Abstract
Forest disturbance causes specialization of plant-frugivore networks and jeopardizes mutualistic interactions through reduction of ecological redundancy. To evaluate how simplification of a forest into an agroecosystem affects plant-disperser mutualistic interactions, we compared bat-fruit interaction indexes of specialization in tropical montane cloud forest fragments (TMCF) and shaded-coffee plantations (SCP). Bat-fruit interactions were surveyed by collection of bat fecal samples. Bat-fruit interactions were more specialized in SCP (mean H2 ' = 0.55) compared to TMCF fragments (mean H2 ' = 0.27), and were negatively correlated to bat abundance in SCP (R = -0.35). The number of shared plant species was higher in the TMCF fragments (mean = 1) compared to the SCP (mean = 0.51) and this was positively correlated to the abundance of frugivorous bats (R= 0.79). The higher specialization in SCP could be explained by lower bat abundance and lower diet overlap among bats. Coffee farmers and conservation policy makers must increase the proportion of land assigned to TMCF within agroecosystem landscapes in order to conserve frugivorous bats and their invaluable seed dispersal service.
Introduction
Seed dispersal by animals constitutes an important ecosystem service in natural forests and agroecosystems [1]. Study of the factors that modify animal-fruit interaction patterns is therefore important to the conservation of ecosystem functionality [2]. In ecology, network theory allows us to understand mutualistic interactions using different indices that describe the structure of interactions [3]. In seed dispersal networks, the specialization (H 2 ') index is one of the main informative network indices, because is related to the complementarity of mutualistic interactions and thus describes the stability of the network by interaction redundancy [4].
Previous studies have found that specialization (H 2 ') is negatively correlated to plant richness, canopy cover and forest disturbance. This is because a reduction in plant richness and canopy forest strata reduces the availability of fruit resources, causing the impoverishment of frugivorous diversity and animal-fruit interactions [5,6]. On the other hand, there is a positive relationship between specialization (H 2 ') and fruit abundance, since the dominance of specific resources promotes the presence of specific frugivorous animals [6]. This information comes from bird-fruit interaction studies [5,6] but it is not known whether specialization of other animal-fruit interactions networks behave in a similar manner to bird-fruit interactions. This is particularly true when we take into consideration the fact that frugivorous birds have low diet overlap with other frugivorous animals, such as bats [7,8].
Frugivorous bats are important seed dispersers in tropical ecosystems, especially in disturbed forest [9]. Neotropical frugivorous bats feed on specific plant taxa; for example, bats of the Carollia and Sturnira genera feed mainly on understory fruits of the genera Piper and Solanum, respectively, while bats of the genus Artibeus feed mainly on canopy fruits of the genera Ficus and Cecropia [10,11]. Agricultural activities cause a reduction in the abundance of these chiropterochoric plants and in the abundance of bats specialized in their consumption [12]. This situation could affect bat-fruit interactions because a negative relationship exists between species abundance and specialization of interactions [13,14].
The mountainous central region of Veracruz, Mexico, offers an opportunity to test the effect of agricultural activities on the specialization of bat-fruit interaction networks. This region was originally occupied by tropical montane cloud forest (TMCF) located within an altitudinal strip at between 700–1800 masl. Landform and climate dictate the extensive cultivation of high-grown coffee between 700–1,400 masl [15], which causes the remaining TMCF fragments to have a higher density of chiropterochoric plants and frugivorous bats compared to shaded-coffee plantations (SCP) [16].
Shaded-coffee plantations are the main agroecosystem in the mountainous region of central Veracruz, Mexico. Due to the presence of an arboreal stratum, this agroecosystem harbors an important proportion of the regional biodiversity [17,18]. However, given the removal of the understory in SCP, chiropterochoric plants are less abundant than is the case in the TMCF [16]. In this study, we compare the specialization of bat-fruit interactions in TMCF and SCP. In addition, we evaluate the relationship between bat abundance and specialization in both vegetation types. We expect a higher specialization in SCP compared to TMCF fragments, due to the low abundance of understory chiropterochoric plants in SCP. In addition, we expect a negative relationship between bat abundance and specialization (H 2 ') in SCP, because of the low abundance of frugivorous bats that produce only occasional bat-fruit interactions in SCP.
Material and Methods
Study area
The study was carried out in the municipalities of Xalapa and San Andrés Tlalnelhuayocan in central Veracruz, Mexico (19°31’19” to 19°29’46” N, 96°59’30” to 96°54’36” W), in two landscapes selected by a geographic information system created in ArcView 3.2. For details, see the map of study sites in [16]. The TMCF landscape (1268.11 ha) was dominated by a cattle ranching matrix (879.8 ha; 69%) with remnant TMCF fragments (388.31 ha; 31%). No SCP exists in this landscape. The SCP landscape (1253.91 ha) was dominated by a heterogeneous matrix comprising secondary TMCF fragments (265.86 ha; 21.2%), pastures and human settlements (645.77 ha; 51.5%). In this landscape, SCP comprised 342.29 ha (27.3%).
Forest cover in both landscapes was calculated using supervised classification from a LANDSAT-7 (2000) satellite image, while SCP cover was calculated using the agricultural census conducted by the Secretaría de Agricultura, Ganadería, Desarrollo Rural y Pesca (2000). We selected four TMCF fragments each in the TMCF and SCP landscapes. We also used the following selection criteria to select bat/seed sampling sites: (1) all forests patches and coffee plantations were chosen within the narrowest possible elevation range (1,300–1,500 m a.s.l.) in order to reduce the uncontrolled effect of altitude and mesoclimate; and (2) TMCF patches and SCP were located at least 7 km apart in order to avoid capturing the same individual bats in different landscapes on the same sample night. TMCF fragments or SCP replicates in each landscape window were located at least 1 km apart.
Chiropterochoric plant species survey
We conducted a survey of chiropterochoric plant species within TMCF fragments and SCP in order to generate a reference collection of the bat-fruit and seeds of the region and assess the potential fruit resource availability in these vegetation types (see Table 1). In addition, this information allowed us to determine whether the bats consume fruits that grow in the plantations or only those from outside the plantations, since the surroundings of the SCP comprised other SCP, secondary forest fragments, and pastures that could contain chiropterochoric plant species.
Table 1. Number of bat-fruit interactions per vegetation type: Tropical montane cloud forest fragment (TMCF) or shaded-coffee plantation (SCP); and number of individuals of chiropterochoric plant species recorded in plant surveys.
| Bat-fruit interactions | Bat plant survey | |||
|---|---|---|---|---|
| Plant species | TMCF | SCP | TMCF | SCP |
| Cecropiaceae | ||||
| Aff. Cecropia | 1 | 2 | ||
| Chlorantaceae | ||||
| Hedyosmum mexicanum | 60 | 1 | ||
| Clusiaceae | ||||
| Vismia mexicana* | 20 | |||
| Melastomataceae | ||||
| Miconia glaberrima^ | 1 | |||
| Miconia mexicana* | 1 | |||
| Moraceae | ||||
| Ficus (Urostigma)* | 1 | |||
| Piperaceae | ||||
| Piper lapathifolium | 85 | 7 | 106 | 3 |
| Piper hispidum | 61 | 3 | 37 | 1 |
| Piper auritum | 17 | 12 | 55 | |
| Piperaceae spp 1^ | 2 | |||
| Piperaceae spp 2* | 1 | |||
| Piperaceae spp 3^ | 1 | |||
| Piperaceae spp 4^ | 2 | |||
| Piperaceae spp 5* | 1 | |||
| Rosaceae | ||||
| Eriobotrya japonica* | 1 | |||
| Solanaceae | ||||
| Solanum aphyodendron | 53 | 19 | ||
| Solanum schlechtendalianum | 44 | 5 | 9 | |
| Solanum acerifolium | 2 | 4 | ||
| Solanum diflorum^ | 2 | |||
| Lycianthes geminifolia | 6 | 12 | ||
| Solanaceae spp 1^ | 3 | |||
| Solanaceae spp 2 | 1 | 1 | ||
| Solanaceae spp 3^ | 4 | |||
| Solanaceae spp 4^ | 7 | |||
| Ulmaceae | ||||
| Trema micrantha^ | 12 | 1 | 2 | 4 |
| Unidentified | ||||
| spp. 1^ | 5 | |||
| spp. 2* | 1 | |||
| spp. 3* | 1 | |||
| spp. 4^ | 1 | |||
| spp. 5^ | 1 | |||
| Total of bat-fruit interactions per vegetation type | 371 | 94 | ||
| Total individual plants | 209 | 8 | ||
| Plant richness | 22 | 19 | 5 | 3 |
^ denotes a plant species exclusive to the forest fragments;
* denotes a plant species exclusive to the coffee plantations.
We recorded the presence of chiropterochoric species along ten strip-transects (50 x 2 m) that were randomly established within each TMCF fragment or SCP, making a total of 0.1 ha of sampling area per site. The plant species survey was based on the database of Neotropical bat/plant interactions [19]. All trees or shrubs of height ≥1 m rooted within transects were recorded and identified. We collected specimens of those plants that could not be identified in the field, for subsequent determination in the XAL herbarium of the Instituto de Ecología, A.C. in Xalapa, Veracruz. All surveys were conducted during the bat sampling seasons (see below).
Bat-fruit interaction data collection
Data on interactions between bats and fruit were obtained through fecal samples. Collection of fecal samples is a reliable method by which to assess frugivorous bat diet whenever samples contain identifiable seed or fruit pulp [20]. One fecal sample could have seeds from more than one plant species [21], but we counted seed deposition per plant species as a bat-fruit interaction. Thus, the number of fecal samples may not necessarily match with the number of bat-fruit interactions.
Sampling was carried out over the course of one year (June 2007-April 2008), covering the three climatic seasons recognized for the region: dry, wet, and the season of northerly cold fronts (“nortes”) [22]. We thus had 12 interaction matrices per vegetation type (four sites, sampled over three seasons). Bats were captured using eight mist nets (9 x 2.4 m, with a 14 x 14 mm mesh size) placed on the ground in each forest fragment and coffee plantation site for six nights, giving 24 nights of sampling per vegetation type. Mist nets were placed at ground level only, since there is no significant difference between canopy and ground mist nets in terms of the detectability of canopy frugivorous bats [23].
Mist nets were set in pairs some 15 to 30 m apart, within each fragment or plantation. Nets were opened at dusk for five hours and checked every 30 minutes. This produced a bat sampling effort of 20,736 m2·h [24] in each vegetation type. To reduce the effects of variable weather, successive sampling nights were alternated between vegetation types. Dates around the full moon were avoided, since frugivorous bat activity declines during this period [25]. In forests, bats move through understory areas with little foliage, which facilitates their movement [26]; mist nets were therefore placed diagonally across man-made trails. In the SCP, mist nets were placed along the lanes that delimit the plantation sections. Captured bats were identified using field keys [27], tagged with a numbered plastic collar and released at the site of capture.
We used two complementary methods to collect fecal samples. First, we placed a plastic sheet (9 x 1 m) below each net in order to gather droppings deposited before the bat was removed from the net [21]. In addition, the canvas bags used for holding bats (< 30 min) were also inspected for feces. To avoid possible confusion in the assignation of the fecal sample to the captured bat we visited the mist-nets every 30 min. On rare occasions, two bats became entangled in the net simultaneously; however, we were always able to identify which individual produced the scats by looking for seeds remaining on the bat and net, by the color or type of seeds on the plastic sheet and/or by the vertical position of the droppings under the bat.
Plant seeds defecated by bats were identified to the lowest possible taxonomic level (family, genus or species) by comparison to our reference collection using a stereoscopic microscope (20×). Seeds of fecal samples that did not match with the reference collection were classified as morphospecies.
Data analysis
We evaluated the completeness of bat-fruit interactions sampling by vegetation type and compared the richness of seed consumed by bats between vegetation types. In the former case, we compared the observed seed richness to the expected richness in each vegetation type. In the latter case, we compared the observed seed richness of the two vegetation types. Since we did not count the number of seeds of plant species in each fecal sample, we selected the bootstrap estimator of richness, which is sensitive to species incidence. Individual bats that produced fecal samples were considered as the sample unit for this analysis [28]. Values for the observed species richness, as well as the bootstrap-estimated species richness with their respective confidence intervals, were obtained by rarefaction of all pooled samples with 100 randomizations without replacement using EstimateS [29]. To determine significant difference in dispersed seed richness between vegetation types, we compared the 84% confidence intervals (CI) that robustly mimic the 0.05 statistical test for asymmetric CI at α ≤ 0.05 [30]. Where the 84% CI overlapped, we considered that seed dispersed richness did not differ statistically.
In order to evaluate spatial autocorrelation and pseudo-replication among sampling/replication sites, we conducted a Mantel test [31]. Originally, this analysis compares the observed correlation between genetic similarity and geographic distance versus the distribution of correlation values of randomized matrices. In our case, the observed correlation was between plant seed richness, number of fecal samples and seed composition similarity among sites of each vegetation type and geographic distance among sampling sites. The Euclidean method was used for the calculation of seed richness, similarity of number of fecal samples and geographic distances matrices, while the Bray-Curtis method was selected for calculating seed composition similarity. The number of randomizations of the matrices values was 9,999.
To compare bat-fruit interactions specialization between vegetation types and their possible interaction with climatic season, we constructed interaction matrices by site and season (see the interaction matrices in S1 Appendix). Two indexes related to specialization of ecological networks were calculated: specialization index (H 2 '), and mean number of shared species consumed by bats of the network. The specialization index (H 2 ') describes the complementarity of interactions among members of a network. Values of H 2 ' range from zero to one; values approaching zero suggest a high complementarity of interactions (low specialization) or high redundancy of interactions in the network, while values approaching one suggest low complementarity (high specialization) of interactions of the network [32]. Compared to highly specialized networks, those with low values of H 2 ' can more easily compensate and maintain their stability in the event of a disturbance or fluctuation in environmental conditions [3]. The underlying equation is the same as the two-dimensional Shannon entropy, however, the value calculated for the given network (H 2) is standardized against the minimum (H 2min) and maximum (H 2max) possible values for the same distribution of interaction:
The mean number of shared species index reveals the mean number of plants consumed by a pair of bat species in the interaction matrix data [33]. To compare the H 2 ' and the mean number of shared species values between TMCF fragments and SCP, we used generalized linear models (GLM). A GLM with a post-hoc χ2 analysis for standardized coefficients and gamma distribution of errors was used for the H 2 ' index because the index values do not follow a normal distribution. For the mean number of shared species index values, we used a normal error distribution (equivalent to a standard ANOVA). The syntax for fitting each linear model was as follows: glm/aov (‘index value’ ~ habitat * season, ‘error’).
We evaluated the dependency of specialization indexes on frugivorous bat abundance using analysis of covariance (ANCOVA) procedures. Included in this analysis were the specialization indexes calculated by each site in turn as the dependent variable, the frugivorous bat abundance of each site as the continuous variable, and vegetation type as a factor. The addition of vegetation type as a factor allows us to detect possible differences among slopes (significant effect of the bat abundance-vegetation interaction) or intercepts (significant effect of the vegetation factor). Since the specialization index residuals were not normally distributed and the relationship between any index and bat abundance was non-linear, we transformed both the indexes and the abundance values to log(x+1). The frugivorous bat abundance data came from all disperser bats species captured at each site, including bats that produced no fecal samples. All analyses were performed using the vegan, ade4, bipartite and stats libraries of the R 2.12.2 software [34–36].
Results
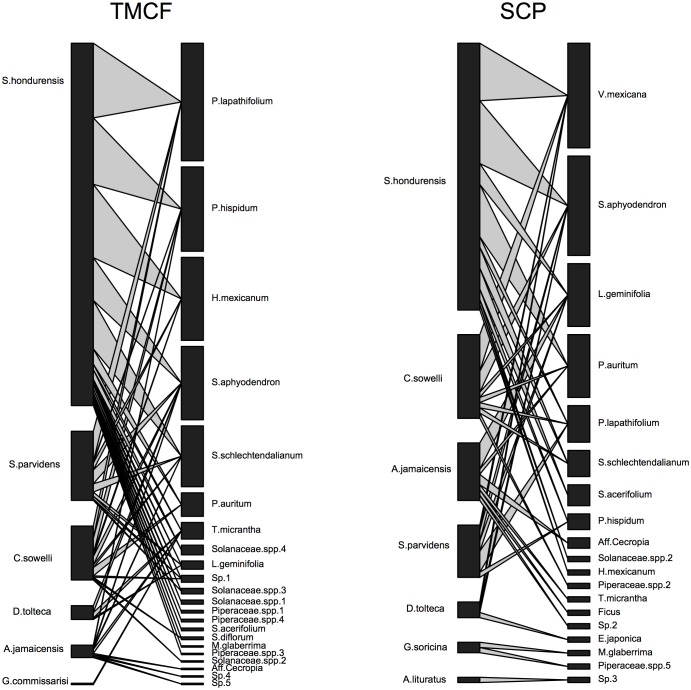
In total, we recorded 768 bats belonging to 16 species in three families (see S1 Table). Eight frugivorous bat species were recorded; six in TMCF fragments and seven in SCP (Fig 1). We recorded fruit consumption in nectarivorous bats of the genus Glossophaga; this genus includes species that are able to consume seasonal fruits in the absence of nectar resources [37]. We observed a total of 465 fecal samples (371 in TMCF vs. 94 in SCP), with seeds of 30 plant species from both vegetation types (Table 1). In the TMCF, 259 fecal samples had seeds of one plant species, 48 had seeds of two plant species, four had seeds of three plant species and one fecal sample contained the seeds of four different plants species. In the SCP, 72 fecal samples had seeds of one plant species and 11 fecal samples had seeds of two plant species. All recaptures occurred in replicates of the same vegetation type, i.e. we did not recapture any individuals in a vegetation type that differed from that in which they were first captured.
Fig 1. Quantitative bipartite plant-bat interaction graph for tropical montane cloud forest fragments (TMCF) and shaded-coffee plantations (SCP).
For each bipartite graph, the right-hand bar size represents the number of plant species in fecal samples and left-hand bar size represents the number of bats for which a fecal sample was obtained. Linkage width indicates the frequency of each trophic interaction, in TMCF were recorded 371 interactions, while in SCP 94.
We found no autocorrelation between ecological and geographic distance of our sampling sites (TMCF richness: r = 0.49, P = 0.17; SCP richness: r = 0.30, P = 0.24; TMCF fecal samples: r = 0.52, P = 0.12; SCP fecal samples: r = –0.11, P = 0.61; and TMCF seed composition: r = 0.50, P = 0.16; SCP seed composition: r = –0.07, P = 0.50). These results indicate that our bat-plant sampling sites provided spatially independent data. Our chiropterochoric plant species survey within the SCP revealed a lower density of chiropterochoric plants compared to that of the TMCF fragments (Table 1). Evaluation of the bat-fruit interaction survey showed no differences between the observed and estimated seed richness in the TMCF fragments, and SCP (Table 2). There were no differences in the observed richness of dispersed seeds between the TMCF fragments and the SCP (Table 2).
Table 2. Observed and estimated seed richness by the bootstrap predictor in tropical montane cloud forest fragments (TMCF), and shaded-coffee plantation (SCP).
| TMCF | SCP | |
|---|---|---|
| Observed richness | 22 ± 3 | 19 ± 5 |
| Estimated richness | 25 ± 0 | 23 ± 1 |
Error values represent a confidence interval of ± 84%.
Bat-fruit interactions networks were more specialized in the SCP than in the TMCF fragments (GLM: χ2 = 0.29, df = 1, P = 0.03, Table 3) and there was neither a seasonal effect nor an interaction between season and vegetation (season: χ2 = 0.18, df = 2, P = 0.15; season-vegetation interaction: χ2 = 0.20, df = 2, P = 0.13). The mean number of species shared by bats was higher in the TMCF fragments than in SCP (F = 10.61, df = 1, P = 0.005, Table 3) and again there was neither a seasonal effect nor an interaction between season and vegetation (season: F = 3.18, df = 1, P = 0.07; season-vegetation interaction: F = 1.04, df = 1, P = 0.37).
Table 3. Specialization index comparisons of bat-fruit interactions in tropical montane cloud forests fragments (TMCF) and shaded-coffee plantations (SCP).
| TMCF | SCP | |
|---|---|---|
| Mean shared plants | 1 ± 0.06 | 0.5 ± 0.16 |
| Specialization (H 2 ') | 0.27 ± 0.05 | 0.55 ± 0.15 |
Values represent the mean and one standard error.
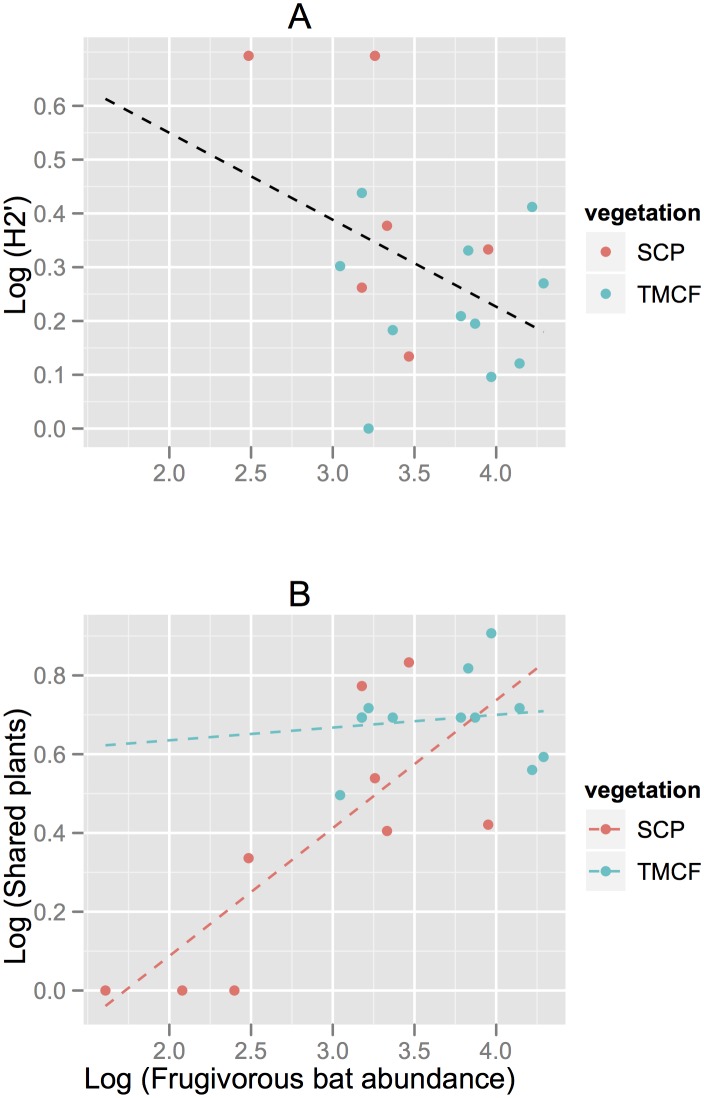
Since it was not justified to assume differences in the linear relationship between vegetation types, SCP vs. TMCF (t = -1.673, P = 0.12 for intercepts; t = 1.482, P = 0.16 for slopes), we simplified the ANCOVA model by retiring the effect of vegetation. After simplifying the model, we found a negative relationship between frugivorous bat abundance and H 2 ' (R = -0.35), although this was not statistically significant (F = 3.19; df = 1, 15; P = 0.09; Fig 2A). On the other hand, there was a significant positive linear relationship between bat abundance and mean number of plant species shared by bats (F = 30.1; df = 17; P = 0.0004); and a difference in the slopes (t = -2.17, P = 0.04) and intercepts (t = -2.42, P = 0.027) of the lines fitted to each vegetation type (TMCF b = 0.57; SCP b = 0.01; t = -2.16; P < 0.05; Fig 2B).
Fig 2. Relationship between frugivorous bat abundance and specialization indices in tropical montane cloud forests fragments (TMCF) and shaded-coffee plantations (SCP).
A: specialization (H 2 '); B: mean shared plants species. Dotted lines represent predicted linear models.
Discussion
We hypothesized that specialization of bat-fruit interactions is affected by the lower chiropterochoric plant and bat abundance in SCP. The higher specialization of SCP bat-fruit interaction networks and the negative relationship between bat abundance and H 2 ' support the hypothesis that specialization is related to bat abundance. In the following sections, we discuss the possible factors that may affect specialization of ecological networks in agroecosystems such as SCP and the consequences for bat-fruit interaction networks. Finally, we discuss the implications of our results for the conservation of frugivorous bats and the valuable seed dispersal service they perform.
Specialization in bat-fruit networks in agroecosystems
The lower values of specialization in TCMF fragments could be due to the high density of chiropterochoric plants and bats in this vegetation type compared to SCP [16]. Low H 2 ' values in TMCF reflect the high complementarity of the bat-fruit interactions (see S1 Appendix). In contrast, the low density of bats in SCP results in a high specialization of networks and less shared plants dispersed by bats (see Table 3 and Fig 2B). These results are consistent with previous studies showing a negative relationship between species abundance and specialization of interactions [13,14].
The values of the specialization index observed in SCP (mean H 2 ' = 0.55) and TCMF fragments (mean H 2 ' = 0.27) are in the range reported previously for Neotropical bat-fruit interactions (H 2 ' = 0.18–0.51). The fluctuation in specialization values is not related to the richness of plants or bats [38]. For example, bat-plant interactions networks with seven species of bats and 12 seed dispersed plants species had H 2 ' value of 0.51, while networks with 14 species of bats and 36 seed dispersed plants species had H 2 ' value of 0.31 [38]. This suggests that the changes in specialization values are better explained by the diet overlap and abundance of frugivorous bats rather than the richness of bats or seed dispersed plants. This hypothesis is supported by the similar bat and chiropterochoric plant richness found in TMCF and SCP and by their significantly different values of H 2 '.
Another interesting result of our study is the large number of plant species detected in fecal samples that were not observed in our plant surveys. While it is possible that our chiropterochoric plant survey may be incomplete, we consider that this does not affect the principal result of the study since the observed richness of dispersed seeds did not differ significantly from the estimated richness (see Table 2). Previous studies have recorded seed dispersed plants that were not observed in our plant survey in primary TMCF, secondary TMCF and SCP [39,40]. This suggests that bats consumed plants in vegetation types similar to those we studied and that the chiropterochoric flora of each plantation is only a reduced portion of the chiropterochoric flora of the fragmented landscape. This is specially true for canopy plants as Hedyosmum mexicanum, Vismia mexicana, and Solanaceae understory plants (see Table 1).
The higher specialization of interactions and the lower bat abundance in SCP could have important consequences for seed dispersal and forest regeneration in coffee landscapes. One consequence could be that the seed dispersal service provided by bats could be less stable in the SCP. For example, bats in SCP on average shared less than one plant (Table 3). If we take into account that networks with high complementarity of interactions can compensate and maintain their stability when a disturbance or fluctuating environmental condition appears [3,32], frugivorous bats in SCP could have a lower probability of maintaining a seed dispersal service compared to those in the TMCF fragments.
Bat-fruit specialization and the conservation of frugivorous bats
SCP could reduce the abundance of frugivorous bats and increase the commuting distance to foraging sites [16,41]. The low abundance of frugivorous bats result in high specialization in bat-fruit interactions and the vulnerability of the interaction network in SCP. Therefore, in order to assist in the conservation efforts of these seed-dispersing bats and to maintain forest regeneration potential in SCP coffee farmers and policy makers should: (1) attempt to increase the proportion of land assigned to forest within the agricultural landscape, and (2) use the measurement of the diversity and abundance of frugivorous bats as an indicator that SCP have sufficient food resources for frugivorous bats around and within the plantations. The first management recommendation is specially important if we take into account that a great number of understory plants consumed by bats came from forest.
In conclusion, in the SCP, bat-plant interaction networks had higher specialization values, bats shared a lower number of plant species and potentially dispersed a lower number of seeds of understory plants. These results are the consequence of low frugivorous bat abundance in the SCP and, under scenarios of coffee plantation expansion, represent trade-offs for the conservation of frugivorous bats and the invaluable seed dispersal service they provide.
Supporting Information
Letter B refers to TMCF, while letter C refers to SCP.
(PDF)
Guild bat classification is based on Rojas et al. 2012.
(PDF)
Acknowledgments
We are grateful to R. Flores, G. Vázquez-Domínguez, A. Tauro, G. Aguilera, L. Avendaño, L. Barradas, E. Barrera, T. Cano, A. Castro-Luna, I. Corrales, G. Cuevas, P. Espinosa, D. Garibay, A. González, N. Hernández, N. Hernández, V. Hernández, D. Jimeno, C. Martínez, P. del Moral, Y. Moreno, A. Pensado, O. Ponce, A. Robles, A. Santa-Anna, G. Solís, A. Tepatlán, M. Toledo, M. Vela and R. Vera for their valuable assistance with fieldwork. We thank C. Gallardo-Hernández from the INECOL XAL-Herbarium and R. Santiago-Gómez from the Laboratorio de Plantas Vasculares, Facultad de Ciencias, UNAM, for their help with the identification of plants. Klaus Mehltreter, Kimberly Williams-Guillén, and Keith MacMillan helped improve the focus and English text of the manuscript. We thank the owners of the coffee plantations and forest fragments for allowing us access to their property.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
JRH-M acknowledges scholarship support from the Programa para el Mejoramiento del Profesorado (PROMEP) of the Secretaría de Educación Pública. RAS-V and VJS were funded by CONACYT-SEMARNAT (grant BIOCAFÉ 2002-01-C01-00194) and FORDECYT (grant 139378). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Forget P-M, Jordano P, Lambert JE, Böhning-Gaese K, Traveset A, Wright SJ (2011) Frugivores and seed dispersal (1985–2010); the 'seeds' dispersed, established and matured. Acta Oecologica 37: 517–520. 10.1016/j.actao.2011.09.008 [DOI] [Google Scholar]
- 2. Valiente-Banuet A, Verdú M (2013) Human impacts on multiple ecological networks act synergistically to drive ecosystem collapse. Frontiers in Ecology and the Environment 11: 408–413. 10.1890/130002 [DOI] [Google Scholar]
- 3. Tylianakis JM, Laliberté E, Nielsen A, Bascompte J (2010) Conservation of species interaction networks. Biological Conservation 143: 2270–2279. 10.1016/j.biocon.2009.12.004 [DOI] [Google Scholar]
- 4. Blüthgen N, Klein A-M (2011) Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic and Applied Ecology 12: 282–291. 10.1016/j.baae.2010.11.001 [DOI] [Google Scholar]
- 5. Menke S, Böhning-Gaese K, Schleuning M (2012) Plant-frugivore networks are less specialized and more robust at forest-farmland edges than in the interior of a tropical forest. Oikos 121: 1553–1566. 10.1111/j.1600-0706.2011.20210.x [DOI] [Google Scholar]
- 6. Chama L, Berens DG, Downs CT, Farwing N (2013) Habitat characteristics of forest fragments determine specialisation of plant-frugivore networks in a mosaic forest landscape. Plos One 8: e54956 10.1371/journal.pone.0054956.s005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gorchov DL, Cornejo F, Ascorra CF, Jaramillo M (1995) Dietary overlap between frugivorous birds and bats in the Peruvian Amazon. Oikos 74: 235–250. [Google Scholar]
- 8. Mello MAR, Marquitti FMD, Guimarães PR, Kalko EKV, Jordano P, Martinez de Aguiar AM (2011) The modularity of seed dispersal: differences in structure and robustness between bat—and bird—fruit networks. Oecologia 167: 131–140. 10.1007/s00442-011-1984-2 [DOI] [PubMed] [Google Scholar]
- 9. Muscarella R, Fleming TH (2007) The role of frugivorous bats in tropical forest succession. Biological Reviews 82: 573–590. [DOI] [PubMed] [Google Scholar]
- 10. Giannini N, Kalko EKV (2004) Trophic structure in a large assemblage of phyllostomid bats in Panama. Oikos 105: 209–220. [Google Scholar]
- 11. Sánchez MS, Giannini NP, Barquez RM (2012) Bat frugivory in two subtropical rain forests of northern Argentina: testing hypotheses of fruit selection in the Neotropics. Mammalian Biology 77: 22–31. 10.1016/j.mambio.2011.06.002 [DOI] [Google Scholar]
- 12. Saldaña-Vázquez RA (2014) Intrinsic and extrinsic factors affecting dietary specialization in Neotropical frugivorous bats. Mammal Review 44: 215–224. [Google Scholar]
- 13. Stang M, Klinkhamer PGL, Meijden E (2006) Asymmetric specialization and extinction risk in plant—flower visitor webs: A matter of morphology or abundance? Oecologia 151: 442–453. 10.1007/s00442-006-0585-y [DOI] [PubMed] [Google Scholar]
- 14. Vázquez DP, Melián C J, Williams NM, Blüthgen N, Krasnov B R, Poulin R (2007) Species abundance and asymmetric interaction strength in ecological networks. Oikos 116: 1120–1127. 10.1111/j.2007.0030-1299.15828.x [DOI] [Google Scholar]
- 15. Muñoz-Villers L, López-Blanco J (2007) Land use/cover changes using Landsat TM/ETM images in a tropical and biodiverse mountainous area of central-eastern Mexico. International Journal of Remote Sensing 29: 71–93. [Google Scholar]
- 16. Saldaña-Vázquez R, Sosa VJ, Hernández-Montero JR, López-Barrera F (2010) Abundance responses of frugivorous bats (Stenodermatinae) to coffee cultivation and selective logging practices in mountainous central Veracruz, Mexico. Biodiversity and Conservation 19: 2111–2124. [Google Scholar]
- 17. Daily GC, Ceballos G, Pacheco J, Suzán G, Sánchez-Azofeifa A (2003) Countryside biogeography of Neotropical mammals: Conservation opportunities in agricultural landscapes of Costa Rica. Conservation Biology 17: 1814–1826. [Google Scholar]
- 18. Philpott SM, Arendt WJ, Armbrecht I, Bichier P, Diestch TV, Gordon C, et al. (2008) Biodiversity loss in Latin American coffee landscapes: Review of the evidence on ants, birds, and trees. Conservation Biology 22: 1093–1093. 10.1111/j.1523-1739.2008.01029.x [DOI] [PubMed] [Google Scholar]
- 19. Lobova TA, Geiselman CK, Scott MA (2009) Seed dispersal by bats in the Neotropics. New York Botanical Garden, Bronx, New York: 471 pp. [Google Scholar]
- 20. Rex K, Czaczkes BI, Michener R, Kunz TH, Voigt CC (2010) Specialization and omnivory in diverse mammalian assemblages. Ecoscience 17: 37–46. 10.2980/17-1-3294 [DOI] [Google Scholar]
- 21. Galindo-González JR, Vázquez-Domínguez G, Saldaña-Vázquez RA, Hernández-Montero JR (2009) A more efficient technique to collect seeds dispersed by bats. Journal of Tropical Ecology 25: 205–209. [Google Scholar]
- 22. Soto-Esparza M, Gómez-Columna M (1990) Atlas climático del municipio de Xalapa. Instituto de Ecología. [Google Scholar]
- 23. Meyer Christoph F J, Aguiar LMS, Aguirre LF, Baumgarten J, Clarke FM, Cosson J- F, et al. (2011) Accounting for detectability improves estimates of species richness in tropical bat surveys. Journal of Applied Ecology 48: 777–787. 10.1111/j.1365-2664.2011.01976.x [DOI] [Google Scholar]
- 24. Straube FC, Bianconi G (2002) Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes de neblina. Chiroptera Neotropical 8: 150–152. [Google Scholar]
- 25. Saldaña-Vázquez R, Munguía-Rosas MA (2013) Lunar phobia in bats and ecological correlates: a meta-analysis. Mammalian Biology 78: 216–219. 10.1016/j.mambio.2012.08.004 [DOI] [Google Scholar]
- 26. Caras T, Korine C (2009) Effect of vegetation density on the use of trails by bats in a secondary tropical rain forest. Journal of Tropical Ecology 25: 97–101. [Google Scholar]
- 27. Medellín R, Arita H, Sánchez O (2008) Identificación de los murciélagos de México: Clave de Campo. 2nd ed D.F., México: Asociación Mexicana de Mastozoología; 78 p. [Google Scholar]
- 28. Gotelli N, Colwell R (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecology Letters 4: 379–391. [Google Scholar]
- 29. Colwell RK (2013) EstimateS: Statistical estimation of species richness and shared species from samples. 9 ed http://purl.oclc.org/estimates. Available: http://purl.oclc.org/estimates. [Google Scholar]
- 30. MacGregor-Fors I, Payton ME (2013) Contrasting diversity values: Statistical inferences based on overlapping confidence intervals. Plos One 8: e56794 10.1371/journal.pone.0056794.t002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCune B, Grace JB, Urban DL (2002) Analysis of ecological communities Mjm Software Design. 300 pp. [Google Scholar]
- 32. Blüthgen N (2010) Why network analysis is often disconnected from community ecology: A critique and an ecologist's guide. Basic and Applied Ecology 11: 185–195. 10.1016/j.baae.2010.01.001 [DOI] [Google Scholar]
- 33. Stone L, Roberts A (1992) Competitive exclusion, or species aggregation? Oecologia 91: 419–424. [DOI] [PubMed] [Google Scholar]
- 34. R Development Core Team, S. (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Viena, Austria: 10.1002/jcc.22917 [DOI] [Google Scholar]
- 35. Dormann C, Gruber B, Fründ J (2008) Introducing the bipartite package: analysing ecological networks. R News 8: 8–9. [Google Scholar]
- 36. Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, et al. (2013) vegan 2nd ed R project. 260 pp. [Google Scholar]
- 37. Kelm D, Schaer J, Ortmann S, Wibbelt G, Speakman J, Voigt CC (2008) Efficiency of facultative frugivory in the nectar-feeding bat Glossophaga commissarisi: the quality of fruits as an alternative food source. Journal of Comparative Physiology B: Biochemical, Systemic, and Environmental Physiology 178: 985–996. 10.1007/s00360-008-0287-3 [DOI] [PubMed] [Google Scholar]
- 38. Mello MAR, Marquitti FMD, Guimarães PR, Kalko EKV, Jordano P, Martinez de Aguiar AM (2011) The missing part of seed dispersal networks: Structure and robustness of bat-fruit interactions. Plos One 6: e17395 10.1371/journal.pone.0017395.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muñiz-Castro MA, Williams-Linera G, Rey Benayas JM (2006) Distance effect from cloud forest fragments on plant community structure in abandoned pastures in Veracruz, Mexico. Journal of Tropical Ecology 22: 431–440. [Google Scholar]
- 40. Castro-Luna AA, Galindo-González J (2011) Enriching agroecosystems with fruit-producing tree species favors the abundance and richness of frugivorous and nectarivorous bats in Veracruz, Mexico. Mammalian Biology 77: 32–40. 10.1016/j.mambio.2011.06.009 [DOI] [Google Scholar]
- 41. Cortés-Delgado N, Sosa VJ (2014) Do bats roost and forage in shade coffee plantations? A perspective from the frugivorous bat Sturnira hondurensis . Biotropica 46: 624–632. 10.1111/btp.12142 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Letter B refers to TMCF, while letter C refers to SCP.
(PDF)
Guild bat classification is based on Rojas et al. 2012.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.