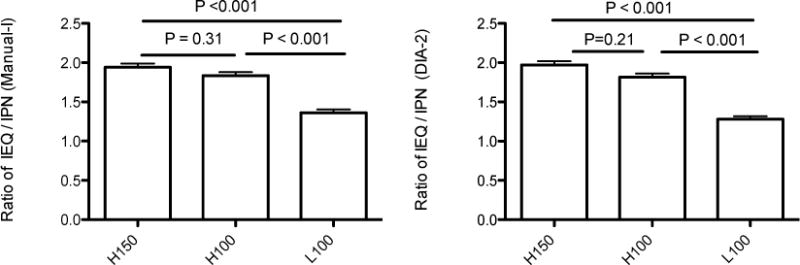
Left: IEQ/IPN ratio assessed by Manual I was statistically higher in high purity samples than in Low purity samples. Right. IEQ/IPN ratio assessed by DIA was statistically higher in high purity samples than in Low purity samples.
DISCUSSION
IEQ, representing pancreatic islet mass, is the most important of all parameters involved in decision making for clinical islet isolation and transplantation. However, the method to determine IEQ has remained the same for the last 20 years. The drawback is that the small amount of islet sample (around 100 to 200 μL collected from 100 to 200 mL of islet product) is counted in a culture dish and discarded thereafter. Later on, it is diffcult to verify the results of manual islet counting mainly due to the difficulty of sample storage after DTZ staining. Moreover, high IEQ counting variations have been noticed by many islet processing laboratories (5). Therefore a better technique is needed to overcome the drawbacks of the IEQ manual counting method and to better comply with the cGMP standards in clinical islet transplantation.
In the study, we used manual islet assessment based on islet image (Manual I) instead of based on islet placed in a dish (Manual D). Applying Manual D would require 5 technicians to analyze 60 islet samples at the same time, which is impractical. Islet stained with DTZ may disassociate after staying in a suspension in a culture dish for a prolonged time. In contrast, Manual I islets are fixed as digital image therefore extensive analysis becomes possible. The Manual I method has more advantages than the Manual D method (Table 4). In Manual D, islets may be easily missed or double-counted due to dish handling errors, leading to the relocation of the islets or due to technician distraction during the counting. The Manual I overcomes this problem. However, both methods follow the same original instruction to group islets by measuring islet size using a 50 μm grid and the same calculation formula for IEQ count.
Table 4.
Comparisons of Manual D and Manual I
| Manual D | Manual I | |
|---|---|---|
| Islets | In a culture dish | In a calibrated image |
| Grid with 50 μm | Located in one ocular | Covering the image |
| Islet position | Movable | Fixed |
| Counted islets | Can NOT be marked | CAN be marked |
| Sample storage | Not available | Permanently stored |
| Verification | Could be done only immediately | Can be done, whenever it is necessary |
DIA and DIA E use calibrated pixel/μm values to measure the islet area. Then, the spherical volume formula was applied in DIA method to calculate IEQ, whereas an ellipsoid volume formula was used in DIA-E. In comparison of Manual I and DIA, the DIA technique (sphere) has an equivalent ability to obtain results close to Manual I IEQ results. In DIA, the islet numbers per size group was not used to calculate IEQ. Instead, the sample IEQ was calculated based on the sum of individual IEQ per sample. The individual IEQ was obtained from an islet volume divided by the volume of a 150 μm size islet, where the islet volume was obtained from measured islet area using the HK template. The standard IEQ formula used in Manual–I is based on defined standardized islet volume for each islet size group, whereas in DIA it is based on actual volume of each islet in the sample, therefore technically IEQ count should be more accurate in DIA than in Manual I. However, the difference in most of IEQ results between Manual I and DIA were not statistically significant in our study.
The significantly lower IEQ results obtained by DIA-E compared to the results of Manual I and DIA was due to the use of different calculation formula based on ellipsoid shape rather than due to measurement error. Although the islet shape on the image resembles an ellipsoid more than a sphere (see random pick-up samples in Figure 1), there is no strong evidence of superiority of either the formula based on ellipsoid or spherical shape in predication of the islet function after the transplant. Historically, we have correlated islet function with IEQ based on spherical shape in manual count so there is no need to change it. Therefore, we propose digital image analysis purely as a quality control tool to supplement rather than replace the established standard manual IEQ count method. The algorithm for calculating the IEQ needs to be the same to guarantee comparability of the results. Of note, the 50 μm grid mask has been used historically as a standard size grid in manual IEQ counting. When we tested 100 μm grid masks, IEQ results were significantly higher than the results using 50 μm grid masks and DIA (data not shown). So using larger grid masks may cause overestimation of the islet count.
IEQ measurement assessed in the same islet sample by five technicians allowed us to calculate Inter-TV that represents the measurement variations. The Inter-TV stayed below 11% for Manual I as well DIA in our study (Table 2). Friberg et al 2011 reported the CVs in manual and DIA were 31% vs 17%, respectively (2). It is very common to see CVs of Inter-TV around 30% or even higher by the Manual D method in most of islet processing centers using Manual D (personal communications). In a multicenter study in which we also participated, the average Inter-TV using Manual I method ranged from 8.4 to 29.3 (5). In our current study, we obtained lower Inter-TV, which is related to the fact that technicians were trained in the same center, following the same rules to perform the manual count as well as DIA. Additionally, all islets in the sample were preserved as a single digital image with a calibrated grid set on the image. By using software annotation function every islet was marked while being counted to prevent being repeated or missed during counting as is possible in the Manual D method.
IEQ count of 20 samples from the same flask performed by 5 technicians allowed for the calculation of the CVs of IEQ as ISV (Inter-Sample Variation). ISV of IEQ in Table 2 had means ranging from 21 to 28%. Those twenty samples were collected from the same purity level of the islet preparation. The mean CV (equal to ISV) obtained from IEQ results of 12 sample pairs of 12 islet preparations was 32% in Manual I and 29% in DIA. Factors contributing to ISV include: biological variation and measurement variation. Biological variation is mainly related to islet size. Islet size always varied from 50 to 400 μm and it is an intrinsically variable factor for any islet preparation. Measurement variation could result from sampling and analysis errors. Sampling method has been introduced in the method section. In this study, sampling error is minimized since two experienced technicians carefully sampled in a consistent manner. Analysis error was around 10 % as indicated from the CVs of IEQ in Inter-TV. Therefore, islet size must be a major factor contributing to the higher CVs in ISV. This biological variation can be minimized by consistent sampling technique but cannot be avoided.
In this study, three technicians investigated intra-technician variation. Three islet samples from each group were analyzed three times per each technician on different days, at least three days apart. Both Manual I and DIA had mean CV of less than 10% without significant difference. In the previous multicenter study, the average of the intra-TV was 7.9 and 9.9 by Manual I method (5). Intra-TV reflects the technical proficiency of individual technician. Achieving mean Intra-TV less than 15% is feasible in Manual I and DIA and should be set as a goal in technician’s training.
Islet purity is a parameter used during islet processing. According to the purity level, islets are grouped in high, middle or low purities during culture. Islet purity is one of the parameters to determine the islet distribution in the infusion bag for transplantation. Rough estimation of islet purity has been a standard way and acceptable in all islet processing facilities (8). In this study, we compared purity estimation by the technicians based on the islet image to the DIA islet purity measurement. Their estimation of the high purity islet samples (group H150) in our study did not differ significantly from the measured purity by DIA. It may be related to the fact that technicians had been well trained in the DIA method prior to the study and they learned to correlate the purity based on DIA measurements with the islet sample image. Despite that, statistically significant differences of purity determination were found between estimation in Manual I and DIA when the other samples where assessed (group H100 and L100) (Table 3). Since purity in DIA is based on precise measurements instead of a rough estimate in manual method, it is obvious that DIA results are more accurate. It is objective, and also easy to be verified afterwards. Therefore, purity measured by DIA certainly has advantage over the traditional estimation method.
In this study DIA and manual method identified similar number of IPN- islet particle number count, there were no statistical difference between Manual I and DIA. Islet distribution pattern per size group was similar as shown in Figure 4.
The IEQ/IPN ratio allows estimate the shift in islet size distribution in the sample. Since one IEQ has a diameter of 150 μm, if IEQ/IPN ratio is 1, it implies the average size of islets in the flask is 150 μm. If the ratio is below 1, it means that islet size distribution is shifted to the lower islet size – relatively more islets are smaller than 150 μm and, if ratio is >1, there is a shift in islet distribution to the large islet size. There are relatively more large islets in the sample. In our experience, IEQ/IPN ratio may be related to pancreas quality: there is a different islet size distribution after isolation from healthy pancreas or pancreas exposed to disease or stress, from an organ well preserved or inappropriately procured. Isolation technique may also influence the IEQ/IPN ratio. Our results also indicate that the IEQ/IPN ratio varies in different purity preparations from a single pancreas processing. In our study, although each of the sample tested came from the same organ and islet isolation, we found significant differences in IEQ/IPN ratio between high and low purity samples regardless of counting methods – Manual or DIA (Fig 5). The IEQ/IPN ratio was found higher in the high purity samples than in low purity ones. This confirms that the IEQ/IPN ratio is not a constant number in the islet preparation from a single donor but it varies depending on the purity, which is resulted from the gradient densities during islet purification.
In this study, we validated our DIA protocol for measurements of islet mass and purity in comparison to the standard manual islet count and purity estimate. We concluded that DIA is a reliable technique. The advantage of DIA is that both islet sample and result produced by DIA can be permanently stored as electronic files and can be easily verified at any time. Retrospective review of islet quality and quantity during different processing stages is available by using DIA, which provides a solid platform for laboratory professional training and promotes exchange of information between islet centers and progress in the field. All together, DIA complies better with cGMP requirements than the manual counting method. It is feasible to be applied routinely in the settings for clinical islet processing and transplantation as a supplemental tool for current manual islet count.
Acknowledgments
The authors thank the cGMP facility staff, Diane Ostrega, Halima Ibrahim and Susan Wilson, for their consistent support to provide high quality work environment for human islet processing. We would like also to acknowledge the generosity and support of Dr. Martin Jendrisak and the entire team of the Gift of Hope Organ & Tissue Donor Network in Chicago for providing the human pancreas tissues used in the this study. The authors also thank Dr. Ted Karrison and Mr. Rangesh Kunnavakkam, Department of Health Sciences, University of Chicago, for their thorough work on statistical analysis. This study was supported by the Illinois Department of Public Health Grant (Pancreatic Islet Transplantation) as well as University of Chicago DRTC Grant # P30 DK020595. Dr. Xiaojun Wang is recipient of a Post-graduate Research Grant provided by China Scholarship Council, China.
Abbreviation List
- cGMP
current good manufacturing practice
- CIT
consortium of islet transplantation
- CV
coefficient of variation
- DIA
digital image analysis
- DMSO
dimethyl sulfoxyde
- DPBS
Dulbecco’s phosphate buffered saline
- DTZ
dithizone
- FDA
food and drug administration
- ICR
islet cell resource
- IEQ
islet equivalent
- IGF-1
insulin-like growth factor-1
- Inter-TV
inter-technician variation
- Intra-TV
intra-technician variation
- IPN
islet particle number
- IRB
institutional review board
- ISV
inter-sample variation
- Manual D
manual dish
- Manual I
manual image
- SD
standard deviation
- USP
United States Pharmacopeia
- OPO
organ procurement organization
- UNOS
United Network for Organ Sharing
Footnotes
The authors of this manuscript have not conflicts of interest to disclose as described by Cell Transplantation.
References
- 1.Buchwald P, Wang X, Khan A, Bernal A, Fraker C, Inverardi L, Ricordi C. Quantitative assessment of islet cell products: estimating the accuracy of the exiting protocol and accounting for islet size distribution. Cell Transplantat. 2009;18(10):1223–35. doi: 10.3727/096368909X476968. [DOI] [PubMed] [Google Scholar]
- 2.Friberg AS, Brandhorst H, Buchwald P, Goto M, Ricordi C, Brandhorst D, Korsgren O. Quantification of the islet product: presentation of a standardized current good manufacturing practices compliant system with minimal variability. Transplantation. 2011;91(6):677–683. doi: 10.1097/TP.0b013e31820ae48e. [DOI] [PubMed] [Google Scholar]
- 3.Girman P, Kriz J, Friedmansky J, Saudek F. Digital imaging as a possible approach in evaluation of islet yield. Cell Transplant. 2003;12(2):129–133. doi: 10.3727/000000003108746713. [DOI] [PubMed] [Google Scholar]
- 4.Gmyr V, Bonner C, Lukowiak B, Pawlowski V, Dellaleau N, Belaich S, Aluka I, Moermann E, Thevenet J, Ezzouaoui R, Queniat G, Pattou F, Kerr-Conte J. Automated digital image analysis of islet cell mass using Nikon’s inverted eclipse Ti microscope and software to improve engraftment may help to advance the therapeutic efficacy and accessibility of islet transplantation across centers. Cell Transplant. 2013 doi: 10.3727/096368913X667493. [DOI] [PubMed] [Google Scholar]
- 5.Kissler HJ, Niland JC, Olack B, Ricordi C, Hering BJ, Naji A, Kandeel F, Oberholzer J, Fernandez L, Contreras J, Stiller T, Sowinski J, Kaufman DB. Validation of methodologies for quantifying isolated human islets: an islet cell resources study. Clin Transplant. 2010;24(2):236–242. doi: 10.1111/j.1399-0012.2009.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niclauss N, Sqroi A, Morel P, Baertschiger R, Armanet M, Wojtusciszyn A, Parnaud G, Muller Y, Berney T, Bosco D. Computer-assisted digital image analysis to quantify the mass and purity of isolated human islets before transplantation. Transplantation. 2008;86(11):1603–9. doi: 10.1097/TP.0b013e31818f671a. [DOI] [PubMed] [Google Scholar]
- 7.Lembert N, Wesche J, Petersen P, Doser M, Becker HD, Ammon HP. Areal density measurement is a convenient method for the determination of porcine islet equivalents without counting and sizing individual islets. Cell Transplant. 2003;12(1):33–41. doi: 10.3727/000000003783985214. [DOI] [PubMed] [Google Scholar]
- 8.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, Lake SP, London NJM, Socci C, Alejandro R, Zeng Y, Scharp DW, Viviani G, Falqui L, Tzakis A, Bretzel RG, Federlin K, Pozza G, James RFL, Rajotte RV, Carlo VD, Morris PJ, Sutherland DER, Starzl TE, Mintz DH, Lacy PE. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27(3):185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Sacchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems J-A, Bretzel RG, Bertuzzi F, Froud T, Kandaswarmy R, Sutherland DER, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JRT. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 10.Stegemann JP, O’Neil JJ, Nicholson DT, Mullon CJ. Improved assessment of isolated islet tissue volume using digital image analysis. Cell Transplant. 1998;7(5):469–478. doi: 10.1177/096368979800700506. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland DE, Gruessner AC, Carlson AM, Blondet JJ, Balamurugan AN, Reigstad KF, Beilman GJ, Bellin MD, Hering BJ. Islet autotransplant outcomes after total pancreatomy: a contrast to islet allograft outcomes. Transplantation. 2008;86(12):1799–1802. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LJ, Kissler HJ, Battle J, Hecyk A, Stuart E, Zhang X, Chen X, Kaufman DB. Digital image analysis to quantify isolated islet mass in human transplant. Am J Transplant. 2010;10(Suppl 4):500. [Google Scholar]


