Abstract
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal disorder that is characterized by intermittent abdominal pain/discomfort, altered bowel habits and abdominal bloating/distension. This review aimed at presenting the recent developments concerning the role of diet in the pathophysiology and management of IBS. There is no convincing evidence that IBS patients suffer from food allergy/intolerance, and there is no evidence that gluten causes the debated new diagnosis of non-coeliac gluten sensitivity (NCGS). The component in wheat that triggers symptoms in NCGS appears to be the carbohydrates. Patients with NCGS appear to be IBS patients who are self-diagnosed and self-treated with a gluten-free diet. IBS symptoms are triggered by the consumption of the poorly absorbed fermentable oligo-, di-, monosaccharides and polyols (FODMAPs) and insoluble fibre. On reaching the distal small intestine and colon, FODMAPS and insoluble fibre increase the osmotic pressure in the large-intestine lumen and provide a substrate for bacterial fermentation, with consequent gas production, abdominal distension and abdominal pain or discomfort. Poor FODMAPS and insoluble fibres diet reduces the symptom and improve the quality of life in IBS patients. Moreover, it changes favourably the intestinal microbiota and restores the abnormalities in the gastrointestinal endocrine cells. Five gastrointestinal endocrine cell types that produce hormones regulating appetite and food intake are abnormal in IBS patients. Based on these hormonal abnormalities, one would expect that IBS patients to have increased food intake and body weight gain. However, the link between obesity and IBS is not fully studied. Individual dietary guidance for intake of poor FODMAPs and insoluble fibres diet in combination with probiotics intake and regular exercise is to be recommended for IBS patients.
Keywords: Appetite, Non-celiac gluten sensitivity, Endocrine cells
Introduction
Irritable bowel syndrome (IBS) is a common chronic gastrointestinal disorder that affects 5–20% of the general population [1-15]. IBS is usually diagnosed at a young age (i.e. <50 years of age) and is more common in females than males [1,3,5,6,16]. This condition reduces considerably the patients’ quality of life, although it is not known to progress to a more serious disease or to cause death [1,17-19].
IBS patients suffer from intermittent abdominal pain/discomfort, altered bowel habits and abdominal bloating/distension [1,2]. Patients believe that their symptoms are triggered by certain food items such as milk and milk products, wheat products, caffeine, cabbage, onion, peas, beans, hot spices, and fried and smoked food [20-23]. Some IBS patients avoiding several foodstuffs, but there does not appear to be any difference between them and the general population regarding the intake of energy, carbohydrates, proteins and fats [23-29]. However, one study found that 62% of IBS patients had either limited or excluded certain food items from their daily diet, and of these 12% were at risk of long-term nutritional deficiencies [30].
The role of diet in the development of IBS symptoms and dietary management as a tool for controlling these symptoms has been the subject of several reviews [20,29,31-36]. The aim of this review was to present the recent developments concerning the role of diet in the pathophysiology and management of IBS.
Diet and the pathophysiology of IBS
It is generally accepted that diet plays an important role in the pathophysiology of IBS [27,36-45]. Several factors have been proposed for explaining how diet influences IBS, such as food allergy/intolerance, poorly absorbed carbohydrates and fibre, and the comorbidity of obesity and IBS.
Food allergy/intolerance
Food allergy occurs in 6–8% of children and 1–4% of adults [46]. The food allergy reaction, which is mediated by immunoglobulin E, occurs within 2 hours of ingesting the offending food item, and manifests as swelling, itching, hives, wheezing, nausea, vomiting, diarrhoea, abdominal pain and collapse. There is no evidence that such an allergic reaction takes place in IBS [47-54]. A large proportion of IBS patients complain of subjective intolerance to various foods [20,21,52,55-60]. Food intolerance is a non-toxic, non-immune-mediated reaction to bioactive chemicals in food such as histamines, sulphites and monosodium glutamate, with symptoms usually manifesting outside the gastrointestinal tract. There is no documented proof that such intolerance occurs in IBS [42,54].
Non-coeliac gluten sensitivity (NCGS) has recently received attention from the mass media and the general public, and has become confused with the popular speculation that the high carbohydrate content of wheat is responsible for negative health aspects such as obesity [61]. NCGS is defined as having gastrointestinal and extra-gastrointestinal IBS-like symptoms without coeliac disease or wheat allergy, but with the symptoms being relieved by a gluten-free diet (GFD) and relapsing on gluten challenge [62-69]. The prevalence of NCGS has been reported as 0.55–6% of the USA population [64,70].
For more than 3 decades, patients with abdominal pain, diarrhoea and small-intestine biopsy findings with no significant changes have experienced symptom relief on a GFD with return of the symptoms after a gluten challenge [71,72]. Similar results have been reported in patients with non-celiac IBS-like symptoms [73-75]. This was confirmed by double-blind randomized placebo-controlled studies [76,77]. There is disagreement as to whether or not NCGS patients have low-grade inflammation and abnormal intestinal permeability [74,77-82].
It is noteworthy that in studies showing beneficial effects on symptoms in NCGS [74,76,77], those effects were actually the result of wheat withdrawal rather than withdrawal of gluten [83]. In a placebo-controlled, crossover study of patients with IBS-like symptoms on a self-imposed GFD [84], the gastrointestinal symptoms consistently and significantly improved when consuming a diet with reduced fermentable oligo-, di-, monosaccharides and polyols (FODMAPs), and these symptoms were not worsened by either a low- or high-dose challenge with gluten. It therefore seems that the carbohydrate content (fructans and galactans) of wheat rather than gluten is responsible for triggering NCGS symptoms. Furthermore, in those who believed that they had NCGS, 24% had uncontrolled symptoms despite consuming a GFD, 27% did not follow a GFD alone, and 65% avoided other foods that contain high levels of FODMAPs [85]. These findings lend further support to the idea that NCGS symptoms are not triggered by the gluten in wheat.
The basic description of NCGS [67] is the same as that of IBS. Both NCGS and IBS patients have the same gastrointestinal and extra-gastrointestinal symptoms that are triggered by wheat consumption. NCGS patients have been reported to have high frequency of immunoglobulin G (IgG)/immunoglobulin A (IgA) antigliadin antibodies (AGA) and a stronger association with human leucocyte antigen-DQ2 (DQ2) and -DQ8 (DQ8) [76]. The prevalence of positivity for IgG/IgA AGA, with negative tissue transglutaminase or deamidated gliadin peptide antibodies, in the blood of IBS patients has been reported to be 5–17% [86-88] or as high as about 50% [65,89]. Serum AGA has been reported to have a good sensitivity but a low specificity for coeliac disease [90], and 12–15% of serum samples from healthy subjects are positive for AGA [86,87,90,91]. Moreover, DQ2 and DQ8 are common in the general population. It appears that NCGS patients are IBS patients with a self-diagnosis and who self-treat with a GFD. It is noteworthy in this context that 20–70% of IBS patients complain of subjective intolerance to various foods [20,21,52,55-60].
Poorly absorbed carbohydrates and fibre
The triggering of symptoms in IBS patients by certain foodstuff has been attributed to indigestible and poorly absorbed short-chain carbohydrates, FODMAPs [42,92-94] and FODMAPs intake is hypothesized to be one factor among others for IBS aetiology. These short-chain sugars include fructose, lactose, sugar alcohols (sorbitol, maltitol, mannitol, xylitol and isomalt), fructans and galactans [42]. FODMAPs occur in a wide range of foods, including wheat, rye, vegetables, fruits and legumes [95-97]. A significant proportion of these carbohydrates enter the distal small intestine and colon, where they exert osmotic effects in the large-intestine lumen, increasing its water content and providing a substrate for bacterial fermentation, with consequent gas production [92,95,98]. The produced gas causes abdominal distension and abdominal pain/discomfort. FODMAPs have been found to trigger gastrointestinal symptoms in IBS, and a low-FODMAPs diet reduces the symptoms and improves the patient’s quality of life [23,29,93,94,99-102]. Recent studies have shown that the mechanisms by which FODMAPs exert their effects are more complicated than originally thought. A low-FODMAPs diet appears to induce favourable changes in the intestinal microbiota [103] and gastrointestinal endocrine cells [104-107].
It has been reported that changing from typical Australian food to a low-FODMAPs diet changed the intestinal microbiota [103]. Thus, a low-FODMAPs diet in healthy subjects and IBS patients reduced the total bacterial abundance, while a typical Australian diet increased the relative abundance of butyrate-producing Clostridiun cluster XIVa and the mucus-associated Akkermansia muciniphia, and reduced Ruminococcus torques [103].
Several types of endocrine cell in all segments of the gastrointestinal tract of IBS patients are abnormal [108-129]. The gastrointestinal endocrine cells interact and integrate with each other, with the enteric nervous system and with the afferent and efferent nerve fibres of the central nervous system, in particular the autonomic nervous system [42,130-132]. These cells regulate several functions of the gastrointestinal tract, including sensation, motility, secretion, absorption, local immune defence and food intake (by affecting appetite) [42,131-134]. The abnormalities in the gastrointestinal endocrine cells are considered to play a major role in the development of symptoms in IBS, and therefore represent future targets for treatment [43,135]. Switching from a typical Norwegian diet to a low-FODMAPs diet was shown to lead to normalization of the endocrine cells in the stomach and large intestines [104-107].
A low intake of dietary fibre was initially believed to be the cause of IBS [136]. In clinical settings the increase in dietary fibre intake in IBS patients has been found to increase abdominal pain, bloating and abdominal distension. A meta-analysis of 12 trials revealed that IBS patients treated with increased fibre intake had no improvement in symptoms compared to placebo or a low-fibre diet [137]. However, it has been reported that water-soluble fibre—but not insoluble fibre—improves the symptoms [138,139].
Obesity and IBS
As mentioned above, IBS patients tend to avoid certain food items that they associate with the onset of their symptoms. There has been some concern that the onset of IBS symptoms upon ingesting certain foods would reduce the amount of food consumed and thereby lead to malnutrition [30]. However, whereas an association between low BMI and IBS in 367 patients with IBS has been reported [140], in another report most of the 330 IBS patients examined were either normal or overweight [20]. In a recent comprehensive review, the association between IBS and obesity was found to be controversial, and the author concluded that obesity and IBS might be linked [141].
Appetite is regulated by a large number of hormones, several of which are secreted by gastrointestinal endocrine cells [142]. The gastrointestinal hormones exert their effects by acting upon the appetite control centre in the hypothalamus [142]. The arcuate nucleus (ARC) lies in the median eminence, which lacks a complete blood barrier, making the ARC particularly susceptible to hormones circulating in the blood [142-145]. The ARC is the centre that integrates the neurological and blood-borne signals [142-145]. The brain reward system in the midbrain controls hedonic feeding (i.e. the consumption of palatable food), which is modulated by blood-borne signals [145].
The following five gastrointestinal endocrine cell types that secrete hormones that regulate appetite are abnormal in patients with IBS: ghrelin, cholecystokinin (CCK), peptide YY (PYY), enteroglucagon (oxyntomodulin) and serotonin (Table 1 and Figures 1, 2, 3, 4 and 5) [108-110,112-115,132,146-149]. The endocrine cells in the oxyntic mucosa are the main source of circulating ghrelin, although small amounts do occur in the small and large intestines as well as in the ARC of the hypothalamus [144,150-153]. Ghrelin has several roles, such as regulating the release of pituitary growth hormone and accelerating gastric and intestinal motility [150-166]. Moreover, ghrelin increases appetite and feeding; central or peripheral administration of ghrelin stimulates the consumption of food and body weight gain [150]. CCK stimulates gallbladder contraction, intestinal motility and pancreatic exocrine secretion, and inhibits gastric motility and food consumption [131]. The anorexigenic action of CCK occurs via the CCK-B (CCK-2) receptor, which is the predominant receptor type in the brain [167-183]. PYY release is proportional to the composition (including calorie content) of a particular meal, and its infusion reduces the consumption of food [184,185]. PYY binds to Y2 receptors localized on the presynaptic terminals of neuropeptide Y and to agouti-related protein neurons in the hypothalamus, which causes inactivation of these neurons, resulting in anorexia [186]. Moreover, PYY is the main regulator of the ileal brake and consequently inhibits the consumption of further food once nutrients reach the terminal ileum [187-194]. Enteroglucagon reduces gastric motility and secretion [195-200] and, similar to PYY, the amount released into the bloodstream is proportional to the calories ingested [201,202]; however, enteroglucagon seems to have only a modest anorexigenic effect [145]. Serotonin has also been reported to have an anorexigenic effect [203].
Table 1.
Abnormalities in the gastrointestinal endocrine cells that regulate appetite in IBS patients
| Gastrointestinal segment | Hormone | Cell density | Hormone function | ||
|---|---|---|---|---|---|
| IBS-D | IBS-M | IBS-C | |||
| Stomach | Ghrelin | Increased | Unchanged | Decreased | Orexigenic (increases appetite) |
| Serotonin | Increased | Unchanged | Decreased | Anorexigenic (decreases appetite) | |
| Duodenum | CCK | Decreased | Unchanged | Unchanged | Anorexigenic (decreases appetite) |
| Serotonin | Unchanged | Unchanged | Unchanged | See above | |
| Ileum | PYY | Unchanged | Unchanged | Increased | Anorexigenic (decreases appetite) |
| Serotonin | Decreased | Decreased | Decreased | See above | |
| Colon | PYY | Decreased | Unknown | Decreased | See above |
| Serotonin | Decreased | Unknown | Decreased | See above | |
| Rectum | PYY | Decreased | Decreased | Decreased | See above |
| Enteroglucagon | Decreased | Unknown | Decreased | Anorexigenic (decreases appetite) | |
| Serotonin | Unchanged | Unknown | Unchanged | See above | |
IBS-D, IBS patients with diarrhoea as the predominant symptom; IBS-M, IBS patients with alternating diarrhea and constipation; IBS-C, IBS patients with constipation as the predominant symptom.
Figure 1.
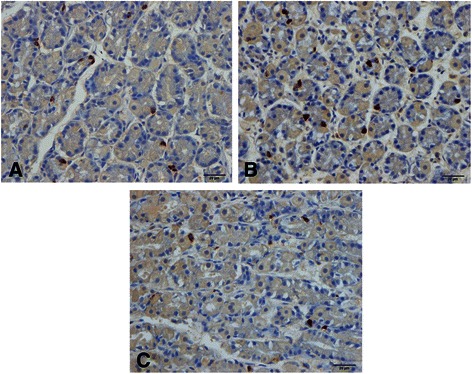
Ghrelin-immunoreactive cells in the oxyntic mucosa of a healthy subject (A), in a patient with IBS-D (B) and in a patient with IBS-C (C).
Figure 2.
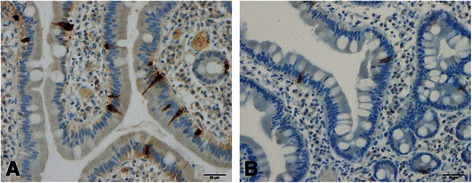
Duodenal CCK cells in a healthy subject (A) and in a patient with IBS (B).
Figure 3.
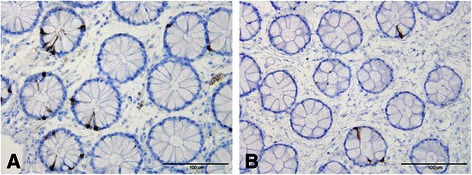
PYY cells in the colon of a healthy subject (A) and in a patient with IBS (B).
Figure 4.
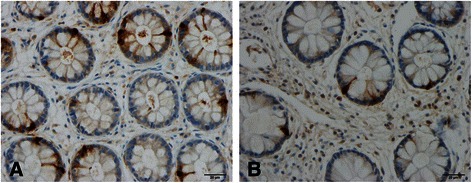
Rectal enteroglucagon cells in a healthy subject (A) and in a patient with IBS (B).
Figure 5.
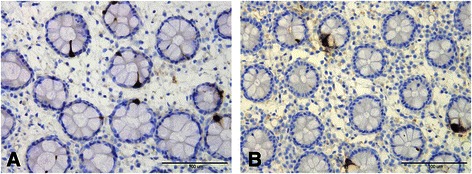
Serotonin cells in the colon of a healthy subject (A) and in a patient with IBS (B).
Whereas the ghrelin cell density is increased in IBS patients with diarrhoea as the predominant symptom (IBS-D), it is reduced in IBS patients with constipation as the predominant symptom (IBS-C). It is therefore reasonable to assume that IBS-D patients would have a greater appetite and food intake than those with IBS-C. In both IBS subtypes, the densities of the four endocrine cell types that produce anorexigenic hormones—namely CCK, PYY, enteroglucagon and serotonin—are reduced. Thus, the changes in the gastrointestinal endocrine cells regulating appetite in IBS patients favour an increase in food consumption. BMI and appetite in IBS patients have not been studied in detail, and the available data are controversial. It is not clear whether IBS patients have a greater appetite, which is opposed by the avoidance of eating because of worsening of symptoms upon eating. Further studies are needed to clarify this issue.
Diet and management of IBS
As mentioned above, it is recommended that IBS patients consume a diet that is poor in FODMAPs and insoluble fibre. IBS patients make a conscious choice to avoid certain food items, some of which are rich in FODMAPs, but they may also consume large amounts of other food items that are rich in FODMAPs and avoid food sources that are important to health maintenance [23]. Dietary guidance is therefore important for IBS patients [23,99]. Furthermore, in our experience individual dietary guidance is preferable due to the wide variety of individual tolerances to different FODMAPs-rich food items, probably due to the intestinal microbiota differing between individuals.
Consuming probiotics increases the tolerance for FODMAPs-rich foodstuffs, and adding regular exercise amplifies the beneficial effects of such a diet [1,204]. People in numerous countries (including Norway) rely on bread and wheat products for a substantial part of their diet [205]. Gluten-free bread (mostly made of rice/corn) contains 0.19 g/100 g fructans, and bread made with spelt flour contains 0.14 g/100 g [96]. Either gluten-free products or spelt products can be consumed. Many of our patients consume spelt bread and spelt products rather than gluten-free products, with satisfactory results. The protein content of spelt flour is also 16% lower in terms of gluten than that in wheat [206].
Conclusion
Diet plays a major role in the pathophysiology of IBS and is a powerful tool for managing IBS. There is no convincing evidence that IBS patients suffer from food allergy/intolerance, and there is evidence that NCGS is caused by the fructans in wheat, rather than by gluten. NCGS patients appear to be IBS patients who have self-diagnosed and self-treated with a GFD. Food items that are rich in poorly absorbed short-chain carbohydrates (FODMAPs) and insoluble fibre trigger IBS symptoms. There appear to be several mechanisms by which these food items exert this effect (Figure 6):
Upon entering the distal small intestine and colon, they increase the osmotic pressure and provide a substrate for bacterial fermentation, resulting in gas production, abdominal distension and abdominal pain/discomfort.
They are prebiotics that favour the colonization of the large intestine with Clostridium bacteria, which produce gas on fermentation.
They affect the gastrointestinal endocrine cells that regulate gastrointestinal sensation, motility, secretion and absorption, as well as local immune defence and food consumption.
Figure 6.
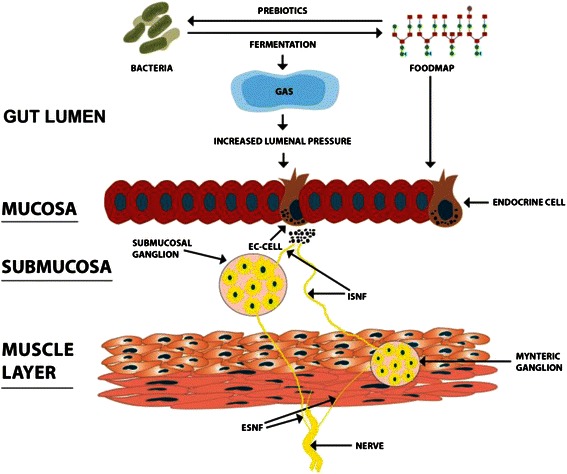
Schematic illustration for the possible mechanisms by which FODMAPs can trigger IBS symptoms. Upon reaching the large intestine FODMAPs can exert direct or indirect effect on the intestinal endocrine cells. They act as prebiotics and change the intestinal flora and they are fermented by the intestinal microbiota with gas production. The production of gas increases the luminal pressure and stimulates the release of serotonin from serotonin (EC) cells. Serotonin act on the intrinsic sensory nerve fibres (ISNF) of the submucosal and myenteric ganglia, which in turn convey the activation to the extrinsic sensory nerve fibres (ESNF) to the central nervous system.
The link between obesity and IBS is an interesting area that needs to be explored further. This is of particular interest since IBS patients have an increased density of ghrelin cells, which increases appetite, stimulates the consumption of food and body weight gain, and have decreased densities of the four endocrine cells that produce anorexigenic hormones, namely CCK, PYY, enteroglucagon and serotonin.
A diet that is poor in FODMAPs and insoluble fibre reduces the symptoms and improves the quality of life of IBS patients. Individual dietary guidance is necessary to identify a suitable diet to which the patient is likely to adhere to in the long term. Combining this diet with probiotics and regular exercise will amplify the effect of such a diet.
Acknowledgements
This work was supported by grants from Helse-Vest and Helse-Fonna.
Abbreviations
- AGA
Anti-gliadin antibodies
- ARC
Arcuate nucleus
- FODMAPs
Fermentable oligo-, di-, monosaccharides and polyols
- GFD
Gluten-free diet
- IBS
Irritable bowel syndrome
- IBS-D
IBS patients with diarrhoea as the predominant symptom
- IBS-C
IBS patients with constipation as the predominant symptom
- NCGS
Non-coeliac gluten sensitivity
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MES delimited the topics, performed the bibliographic search and drafted the manuscript. GD contributed to the planning of the review and made comments that improved the manuscript. Both authors read and approved the final manuscript
Contributor Information
Magdy El-Salhy, Email: magdy.el-salhy@helse-fonna.no.
Doris Gundersen, Email: Doris.Irene.Gundersen@helse-fonna.no.
References
- 1.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: diagnosis, pathogenesis and treatment options. New York: Nova Science Publishers, Inc.; 2012. [Google Scholar]
- 2.Thompson WG. A World View of IBS. In: Camilleri M, Spiller RC, editors. Irritable Bowel Syndrome. Philadelphia and London: Saunders; 2002. pp. 17–26. [Google Scholar]
- 3.Agreus L, Svardsudd K, Nyren O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–80. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WG, Heaton KW. Functional bowel disorders in apparently healthy people. Gastroenterology. 1980;79:283–8. [PubMed] [Google Scholar]
- 5.Kennedy TM, Jones RH, Hungin AP, O’Flanagan H, Kelly P. Irritable bowel syndrome, gastro-oesophageal reflux, and bronchial hyper-responsiveness in the general population. Gut. 1998;43:770–4. doi: 10.1136/gut.43.6.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. U.S. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993;38:1569–80. doi: 10.1007/BF01303162. [DOI] [PubMed] [Google Scholar]
- 7.Talley NJ, Gabriel SE, Harmsen WS, Zinsmeister AR, Evans RW. Medical costs in community subjects with irritable bowel syndrome. Gastroenterology. 1995;109:1736–41. doi: 10.1016/0016-5085(95)90738-6. [DOI] [PubMed] [Google Scholar]
- 8.Hungin AP, Whorwell PJ, Tack J, Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17:643–50. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones R, Lydeard S. Irritable bowel syndrome in the general population. BMJ. 1992;304:87–90. doi: 10.1136/bmj.304.6819.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordie AK. Functional disorders of the colon. J Indian Med Assoc. 1972;58:451–6. [PubMed] [Google Scholar]
- 11.O’Keefe EA, Talley NJ, Zinsmeister AR, Jacobsen SJ. Bowel disorders impair functional status and quality of life in the elderly: a population-based study. J Gerontol A Biol Sci Med Sci. 1995;50:M184–9. doi: 10.1093/gerona/50A.4.M184. [DOI] [PubMed] [Google Scholar]
- 12.Everhart JE, Renault PF. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology. 1991;100:998–1005. doi: 10.1016/0016-5085(91)90275-p. [DOI] [PubMed] [Google Scholar]
- 13.Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- 14.Quigley EM, Locke GR, Mueller-Lissner S, Paulo LG, Tytgat GN, Helfrich I, et al. Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther. 2006;24:411–9. doi: 10.1111/j.1365-2036.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- 15.Harvey RF, Salih SY, Read AE. Organic and functional disorders in 2000 gastroenterology outpatients. Lancet. 1983;1:632–4. doi: 10.1016/S0140-6736(83)91802-0. [DOI] [PubMed] [Google Scholar]
- 16.Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002;47:225–35. doi: 10.1023/A:1013208713670. [DOI] [PubMed] [Google Scholar]
- 17.Miller V, Whitaker K, Morris JA, Whorwell PJ. Gender and irritable bowel syndrome: the male connection. J Clin Gastroenterol. 2004;38:558–60. doi: 10.1097/00004836-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Whitehead WE, Burnett CK, Cook EW, 3rd, Taub E. Impact of irritable bowel syndrome on quality of life. Dig Dis Sci. 1996;41:2248–53. doi: 10.1007/BF02071408. [DOI] [PubMed] [Google Scholar]
- 19.Gralnek IM, Hays RD, Kilbourne A, Naliboff B, Mayer EA. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology. 2000;119:654–60. doi: 10.1053/gast.2000.16484. [DOI] [PubMed] [Google Scholar]
- 20.Simren M, Mansson A, Langkilde AM, Svedlund J, Abrahamsson H, Bengtsson U, et al. Food-related gastrointestinal symptoms in the irritable bowel syndrome. Digestion. 2001;63:108–15. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- 21.Nanda R, James R, Smith H, Dudley CR, Jewell DP. Food intolerance and the irritable bowel syndrome. Gut. 1989;30:1099–104. doi: 10.1136/gut.30.8.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simren M. Self-reported food-related gastrointestinal symptoms in IBS are common and associated with more severe symptoms and reduced quality of life. Am J Gastroenterol. 2013;108:634–41. doi: 10.1038/ajg.2013.105. [DOI] [PubMed] [Google Scholar]
- 23.Ostgaard H, Hausken T, Gundersen D, El-Salhy M. Diet and effects of diet management on quality of life and symptoms in patients with irritable bowel syndrome. Mol Med Report. 2012;5:1382–90. doi: 10.3892/mmr.2012.843. [DOI] [PubMed] [Google Scholar]
- 24.Jarrett M, Heitkemper MM, Bond EF, Georges J. Comparison of diet composition in women with and without functional bowel disorder. Gastroenterol Nurs. 1994;16:253–8. doi: 10.1097/00001610-199406000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Saito YA, Locke GR, 3rd, Weaver AL, Zinsmeister AR, Talley NJ. Diet and functional gastrointestinal disorders: a population-based case-control study. Am J Gastroenterol. 2005;100:2743–8. doi: 10.1111/j.1572-0241.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 26.Williams EA, Nai X, Corfe BM. Dietary intakes in people with irritable bowel syndrome. BMC Gastroenterol. 2011;11:9. doi: 10.1186/1471-230X-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohn L, Storsrud S, Simren M. Nutrient intake in patients with irritable bowel syndrome compared with the general population. Neurogastroenterol Motil. 2013;25:23–e21. doi: 10.1111/nmo.12001. [DOI] [PubMed] [Google Scholar]
- 28.Ligaarden SC, Lydersen S, Farup PG. Diet in subjects with irritable bowel syndrome: a cross-sectional study in the general population. BMC Gastroenterol. 2012;12:61. doi: 10.1186/1471-230X-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Salhy M, Ostgaard H, Gundersen D, Hatlebakk JG, Hausken T. The role of diet in the pathogenesis and management of irritable bowel syndrome (Review) Int J Mol Med. 2012;29:723–31. doi: 10.3892/ijmm.2012.926. [DOI] [PubMed] [Google Scholar]
- 30.Monsbakken KW, Vandvik PO, Farup PG. Perceived food intolerance in subjects with irritable bowel syndrome– etiology, prevalence and consequences. Eur J Clin Nutr. 2006;60:667–72. doi: 10.1038/sj.ejcn.1602367. [DOI] [PubMed] [Google Scholar]
- 31.Wald A, Rakel D. Behavioral and complementary approaches for the treatment of irritable bowel syndrome. Nutr Clin Pract. 2008;23:284–92. doi: 10.1177/0884533608318677. [DOI] [PubMed] [Google Scholar]
- 32.Heizer WD, Southern S, McGovern S. The role of diet in symptoms of irritable bowel syndrome in adults: a narrative review. J Am Diet Assoc. 2009;109:1204–14. doi: 10.1016/j.jada.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Morcos A, Dinan T, Quigley EM. Irritable bowel syndrome: role of food in pathogenesis and management. J Dig Dis. 2009;10:237–46. doi: 10.1111/j.1751-2980.2009.00392.x. [DOI] [PubMed] [Google Scholar]
- 34.Eswaran S, Tack J, Chey WD. Food: the forgotten factor in the irritable bowel syndrome. Gastroenterol Clin North Am. 2011;40:141–62. doi: 10.1016/j.gtc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Austin GL, Dalton CB, Hu Y, Morris CB, Hankins J, Weinland SR, et al. A very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7:706–8. doi: 10.1016/j.cgh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Interaction between ingested nutrients and gut endocrine cells in patients with irritable bowel syndrome (Review) Int J Mol Med. 2014;34:363–71. doi: 10.3892/ijmm.2014.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee YJ, Park KS. Irritable bowel syndrome: Emerging paradigm in pathophysiology. World J Gastroenterol. 2014;20:2456–69. doi: 10.3748/wjg.v20.i10.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asare F, Storsrud S, Simren M. Meditation over medication for irritable bowel syndrome? On exercise and alternative treatments for irritable bowel syndrome. Curr Gastroenterol Rep. 2012;14:283–9. doi: 10.1007/s11894-012-0268-2. [DOI] [PubMed] [Google Scholar]
- 39.Gibson PR. Food intolerance in functional bowel disorders. J Gastroenterol Hepatol. 2011;26(Suppl 3):128–31. doi: 10.1111/j.1440-1746.2011.06650.x. [DOI] [PubMed] [Google Scholar]
- 40.Gibson PR, Shepherd SJ. Food choice as a key management strategy for functional gastrointestinal symptoms. Am J Gastroenterol. 2012;107:657–66. doi: 10.1038/ajg.2012.49. [DOI] [PubMed] [Google Scholar]
- 41.Soares RL. Irritable bowel syndrome: A clinical review. World J Gastroenterol. 2014;20:12144–60. doi: 10.3748/wjg.v20.i34.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El-Salhy M. Irritable bowel syndrome: diagnosis and pathogenesis. World J Gastroenterol. 2012;18:5151–63. doi: 10.3748/wjg.v18.i42.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. 2014;20:384–400. doi: 10.3748/wjg.v20.i2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Diet and Irritable Bowel Syndrome, with a Focus on Appetite-Regulating Hormones. In: Watson RR, editor. Nutrition in the Prevention and Treatment of Abdominal Obesity. San Diego: Elsevier; 2014. pp. 5–16. [Google Scholar]
- 45.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Irritable bowel syndrome: recent developments in diagnosis, pathophysiology, and treatment. Expert Rev Gastroenterol Hepatol. 2014;8:435–43. doi: 10.1586/17474124.2014.888952. [DOI] [PubMed] [Google Scholar]
- 46.Stefanini GF, Saggioro A, Alvisi V, Angelini G, Capurso L, di Lorenzo G, et al. Oral cromolyn sodium in comparison with elimination diet in the irritable bowel syndrome, diarrheic type. Multicenter study of 428 patients. Scand J Gastroenterol. 1995;30:535–41. doi: 10.3109/00365529509089786. [DOI] [PubMed] [Google Scholar]
- 47.Whorwell PJ. The growing case for an immunological component to irritable bowel syndrome. Clin Exp Allergy. 2007;37:805–7. doi: 10.1111/j.1365-2222.2007.02736.x. [DOI] [PubMed] [Google Scholar]
- 48.Zar S, Benson MJ, Kumar D. Food-specific serum IgG4 and IgE titers to common food antigens in irritable bowel syndrome. Am J Gastroenterol. 2005;100:1550–7. doi: 10.1111/j.1572-0241.2005.41348.x. [DOI] [PubMed] [Google Scholar]
- 49.Park MI, Camilleri M. Is there a role of food allergy in irritable bowel syndrome and functional dyspepsia? A systematic review. Neurogastroenterol Motil. 2006;18:595–607. doi: 10.1111/j.1365-2982.2005.00745.x. [DOI] [PubMed] [Google Scholar]
- 50.Uz E, Turkay C, Aytac S, Bavbek N. Risk factors for irritable bowel syndrome in Turkish population: role of food allergy. J Clin Gastroenterol. 2007;41:380–3. doi: 10.1097/01.mcg.0000225589.70706.24. [DOI] [PubMed] [Google Scholar]
- 51.Dainese R, Galliani EA, De Lazzari F, Di Leo V, Naccarato R. Discrepancies between reported food intolerance and sensitization test findings in irritable bowel syndrome patients. Am J Gastroenterol. 1999;94:1892–7. doi: 10.1111/j.1572-0241.1999.01226.x. [DOI] [PubMed] [Google Scholar]
- 52.Bischoff S, Crowe SE. Gastrointestinal food allergy: new insights into pathophysiology and clinical perspectives. Gastroenterology. 2005;128:1089–113. doi: 10.1053/j.gastro.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 53.McKee AM, Prior A, Whorwell PJ. Exclusion diets in irritable bowel syndrome: are they worthwhile? J Clin Gastroenterol. 1987;9:526–8. doi: 10.1097/00004836-198710000-00007. [DOI] [PubMed] [Google Scholar]
- 54.Boettcher E, Crowe SE. Dietary proteins and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:728–36. doi: 10.1038/ajg.2013.97. [DOI] [PubMed] [Google Scholar]
- 55.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343:1127–30. doi: 10.1016/S0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 56.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ. Risk factors for irritable bowel syndrome: role of analgesics and food sensitivities. Am J Gastroenterol. 2000;95:157–65. doi: 10.1111/j.1572-0241.2000.01678.x. [DOI] [PubMed] [Google Scholar]
- 57.Bischoff SC, Herrmann A, Manns MP. Prevalence of adverse reactions to food in patients with gastrointestinal disease. Allergy. 1996;51:811–8. doi: 10.1111/j.1398-9995.1996.tb04471.x. [DOI] [PubMed] [Google Scholar]
- 58.Jones JG, Elmes ME. The measurement of mucosal non-myelinated nerve fibre area and endocrine cell area in coeliac disease using morphometric analysis. Diagn Histopathol. 1982;5:183–8. [PubMed] [Google Scholar]
- 59.Bhat K, Harper A, Gorard DA. Perceived food and drug allergies in functional and organic gastrointestinal disorders. Aliment Pharmacol Ther. 2002;16:969–73. doi: 10.1046/j.1365-2036.2002.01256.x. [DOI] [PubMed] [Google Scholar]
- 60.Bijkerk CJ, de Wit NJ, Stalman WA, Knottnerus JA, Hoes AW, Muris JW. Irritable bowel syndrome in primary care: the patients’ and doctors’ views on symptoms, etiology and management. Can J Gastroenterol. 2003;17:363–8. doi: 10.1155/2003/532138. [DOI] [PubMed] [Google Scholar]
- 61.Davis W. Wheat belly: lose the wheat, loss the weight and find your path back to health. New York: Rodale; 2011. [Google Scholar]
- 62.Lundin KE. Non-celiac gluten sensitivity - why worry? BMC Med. 2014;12:86. doi: 10.1186/1741-7015-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aziz I, Sanders DS. Emerging concepts: from coeliac disease to non-coeliac gluten sensitivity. Proc Nutr Soc. 2012;71:576–80. doi: 10.1017/S002966511200081X. [DOI] [PubMed] [Google Scholar]
- 64.Mansueto P, Seidita A, D’Alcamo A, Carroccio A. Non-celiac gluten sensitivity: literature review. J Am Coll Nutr. 2014;33:39–54. doi: 10.1080/07315724.2014.869996. [DOI] [PubMed] [Google Scholar]
- 65.Volta U, De Giorgio R. New understanding of gluten sensitivity. Nat Rev Gastroenterol Hepatol. 2012;9:295–9. doi: 10.1038/nrgastro.2012.15. [DOI] [PubMed] [Google Scholar]
- 66.Sapone A, Bai JC, Ciacci C, Dolinsek J, Green PH, Hadjivassiliou M, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Med. 2012;10:13. doi: 10.1186/1741-7015-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Catassi C, Bai JC, Bonaz B, Bouma G, Calabro A, Carroccio A, et al. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839–53. doi: 10.3390/nu5103839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sestak K, Fortgang I. Celiac and non-celiac forms of gluten sensitivity: shifting paradigms of an old disease. Br Microbiol Res. 2013;3:585–9. doi: 10.9734/BMRJ/2013/6083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Czaja-Bulsa G. Non coeliac gluten sensitivity - a new disease with gluten intolerance. Clin Nutr. 2015;34:189–194. doi: 10.1016/j.clnu.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 70.Piston F, Gil-Humanes J, Barro F. Integration of promoters, inverted repeat sequences and proteomic data into a model for high silencing efficiency of coeliac disease related gliadins in bread wheat. BMC Plant Biol. 2013;13:136. doi: 10.1186/1471-2229-13-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ellis A, Linaker BD. Non-coeliac gluten sensitivity? Lancet. 1978;1:1358–9. doi: 10.1016/S0140-6736(78)92427-3. [DOI] [PubMed] [Google Scholar]
- 72.Cooper BT, Holmes GK, Ferguson R, Thompson RA, Allan RN, Cooke WT. Gluten-sensitive diarrhea without evidence of celiac disease. Gastroenterology. 1980;79:801–6. [PubMed] [Google Scholar]
- 73.Campanella J, Biagi F, Bianchi PI, Zanellati G, Marchese A, Corazza GR. Clinical response to gluten withdrawal is not an indicator of coeliac disease. Scand J Gastroenterol. 2008;43:1311–4. doi: 10.1080/00365520802200036. [DOI] [PubMed] [Google Scholar]
- 74.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–11. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kaukinen K, Turjanmaa K, Maki M, Partanen J, Venalainen R, Reunala T, et al. Intolerance to cereals is not specific for coeliac disease. Scand J Gastroenterol. 2000;35:942–6. doi: 10.1080/003655200750022995. [DOI] [PubMed] [Google Scholar]
- 76.Carroccio A, Mansueto P, Iacono G, Soresi M, D’Alcamo A, Cavataio F, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. Am J Gastroenterol. 2012;107:1898–906. doi: 10.1038/ajg.2012.236. [DOI] [PubMed] [Google Scholar]
- 77.Biesiekierski JR, Newnham ED, Irving PM, Barrett JS, Haines M, Doecke JD, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–14. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 78.Verdu EF, Armstrong D, Murray JA. Between celiac disease and irritable bowel syndrome: the “no man’s land” of gluten sensitivity. Am J Gastroenterol. 2009;104:1587–94. doi: 10.1038/ajg.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carroccio A, Brusca I, Mansueto P, Pirrone G, Barrale M, Di Prima L, et al. A cytologic assay for diagnosis of food hypersensitivity in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2010;8:254–60. doi: 10.1016/j.cgh.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 80.Carroccio A, Brusca I, Mansueto P, D’Alcamo A, Barrale M, Soresi M, et al. A comparison between two different in vitro basophil activation tests for gluten- and cow’s milk protein sensitivity in irritable bowel syndrome (IBS)-like patients. Clin Chem Lab Med. 2013;51:1257–63. doi: 10.1515/cclm-2012-0609. [DOI] [PubMed] [Google Scholar]
- 81.Bucci C, Zingone F, Russo I, Morra I, Tortora R, Pogna N, et al. Gliadin does not induce mucosal inflammation or basophil activation in patients with nonceliac gluten sensitivity. Clin Gastroenterol Hepatol. 2013;11:1294–9. doi: 10.1016/j.cgh.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa M, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nijeboer P, Bontkes HJ, Mulder CJ, Bouma G. Non-celiac gluten sensitivity. Is it in the gluten or the grain? J Gastrointestin Liver Dis. 2013;22:435–40. [PubMed] [Google Scholar]
- 84.Biesiekierski JR, Peters SL, Newnham ED, Rosella O, Muir JG, Gibson PR. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterology. 2013;145:320–8. doi: 10.1053/j.gastro.2013.04.051. [DOI] [PubMed] [Google Scholar]
- 85.Biesiekierski JR, Newnham ED, Shepherd SJ, Muir JG, Gibson PR. Characterization of Adults With a Self-Diagnosis of Nonceliac Gluten Sensitivity. Nutr Clin Pract. 2014;29:504–9. doi: 10.1177/0884533614529163. [DOI] [PubMed] [Google Scholar]
- 86.Sanders DS, Carter MJ, Hurlstone DP, Pearce A, Ward AM, McAlindon ME, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–8. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 87.Sanders DS, Patel D, Stephenson TJ, Ward AM, McCloskey EV, Hadjivassiliou M, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15:407–13. doi: 10.1097/00042737-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 88.Elloumi H, El Assoued Y, Ghedira I, Ben Abdelaziz A, Yacoobi MT, Ajmi S. [Immunological profile of coeliac disease in a subgroup of patients with symptoms of irritable bowel syndrome] Tunis Med. 2008;86:802–5. [PubMed] [Google Scholar]
- 89.Volta U, Tovoli F, Cicola R, Parisi C, Fabbri A, Piscaglia M, et al. Serological tests in gluten sensitivity (nonceliac gluten intolerance) J Clin Gastroenterol. 2012;46:680–5. doi: 10.1097/MCG.0b013e3182372541. [DOI] [PubMed] [Google Scholar]
- 90.Ruuskanen A, Kaukinen K, Collin P, Huhtala H, Valve R, Maki M, et al. Positive serum antigliadin antibodies without celiac disease in the elderly population: does it matter? Scand J Gastroenterol. 2010;45:1197–202. doi: 10.3109/00365521.2010.496491. [DOI] [PubMed] [Google Scholar]
- 91.Hadjivassiliou M, Gibson A, Davies-Jones GA, Lobo AJ, Stephenson TJ, Milford-Ward A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet. 1996;347:369–71. doi: 10.1016/S0140-6736(96)90540-1. [DOI] [PubMed] [Google Scholar]
- 92.Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874–82. doi: 10.1111/j.1365-2036.2010.04237.x. [DOI] [PubMed] [Google Scholar]
- 93.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261–8. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252–8. doi: 10.1111/j.1440-1746.2009.06149.x. [DOI] [PubMed] [Google Scholar]
- 95.Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707–17. doi: 10.1038/ajg.2013.96. [DOI] [PubMed] [Google Scholar]
- 96.Biesiekierski JR, Rosella O, Rose R, Liels K, Barrett JS, Shepherd SJ, et al. Quantification of fructans, galacto-oligosacharides and other short-chain carbohydrates in processed grains and cereals. J Hum Nutr Diet. 2011;24:154–76. doi: 10.1111/j.1365-277X.2010.01139.x. [DOI] [PubMed] [Google Scholar]
- 97.Muir JG, Rose R, Rosella O, Liels K, Barrett JS, Shepherd SJ, et al. Measurement of short-chain carbohydrates in common Australian vegetables and fruits by high-performance liquid chromatography (HPLC) J Agric Food Chem. 2009;57:554–65. doi: 10.1021/jf802700e. [DOI] [PubMed] [Google Scholar]
- 98.Marcason W. What is the FODMAP diet? J Acad Nutr Diet. 2012;112:1696. doi: 10.1016/j.jand.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 99.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effects of dietary guidance on the symptoms, quality of life and habitual dietary intake of patients with irritable bowel syndrome. Mol Med Rep. 2013;8:845–52. doi: 10.3892/mmr.2013.1565. [DOI] [PubMed] [Google Scholar]
- 100.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 101.Ong DK, Mitchell SB, Barrett JS, Shepherd SJ, Irving PM, Biesiekierski JR, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25:1366–73. doi: 10.1111/j.1440-1746.2010.06370.x. [DOI] [PubMed] [Google Scholar]
- 102.Gibson PR, Shepherd SJ. Personal view: food for thought–western lifestyle and susceptibility to Crohn’s disease. FODMAP hypothesis Aliment Pharmacol Ther. 2005;21:1399–409. doi: 10.1111/j.1365-2036.2005.02506.x. [DOI] [PubMed] [Google Scholar]
- 103.Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64:93–100. doi: 10.1136/gutjnl-2014-307264. [DOI] [PubMed] [Google Scholar]
- 104.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. 2014;69:519–24. doi: 10.1038/ejcn.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. 2014;10:2322–6. doi: 10.3892/mmr.2014.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased chromogranin A cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. 2015;ID 269831:8. doi: 10.1155/2015/823897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Normalization of large intestinal endocrine cells following dietary management in patients with irritable bowel syndrome. Eur J Clin Nutr. in press 2015. [DOI] [PMC free article] [PubMed]
- 108.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Duodenal chromogranin a cell density as a biomarker for the diagnosis of irritable bowel syndrome. Gastroenterol Res Pract. 2014;2014:462856. doi: 10.1155/2014/462856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.El-Salhy M, Gilja OH, Gundersen D, Hatlebakk JG, Hausken T. Endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroenterol. 2014;20:2383–91. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.El-Salhy M, Gilja OH, Gundersen D, Hausken T. Endocrine cells in the oxyntic mucosa of the stomach in patients with irritable bowel syndrome. World J Gastrointest Endosc. 2014;6:176–85. doi: 10.4253/wjge.v6.i5.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.El-Salhy M, Gilja OH, Hatlebakk JG, Hausken T. Stomach antral endocrine cells in patients with irritable bowel syndrome. Int J Mol Med. 2014;34:967–74. doi: 10.3892/ijmm.2014.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. 2014;188:60–5. doi: 10.1016/j.regpep.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 113.El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. 2012;57:873–8. doi: 10.1007/s10620-011-1948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.El-Salhy M, Hatlebakk JG, Gilja OH, Hausken T. Densities of rectal peptide YY and somatostatin cells as biomarkers for the diagnosis of irritable bowel syndrome. Peptides. 2015;67:12–9. doi: 10.1016/j.peptides.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 115.El-Salhy M, Vaali K, Dizdar V, Hausken T. Abnormal small-intestinal endocrine cells in patients with irritable bowel syndrome. Dig Dis Sci. 2010;55:3508–13. doi: 10.1007/s10620-010-1169-6. [DOI] [PubMed] [Google Scholar]
- 116.El-Salhy M, Wendelbo I, Gundersen D. Serotonin and serotonin transporter in the rectum of patients with irritable bowel disease. Mol Med Rep. 2013;8:451–5. doi: 10.3892/mmr.2013.1525. [DOI] [PubMed] [Google Scholar]
- 117.El-Salhy M, Wendelbo IH, Gundersen D. Reduced chromogranin A cell density in the ileum of patients with irritable bowel syndrome. Mol Med Rep. 2013;7:1241–4. doi: 10.3892/mmr.2013.1325. [DOI] [PubMed] [Google Scholar]
- 118.Wendelbo I, Mazzawi T, El-Salhy M. Increased serotonin transporter immunoreactivity intensity in the ileum of patients with irritable bowel disease. Mol Med Rep. 2014;9:180–4. doi: 10.3892/mmr.2013.1784. [DOI] [PubMed] [Google Scholar]
- 119.Wang SH, Dong L, Luo JY, Gong J, Li L, Lu XL, et al. Decreased expression of serotonin in the jejunum and increased numbers of mast cells in the terminal ileum in patients with irritable bowel syndrome. World J Gastroenterol. 2007;13:6041–7. doi: 10.3748/wjg.13.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Park JH, Rhee PL, Kim G, Lee JH, Kim YH, Kim JJ, et al. Enteroendocrine cell counts correlate with visceral hypersensitivity in patients with diarrhoea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2006;18:539–46. doi: 10.1111/j.1365-2982.2006.00771.x. [DOI] [PubMed] [Google Scholar]
- 121.Coates MD, Mahoney CR, Linden DR, Sampson JE, Chen J, Blaszyk H, et al. Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology. 2004;126:1657–64. doi: 10.1053/j.gastro.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 122.Wang LH, Fang XC, Pan GZ. Bacillary dysentery as a causative factor of irritable bowel syndrome and its pathogenesis. Gut. 2004;53:1096–101. doi: 10.1136/gut.2003.021154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dizdar V, Spiller R, Singh G, Hanevik K, Gilja OH, El-Salhy M, et al. Relative importance of abnormalities of CCK and 5-HT (serotonin) in Giardia-induced post-infectious irritable bowel syndrome and functional dyspepsia. Aliment Pharmacol Ther. 2010;31:883–91. doi: 10.1111/j.1365-2036.2010.04251.x. [DOI] [PubMed] [Google Scholar]
- 124.Coleman NS, Foley S, Dunlop SP, Wheatcroft J, Blackshaw E, Perkins AC, et al. Abnormalities of serotonin metabolism and their relation to symptoms in untreated celiac disease. Clin Gastroenterol Hepatol. 2006;4:874–81. doi: 10.1016/j.cgh.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 125.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:349–57. doi: 10.1016/S1542-3565(04)00726-8. [DOI] [PubMed] [Google Scholar]
- 126.Dunlop SP, Jenkins D, Neal KR, Spiller RC. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology. 2003;125:1651–9. doi: 10.1053/j.gastro.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 127.Lee KJ, Kim YB, Kim JH, Kwon HC, Kim DK, Cho SW. The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol. 2008;23:1689–94. doi: 10.1111/j.1440-1746.2008.05574.x. [DOI] [PubMed] [Google Scholar]
- 128.Kim HS, Lim JH, Park H, Lee SI. Increased immunoendocrine cells in intestinal mucosa of postinfectious irritable bowel syndrome patients 3 years after acute Shigella infection–an observation in a small case control study. Yonsei Med J. 2010;51:45–51. doi: 10.3349/ymj.2010.51.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spiller RC, Jenkins D, Thornley JP, Hebden JM, Wright T, Skinner M, et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47:804–11. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes. 2013;20:14–21. doi: 10.1097/MED.0b013e32835bc703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed) 2012;4:2783–800. doi: 10.2741/e583. [DOI] [PubMed] [Google Scholar]
- 132.Seim I, El-Salhy M, Hausken T, Gundersen D, Chopin L. Ghrelin and the brain-gut axis as a pharmacological target for appetite control. Curr Pharm Des. 2012;18:768–75. doi: 10.2174/138161212799277806. [DOI] [PubMed] [Google Scholar]
- 133.May CL, Kaestner KH. Gut endocrine cell development. Mol Cell Endocrinol. 2010;323:70–5. doi: 10.1016/j.mce.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. 2011;92:219–31. doi: 10.1111/j.1365-2613.2011.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Camilleri M. Physiological underpinnings of irritable bowel syndrome: neurohormonal mechanisms. J Physiol. 2014;592:2967–80. doi: 10.1113/jphysiol.2014.270892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, et al. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Francis CY, Whorwell PJ. Bran and irritable bowel syndrome: time for reappraisal. Lancet. 1994;344:39–40. doi: 10.1016/S0140-6736(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 138.Bijkerk CJ, Muris JW, Knottnerus JA, Hoes AW, de Wit NJ. Systematic review: the role of different types of fibre in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2004;19:245–51. doi: 10.1111/j.0269-2813.2004.01862.x. [DOI] [PubMed] [Google Scholar]
- 139.Bijkerk CJ, de Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo Cont Trial BMJ. 2009;339:b3154. doi: 10.1136/bmj.b3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kubo M, Fujiwara Y, Shiba M, Kohata Y, Yamagami H, Tanigawa T, et al. Differences between risk factors among irritable bowel syndrome subtypes in Japanese adults. Neurogastroenterol Motil. 2011;23:249–54. doi: 10.1111/j.1365-2982.2010.01640.x. [DOI] [PubMed] [Google Scholar]
- 141.Pickett-Blakely O. Obesity and irritable bowel syndrome: a comperhensive review. Gastroenterologt Hepatology. 2014;10:411–216. [PMC free article] [PubMed] [Google Scholar]
- 142.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, et al. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- 144.Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S63–7. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 145.Yu JH, Kim MS. Molecular mechanisms of appetite regulation. Diabetes Metab J. 2012;36:391–8. doi: 10.4093/dmj.2012.36.6.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.El-Salhy M. Ghrelin in gastrointestinal diseases and disorders: a possible role in the pathophysiology and clinical implications (review) Int J Mol Med. 2009;24:727–32. doi: 10.3892/ijmm_00000285. [DOI] [PubMed] [Google Scholar]
- 147.El-Salhy M, Gundersen D, Hatlebakk JG, Hausken T. Abnormal endocrine cells in the ileum of patients with irritable bowel syndrome. World J Gastroentrol. 2013;20:2383–91. doi: 10.3748/wjg.v20.i9.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.El-Salhy M, Lillebo E, Reinemo A, Salmelid L. Ghrelin in patients with irritable bowel syndrome. Int J Mol Med. 2009;23:703–7. doi: 10.3892/ijmm_00000183. [DOI] [PubMed] [Google Scholar]
- 149.El-Salhy M, Mazzawi T, Gundersen D, Hatlebakk JG, Hausken T. The role of peptide YY in gastrointestinal diseases and disorders (Review) Int J Mol Med. 2013;31:275–82. doi: 10.3892/ijmm.2012.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 151.Kojima M, Hosoda H, Kangawa K. Purification and distribution of ghrelin: the natural endogenous ligand for the growth hormone secretagogue receptor. Horm Res. 2001;56(Suppl 1):93–7. doi: 10.1159/000048143. [DOI] [PubMed] [Google Scholar]
- 152.Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–22. doi: 10.1016/S1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- 153.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 154.Masuda Y, Tanaka T, Inomata N, Ohnuma N, Tanaka S, Itoh Z, et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun. 2000;276:905–8. doi: 10.1006/bbrc.2000.3568. [DOI] [PubMed] [Google Scholar]
- 155.Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–40. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Asakawa A, Ataka K, Fujino K, Chen CY, Kato I, Fujimiya M, et al. Ghrelin family of peptides and gut motility. J Gastroenterol Hepatol. 2011;26(Suppl 3):73–4. doi: 10.1111/j.1440-1746.2011.06638.x. [DOI] [PubMed] [Google Scholar]
- 157.Dornon ville de laCour C, Lindstrom E, Norlen P, Hakanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 158.Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, et al. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol. 2004;39:1209–14. doi: 10.1080/00365520410007908. [DOI] [PubMed] [Google Scholar]
- 159.Edholm T, Levin F, Hellstrom PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25–30. doi: 10.1016/j.regpep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 160.Levin F, Edholm T, Schmidt PT, Gryback P, Jacobsson H, Degerblad M, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91:3296–302. doi: 10.1210/jc.2005-2638. [DOI] [PubMed] [Google Scholar]
- 161.Tack J, Depoortere I, Bisschops R, Delporte C, Coulie B, Meulemans A, et al. Influence of ghrelin on interdigestive gastrointestinal motility in humans. Gut. 2006;55:327–33. doi: 10.1136/gut.2004.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol Motil. 2007;19:675–80. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 163.Tumer C, Oflazoglu HD, Obay BD, Kelle M, Tasdemir E. Effect of ghrelin on gastric myoelectric activity and gastric emptying in rats. Regul Pept. 2008;146:26–32. doi: 10.1016/j.regpep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 164.Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schafer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570–6. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 165.Fujimiya M, Ataka K, Asakawa A, Chen CY, Kato I, Inui A. Regulation of gastroduodenal motility: acyl ghrelin, des-acyl ghrelin and obestatin and hypothalamic peptides. Digestion. 2012;85:90–4. doi: 10.1159/000334654. [DOI] [PubMed] [Google Scholar]
- 166.Ogiso K, Asakawa A, Amitani H, Inui A. Ghrelin: a gut hormonal basis of motility regulation and functional dyspepsia. J Gastroenterol Hepatol. 2011;26(Suppl 3):67–72. doi: 10.1111/j.1440-1746.2011.06630.x. [DOI] [PubMed] [Google Scholar]
- 167.Corwin RL, Gibbs J, Smith GP. Increased food intake after type A but not type B cholecystokinin receptor blockade. Physiol Behav. 1991;50:255–8. doi: 10.1016/0031-9384(91)90529-W. [DOI] [PubMed] [Google Scholar]
- 168.Gibbs J, Falasco JD, McHugh PR. Cholecystokinin-decreased food intake in rhesus monkeys. Am J Physiol. 1976;230:15–8. doi: 10.1152/ajplegacy.1976.230.1.15. [DOI] [PubMed] [Google Scholar]
- 169.Gibbs J, Smith GP. Cholecystokinin and satiety in rats and rhesus monkeys. Am J Clin Nutr. 1977;30:758–61. doi: 10.1093/ajcn/30.5.758. [DOI] [PubMed] [Google Scholar]
- 170.Gibbs J, Smith GP. Satiety: the roles of peptides from the stomach and the intestine. Fed Proc. 1986;45:1391–5. [PubMed] [Google Scholar]
- 171.Smith GP, Falasco J, Moran TH, Joyner KM, Gibbs J. CCK-8 decreases food intake and gastric emptying after pylorectomy or pyloroplasty. Am J Physiol. 1988;255:R113–6. doi: 10.1152/ajpregu.1988.255.1.R113. [DOI] [PubMed] [Google Scholar]
- 172.Smith GP, Gibbs J. Role of CCK in satiety and appetite control. Clin Neuropharmacol. 1992;15(1):Pt A:476A. doi: 10.1097/00002826-199201001-00248. [DOI] [PubMed] [Google Scholar]
- 173.Smith GP, Gibbs J. Gut peptides and postprandial satiety. Fed Proc. 1984;43:2889–92. [PubMed] [Google Scholar]
- 174.Smith GP, Gibbs J. Cholecystokinin: a putative satiety signal. Pharmacol Biochem Behav. 1975;3:135–8. [PubMed] [Google Scholar]
- 175.Smith GP, Tyrka A, Gibbs J. Type-A CCK receptors mediate the inhibition of food intake and activity by CCK-8 in 9- to 12-day-old rat pups. Pharmacol Biochem Behav. 1991;38:207–10. doi: 10.1016/0091-3057(91)90612-6. [DOI] [PubMed] [Google Scholar]
- 176.Stallone D, Nicolaidis S, Gibbs J. Cholecystokinin-induced anorexia depends on serotoninergic function. Am J Physiol. 1989;256:R1138–41. doi: 10.1152/ajpregu.1989.256.5.R1138. [DOI] [PubMed] [Google Scholar]
- 177.Weller A, Smith GP, Gibbs J. Endogenous cholecystokinin reduces feeding in young rats. Science. 1990;247:1589–91. doi: 10.1126/science.2321020. [DOI] [PubMed] [Google Scholar]
- 178.Woods SC, Gibbs J. The regulation of food intake by peptides. Ann N Y Acad Sci. 1989;575:236–43. doi: 10.1111/j.1749-6632.1989.tb53246.x. [DOI] [PubMed] [Google Scholar]
- 179.Huppi K, Siwarski D, Pisegna JR, Wank S. Chromosomal localization of the gastric and brain receptors for cholecystokinin (CCKAR and CCKBR) in human and mouse. Genomics. 1995;25:727–9. doi: 10.1016/0888-7543(95)80018-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Silvente-Poirot S, Wank SA. A segment of five amino acids in the second extracellular loop of the cholecystokinin-B receptor is essential for selectivity of the peptide agonist gastrin. J Biol Chem. 1996;271:14698–706. doi: 10.1074/jbc.271.25.14698. [DOI] [PubMed] [Google Scholar]
- 181.Tarasova NI, Stauber RH, Choi JK, Hudson EA, Czerwinski G, Miller JL, et al. Visualization of G protein-coupled receptor trafficking with the aid of the green fluorescent protein. Endocytosis and recycling of cholecystokinin receptor type A. J Biol Chem. 1997;272:14817–24. doi: 10.1074/jbc.272.23.14817. [DOI] [PubMed] [Google Scholar]
- 182.Tarasova NI, Wank SA, Hudson EA, Romanov VI, Czerwinski G, Resau JH, et al. Endocytosis of gastrin in cancer cells expressing gastrin/CCK-B receptor. Cell Tissue Res. 1997;287:325–33. doi: 10.1007/s004410050757. [DOI] [PubMed] [Google Scholar]
- 183.Wank SA. Cholecystokinin receptors. Am J Physiol. 1995;269:G628–46. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 184.Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349:941–8. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- 185.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 186.Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, et al. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–50. [PubMed] [Google Scholar]
- 187.Lin HC, Zhao XT, Wang L. Intestinal transit is more potently inhibited by fat in the distal (ileal brake) than in the proximal (jejunal brake) gut. Dig Dis Sci. 1997;42:19–25. doi: 10.1023/A:1018816517404. [DOI] [PubMed] [Google Scholar]
- 188.Lin HC, Zhao XT, Wang L. Jejunal brake: inhibition of intestinal transit by fat in the proximal small intestine. Dig Dis Sci. 1996;41:326–9. doi: 10.1007/BF02093823. [DOI] [PubMed] [Google Scholar]
- 189.Van Citters GW, Lin HC. The ileal brake: a fifteen-year progress report. Curr Gastroenterol Rep. 1999;1:404–9. doi: 10.1007/s11894-999-0022-6. [DOI] [PubMed] [Google Scholar]
- 190.Ohtani N, Sasaki I, Naito H, Shibata C, Matsuno S. Mediators for fat-induced ileal brake are different between stomach and proximal small intestine in conscious dogs. J Gastrointest Surg. 2001;5:377–82. doi: 10.1016/S1091-255X(01)80065-2. [DOI] [PubMed] [Google Scholar]
- 191.Pironi L, Stanghellini V, Miglioli M, Corinaldesi R, De Giorgio R, Ruggeri E, et al. Fat-induced ileal brake in humans: a dose-dependent phenomenon correlated to the plasma levels of peptide YY. Gastroenterology. 1993;105:733–9. doi: 10.1016/0016-5085(93)90890-O. [DOI] [PubMed] [Google Scholar]
- 192.Maljaars J, Peters HP, Masclee AM. Review article: The gastrointestinal tract: neuroendocrine regulation of satiety and food intake. Aliment Pharmacol Ther. 2007;26(Suppl 2):241–50. doi: 10.1111/j.1365-2036.2007.03550.x. [DOI] [PubMed] [Google Scholar]
- 193.Maljaars PW, Peters HP, Mela DJ, Masclee AA. Ileal brake: a sensible food target for appetite control. A Rev Physiol Behav. 2008;95:271–81. doi: 10.1016/j.physbeh.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 194.Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes (Lond) 2008;32:1633–9. doi: 10.1038/ijo.2008.166. [DOI] [PubMed] [Google Scholar]
- 195.Dubrasquet M, Bataille D, Gespach C. Oxyntomodulin (glucagon-37 or bioactive enteroglucagon): a potent inhibitor of pentagastrin-stimulated acid secretion in rats. Biosci Rep. 1982;2:391–5. doi: 10.1007/BF01119301. [DOI] [PubMed] [Google Scholar]
- 196.Dubrasquet M, Roze C, Ling N, Florencio H. Inhibition of gastric and pancreatic secretions by cerebroventricular injections of gastrin-releasing peptide and bombesin in rats. Regul Pept. 1982;3:105–12. doi: 10.1016/0167-0115(82)90087-8. [DOI] [PubMed] [Google Scholar]
- 197.Schjoldager BT, Baldissera FG, Mortensen PE, Holst JJ, Christiansen J. Oxyntomodulin: a potential hormone from the distal gut. Pharmacokinetics and effects on gastric acid and insulin secretion in man. Eur J Clin Invest. 1988;18:499–503. doi: 10.1111/j.1365-2362.1988.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 198.Schjoldager B, Mortensen PE, Myhre J, Christiansen J, Holst JJ. Oxyntomodulin from distal gut. Role in regulation of gastric and pancreatic functions. Dig Dis Sci. 1989;34:1411–9. doi: 10.1007/BF01538078. [DOI] [PubMed] [Google Scholar]
- 199.Dakin CL, Small CJ, Batterham RL, Neary NM, Cohen MA, Patterson M, et al. Peripheral oxyntomodulin reduces food intake and body weight gain in rats. Endocrinology. 2004;145:2687–95. doi: 10.1210/en.2003-1338. [DOI] [PubMed] [Google Scholar]
- 200.Wynne K, Park AJ, Small CJ, Patterson M, Ellis SM, Murphy KG, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes. 2005;54:2390–5. doi: 10.2337/diabetes.54.8.2390. [DOI] [PubMed] [Google Scholar]
- 201.Le Quellec A, Kervran A, Blache P, Ciurana AJ, Bataille D. Oxyntomodulin-like immunoreactivity: diurnal profile of a new potential enterogastrone. J Clin Endocrinol Metab. 1992;74:1405–9. doi: 10.1210/jcem.74.6.1592887. [DOI] [PubMed] [Google Scholar]
- 202.Ghatei MA, Uttenthal LO, Christofides ND, Bryant MG, Bloom SR. Molecular forms of human enteroglucagon in tissue and plasma: plasma responses to nutrient stimuli in health and in disorders of the upper gastrointestinal tract. J Clin Endocrinol Metab. 1983;57:488–95. doi: 10.1210/jcem-57-3-488. [DOI] [PubMed] [Google Scholar]
- 203.Fang XL, Shu G, Yu JJ, Wang LN, Yang J, Zeng QJ, et al. The Anorexigenic Effect of Serotonin Is Mediated by the Generation of NADPH Oxidase-Dependent ROS. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.El-Salhy M, Lilbo E, Reinemo A, Salmeøid L, Hausken T. Effects of a health program comprising reassurance, diet management, probiotic administration and regular exercise on symptoms and quality of life in patients with irritable bowel syndrome. Gastroenterology Insights. 2010;2:21–6. doi: 10.4081/gi.2010.e6. [DOI] [Google Scholar]
- 205.Shewry PR. Wheat. J Exp Bot. 2009;60:1537–53. doi: 10.1093/jxb/erp058. [DOI] [PubMed] [Google Scholar]
- 206.Pattison AL, Appelbee M, Trethowan RM. Characteristics of modern triticale quality: glutenin and secalin subunit composition and mixograph properties. J Agric Food Chem. 2014;62:4924–31. doi: 10.1021/jf405138w. [DOI] [PubMed] [Google Scholar]


