Editor’s Note:
What processes in the brain are responsible for individuality? Mounting imaging evidence suggests that brain circuits involved in our emotional responses are highly plastic and change with experience, affecting our temperament. Moreover, new research suggests that psychological interventions can further harness brain plasticity to promote positive behavioral changes that increase resilience and well-being.
When we reflect on the people we know—family members and individuals in our immediate social or occupational groups—we are often struck by the diversity in personality, temperament, and responses to everyday challenges. Individual variation is perhaps most salient in the realm of emotion, given that our emotions primarily determine how we respond to life’s slings and arrows and where we stand on the continuum of psychopathology and resilience. Increasing evidence also suggests that this variation in the emotional response of individuals to common challenges in everyday life is related to peripheral biology—biology below the neck—that may be consequential for physical health.1
When attempting to identify the causes of variations in individuality, we must distinguish between distal and proximal causes. Proximal causes typically feature specific brain mechanisms, neural circuits, and molecular processes that underlie the behavioral phenotypes (composites of observable traits) that we measure. Distal causes might include early learning or genetic factors that modulate neural circuits and specify starting conditions or baseline levels of activation in the proximal neural networks that directly control the behavioral phenotypes.
Studies of intra-pair variation in monozygotic (MZ; identical) twins afford an unusual opportunity to characterize variation that is due entirely to non-genetic causes since the two co-twins are genetically identical. A novel study involving 80 MZ twins examined epigenetic variation as a function of age.2 The researchers found that early in life the co-twins were virtually indistinguishable epigenetically. However, with age, increasingly pronounced epigenetic differences emerged. The authors noted that the fact that epigenetic markers were more distinct in MZ twins who were older, had different lifestyles, and spent less of their lives together underscores a significant role for environmental factors in shaping a common genotype into a different phenotype.
Other prominent behavioral phenotypes have also received extensive scientific attention. Behavioral inhibition, sometimes called anxious temperament, is a phenotype that has been studied in rodents, nonhuman primates, and humans, and is typically associated with high levels of freezing (inhibition of behavior, with the organism remaining relatively fixed in position, not moving and exhibiting high levels of vigilance), decreased vocalizations, and an increase in the stress hormone cortisol release (or corticosterone in rodents).2 Behavioral inhibition early in life is a predictor of later psychopathology and of increased activation in limbic circuits that play a role in adult anxiety.3 A related phenotype is associated with rapid recovery from a negative event. Individuals with a slow recovery rate are considered vulnerable, while those with a rapid recovery rate are thought to be resilient.4 Finally, a third phenotype involves the extent to which a person maintains a positive affect, a characteristic that is central to understanding the underlying affective dynamics of depression.5
Nonhumans and Individuality
Studies in nonhuman species have been extremely important in helping scientists to identify some of the causes and consequences of individual differences in aspects of emotional processing. In a unique recent study, Julia Freund and colleagues studied 40 inbred genetically identical mice that lived in a highly enriched environment for three months beginning at four weeks of age.6 With the goal of studying exploratory behavior, the team computed a measure that they called roaming entropy. High roaming entropy relates to exploring a wide range in a more complex environment. Low roaming entropy is associated with returning to the same location on repeated occasions.
At the end of the experiment, the team assessed hippocampal neurogenesis by counting proliferating precursor cells that they had labeled with bromodeoxyuridine (BUdR) three weeks earlier. Mice raised in this highly enriched environment displayed substantially increased neurogenesis compared with a control group. Most important, mice showing higher levels of roaming entropy also showed greater levels of neurogenesis. The fact that genetically identical mice were used in this study and that their behavior was quite similar across animals at the start of the experiment suggest that experience-dependent changes can induce profound alterations in brain function and structure. The team did not examine why some mice displayed increased roaming entropy over time and others did not, but the study underscores the potency of experience-dependent plasticity in producing individual differences in emotion-related behavior.
In a series of studies of rats bred to show either high levels of locomotor activity to novelty or low levels of locomotor activity in response to the same novel environment (an animal index of exploration/anxiety), Huda Akil and her colleagues established that low responders exhibit significantly fewer ultrasonic vocalizations, which are markers of positive affect; upon repeated exposure to environmental complexity, the low responders change their phenotype and show increases in ultrasonic vocalizations.7 Akil’s study also demonstrated microRNA differences in limbic brain regions between the high and low responder groups.8 Such microRNAs are known to be potent regulators of gene expression. These findings suggest ways in which these two phenotypes might be associated with differences in gene expression in specific limbic regions.
In a series of collaborative studies with Ned Kalin, M.D., at the University of Wisconsin, we developed a nonhuman primate model of behavioral inhibition. We found that if rhesus monkeys are exposed to the profile of an unfamiliar human, they exhibit freezing. Substantial individual differences exist in the duration of freezing. Rhesus monkeys and human toddlers share two additional features of this phenotype: vocalizations and the steroid hormone cortisol. High freezers show fewer vocalizations, while the stress hormone cortisol demonstrates freezing’s positive effects. We created a composite score of behavioral inhibition by standardizing these three metrics and averaging them. The distribution of the composite scores in a large sample is approximately normal.
We also found that individual differences in this composite are reasonably stable over time in rhesus monkeys.9 By injecting a radiolabeled glucose tracer while exposing the animal to the natural stress of the profile of a stranger, and then placing the monkey in a special nonhuman primate positron-emission tomography (PET) scanner after approximately 30 minutes, we were able to measure brain function during exposure to the stressor, since the images we obtained reflected the integrated activity from the previous 30-minute period. Using this method, we found that metabolic rate in both the amygdala and the anterior hippocampus predicted the extent of anxious temperament and behavioral inhibition.3
More recently, in a very large sample, we replicated these basic effects and established that metabolic rate only in the anterior hippocampus (not in the amygdala) was significantly heritable, a finding that was initially surprising.4 In retrospect, however, we reasoned that it is likely that the amygdala is highly plastic and responsible for various aspects of emotional learning, therefore showing a weaker heritability signal than the hippocampus.11
In other recent work, scientists found that animals with stronger resting-state connectivity (measured with resting-state functional MRI) between several regions of the prefrontal cortex, including medial and dorsolateral prefrontal sectors and the amygdala, show lower levels of glucose metabolism in the amygdala. In turn, higher levels of dorsolateral-prefrontal-amygdala connectivity are associated with decreased anxious temperament. This relationship is mediated through decreased glucose metabolism in the amygdala.12 The collective findings in studies in monkeys indicate that anxious temperament is brought about by a distributed neural network that includes the amygdala and the prefrontal cortex. Several regions of the prefrontal cortex play an important role in emotion regulation while also modulating activation in the amygdala. It is also likely that these regions modulate the time course of amygdala response.
I believe that future researchers would profit by examining experience-dependent amygdala plasticity in humans. Our studies in nonhuman primates suggest that individual differences in amygdala function and the associated circuitry directly interconnected with the amygdala, including the prefrontal cortex, the bed nucleus of the stria terminalis, the anterior hippocampus, and the periaqueductal gray play a key role in determining individual differences in anxious temperament. All of these areas likely play an important role in governing individual differences in both reactivity to and recovery from negative events.
Our studies in humans, which have begun to parse the temporal course of emotional response, are predicated on the intuition that resilience is, at least in part, associated with rapid recovery following adversity, while vulnerability is associated with the opposite—a difficulty in recovering from negative events. Temporal dynamics are also important in the realm of positive affect. Individuals who can savor and sustain positive affect may show higher levels of well-being than those who cannot.
A Matter of Timing
One central characteristic of resilience may be more rapid recovery following negative events. The recovery may occur in any of several different systems that show stress-related reactivity. Two individuals may respond equally but at different rates. This is illustrated below in the hypothetical curves in figure 1. We have measured the time course of response to emotional stimuli with both peripheral physiological measures and more direct measures of brain function.
Figure 1:
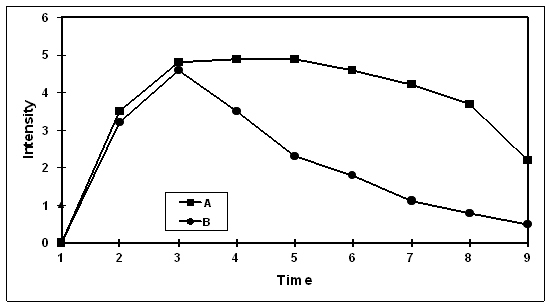
Hypothetical curves showing reactivity and recovery for two individuals, with each person showing comparable amplitude of response but with person B recovering more quickly than person A.
In a recent study, we showed that people who reported higher levels of well-being, particularly regarding “purpose in life,” faster and more complete recovery following negative stimuli.5 In another study, we found that those who recover more quickly also report higher levels of conscientiousness.6 In each of these studies, we controlled for how much reactivity the person showed to the emotional stimulus and thus we were able to obtain a “pure” measure of recovery. In yet another recent study, we used functional magnetic resonance imaging (fMRI) to identify neural correlates of recovery and found that variations in the time course of recovery of activation in the amygdala was a predictor of individual differences in neuroticism, one of the best-studied traits reflecting negative emotion.7 The most important finding in our study was that variations in reactivity—the initial responses to the negative stimuli—did not predict neuroticism. Collectively these findings underscore the importance of individual differences in recovery from negative events as a key constituent of variations in emotional style and suggest that the time course of amygdala response likely plays an important role in moderating these effects.
In another series of studies, we have examined variations in positive affect, with specific interest in the extent to which individuals persist in positive emotional response. Some have called this persistence “savoring” or “sustainment.”8 We found that patients with major depression show normal levels of initial activation in ventral striatal regions but fail to sustain this activation over time. The less the depressed patients were able to sustain ventral striatal activation over time, the lower were their reported levels of positive affect. 5 Patients who show increases in sustained activation of the ventral striatum due after two months of antidepressant treatment also show increases in treatment-related gains in positive affect.16 And in a community sample, individuals with greater sustained activation of the ventral striatum report higher levels of psychological well-being and have lower basal levels of cortisol.16 This latter finding indicates an important association between patterns of central nervous system activity that are associated with emotional styles and peripheral biological processes that are implicated in physical health.
An abundance of evidence suggests that high levels of well-being are associated with better physical health.18 The mechanisms by which this association arises are unknown, but its existence is now well documented. These findings raise the possibility that interventions that are designed both to promote well-being and to influence the central circuitry of emotional style may also have peripheral biological benefits on physical health.
Our findings also indicate that one important constituent of individual differences in positive affect and well-being is the ability to sustain positive affect following a positive incentive. Depressed patients show activation in circuits important for positive affect following the initial presentations of positive stimuli, but they fail to sustain this activation. Those who can sustain such activation report higher levels of positive affect and higher levels of well-being. In addition, in a community sample, such individuals have lower serum levels of cortisol. The temporal dynamics of emotional response play an important proximal role in modulating individual differences in emotional response. We do not yet know the distal causes of these individual differences, though some combination of genetic and environmental factors clearly plays an important role.
Plasticity and Individuality
The brain circuits that underlie individual differences in emotional response and emotion regulation are highly plastic and can be altered in an experience-dependent fashion—that is, they change in response to interventions.19 Scientists have found that several nonpharmacological interventions that are designed to reduce anxiety and depression and to promote well-being change the central circuitry of emotion both functionally and structurally.19 For example, Britta Hölzel and her colleagues found that among participants going through mindfulness-based stress reduction, the greater the decrease in perceived stress, the greater the reduction in amygdala volume over the course of the eight-week intervention.20
A recent study randomized patients with Parkinson’s disease (PD) either to an eight-week mindfulness meditation intervention or to usual care. Structural MRI was obtained before and after the eight weeks. Mindfulness meditation was associated with increases in gray-matter volume in the caudate and other related regions implicated in PD compared with the control condition.9 Thus, even among patients with a frank neurological disorder, benefits may result from a nonpharmacological intervention that targets some of the key circuitry of the regulation of attention and emotion. Unfortunately, measures of emotional style were not obtained in this study, and so I prefer not to comment on the relationship between structural brain changes and emotional aspects of individuality.
Our lab recently published a study that evaluated the impact of a short-term intervention designed to cultivate compassion and the associated positive emotions linked to compassion.10 Participants were randomly assigned to either a compassion-training intervention or a cognitive-reappraisal training intervention that was structurally matched to the compassion training. Functional MRI was obtained before and after the two-week interventions. In addition, at the conclusion of the interventions, both groups were administered an economic decision-making task to assess individual differences in altruistic behavior. We found that after two weeks of compassion training, those assigned to the compassion-training group showed significantly more altruistic behavior compared with the group assigned to the cognitive training. In addition, we found systematic alterations in brain function that predicted the increase in altruistic behavior. One key change in brain function was an increase in connectivity between the dorsolateral prefrontal cortex and the nucleus accumbens, which showed enhanced connectivity for the compassion group compared with the cognitive-reappraisal-training group. This enhanced connectivity in the compassion group predicted increased altruistic behavior. This prefrontal-striatal network is the same as the one implicated as deficient in depression, and, again indicates that the circuits underlying emotional styles are at least somewhat plastic and can be altered through training.
Individuality, particularly in the realm of emotional responding, provides color to our everyday life and infuses our interpersonal relationships with meaning. The fact that the brain networks that underlie such individuality exhibit plasticity is not surprising, for we all recognize that early adversity can result in long-term deleterious effects for a person. However, the very plasticity that can cause pathology is also the source of potential positive change. We can harness the potential of plasticity to shape the brain in more intentional ways to cultivate healthy habits of mind that can confer resilience. The prospects of having this perspective be widely recognized and adopted is personally very significant to me, for I believe that if we all took more responsibility for our minds and brains in these ways by intentionally cultivating healthy habits of mind, we can exercise the brain in ways that are similar to exercising the body and potentially promote positive behavioral changes that might increase resilience and well-being in a large fraction of the population.
Bio
Richard J. Davidson, Ph.D., is the William James and Vilas Research Professor of Psychology and Psychiatry, director of the Waisman Laboratory for Brain Imaging and Behavior, and founder of the Center for Investigating Healthy Minds, Waisman Center, at the University of Wisconsin-Madison. Named one of the 100 most influential people in the world by Time magazine in 2006, Davidson is co-author (with Sharon Begley) of the New York Times best seller, The Emotional Life of Your Brain (Penguin, 2012) and founding co-editor of the new American Psychological Association journal Emotion. He is the recipient of a National Institute of Mental Health Research Scientist Award, a MERIT Award from NIMH, an Established Investigator Award from the National Alliance for Research in Schizophrenia and Affective Disorders (NARSAD), a Distinguished Investigator Award from NARSAD, the William James Fellow Award from the American Psychological Society, and the Hilldale Award from the University of Wisconsin-Madison. He received his Ph.D. from Harvard University in psychology and has been at Wisconsin since 1984.
References
- 1.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci U S A. 2005;102:6508–12. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fraga MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS One. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oler JA, et al. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–8. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer SM, et al. Purpose in life predicts better emotional recovery from negative stimuli. PLoS One. 2013;8:e80329. doi: 10.1371/journal.pone.0080329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Javaras KN, et al. Conscientiousness predicts greater recovery from negative emotion. Emotion. 2012;12:875–81. doi: 10.1037/a0028105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuyler BS, et al. Temporal dynamics of emotional responding: amygdala recovery predicts emotional traits. Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McMakin DL, Siegle GJ, Shirk SR. Positive Affect Stimulation and Sustainment (PASS) Module for Depressed Mood: A preliminary investigation of treatment-related effects. Cognit Ther Res. 2011;35:217–226. doi: 10.1007/s10608-010-9311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pickut Ba, et al. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin Neurol Neurosurg. 2013;115:2419–25. doi: 10.1016/j.clineuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Weng HY, et al. Compassion Training Alters Altruism and Neural Responses to Suffering. Psychol Sci. 2013;24:1171–80. doi: 10.1177/0956797612469537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–76. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birn RM, et al. Evolutionarily-conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schaefer SM, et al. Purpose in life predicts better emotional recovery from negative stimuli. PLoS One. 2013;8:e80329. doi: 10.1371/journal.pone.0080329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Javaras KN, et al. Conscientiousness predicts greater recovery from negative emotion. Emotion. 2012;12:875–81. doi: 10.1037/a0028105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMakin DL, Siegle GJ, Shirk SR. Positive Affect Stimulation and Sustainment (PASS) Module for Depressed Mood: A preliminary investigation of treatment-related effects. Cognit Ther Res. 2011;35:217–226. doi: 10.1007/s10608-010-9311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heller AS, et al. Relationships Between Changes in Sustained Fronto-Striatal Connectivity and Positive Affect in Major Depression Resulting From Antidepressant Treatment. Am J Psychiatry. 2013;170:197–206. doi: 10.1176/appi.ajp.2012.12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heller AS, et al. Sustained ventral striatal activity predicts eudaimonic well-being and cortisol output. Psychol Sci. 2013;24:2191–2200. doi: 10.1177/0956797613490744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steptoe A, Dockray S, Wardle J. Positive affect and psychobiological processes relevant to health. J Pers. 2009;77:1747–76. doi: 10.1111/j.1467-6494.2009.00599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davidson RJ, McEwen BS. Social influences on neuroplasticity: stress and interventions to promote well-being. Nat Neurosci. 2012;15:689–95. doi: 10.1038/nn.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hölzel BK, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–7. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickut Ba, et al. Mindfulness based intervention in Parkinson’s disease leads to structural brain changes on MRI: a randomized controlled longitudinal trial. Clin Neurol Neurosurg. 2013;115:2419–25. doi: 10.1016/j.clineuro.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Weng HY, et al. Compassion Training Alters Altruism and Neural Responses to Suffering. Psychol Sci. 2013;24:1171–80. doi: 10.1177/0956797612469537. [DOI] [PMC free article] [PubMed] [Google Scholar]


