Abstract
For nearly two centuries, the ophthalmoscope has permitted examination of the retina and optic nerve—the only axons directly visualized by the physician. The retinal ganglion cells project their axons, which travel along the innermost retina to form the optic nerve, marking the beginning of the anterior visual pathway. Both the structure and function of the visual pathway are essential components of the neurologic examination as it can be involved in numerous acquired, congenital and genetic central nervous system conditions. The development of optical coherence tomography now permits the pediatric neuroscientist to visualize and quantify the optic nerve and retinal layers with unprecedented resolution. As optical coherence tomography becomes more accessible and integrated into research and clinical care, the pediatric neuroscientist may have the opportunity to utilize and/or interpret results from this device. This review describes the basic technical features of optical coherence tomography and highlights its potential clinical and research applications in pediatric clinical neuroscience including optic nerve swelling, optic neuritis, tumors of the visual pathway, vigabatrin toxicity, nystagmus, and neurodegenerative conditions.
Keywords: optical coherence tomography, optic neuritis, papilledema, optic pathway gliomas
Optical Coherence Tomography
Background
Optical coherence tomography (OCT) uses a near-infrared laser to provide high resolution, cross-sectional images of retinal structures, and the optic nerve.1 These images can distinguish between multiple retinal layers around the optic nerve head and macula, permitting axial thickness and total volume measures at specific anatomic locations. The OCT acquisitions are safe and the device does not touch the patient’s eye. OCT is commonly compared with ultrasound as both provide cross-sectional views of the eye. The primary difference is that OCT uses reflected light waves ranging from 820 to 870 nm whereas ultrasound uses sound waves. Similarly, both systems are technically comprised of A-scans and B-scans. An A-scan is an axial scan at a single point along the retina and a B-scan is a collection of axial scans to create cross-sectional “slices” on a transverse plane (Fig. 1). Scan resolution can be improved by increasing the number of A-scans per B-scan and the total number of B-scans, as well as decreasing the distance between B-scans. Newer OCT systems can produce volume scans by averaging numerous B-scans at different planes along the retina.
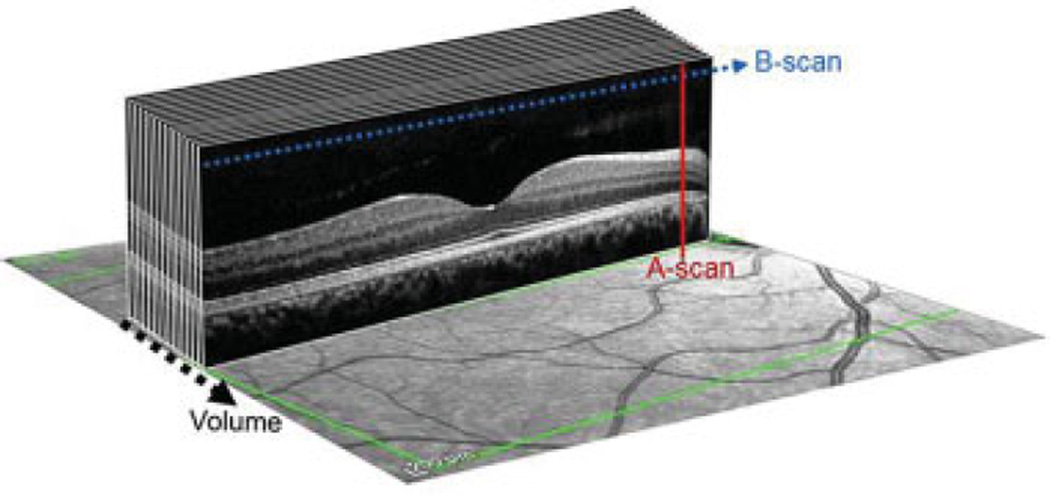
Fig. 1.
Composition of a spectral domain optical coherence tomographic volume scan. Red line demonstrates the axial scan (A-scan). Blue dotted line represents the compilation of A-scans to create a B-scan and black dotted line is the collection of B-scans to create a volume.
OCT technology has continued to evolve since its inception almost 30 years ago. The first commercially available systems were based on time-domain optical coherence tomography (TD–OCT) and were quickly adopted by the retina and glaucoma specialists because it was one of the first instruments to provide an in vivo view of retinal tissue.2 The biggest limitation of TD–OCTwas its slow acquisition speed of 400 A-scans per second, making it susceptible to eye motion artifacts. Currently, systems are based on spectral domain (or Fourier domain, referred to as SD–OCT throughout) technology which relies on real-time measurements of reflected light at different wavelengths. This results in a higher acquisition speed, better accuracy, and resolution at a near histological level (i.e., 3–5 µm). Most SD–OCT systems are capable of acquiring up to 40,000 A-scans per second for greater precision and reduced motion artifact.
Optic Nerve
Ganglion cell axons travel along the innermost layer of the retina, commonly referred to as the retinal nerve fiber layer (RNFL), and combine to form the optic nerve (Fig. 2A). The RNFL thickness is greatest in the superior and inferior quadrants because of the size of the axons, compared with the temporal and nasal aspects of the optic nerve head (Fig. 2B). To measure specific anatomic regions, OCT devices measure the circumpapillary RNFL (cpRNFL) thickness by placing a 3.45-mm circle centered over the optic nerve (Fig. 2C). cpRNFL measures are made around the entire circle and thickness is reported in clock hours, quadrants, subquadrants, or an average of the entire circle (Fig. 2D). Thickness measures are compared with the manufacturer’s reference database and values falling below the lower 5th and 1st percentile are labeled as abnormal. Reduced cpRNFL thickness in specific locations around this circle correspond to focal deficits in the visual field.3 cpRNFL measures in healthy children have been reported to be thicker than in adults.4 Unfortunately, the reference values used in the manufacturer’s software are based on adults 18 years and older, and thus cannot be strictly applied to children.4,5 However, some investigators have published reference values for children that may be helpful in interpreting pediatric results.4,5
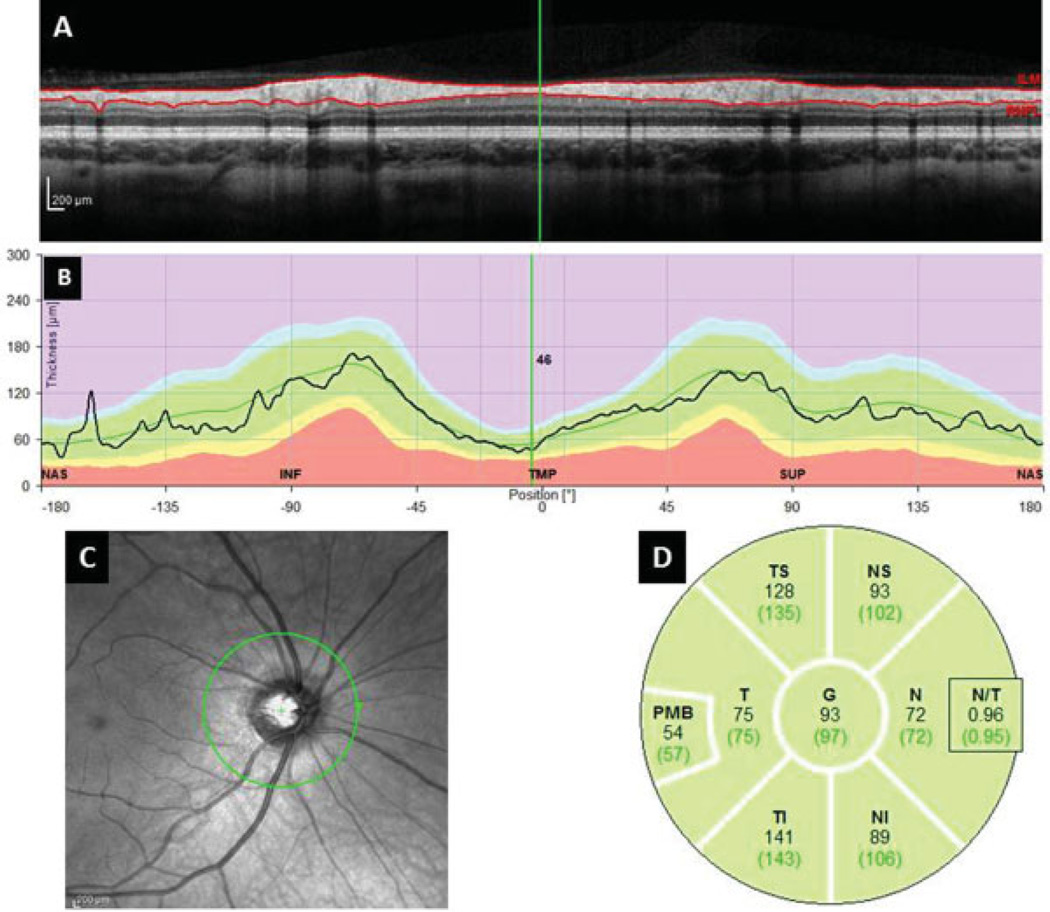
Fig. 2.
SD–OCT image of the Circumpapillary Retinal Nerve Fiber Layer Thickness Measures. (A) Single B-scan of the circumpapillary SD–OCT acquisition. Solid red lines demonstrate automated segmentation of the RNFL. (B) RNFL thickness measures of the OCT image at anatomic locations. (C) Near-infrared image demonstrating en face view of the 3.45 mm circle centered over the optic nerve. (D) Quadrant, subquadrant, and global average RNFL thickness measures. N, nasal; NI, nasal inferior; NS, nasal-superior; PMB, papillomacular bundle; SD–OCT, spectral domain optical coherence tomography; T, temporal; TS, temporal-superior; TI, temporal inferior.
Despite numerous publications demonstrating excellent reproducibility of thickness measures,6–11 clinicians should always visually inspect the automated segmentation for artifacts. Blood vessels within the RNFL, optic nerve swelling, large refractive error, poor image focus, off-center circumpapillary circle and the position of the superior and inferior vascular arcades may falsely elevate or reduce the thickness measures. Despite our ability to map visual field regions to cpRNFL locations,3 it is important to remember that these values represent the accumulation of axons from across those visual field sectors.
Macula
OCT imaging of the macula permits visualization of multiple retinal layers (Fig. 3A) by acquiring a large rectangular volume scan centered over the fovea (Fig. 3B). The macula is densely populated with photoreceptors that produce high-resolution visual acuity via the parvocellular pathway. Most neuroscientists interested in visual acuity and visual deficits have focused on measuring the combined ganglion cell layer-inner plexiform layer (GCL–IPL) using OCT.12–16 There are advantages in measuring the macular GCL–IPL instead of the cpRNFL as this region is not affected by optic nerve swelling, has few blood vessels to create artifact, and the measurement of GCL–IPL is region specific and is not cumulative across the macula. Both the total volume and average thickness can be measured and highlighted using a color map (Fig. 3C,D). The importance of other retinal layers in the diagnosis of neurologic conditions has recently proved to be beneficial.17
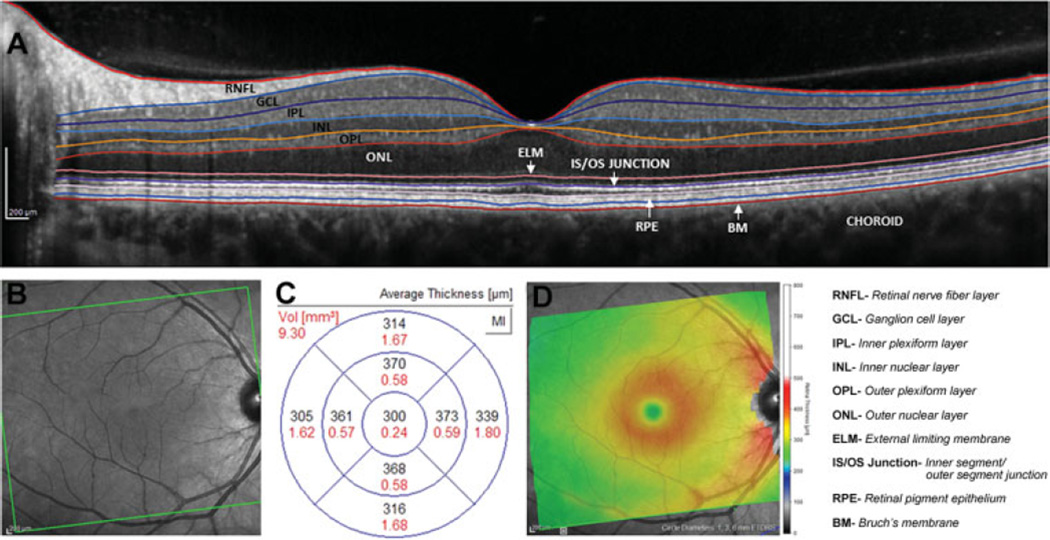
Fig. 3.
(A) Spectral domain optical coherence tomography image of a single B-scan centered on the macula. Solid colored lines delineate border between individual retinal layers. (B) Infrared OCT image of the entire acquisition area. (C) Thickness (black numbers) and volume (red numbers) measures across macula sectors. (D) Heat map of macula thickness measures with red/yellow indicating greater thickness and green/blue representing thinner regions.
Technologic Improvements Benefiting Children
As with many medical devices, OCT was originally designed for adults and common ophthalmologic conditions such as glaucoma.2 Applications of OCT to pediatric conditions were limited by the child’s ability to cooperate with OCT testing. To improve image quality and reproducibility in adults and children, devices such as the Spectralis (Heidelberg Engineering GmbH, Heidelberg, Germany) incorporate eye tracking.4,9–11,18 This tracking technology utilizes two light beams—one to “track” the fundus (once it is in a locked position), the other to align the OCT scan to this tracked position. This is an essential tool when imaging children, patients with nystagmus, and patients with cognitive impairment because the system will pause scanning once it detects misalignment.
For young children who cannot cooperate with traditional table-mounted OCT, a handheld OCT (HH–OCT) can acquire high-resolution images in awake neonates or young children who are sedated.6,7,19–29 The flexibility of imaging protocols provided by the HH–OCT has also been useful in imaging awake children with nystagmus.17,30
Clinical and Research Applications
Optic Nerve Swelling
Determining if optic nerve head elevation is secondary to increased intracranial pressure (i.e., papilledema) or due other congenital/structural anomalies (i.e., pseudopapilledema) remains a clinical challenge for many Neurologists and Ophthalmologists. As mild papilledema and pseudopapilledema may have a similar appearance, it can be difficult to differentiate between them, especially when examining young and uncooperative children. Numerous investigators have used OCT to measure the cpRNFL, total retinal volume, and displacement of the outer retinal layers as a surrogate marker to distinguish between pseudopapilledema and papilledema.31–36 cpRNFL thickness measures have not been found to be useful when trying to distinguish between mild papilledema and optic nerve head drusen.36,37 Outer peripapillary total retinal volume has been found to be the greatest in those with papilledema, although there was some overlap with control and pseudopapilledema patients.31 Kupersmith et al have noted an upward angulation of the retinal pigmented epithelium/basement membrane layer in 67% of patient eyes with papilledema.35 While this angle decreased following treatment for papilledema,38 other investigators have not demonstrated similar findings.36
To better visualize the deep structures of the optic nerve, enhanced depth imaging with OCT has been reported to provide better confirmation of optic nerve head drusen compared with other conventional methods of diagnosis.39 However, this method has not been directly compared between subjects with papilledema and pseudopapilledema.39 Using the infrared image acquired with some OCT devices, Moss et al have found that retinal veins demonstrate a significantly greater diameter in those with papilledema compared with normal subjects and those with pseudopapilledema.40
Until OCT can demonstrate adequate sensitivity and specificity in differentiating between papilledema and pseudopapilledema, its clinical utility remains tenuous. However, OCT might be a useful clinical research tool to provide an objective quantitative rather than subjective qualitative measure of optic nerve swelling in patients being treated for papilledema. The Idiopathic Intracranial Hypertension Treatment Trial has included OCT measures of optic swelling and ganglion cell thickness to monitor treatment response and visual outcomes using custom-designed segmentation software.32,41 Furthermore, ganglion cell loss detected at diagnosis of papilledema has been proposed as a potential method to stratify patients into more aggressive treatment options.41
Optic Neuritis
Optic neuritis is an inflammatory disease of the anterior visual pathway. The pathophysiology is multifactorial, likely involving demyelination, sodium channel dysregulation, and axonal loss. Although demyelination of the optic nerve causes visual dysfunction, permanent visual loss is likely caused by axonal and neuronal degeneration. Given its unique ability to visualize unmyelinated retinal axons, OCT has been used by neurologists, ophthalmologists, and neuro-ophthalmologists to quantify axonal loss in many inflammatory conditions of the central nervous system, such as, optic neuritis, multiple sclerosis (MS), and neuromyelitis optica (NMO).
Most studies of OCT in optic neuritis have been performed in the adult MS population. While optic neuritis may be an isolated and monophasic inflammatory disease in some children and adults, optic neuritis is a common manifestation of MS. Acutely, the cpRNFL appears “thicker” in eyes with optic neuritis compared with unaffected eyes because of disc edema. Within 3 to 6 months of the onset of optic neuritis in adults, cpRNFL thinning is detectable in 85% of affected eyes.42 In the eyes of adults with MS and a history of optic neuritis in that eye, the cpRNFL thickness is decreased by a mean of 20 µm compared with healthy control eyes43; however, thinning also occurs in the non–optic neuritis eyes of patients with MS (mean loss of 7 µm compared with controls). This may reflect retrograde transsynaptic degeneration from postchiasmal lesions involving the lateral geniculate nucleus of the thalamus, optic radiations, or visual cortex.44 Alternatively, cpRNFL thinning in nonoptic neuritis eyes in adults with MS may reflect a global process as suggested by correlations between cpRNFL thickness (using TD–OCT) and whole brain atrophy (as measured by brain parenchymal fraction).45
Three studies using TD–OCT in pediatric MS demonstrate cpRNFL thinning in children with MS and optic neuritis,46–48 with a mean loss of approximately 23 to 24 µm compared with pediatric control eyes. The same studies report conflicting data regarding cpRNFL thickness in the non–optic neuritis eyes of children with MS. Waldman et al demonstrated preserved cpRNFL thickness in children with MS who did not have a history of optic neuritis in either eye,47 whereas other studies have shown cpRNFL thinning in these unaffected eyes.46,48 The pediatric studies have been limited by small sample sizes and short disease duration, which may account for the preserved cpRNFL thickness in eyes unaffected by optic neuritis in the one study. A longitudinal study in adults using TD–OCT showed a decline in cpRNFL thickness with time in MS eyes.49 Of note, in adult MS eyes without optic neuritis, significant cpRNFL thinning was detected in this study after 2 to 3 years of follow-up. Using SD–OCT in adults, Huhn et al compared cpRNFL thickness and total macular volume between subjects with disease onset younger than 18 years (pediatric-onset MS) to adult-onset MS, matched for age or disease duration, and healthy controls.50 cpRNFL thickness did not differ between the pediatric-onset and adult-onset MS eyes, either matched for age or disease duration; however, total macular volume was significantly decreased between subjects with pediatric-onset MS compared with disease duration–matched adults.
Current research using SD–OCT allows for the segmentation of the retinal layers, with a recent focus on the macular GCL–IPL and inner and outer nuclear layers. Thinning of the macular GCL–IPL, a potential marker for neuronal degeneration, has been reported in adults with MS and may decline before cpRNFL.13,51,52 Using SD–OCT, Yeh et al demonstrated cpRNFL and GCL–IPL thinning in 37 children with various demyelinating diseases, including 16 children with MS, compared with healthy control eyes.53 The GCL–IPL thickness was decreased in all demyelinating disease eyes, with and without a history of optic neuritis. The authors hypothesized that GCL–IPL thinning occurs at the time of a first clinical event, unlike cpRNFL thinning which occurs after the edema has resolved, and may implicate a primary neuronal pathology in pediatric demyelination. In adults, correlations between macular GCL–IPL thickness and gray matter structures using MRI provide further support for a degenerative biology beyond the anterior visual pathway in MS.54 Additional pediatric studies are needed to determine the timing of retinal and macular injury and their relationship to clinical and subclinical optic neuritis and MRI markers of brain atrophy.
OCT has also been used in adults with NMO, with notable differences between cpRNFL thickness in NMO compared with MS eyes. In NMO, there is greater cpRNFL thinning, averaging 30 to 40 µmin ON eyes.55 In NMO–ON eyes, cpRNFL thinning affects all the quadrants, including the superior and inferior quadrants, which are less affected in MS. In adults with NMO spectrum disorder without optic neuritis, the cpRNFL thickness does not differ from controls, suggesting that subclinical optic neuritis does not occur in these adults. OCT has not been reported in pediatric NMO.56
The above research studies have led some clinicians to incorporate the use of OCT into the management of children and adults with optic neuritis, MS, and NMO, although its utility in the clinical setting is debated. Although OCT permits the ability to quantify axonal damage after optic neuritis, cpRNFL thinning is a late effect of optic neuritis. Still, OCT has been recommended by some to obtain a “baseline” cpRNFL thickness performed 6 months after the optic neuritis attack.57 Subsequent scans could be obtained to assess the effects of additional attacks or for disease progression; however, such data should be interpreted with caution as longitudinal studies are limited in adults and have not been published in pediatrics.
Others clinicians have suggested using OCT to detect subclinical optic nerve involvement, similar to the use of visual-evoked potentials. For example, if a child had visual complaints in the past or failed a vision screen but does not have objective findings of visual acuity or field deficits, abnormal color vision, a relative afferent pupillary defect, or optic atrophy, an OCT could be used to assess for deviations from normative mean values in an eye that might suggest previous subclinical optic neuritis. However, despite the ability of OCT to quantify retinal layers, the clinical use of OCT is challenged by uncertainty around the interpretation of research data for the individual patient.
Combining the clinical and research data, OCT may have a role in the future in identifying disease subtypes, such as, the macular thinning predominant subtype, which has been defined in adults (without a history of optic neuritis) by a normal cpRNFL thickness but with macular thickness < 5% using reference data.58 Adults with this phenotype have more pronounced photophobia and excessive glare as well as higher disability scores, possibly because of the primary neuronal pathology as suggested by the disproportionate involvement of the macula in the absence of optic nerve pathology. Similarly, microcystic macular edema (thickening of the inner nuclear layer) may be identified using SD–OCT.59,60 This subtype has been associated with greater inflammation in adults (as measured by new T2 lesions and gadolinium enhancing lesions on MRI) and may be relevant in pediatric MS which is hypothesized to be more inflammatory and less neurodegenerative. In comparison to adults, children with MS have a greater number of clinical relapses early in the disease course and an increased number of T2 lesions in this age group.61,62 Future studies are required to determine if these phenotypes are present in children and whether they correlate with other markers of disease severity, inflammation, or progression.
Tumors of the Visual Pathway
A variety of childhood tumors are located along the anterior visual pathway including craniopharyngiomas, prolactinomas, germ cell tumors, and low-grade gliomas. In adults with surgically resectable parachiasmal tumors, the preoperative cpRNFL thickness measured by OCT was predictive of postoperative visual acuity and visual field outcomes.63 Many craniopharyngiomas, prolactinomas, and germ cell tumors are diagnosed after the child has experienced vision loss; therefore, OCT may only provide prognostic information about long-term visual outcomes as these tumors respond well to treatment and have a low-recurrence rate.
On the contrary, low-grade gliomas (referred to as optic pathway gliomas, OPGs) are unique tumors that are not amenable to surgical resection, can remain stable for years before progression, and may involve multiple but separate treatment courses.56 Furthermore, changes in OPG size and contrast enhancement as visualized on MRI are known to have a poor correlation to changes in vision.64,65 Given the lack of MRI and clinical features to predict an impending visual decline, OCT may have the potential to alter clinical care by identifying a declining cpRNFL thickness and or GCL–IPL thickness before vision loss has occurred.12,19,66 No longitudinal studies have proven that cpRNFL or GCL–IPL precedes or concurrently declines with vision loss, but the current studies are underway as these measures are highly reproducible in patients without clinical progression.6,7,19 cpRNFL and GCL-IPL thickness measures have shown excellent discrimination between children who have experienced vision loss from their OPGs and those who have normal vision.12,19,66 For children unable to complete formal visual field testing, OCT measures may be able to provide added information about the child’s visual status (Fig. 4). Having quantitative structural information from OCT at diagnosis may also help stratify patients to more or less aggressive treatment plans depending on the presence, absence, or severity of visual pathway damage.
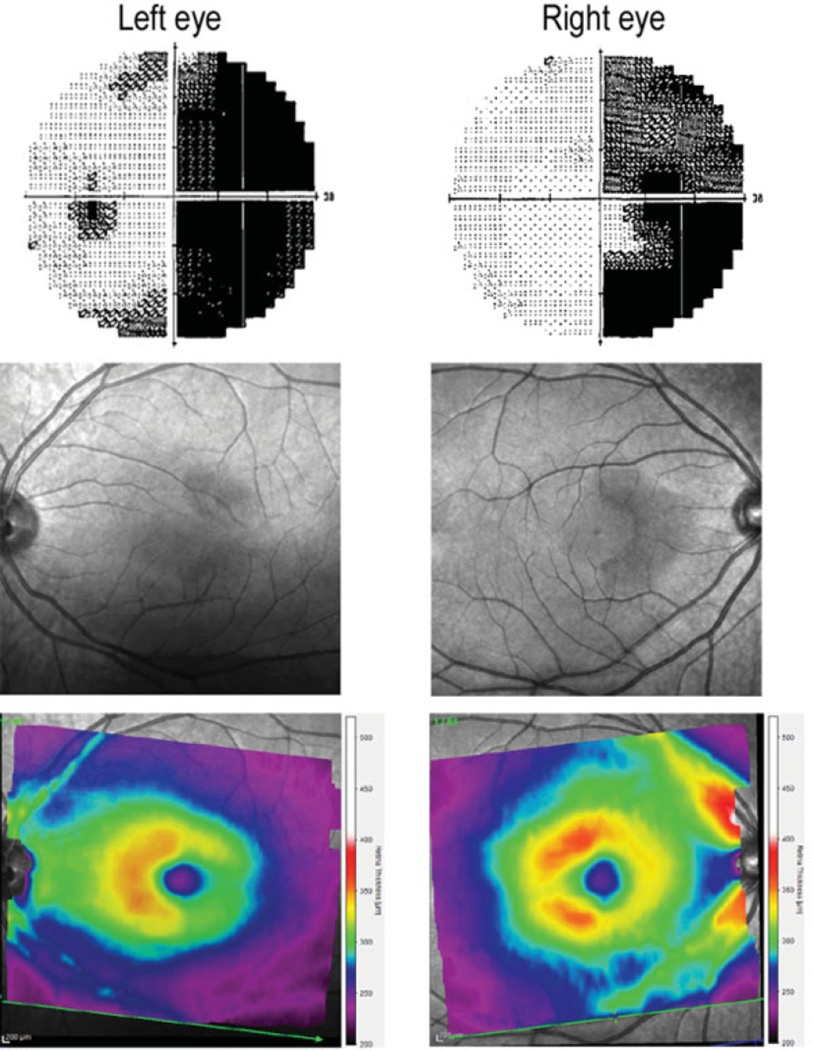
Fig. 4.
Automated visual field demonstrating a right homonymous hemianopsia (top), infrared optical coherence tomography image of macula (middle), and heat map of the macula thickness (red/yellow indicating greater thickness and green/blue/magenta representing thinner regions) corresponding to regions of visual field loss (bottom).
Most children with OPGs are younger than 6 years, especially those with neurofibromatosis type 1 (NF1), so they are less likely to cooperate with traditional table top OCT. The advent of the HH–OCT now permits OCT imaging when children are sedated for their MRI.6,7,12,19 Also, many of the younger children cannot reliably complete an accurate assessment of their visual acuity and/or visual fields,67 therefore, the HH–OCT may be particularly helpful in making management decisions.19 For example, HH–OCT measures that are within normal limits and do not show a progressive decline could be reassuring that the tumor is not causing progressive vision loss, thus deferring the need to initiate treatment. Whereas a child with stable or even untestable visual acuity that demonstrates a progressive decline in their cpRNFL/GCL–IPL thickness measure may be a candidate for initiating treatment before they manifest vision loss. As children with NF1-related OPGs and normal vision have cpRNFL/GCL–IPL thickness measures similar to healthy controls, OCT should not be used as a screening tool for OPGs.19 Despite the many promising applications of OCT in children with visual pathway tumors, the previously mentioned studies need further confirmation along with larger subject numbers followed over time to determine if this technology can improve the care of these children.
Vigabatrin
Vigabatrin has demonstrated good efficacy for treating infantile spasms, especially in children with tuberous sclerosis.68 However, vigabatrin also causes retinal toxicity resulting primarily in concentric peripheral visual field loss.69 Visual field changes can occur without the clinician being able to visualize changes of the optic nerve during ophthalmoscopy.70 Multiple studies have utilized OCT to measure cpRNFL, primarily in adults taking vigabatrin, and demonstrated decreased cpRNFL thickness in subjects with visual field loss.69,71–75 Unfortunately, none of these studies have performed longitudinal OCT imaging before, during, and after vigabatrin exposure, thus failing to establish the temporal relationship between visual field loss and the decreased cpRNFL. It is conceivable that the visual field loss precedes the OCT changes.
While several investigators have acquired OCT measures in young children taking vigabatrin, we are unaware of any published research demonstrating its ability to improve visual outcomes in these patients. OCT imaging has not been established as the standard of care in pediatric patients, so we do not recommend sedating young children on vigabatrin for the sole purpose of acquiring OCT measures. In an expert review, Sergott did not recommend OCT imaging for children younger than 2 years as this was an exploratory test, and the risks of sedation/anesthesia may outweigh any potential benefit.70
Nystagmus
Determining the etiology of infant and childhood onset nystagmus can be challenging to both the ophthalmologist and neurologist as both congenital and acquired etiologies must be considered. In some cases, the subtle structural changes responsible for the nystagmus may not be readily visualized during examination, especially when the nystagmus is pronounced. Several investigators have utilized both traditional table top OCT and HH–OCT to examine the foveal morphology of children with nystagmus.17,30,76–81 Investigators at University of Leicester have imaged a large number of children with various types of nystagmus and have determined that a foveal grading system provides excellent diagnostic accuracy as well as the ability to predict visual outcomes.17,78 Using HH–OCT on awake young children (mean age, 3.2 years) with nystagmus, Lee et al frequently achieved a diagnostic sensitivity above 90% in subjects with typical foveal hypoplasia, atypical foveal hypoplasia, retinal dystrophies, and healthy controls.17 Their ability to establish a strong genotype–phenotype relationship using HH–OCT in nystagmus patients may ultimately improve clinical care and decrease the number of unnecessary ancillary tests needed to confirm the diagnosis.
Neurodegenerative Conditions
Many neurodegenerative conditions frequently involve visual symptoms, thus, OCT findings have been proposed to be a biomarker of disease progression.82–88 In presymptomatic children with X-linked adrenoleukodystrophy, cpRNFL, and total macular thickness were not different from healthy controls.83 However, in adults with adrenoleukodystrophy and vision loss, cpRNFL is markedly reduced.82 Reduced cpRNFL thickness in Friedreich ataxia demonstrates a strong relationship to decreased visual acuity, low-contrast visual acuity, quality of life, and GAA repeat length.84,85 Qualitative OCT analysis has also provided insight into the retinal findings associated with pediatric storage disorders.86,87 In late infantile neuronal ceroid lipofuscinosis, OCT along with other ophthalmologic signs has been proposed as an objective marker of disease severity.88 Neurodegenerative and storage disorders whose patients manifest significant cognitive deficits that limit functional assessments provide a unique opportunity for OCT measures to serve as a biomarker of disease severity and progression.
Future Considerations
OCT now permits the pediatric neuroscientist to visualize and quantify the optic nerve and retinal layers with unprecedented resolution—providing an in vivo assessment of the anterior visual pathway. The aforementioned research studies have provided invaluable insight into the clinical and histologic changes accompanying these conditions which will undoubtedly lead to a better understanding of the disease course and treatment outcomes. OCT results should never be interpreted in isolation, but instead considered supplementary information to a complete history and clinical examination. The accurate interpretation of OCT imaging requires an experienced imaging specialist who is well trained in detecting pathologic changes, determining appropriate image acquisition protocols and most importantly, differentiating between true tissue changes and imaging artifacts. Prospectively designed studies that examine large pediatric patient cohorts are needed to accurately interpret OCT data for the individual patient and, more importantly, determine its impact on clinical care.
Acknowledgment
None.
Funding/Support
This article was supported by the National Institutes of Health grants K23-EY022673 (R. A. A.) and K23-NS069806 (A.T.W.), the National Institutes of Health Pediatric Research Loan repayment program (R.A.A and A.T.W.) and the Gilbert Family Neurofibromatosis Institute (R.A.A). Ms Rajjoub was supported by the Gill Fellowship, George Washington University School of Medicine, Washington, DC.
Footnotes
Financial Disclosures
None.
Contribution of Authors
Design of the study (R.A.A., R.R., C.T.-H., and A.T.W.), conduct of the study (R.A.A., R.R., C.T.-H., and A.T.W.), collection, management, analysis, and interpretation of the data (R.A.A., R.R., C.T.-H., and A.T.W.), preparation, approval, and review of the article (R.A.A., R.R., C.T.-H., and A.T.W.).
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuman JS, Hee MR, Puliafito CA, et al. Quantification of nerve fiber layer thickness in normal and glaucomatous eyes using optical coherence tomography. Arch Ophthalmol. 1995;113(5):586–596. doi: 10.1001/archopht.1995.01100050054031. [DOI] [PubMed] [Google Scholar]
- 3.Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000;107(10):1809–1815. doi: 10.1016/s0161-6420(00)00284-0. [DOI] [PubMed] [Google Scholar]
- 4.Yanni SE, Wang J, Cheng CS, et al. Normative reference ranges for the retinal nerve fiber layer, macula, and retinal layer thicknesses in children. Am J Ophthalmol. 2013;155(2):354–360. doi: 10.1016/j.ajo.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turk A, Ceylan OM, Arici C, et al. Evaluation of the nerve fiber layer and macula in the eyes of healthy children using spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153(3):552–559. doi: 10.1016/j.ajo.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 6.Avery RA, Cnaan A, Schuman JS, et al. Reproducibility of circumpapillary retinal nerve fiber layer measurements using handheld optical coherence tomography in sedated children. Am J Ophthalmol. 2014;158(4):780–787. doi: 10.1016/j.ajo.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avery RA, Cnaan A, Schuman JS, et al. Intra- and inter-visit reproducibility of ganglion cell-inner plexiform layer measurements using handheld optical coherence tomography in children with optic pathway gliomas. Am J Ophthalmol. 2014;158(5):916–923. doi: 10.1016/j.ajo.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Syc SB, Warner CV, Hiremath GS, et al. Reproducibility of high-resolution optical coherence tomography in multiple sclerosis. Mult Scler. 2010;16(7):829–839. doi: 10.1177/1352458510371640. [DOI] [PubMed] [Google Scholar]
- 9.Altemir I, Pueyo V, Elía N, Polo V, Larrosa JM, Oros D. Reproducibility of optical coherence tomography measurements in children. Am J Ophthalmol. 2013;155(1):171–176. doi: 10.1016/j.ajo.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Langenegger SJ, Funk J, Töteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci. 2011;52(6):3338–3344. doi: 10.1167/iovs.10-6611. [DOI] [PubMed] [Google Scholar]
- 11.Serbecic N, Beutelspacher SC, Aboul-Enein FC, Kircher K, Reitner A, Schmidt-Erfurth U. Reproducibility of high-resolution optical coherence tomography measurements of the nerve fibre layer with the new Heidelberg Spectralis optical coherence tomography. Br J Ophthalmol. 2011;95(6):804–810. doi: 10.1136/bjo.2010.186221. [DOI] [PubMed] [Google Scholar]
- 12.Gu S, Glaug N, Cnaan A, Packer RJ, Avery RA. Ganglion cell layer-inner plexiform layer thickness and vision loss in young children with optic pathway gliomas. Invest Ophthalmol Vis Sci. 2014;55(3):1402–1408. doi: 10.1167/iovs.13-13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter SD, Ishikawa H, Galetta KM, et al. Ganglion cell loss in relation to visual disability in multiple sclerosis. Ophthalmology. 2012;119(6):1250–1257. doi: 10.1016/j.ophtha.2011.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kardon RH. Role of the macular optical coherence tomography scan in neuro-ophthalmology. J Neuroophthalmol. 2011;31(4):353–361. doi: 10.1097/WNO.0b013e318238b9cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barboni P, Savini G, Cascavilla ML, et al. Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am J Ophthalmol. 2014;158(3):628–36. doi: 10.1016/j.ajo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal D, Tan O, Huang D, Sadun AA. Patterns of ganglion cell complex and nerve fiber layer loss in nonarteritic ischemic optic neuropathy by Fourier-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(8):4539–4545. doi: 10.1167/iovs.11-9300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H, Sheth V, Bibi M, et al. Potential of handheld optical coherence tomography to determine cause of infantile nystagmus in children by using foveal morphology. Ophthalmology. 2013;120(12):2714–2724. doi: 10.1016/j.ophtha.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 18.Rajjoub RD, Trimboli-Heidler C, Packer RJ, Avery RA. Reproducibility of retinal nerve fiber layer thickness measures using eye tracking in children with nonglaucomatous optic neuropathy. Am J Ophthalmol. 2015;159(1):71–77. doi: 10.1016/j.ajo.2014.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Avery RA, Hwang EI, Ishikawa H, et al. Handheld optical coherence tomography during sedation in young children with optic pathway gliomas. JAMA Ophthalmol. 2014;132(3):265–271. doi: 10.1001/jamaophthalmol.2013.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno TA, O’Connell RV, Chiu SJ, et al. Choroid development and feasibility of choroidal imaging in the preterm and term infants utilizing SD-OCT. Invest Ophthalmol Vis Sci. 2013;54(6):4140–4147. doi: 10.1167/iovs.12-11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabrera MT, O’Connell RV, Toth CA, et al. Macular findings in healthy full-term Hispanic newborns observed by hand-held spectral-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging Retina. 2013;44(5):448–454. doi: 10.3928/23258160-20130801-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cabrera MT, Maldonado RS, Toth CA, et al. Subfoveal fluid in healthy full-term newborns observed by handheld spectral-domain optical coherence tomography. Am J Ophthalmol. 2012;153(1):167–175. doi: 10.1016/j.ajo.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maldonado RS, O’Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315–2325. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maldonado RS, Izatt JA, Sarin N, et al. Optimizing hand-held spectral domain optical coherence tomography imaging for neonates, infants, and children. Invest Ophthalmol Vis Sci. 2010;51(5):2678–2685. doi: 10.1167/iovs.09-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerth C, Zawadzki RJ, Héon E, Werner JS. High-resolution retinal imaging in young children using a handheld scanner and Fourier-domain optical coherence tomography. J AAPOS. 2009;13(1):72–74. doi: 10.1016/j.jaapos.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerth C, Zawadzki RJ, Werner JS, Héon E. Retinal morphology in patients with BBS1 and BBS10 related Bardet-Biedl Syndrome evaluated by Fourier-domain optical coherence tomography. Vision Res. 2008;48(3):392–399. doi: 10.1016/j.visres.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muni RH, Kohly RP, Charonis AC, Lee TC. Retinoschisis detected with handheld spectral-domain optical coherence tomography in neonates with advanced retinopathy of prematurity. Arch Ophthalmol. 2010;128(1):57–62. doi: 10.1001/archophthalmol.2009.361. [DOI] [PubMed] [Google Scholar]
- 29.Muni RH, Kohly RP, Sohn EH, Lee TC. Hand-held spectral domain optical coherence tomography finding in shaken-baby syndrome. Retina. 2010;30(4) Suppl:S45–S50. doi: 10.1097/IAE.0b013e3181dc048c. [DOI] [PubMed] [Google Scholar]
- 30.Lee H, Proudlock F, Gottlob I. Is handheld optical coherence tomography reliable in infants and young children with and without nystagmus? Invest Ophthalmol Vis Sci. 2013;54(13):8152–8159. doi: 10.1167/iovs.13-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fard MA, Fakhree S, Abdi P, Hassanpoor N, Subramanian PS. Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol. 2014;158(1):136–143. doi: 10.1016/j.ajo.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 32.Auinger P, Durbin M, Feldon S, et al. OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci. 2014;55(12):8180–8188. doi: 10.1167/iovs.14-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JK, Kardon RH, Kupersmith MJ, Garvin MK. Automated quantification of volumetric optic disc swelling in papilledema using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(7):4069–4075. doi: 10.1167/iovs.12-9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sibony P, Kupersmith MJ, Rohlf FJ. Shape analysis of the peripapillary RPE layer in papilledema and ischemic optic neuropathy. Invest Ophthalmol Vis Sci. 2011;52(11):7987–7995. doi: 10.1167/iovs.11-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kupersmith MJ, Sibony P, Mandel G, Durbin M, Kardon RH. Optical coherence tomography of the swollen optic nerve head: deformation of the peripapillary retinal pigment epithelium layer in papilledema. Invest Ophthalmol Vis Sci. 2011;52(9):6558–6564. doi: 10.1167/iovs.10-6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kulkarni KM, Pasol J, Rosa PR, Lam BL. Differentiating mild papilledema and buried optic nerve head drusen using spectral domain optical coherence tomography. Ophthalmology. 2014;121(4):959–963. doi: 10.1016/j.ophtha.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KM, Woo SJ, Hwang JM. Differentiation of optic nerve head drusen and optic disc edema with spectral-domain optical coherence tomography. Ophthalmology. 2011;118(5):971–977. doi: 10.1016/j.ophtha.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Wall M, McDermott MP, Kieburtz KD, et al. NORDIC Idiopathic Intracranial Hypertension Study Group Writing Committee. Effect of acetazolamide on visual function in patients with idiopathic intracranial hypertension and mild visual loss: the idiopathic intracranial hypertension treatment trial. JAMA. 2014;311(16):1641–1651. doi: 10.1001/jama.2014.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merchant KY, Su D, Park SC, et al. Enhanced depth imaging optical coherence tomography of optic nerve head drusen. Ophthalmology. 2013;120(7):1409–1414. doi: 10.1016/j.ophtha.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 40.Moss HE, Treadwell G, Wanek J, DeLeon S, Shahidi M. Retinal vessel diameter assessment in papilledema by semi-automated analysis of SLO images: feasibility and reliability. Invest Ophthalmol Vis Sci. 2014;55(4):2049–2054. doi: 10.1167/iovs.13-13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Auinger P, Durbin M, Feldon S, et al. OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part II: correlations and relationship to clinical features. Invest Ophthalmol Vis Sci. 2014;55(12):8173–8179. doi: 10.1167/iovs.14-14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costello F, Coupland S, Hodge W, et al. Quantifying axonal loss after optic neuritis with optical coherence tomography. Ann Neurol. 2006;59(6):963–969. doi: 10.1002/ana.20851. [DOI] [PubMed] [Google Scholar]
- 43.Petzold A, de Boer JF, Schippling S, et al. Optical coherence tomography in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2010;9(9):921–932. doi: 10.1016/S1474-4422(10)70168-X. [DOI] [PubMed] [Google Scholar]
- 44.Jindahra P, Petrie A, Plant GT. Retrograde trans-synaptic retinal ganglion cell loss identified by optical coherence tomography. Brain. 2009;132(Pt 3):628–634. doi: 10.1093/brain/awp001. [DOI] [PubMed] [Google Scholar]
- 45.Gordon-Lipkin E, Chodkowski B, Reich DS, et al. Retinal nerve fiber layer is associated with brain atrophy in multiple sclerosis. Neurology. 2007;69(16):1603–1609. doi: 10.1212/01.wnl.0000295995.46586.ae. [DOI] [PubMed] [Google Scholar]
- 46.Yeh EA, Weinstock-Guttman B, Lincoff N, et al. Retinal nerve fiber thickness in inflammatory demyelinating diseases of childhood onset. Mult Scler. 2009;15(7):802–810. doi: 10.1177/1352458509104586. [DOI] [PubMed] [Google Scholar]
- 47.Waldman AT, Hiremath G, Avery RA, et al. Monocular and binocular low-contrast visual acuity and optical coherence tomography in pediatric multiple sclerosis. Mult Scler Relat Disord. 2013;3(3):326–334. doi: 10.1016/j.msard.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yilmaz Ü, Gücüyener K, Erin DM, et al. Reduced retinal nerve fiber layer thickness and macular volume in pediatric multiple sclerosis. J Child Neurol. 2012;27(12):1517–1523. doi: 10.1177/0883073812447683. [DOI] [PubMed] [Google Scholar]
- 49.Talman LS, Bisker ER, Sackel DJ, et al. Longitudinal study of vision and retinal nerve fiber layer thickness in multiple sclerosis. Ann Neurol. 2010;67(6):749–760. doi: 10.1002/ana.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huhn K, Lämmer R, Oberwahrenbrock T, et al. Optical coherence tomography in patients with a history of juvenile multiple sclerosis reveals early retinal damage. Eur J Neurol. 2015;22(1):86–92. doi: 10.1111/ene.12532. [DOI] [PubMed] [Google Scholar]
- 51.Saidha S, Syc SB, Durbin MK, et al. Visual dysfunction in multiple sclerosis correlates better with optical coherence tomography derived estimates of macular ganglion cell layer thickness than peripapillary retinal nerve fiber layer thickness. Mult Scler. 2011;17(12):1449–1463. doi: 10.1177/1352458511418630. [DOI] [PubMed] [Google Scholar]
- 52.Garcia-Martin E, Polo V, Larrosa JM, et al. Retinal layer segmentation in patients with multiple sclerosis using spectral domain optical coherence tomography. Ophthalmology. 2014;121(2):573–579. doi: 10.1016/j.ophtha.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 53.Yeh EA, Marrie RA, Reginald YA, et al. Canadian Pediatric Demyelinating Disease Network. Functional-structural correlations in the afferent visual pathway in pediatric demyelination. Neurology. 2014;83(23):2147–2152. doi: 10.1212/WNL.0000000000001046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saidha S, Sotirchos ES, Oh J, et al. Relationships between retinal axonal and neuronal measures and global central nervous system pathology in multiple sclerosis. JAMA Neurol. 2013;70(1):34–43. doi: 10.1001/jamaneurol.2013.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bennett J, de Seze J, Lana-Peixoto M, et al. with the GJCF-ICC&BR, Neuromyelitis optica and multiple sclerosis: Seeing differences through optical coherence tomography. Mult Scler. 2015 doi: 10.1177/1352458514567216. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avery RA, Fisher MJ, Liu GT. Optic pathway gliomas. J Neuroophthalmol. 2011;31(3):269–278. doi: 10.1097/WNO.0b013e31822aef82. [DOI] [PubMed] [Google Scholar]
- 57.Costello F, Van Stavern GP. Should optical coherence tomography be used to manage patients with multiple sclerosis? J Neuroophthalmol. 2012;32(4):363–371. doi: 10.1097/WNO.0b013e318261f7e7. [DOI] [PubMed] [Google Scholar]
- 58.Saidha S, Syc SB, Ibrahim MA, et al. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011;134(Pt 2):518–533. doi: 10.1093/brain/awq346. [DOI] [PubMed] [Google Scholar]
- 59.Gelfand JM, Nolan R, Schwartz DM, Graves J, Green AJ. Microcystic macular oedema in multiple sclerosis is associated with disease severity. Brain. 2012;135(Pt 6):1786–1793. doi: 10.1093/brain/aws098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11(11):963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T. Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol. 2009;66(1):54–59. doi: 10.1001/archneurol.2008.505. [DOI] [PubMed] [Google Scholar]
- 62.Waubant E, Chabas D, Okuda DT, et al. Difference in disease burden and activity in pediatric patients on brain magnetic resonance imaging at time of multiple sclerosis onset vs adults. Arch Neurol. 2009;66(8):967–971. doi: 10.1001/archneurol.2009.135. [DOI] [PubMed] [Google Scholar]
- 63.Danesh-Meyer HV, Papchenko T, Savino PJ, Law A, Evans J, Gamble GD. In vivo retinal nerve fiber layer thickness measured by optical coherence tomography predicts visual recovery after surgery for parachiasmal tumors. Invest Ophthalmol Vis Sci. 2008;49(5):1879–1885. doi: 10.1167/iovs.07-1127. [DOI] [PubMed] [Google Scholar]
- 64.Dalla Via P, Opocher E, Pinello ML, et al. Visual outcome of a cohort of children with neurofibromatosis type 1 and optic pathway glioma followed by a pediatric neuro-oncology program. Neurooncol. 2007;9(4):430–437. doi: 10.1215/15228517-2007-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fisher MJ, Loguidice M, Gutmann DH, et al. Visual outcomes in children with neurofibromatosis type 1-associated optic pathway glioma following chemotherapy: a multicenter retrospective analysis. Neuro-oncol. 2012;14(6):790–797. doi: 10.1093/neuonc/nos076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avery RA, Liu GT, Fisher MJ, et al. Retinal nerve fiber layer thickness in children with optic pathway gliomas. Am J Ophthalmol. 2011;151(3):542–549. doi: 10.1016/j.ajo.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Avery RA, Bouffet E, Packer RJ, Reginald A. Feasibility and comparison of visual acuity testing methods in children with neurofibromatosis type 1 and/or optic pathway gliomas. Invest Ophthalmol Vis Sci. 2013;54(2):1034–1038. doi: 10.1167/iovs.12-11385. [DOI] [PubMed] [Google Scholar]
- 68.Lux AL, Edwards SW, Hancock E, et al. The United Kingdom Infantile Spasms Study comparing vigabatrin with prednisolone or tetracosactide at 14 days: a multicentre, randomised controlled trial. Lancet. 2004;364(9447):1773–1778. doi: 10.1016/S0140-6736(04)17400-X. [DOI] [PubMed] [Google Scholar]
- 69.Plant GT, Sergott RC. Understanding and interpreting vision safety issues with vigabatrin therapy. Acta Neurol Scand Suppl. 2011;192:57–71. doi: 10.1111/j.1600-0404.2011.01601.x. [DOI] [PubMed] [Google Scholar]
- 70.Sergott RC. Recommendations for visual evaluations of patients treated with vigabatrin. Curr Opin Ophthalmol. 2010;21(6):442–446. doi: 10.1097/ICU.0b013e32833f0085. [DOI] [PubMed] [Google Scholar]
- 71.Miller NR, Johnson MA, Paul SR, et al. Visual dysfunction in patients receiving vigabatrin: clinical and electrophysiologic findings. Neurology. 1999;53(9):2082–2087. doi: 10.1212/wnl.53.9.2082. [DOI] [PubMed] [Google Scholar]
- 72.Wild JM, Robson CR, Jones AL, Cunliffe IA, Smith PE. Detecting vigabatrin toxicity by imaging of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 2006;47(3):917–924. doi: 10.1167/iovs.05-0854. [DOI] [PubMed] [Google Scholar]
- 73.Lawthom C, Smith PE, Wild JM. Nasal retinal nerve fiber layer attenuation: a biomarker for vigabatrin toxicity. Ophthalmology. 2009;116(3):565–571. doi: 10.1016/j.ophtha.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 74.Clayton LM, Devile M, Punte T, et al. Patterns of peripapillary retinal nerve fiber layer thinning in vigabatrin-exposed individuals. Ophthalmology. 2012;119(10):2152–2160. doi: 10.1016/j.ophtha.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Clayton LM, Dévilé M, Punte T, et al. Retinal nerve fiber layer thickness in vigabatrin-exposed patients. Ann Neurol. 2011;69(5):845–854. doi: 10.1002/ana.22266. [DOI] [PubMed] [Google Scholar]
- 76.Cronin TH, Hertle RW, Ishikawa H, Schuman JS. Spectral domain optical coherence tomography for detection of foveal morphology in patients with nystagmus. J AAPOS. 2009;13(6):563–566. doi: 10.1016/j.jaapos.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas MG, Kumar A, Thompson JR, Proudlock FA, Straatman K, Gottlob I. Is high-resolution spectral domain optical coherence tomography reliable in nystagmus? Br J Ophthalmol. 2013;97(4):534–536. doi: 10.1136/bjophthalmol-2011-300476. [DOI] [PubMed] [Google Scholar]
- 78.Thomas MG, Kumar A, Mohammad S, et al. Structural grading of foveal hypoplasia using spectral-domain optical coherence tomography a predictor of visual acuity? Ophthalmology. 2011;118(8):1653–1660. doi: 10.1016/j.ophtha.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas MG, Kumar A, Kohl S, Proudlock FA, Gottlob I. High-resolution in vivo imaging in achromatopsia. Ophthalmology. 2011;118(5):882–887. doi: 10.1016/j.ophtha.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 80.Papageorgiou E, McLean RJ, Gottlob I. Nystagmus in childhood. Pediatr Neonatol. 2014;55(5):341–351. doi: 10.1016/j.pedneo.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 81.Thomas S, Thomas MG, Andrews C, et al. Autosomal-dominant nystagmus, foveal hypoplasia and presenile cataract associated with a novel PAX6 mutation. Eur J Hum Genet. 2014;22(3):344–349. doi: 10.1038/ejhg.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Grainger BT, Papchenko TL, Danesh-Meyer HV. Optic nerve atrophy in adrenoleukodystrophy detectable by optic coherence tomography. J Clin Neurosci. 2010;17(1):122–124. doi: 10.1016/j.jocn.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 83.Aquino JJ, Sotirchos ES, Saidha S, Raymond GV, Calabresi PA. Optical coherence tomography in x-linked adrenoleukodystrophy. Pediatr Neurol. 2013;49(3):182–184. doi: 10.1016/j.pediatrneurol.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 84.Noval S, Contreras I, Sanz-Gallego I, Manrique RK, Arpa J. Ophthalmic features of Friedreich ataxia. Eye (Lond) 2012;26(2):315–320. doi: 10.1038/eye.2011.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seyer LA, Galetta K, Wilson J, et al. Analysis of the visual system in Friedreich ataxia. J Neurol. 2013;260(9):2362–2369. doi: 10.1007/s00415-013-6978-z. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg R, Halimi E, Mention-Mulliez K, Cuisset JM, Holder M, Defoort-Dhellemmes S. Five year follow-up of two sisters with type II sialidosis: systemic and ophthalmic findings including OCT analysis. J Pediatr Ophthalmol Strabismus. 2013;50 doi: 10.3928/01913913-20130625-02. Online: e33–e36. [DOI] [PubMed] [Google Scholar]
- 87.Rudich DS, Curcio CA, Wasserstein M, Brodie SE. Inner macular hyperreflectivity demonstrated by optical coherence tomography in niemann-pick disease. JAMA Ophthalmol. 2013;131(9):1244–1246. doi: 10.1001/jamaophthalmol.2013.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Orlin A, Sondhi D, Witmer MT, et al. Spectrum of ocular manifestations in CLN2-associated batten (Jansky-Bielschowsky) disease correlate with advancing age and deteriorating neurological function. PLoS ONE. 2013;8(8):e73128. doi: 10.1371/journal.pone.0073128. [DOI] [PMC free article] [PubMed] [Google Scholar]