Abstract
The antibacterial and anti-inflammatory activities, and protective effects of extracts (flavonoid glycosides) of Polygonum capitatum were investigated to detect the evidence for the utilization of the herb in the clinical therapy of gastritis caused by H. pylori. A mouse gastritis model was established using H. pylori. According to treating methods, model mice were random assigned into a model group (MG group), a triple antibiotics group (TG group, clarithromycin, omeprazole and amoxicillin), low/middle/high concentrations of flavonoid glycosides groups (LF, MF and HF groups) and low/middle/high concentrations of flavonoid glycosides and amoxicillin groups (LFA, MFA and HFA groups). A group with pathogen-free mice was regarded as a control group (CG group). The eradicate rates of H. pylori were 100%, 93%, 89% in TG, MFA and HF groups. The serum levels of IFN-gamma and gastrin were higher in a MG group than those from all other groups (P < 0.05). The serum levels of IFN-gamma and gastrin were reduced significantly in LF, MF and HF groups (P < 0.05) while little changes were observed in LFA, MFA and HFA groups. In contrast, the serum levels of IL-4 were lower and higher in MG and CG groups compared with other groups (P<0.05). The serum levels of IL-4 were increased significantly in LF, MF and HF groups (P < 0.05) while little changes were found in LFA, MFA and HFA groups. According to pathological scores, flavonoid glycosides therapy showed better protection for gastric injuries than the combination of flavonoid glycoside and amoxicillin (P < 0.05). The results suggested that flavonoid glycoside has repairing functions for gastric injuries. The results suggest that the plant can treat gastritis and protect against gastric injuries. The flavonoid glycosides from Polygonum capitatum should be developed as a potential drug for the therapy of gastritis caused by H. pylori.
Introduction
Helicobacter pylori (H. pylori) infection is the main cause of chronic inflammation (gastritis), which is the second leading cause of gastric cancer in the world [1]. H. pylori infection also causes gastrointestinal lymphoma, which develop in stomach[2]. Mucosa-associated lymphoid tissue (MALT) and diffusing large B-cell lymphoma are the common histologic characters of gastric lymphoma[3]. Additionally, H. pylori infection is closely associated with the development of MALT lymphoma, which also results in gastric cancer[4]. The occurrence of selected genes such as gastrin and somatostatin[5], determine the pathogenicity of H. pylori. These proteins are pathogens contributing to peptic inflammation, ulceration, and cancer [6–8]. H. pylori eradication can completely control the development of MALT lymphomas[9] and has become the main focus for the prevention of gastric disease.
Presently, metronidazole, clarithromycine and amoxicillin are mostly used medicine for the therapy of H. pylori [10, 11]. However, a high prevalence of medicine resistance has been widely reported in H. pylori and the mechanisms for causing medicine resistance are complex [12–14]. The rate for the eradication of H. pylori is even less than 50% in most places[15]. Amoxicillin is the most powerful medicine for the therapy of the bacteria, but the high prevalence of medicine resistance in H. pylori also limits its utilization [12, 16]. There is increasing evidence that amoxicillin causes severe adverse effects in most patients [17].
Traditional Chinese medicine (TCM) has been implied in the Chinese health care for more than two thousand years[18]. The side effects and adverse events of TCM are often regarded as generally mild and infrequent[19]. Polygonum capitatum (P. capitatum), a traditional Chinese Miao-nationality herb, has been widely used in the treatment of urologic diseases [20]. Recent pharmacological studies have demonstrated that the antibacterial and anti-inflammatory activities of P. capitatum can be used for treating urinary tract infections at a clinical stage [21]. Further work extends the application of P. capitatum in treating diseases caused by H. pylori (Chinese patent No. CN102824417A), but the molecular mechanism remains unknown.
In immune system, interferon (IFN)-gamma can stimulate macrophage release and plays a critical role in the immune response against infection and controlling intracellular pathogens [22, 23]. Aberrant IFN-gamma expression is linked to many autoinflammatory and autoimmune diseases. The importance of IFN-gamma in the immune system stems is mostly due to its immunostimulatory and immunomodulatory effects [24]. IFN-gamma is mainly produced by natural killer and natural killer T cells in immune response, and by cluster of differentiation CD4 Th1 and cytotoxic CD8 T cells when antigen-mediated immunity develops[25]. H. pylori infection is one of the major causes for gastroduodenal pathologies. The long-term persistence of bacterial infection and immune and inflammatory response will affect the levels of IFN-gamma. IFN-gamma promotes the severity of the induced gastric lesions during the host response to H. pylori [26].
The interleukin 4 (IL4) is a kind of cytokine inducing the differentiation of naive helper T cells to Th2 cells[27]. Upon activation by IL-4, Th2 cell produces additional IL-4 in a positive feedback way. The function of IL-4 is similar to that of Interleukin 13[28]. It has many biological roles, such as activating the proliferation of B cells and T cells. It plays an important role in humoral and adaptive immunity. IL-4 can induce B-cell class switching to the antibody IgE, and up-regulate the levels of MHC class II. IL-4 can decrease the levels of Th1 cells, macrophages, IFN-gamma, and dendritic cell IL-12. Elevated IL-4 is associated with inflammation and wound repair[29]. IL-4 can modulate macrophage activation and polarization into M2 and inhibit activation of macrophages into M1 cells[30]. An increase in M2 macrophages is accompanied by the production of IL-10 and TGF-β that result in a decrease of pathological inflammation[31].
Gastrin, a kind of peptide hormone, stimulates the production of gastric acid by the parietal cells from the stomach and promotes gastric motility. Gastrin is secreted by G cells from the pyloric antrum of the stomach[32]. Decreased levels of gastrin were found in healthy adults because of the change of H. pylori infection. The control of gastrin can prevent H. pylori-induced gastritis. Gastrin plays a critical role in the inflammatory reaction of the gastric mucosa caused by H. pylori infection [33]. Somatostatin, a kind of growth hormone-inhibiting hormone[34], is a peptide hormone regulating the endocrine system and affecting cell proliferation by interacting with G protein-coupled somatostatin receptors and inhibiting the release of secondary hormones. Somatostatin cell is an important regulator of gastric acid secretion and alteration in its numbers plays a key role in gastroduodenal disease. The alterations may correlate with the severity of inflammation and certain peptide-immune interactions in the gastric mucosa caused by H. pylori infection [35].
Flavonoid glycoside is one of main components of P. capitatum and shows anti-bacterial activities [21]. Furthermore, amoxicillin is often combined with other medicine to treat H. pylori related disease. Therefore, we want to know the effects of flavonoid glycoside, which were compared with effects of combination therapy of flavonoid glycoside and amoxicillin on H. pylori infected disease. Meanwhile, the levels of IFN-gamma, IL-4, Gastrin and Somatostatin were measured.
Results
Sensitivity test of H. pylori to flavonoid glycoside
In vitro antibacterial test, 40 ug/mL or higher concentrations of flavonoid glycoside inhibited the growth of H. pylori (Fig 1). Therefore, the MIC of flavonoid glycoside was 40 ug/mL, which was much higher than MIC of amoxicillin (1 ug/mL). The resistance of MIC of flavonoid glycoside was regarded as >40.0 μg/mL. The resistance of MIC of amoxicillin was regarded as >1.0 μg/ml.
Fig 1. Sensitivity test of H. pylori to flavonoid glycoside.

A: blank control. B: 40 ug/mL flavonoid glycoside. C: 80 ug/mL flavonoid glycoside.
The toxicity of flavonoid glycoside on mice lymphocyte
High levels of flavonoid glycoside may inhibit the bioactivity of lymphocytes although flavonoid glycoside shows antibacterial activities [36]. There were not significant differences for mean OD values of lymphocytes when the concentrations were less than 64 ug/ml (P < 0.05). The OD values of lymphocytes were reduced significantly when the concentrations were more than 128 ug/ml (P < 0.05). The OD of lymphocytes reached the lowest level when the concentration of flavonoid glycoside was 512 ug/mL (Table 1).The results suggest that certain concentrations of flavonoid glycoside will not affect the proliferation of lymphocytes while the high concentrations of flavonoid glycoside will inhibit the proliferation of lymphocytes by its toxicity.
Table 1. CCK-8 colorimetric assay for detecting lymphocyte stimulation index after mice model treated with different concentrations of Flavonoid glycoside in vitro after 72 h.
| Concentrations of Flavonoid glycoside | The OD of lymphocyte |
|---|---|
| 0 μ /mL | 0.673+0.0213 |
| 1 μ /mL | 0.659+0.0138 |
| 2 μ /mL | 0.712+0.0135 |
| 4 μ /mL | 0.685+0.0165 |
| 8 μ /mL | 0.598+0.0126 |
| 16 μ6/mL | 0.647+0.0219 |
| 32 μ/mL | 0.655+0.0240 |
| 64 μ/mL | 0.542+0.0361 |
| 128 μ/mL | 0.495+0.0271* |
| 256 μ/mL | 0.321±0.0230* |
| 512 μ/mL | 0.201±0.0172* |
| 1024 μ/mL | 0.225±0.0272* |
Note:
*P<0.05 vs 0 ug/mL group.
The establishment of a mouse model infected with H. pylori
The establishment of H. pylori-infected mice model was evaluated from two aspects: the identification of H. pylori isolated from gastric tissues of mice models and analysis of histochemistry and histopathology. H. pylori were identified via Gram staining, rapid urease test, oxidase test and catalase test. Gram staining differentiates bacteria by detecting peptidoglycan[37], which locates in the cell walls of gram-positive bacteria. After a Gram stain test, gram-positive bacteria showed the crystal violet dye, while a counterstain gave gram-negative bacteria a pink color (Fig 2A).Rapid urease test, is a rapid diagnostic test for H. pylori[38]. H. pylori secreted the urease enzyme, which catalyzed the conversion of urea to NH3 and raised the pH of the medium. The medium contained urea and an indicator phenol red, and thus the raised pH changed the color of the medium from yellow (a blank control) to red (an experimental group) (Fig 2B). The oxidase test was used to determine if a bacterium produced certain cytochrome c oxidases [39]. The disks were impregnated with a reagent such TMPD, which was a redox indicator. The reagent changed from dark-blue to maroon when it was oxidized (Fig 2C). The catalase test is one of main tests to identify H. pylori[40]. The presence of catalase enzyme in the species was detected via hydrogen peroxide. If the bacteria possessed catalase, bubbles of oxygen would be observed when bacterial isolate was added to hydrogen peroxide (Fig 2D).
Fig 2. Identification of H. pylori.
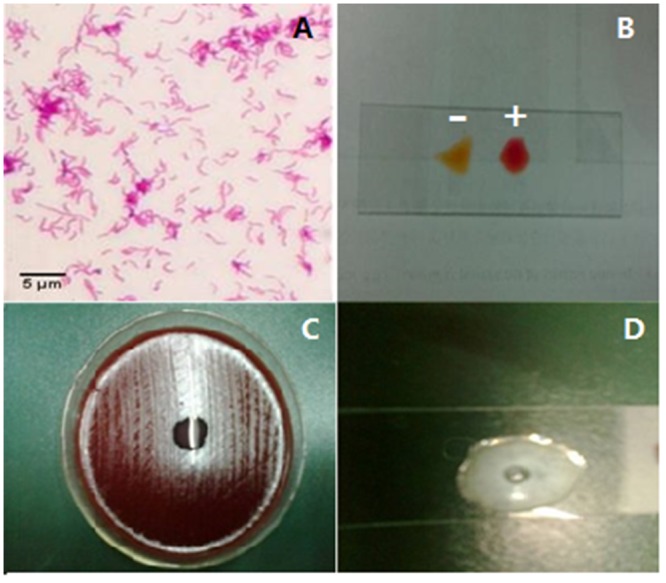
A: Gram staining, Gram staining differentiates bacteria by detecting peptidoglycan, which exists in the cell walls of gram-positive bacteria. After a Gram stain test, gram-positive bacteria shows the crystal violet dye, while a counterstain (safranin) added, gives gram-negative bacteria a pink coloring. B, rapid urease test, is a rapid diagnostic test for H. pylori. H. pylori secrete the urease enzyme, which can catalyze the conversion of urea to NH3 and raises the pH of the medium. The medium contains urea and an indicator such as phenol red, and thus the raised pH changes the color of the medium from yellow (a blank control) to red (an experimental group). C, the oxidase test is used to determine if a bacterium produces certain cytochrome c oxidases. It uses disks impregnated with a reagent such as TMPD, which is a redox indicator. The reagent can be changed from a dark-blue to maroon color when it is oxidized. D, the catalase test is one of main tests to identify H. pylori. The presence of catalase enzyme in the species is detected via hydrogen peroxide. If the bacteria possess catalase, when bacterial isolate is added to hydrogen peroxide, bubbles of oxygen will be observed. If the mixture produces bubbles, the organism is regarded as 'catalase-positive'.
Analysis of histochemistry and histopathology was performed via Giemsa staining, HE staining of mice gastric mucosa, microaerophilic culture and Immunohistochemical staining of G and D cells in Gastric mucosa-associated lymphoid tissue (MALT) lymphoma between control and model groups. Giemsa stain can be used to study the adherence of pathogenic bacteria to mammalians cells. In the control groups, no H. pylori were identified in the gastric mucosa. In contrast, H. pylori were observed in the gastric mucosa from mice models (Fig 3). Microaerophilic culture conditions are necessary for the growth of H. pylori[41]. Gastric biopsies were subjected to microaerophilic culture and then identified by the growth of H. pylori in mice models. In contrast, no colonies were observed in control groups (Fig 3). H&E staining was conducted to observe morphological alterations in gastric mucosa after H. pylori infection. Mucosal destruction in the gastric mucosa were observed in mice models. Representative inflammatory infiltrates were found at the base of the mucosa with lymphocytes and polymorphonuclears in the mice infected by H. pylori. In contrast, the mice showed normal gastric morphology without mucosal destruction and inflammatory infiltrates (Fig 3). Immunohistochemical staining of G cells in MALT lymphoma showed that the number of G cells in model groups was more that in a control group. Similarly, immunohistochemical staining of D cells in MALT lymphoma showed that the number of D cells in model groups was more that in a control group (Fig 3). All above information indicated that a H. pylori-infected model was successfully established.
Fig 3. The comparision for histochemistry and histopathology between a control and a model group.
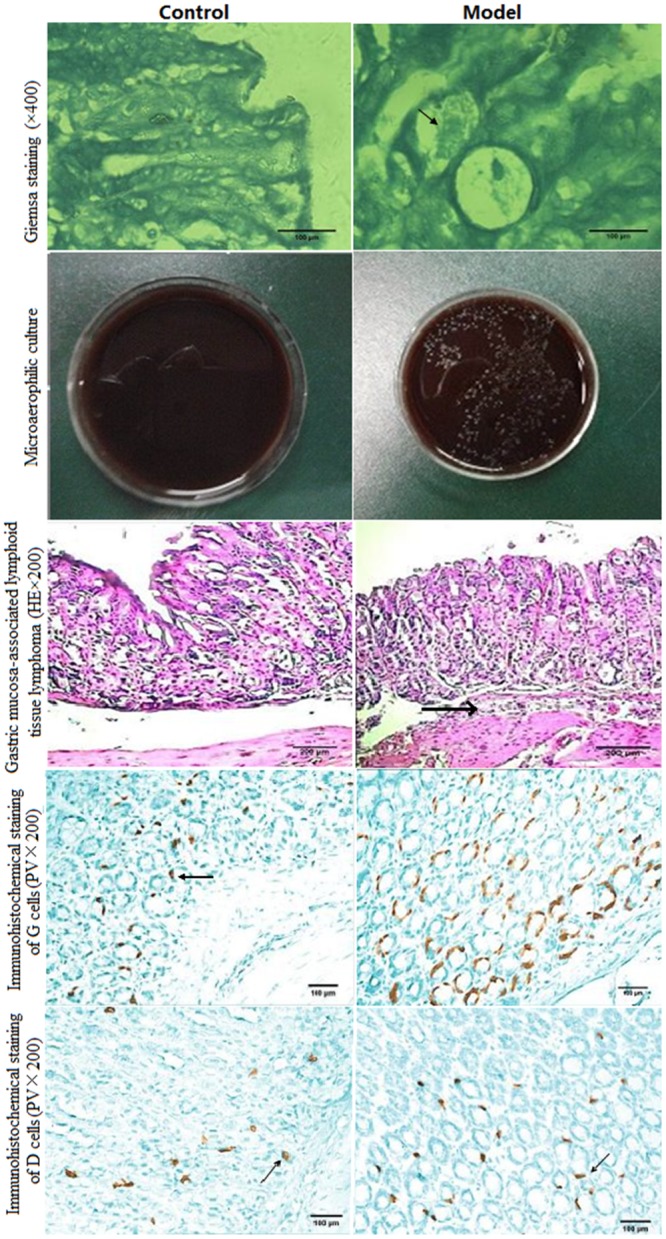
Giemsa staining (×400), Giemsa stain was used to observe the adherence of pathogenic bacteria to gastric cells. Microaerophilic culturing of H. pylori, the extracellular H. pylori are microaerophilic. Hematoxylin and Eosin staining of gastric tissues was used for the detection of H. pylori (HE×200). The staining of G cells of Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (PV×200). The staining of D cells of Gastric MALT lymphoma (PV×200).
The effects of flavonoid glycoside on the eradication rate of H. pylori
Different groups showed different eradication rate of H. pylori (Table 2). Three kinds of antibiotics eradicated H. pylori completely in a TG group. The combination of flavonoid glycoside and amoxicillin eradicated H. pylori by more than 93% in a MFA group while only flavonoid glycoside eradicated H. pylori by 89% at most. According to the relative amounts of H. pylori-specific CagA gene determined by qRT-PCR[42], the log cells of per g of tissues were 0 in a TG group while the number is 6.6 ± 0.5 in a MG group. Comparatively, the numbers were 5.4 ± 0.3 in a MFA group and 5.8 ± 0.3 in a HF group. Flavonoid glycoside showed the similar antibacterial activities compared with the combination therapy of flavonoid glycoside and amoxicillin.
Table 2. Analysis of H. pyloriclearance between model group and other treatment groups.
| Colonization density(Ig cfu/g) | H. pylori clearance | a Log cells of per g of wet tissues | |
|---|---|---|---|
| MG | 7.27±0.94 | 0 | 6.6 ± 0.5 |
| TG | 6.35 ± 0.78 | 100% | 0 |
| LF | 6.31±0.89 | 76.66% | 6.1 ± 0.4 |
| MF | 6.56±0.41 | 76.15% | 6.1 ± 0.4 |
| HF | 6.64±0.42 | 89.07% | 5.8 ± 0.3 |
| LFA | 6.15±1.03* | 92.09% | 5.5 ± 0.5 |
| MFA | 6.10±0.59* | 93.32% | 5.4 ± 0.3 |
| HFA | 6.17±0.85 | 92.57% | 5.5 ± 0.4 |
Note:
aThe number is determined by the relative amounts of H. pylori-specific CagA DNA.
*P<0.05 vs model group (n = 8).
Flavonoid glycoside improves the pathology of H. pylori-infected mice model
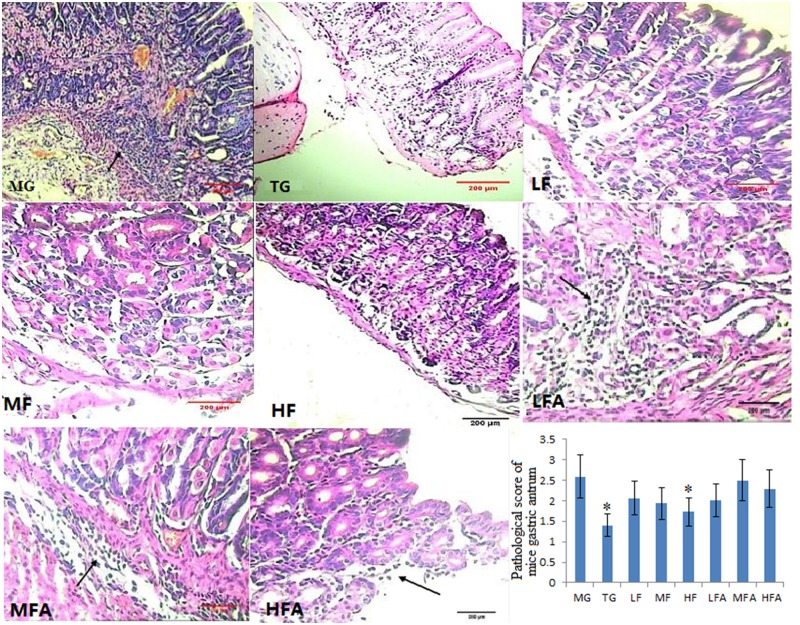
We established a mouse gastritis model using H. pylori and the degree of infection was evaluated by H&E staining (Fig 4). In a CG group, gastric mucosa was found with a common morphology, and no inflammation was observed. These histopathological varies showed that a mouse model was established successfully. In a MG group, the structures of normal gastric mucosa were destroyed, and mucosal destruction was observed. The degree of infection was significantly alleviated and inflammatory cells (lymphocytes, monocytes, neutrophils) were reduced in TG and HF groups compared with those in MF, HF, LFA, MFA and HFA groups (Fig 4). In TG and HF groups, the gastric mucosa was in normal morphology and the pathological scores were also lower than other groups. In contrast, the gastric mucosa were destroyed with different degrees in MG, LF, MF, LFA, MFA and HFA groups, especially the pathological scores were the highest in a MG group. The results showed that flavonoid glycoside could improve the pathology of H. pylori infection significantly. More importantly, according to pathological scores, flavonoid glycoside showed better protect gastric tissues than the combination of flavonoid glycoside and amoxicillin (P < 0.05) (Fig 4). The results suggested that flavonoid glycoside has repairing functions for gastric injuries.
Fig 4. Hematoxylin and Eosin staining of gastric tissues (HE×200).
Normal mice (without H. pylori infection) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group, only treated with saline solution), triple combination therapy group (TG group, the daily medicine intake is 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low/middle/high concentrations of flavonoid glycoside group (LF/MF/HF group, treated with one daily dose of Flavonoid glycoside at 32/64/128 ug), low/middle/high concentrations of flavonoid glycoside and common concentration of amoxicillin group (LFA/MFA/HFA group, treated with one daily dose of flavonoid glycoside at 32/64/128 ug and amoxicillin at 1 ug). Pathological score of mice gastric antrum in each group (±SD, n = 10). *P < 0.05 vs model group.
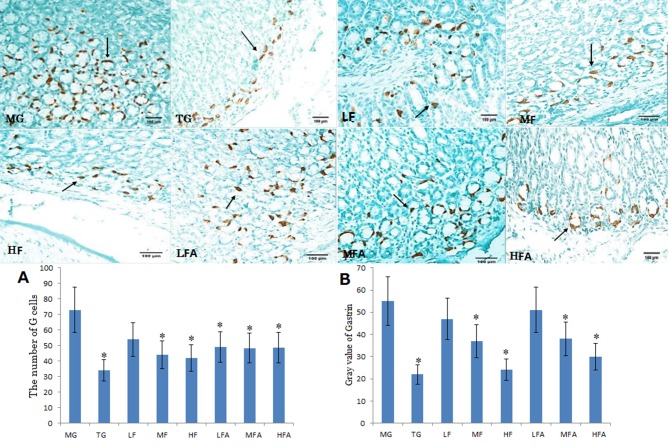
Immunohistochemical staining of G cells MALT lymphoma was restricted to gastric cells in MG and LF groups, and a stronger immune staining was observed in the two groups. In contrast, G cells were weaker immune staining with a little light brown color in TG, MF, HF, LFA, MFA and HFA groups (Fig 5). A G cell staining was positively related with the degrees of H. pylori infection. Thus, the infection could be controlled well in TG, MF, HF, LFA, MFA and HFA groups. The results suggested that certain concentrations of flavonoid glycoside controlled the H. pylori infection well.
Fig 5. The staining of G cells of Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (PV×200).
Normal mice (without H. pylori infection) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group, only treated with saline solution), triple combination therapy group (TG group, the daily medicine intake is 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low/middle/high concentrations of flavonoid glycoside group (LF/MF/HF group, treated with one daily dose of Flavonoid glycoside at 32/64/128 ug), low/middle/high concentrations of flavonoid glycoside and common concentration of amoxicillin group (LFA/MFA/HFA group, treated with one daily dose of Flavonoid glycoside at 32/64/128 ug and amoxicillin at 1 ug). Comparison of G cells and gastrin gray value in each group (±s, n = 10). *P < 0.05 vs model group.
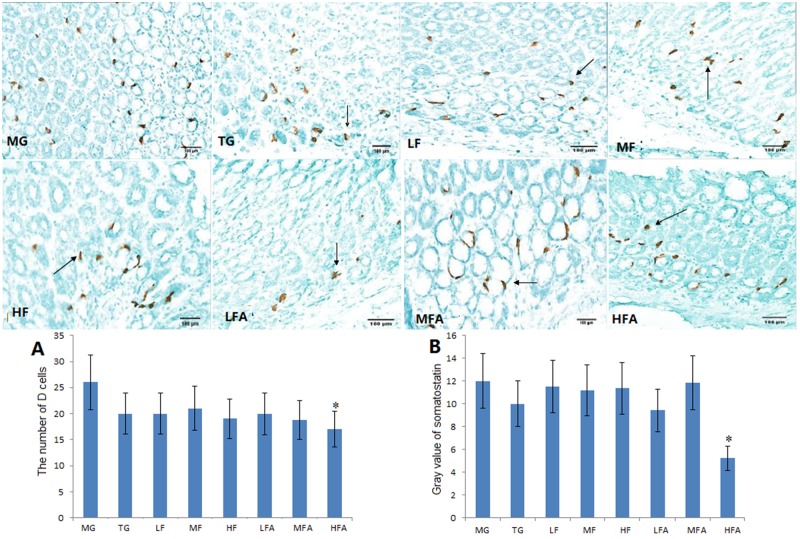
Comparably, immunohistochemical staining of D cells MALT lymphoma was restricted to gastric cells in MG, TG, LF, MF, HF, LFA and MFA groups, and a stronger immune staining was observed in these groups. In contrast, D cells were weaker immune staining with a little light brown color in a HFA group (Fig 6). All the results suggested that the combination therapy of high concentration of flavonoid glycoside and amoxicillin affected the number of D cells significantly, which was different from the effect on G cells.
Fig 6. The staining of D cells of Gastric mucosa-associated lymphoid tissue (MALT) lymphoma (PV×200).
Normal mice (without H. pylori infection) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group, only treated with saline solution), triple combination therapy group (TG group, the daily medicine intake is 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low/middle/high concentrations of flavonoid glycoside group (LF/MF/HF group, treated with one daily dose of flavonoid glycoside at 32/64/128 ug), low/middle/high concentrations of flavonoid glycoside and common concentration of amoxicillin group (LFA/MFA/HFA group, treated with one daily dose of Flavonoid glycoside at 32/64/128 ug and amoxicillin at 1 ug). Analysis of G cells and somatostatin gray value in each group (n = 10). *P < 0.05 vs model group.
Effects of flavonoid glycoside and amoxicillin on the mRNA levels of IFN-gamma, IL-4, Gastrin and Somatostatin
We investigated the effects of flavonoid glycoside and amoxicillin on the mRNA levels of IFN-gamma, IL-4, gastrin and somatostatin, which can reflect the degrees of H. pylori infection [33, 43–45]. The mRNA levels of IFN-gamma were significantly higher in a MG group than those in all other groups (P < 0.05) (Table 3). The mRNA levels of IFN-gamma were significantly lower in CG, TG, HF and MF groups than those in all other groups (P < 0.05) (Table 4). All the results suggested that flavonoid glycoside affected the mRNA levels of IFN-gamma. In contrast, the mRNA levels of IL-4 were significantly lower in a MG group than those in all other groups (P < 0.05) (Table 4). The mRNA levels of IL-4 were significantly increased in CG, TG, HF and MF groups than those in all other groups (P < 0.05) (Table 4). All the results suggested that the treatment of flavonoid glycoside increased the mRNA levels of IL-4. Gastrin (GAS) is produced by G cells in mice stomach lining and associated with the number of G cells. Real-time qRT-PCR analysis showed that the levels of GAS were higher in a MG group than all other groups (P < 0.05) (Table 5). Similarly, somatostain (SS) is produced by D cells in mice stomach and associated with the number of D cells. Real-time qRT-PCR analysis showed that the levels of SS were higher in a MG group than all other groups (P < 0.05) (Table 6).
Table 3. The mRNA levels of IFN-gamma in the gastric mucosa of H. pylori infected mice in different groups (n = 6).
| Groups | β-actin | IFN-gamma CT value | 2-△△CT |
|---|---|---|---|
| MG | 32.831±0.428 | 19.523±2.410 | 1▲ |
| CG | 35.000±1.004 | 18.513±3.261 | 0.110* |
| TG | 33.796±2.416 | 17.768±1.945 | 0.150* |
| HF | 33.093±1.024 | 16.995±3.584 | 0.166* |
| MF | 33.964±3.739 | 18.669±3.105 | 0.269* |
| LF | 35.000±0.692 | 21.377±5.042 | 0.451 |
| HFA | 31.179±1.296 | 18.513±2.178 | 0.874 |
| MFA | 30.198±1.397 | 17.029±3.791 | 0.912 |
▲ P < 0.05 model group vs blank group;
*P < 0.05 vs model group.
Table 4. The mRNA levels of IL-4 in the gastric mucosa of H. pylori infected mice in different groups (n = 6).
| Groups | β-actin CT values | IL-4 CT values | 2-ΔΔCT |
|---|---|---|---|
| MG | 32.501±0.138 | 17.283±1.383 | 1 ▲ |
| CG | 30.651±1.027 | 18.388±1.972 | 7.755* |
| TG | 31.872±0.987 | 18.837±2.936 | 4.831* |
| HF | 31.198±0.237 | 17.988±1.097 | 4.022* |
| MF | 30.868±0.487 | 17.390±2.945 | 3.324* |
| LF | 31.989±1.002 | 18.292±0.843 | 2.869* |
| HFA | 33.946±0.948 | 18.387±1.075 | 1.257 |
| MFA | 32.202±0.739 | 17.639±2.382 | 1.354 |
| LFA | 35.662±1.024 | 21.377±2.261 | 1.669 |
Note:
▲ P < 0.05 via a CG group,
*P < 0.05 via a MG group.
Table 5. The mRNA levels of Gastrin in the gastric mucosa of H. pylori infected mice in different groups (n = 6).
| Groups | β-actin | GAS(CT) | 2-ΔΔCT | LOG(2-ΔΔCT) |
|---|---|---|---|---|
| MG | 20.68±0.97 | 20.56±0.79 | 1 | 0 |
| CG | 19.79±0.02 | 26.51±0.13 | 0.0093±0.0001* | -2.03 |
| TG | 20.01±0.55 | 25.45±0.72 | 0.0225±0.0001* | -1.65 |
| HF | 20.28±0.14 | 25.38±0.29 | 0.0286±0.0006* | -1.54 |
| MF | 20.48±0.23 | 26.16±0.03 | 0.0192±0.0010* | -1.72 |
| LF | 20.79±0.38 | 26.96±0.36 | 0.0135±0.0015* | -1.87 |
| HFA | 20.52±0.33 | 25.29±0.07 | 0.0361±0.0053* | -1.44 |
| MFA | 20.07±0.08 | 20.54±0.22 | 0.7095±0.0199 | -0.15 |
*P < 0.05 vs model group
Table 6. The mRNA levels of Somatostatinin the gastric mucosa of H. pylori infected mice in different groups (n = 6).
| Groups | β-actin (CT) | SS (CT) | 2-ΔΔCT | LOG(2-ΔΔCT) |
|---|---|---|---|---|
| MG | 20.68±0.97 | 19.07±1.04 | 1 | 0 |
| CG | 19.79±0.02 | 20.92±0.02 | 0.150±0.013* | -0.82 |
| TG | 20.01±0.55 | 20.61±0.37 | 0.217±0.015* | -0.66 |
| HF | 20.28±0.14 | 20.40±0.05 | 0.303±0.004* | -0.52 |
| MF | 20.48±0.23 | 19.86±0.25 | 0.506±0.018* | -0.30 |
| LF | 20.79±0.38 | 21.27±0.38 | 0.236±0.012* | -0.63 |
| HFA | 20.52±0.33 | 20.38±0.43 | 0.362±0.005* | -0.44 |
| MFA | 20.07±0.08 | 19.05±0.20 | 0.556±0.064* | -0.25 |
*P < 0.05 vs model group
Effects of flavonoid glycoside and amoxicillin on the protein levels of inflammatory biomarkers
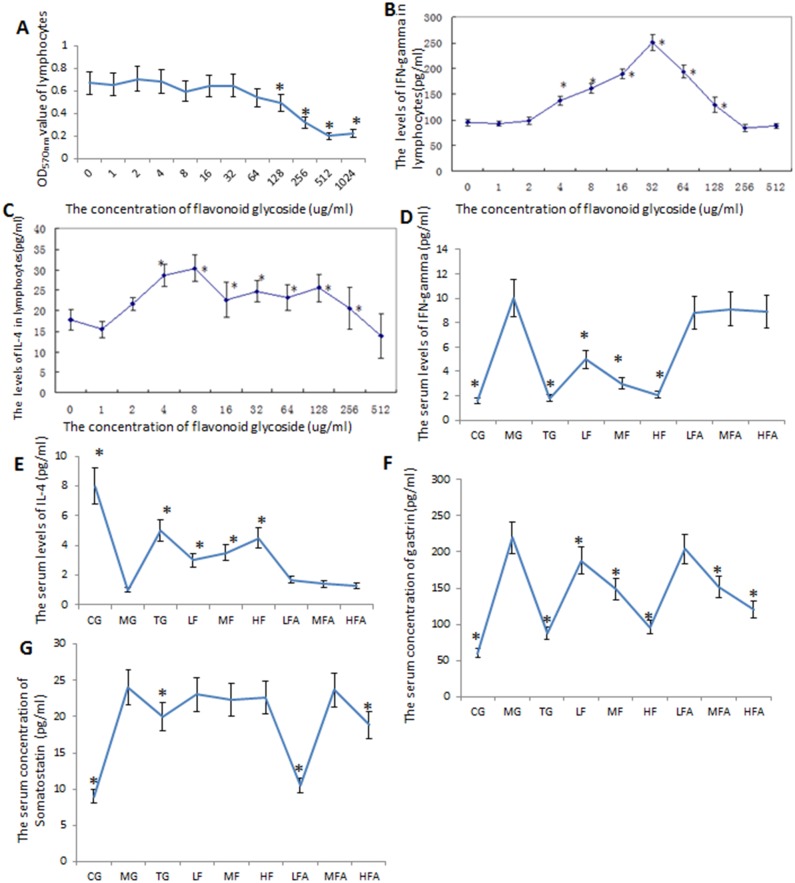
Before the study, the effects of flavonoid glycoside on lymphocytes were measured. The results showed that the OD values of lymphocytes were affected by the concentration of flavonoid glycoside. The OD values were the highest when no flavonoid glycoside was used and the values reached the lowest level when 512 ug/ml of flavonoid glycoside was used (P < 0.05) (Fig 7A). The levels of IFN-gamma from lymphocytes were also affected by flavonoid glycoside and the levels reached the highest point when 32 ug/ml of flavonoid glycoside was used (P < 0.05) (Fig 7B). Comparatively, the levels of IL-4 reached the highest point when 8 ug/ml of flavonoid glycoside was used (P < 0.05) (Fig 7C). To explore the effects of flavonoid glycoside and amoxicillin on IFN-gamma and IL-4, the serum levels of IFN-gamma and IL-4 were measured in different groups. The results indicated that the serum levels of IFN-gamma were higher in a MG group than those in all other groups (P < 0.05) (Fig 7D). The groups only with flavonoid glycoside could reduce the serum levels of IFN-gamma significantly (P < 0.05) while the combination of flavonoid glycoside and amoxicillin caused no obvious changes. In contrast, the serum levels of IL-4 were lower in a MG group than those from all other groups while the levels in a CG group were higher than those from all other groups (P < 0.05) (Fig 7E). The groups only with flavonoid glycoside could increase the serum levels of IL-4 significantly (P < 0.05) while the combination of flavonoid glycoside and amoxicillin caused no obvious changes. For gastrin, the serum levels were higher in a MG group than those in all other groups except a LF group (P < 0.05) (Fig 7F). The concentrations of serum gastrin reached the lowest level in HF, TG and CG groups. Comparatively, the serum levels of somatostatin were higher in all groups only with flavonoid glycoside, a MFA group and a MG group than those from other groups (P < 0.05) (Fig 7G). The concentrations of serum somatostatin reached the lowest level in LFA and CG groups.
Fig 7. The effects of flavonoid glycoside on the number of lymphocytes, protein levels of inflammatory biomarkers.
A, the effects of different concentrations of flavonoid glycoside on the number of lymphocytes. B, the effects of different concentrations of flavonoid glycoside on protein levels of INF-gamma. C, the effects of different concentrations of flavonoid glycoside on protein levels of IL-4. D, the serum levels of INF-gamma in different groups. E, the serum levels of IL-4 in different groups. F, the serum levels of gastrin in different groups. G, the serum levels of somatostatin in different groups. Normal mice (without H. pylori infection) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group, only treated with saline solution), triple combination therapy group (TG group, the daily medicine intake is 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low/middle/high concentrations of flavonoid glycoside group (LF/MF/HF group, treated with one daily dose of flavonoid glycoside at 32/64/128 ug), low/middle/high concentrations of flavonoid glycoside and common concentration of amoxicillin group (LFA/MFA/HFA group, treated with one daily dose of flavonoid glycoside at 32/64/128 ug and amoxicillin at 1 ug). *P < 0.05 vs a model group (n = 10).
The effects of flavonoid glycosides on Gastric cells infected by H. pylori
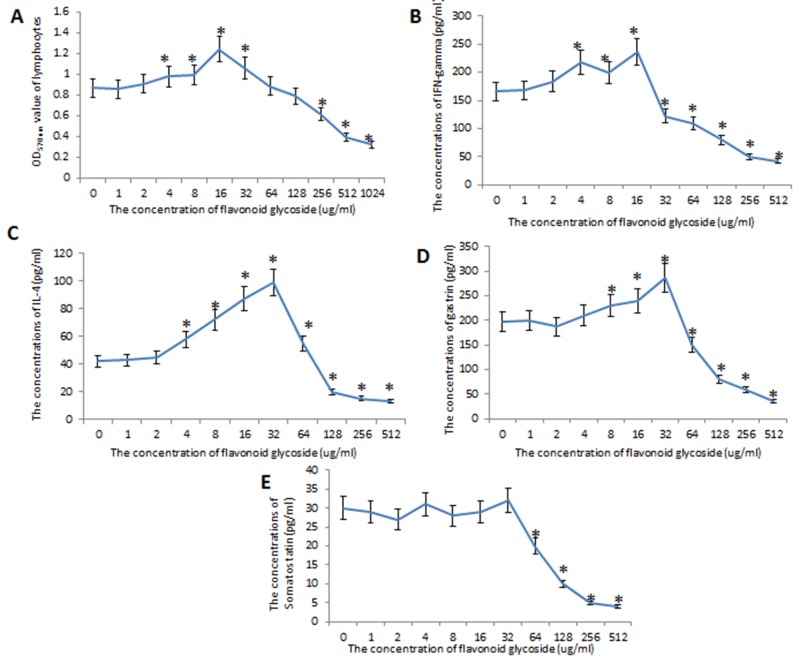
Before the study, the effects of flavonoid glycoside on gastric cells were measured. The results showed that the OD values of gastric cells were affected by the concentrations of flavonoid glycoside. The OD values were the highest when 16 flavonoid glycoside was used and the values reached the lowest level when the concentrations were more than 512 ug/ml (P < 0.05) (Fig 8A). The levels of IFN-gamma from lymphocytes were also affected by flavonoid glycoside and the levels reached the highest point when 16 ug/ml of flavonoid glycoside was used (P < 0.05) (Fig 8B). Comparatively, the levels of IL-4 and gastrin reached the highest point when 32 ug/ml of flavonoid glycoside was used (P < 0.05) (Fig 8C and 8D). The levels of somatostatin were not affected by flavonoid glycoside if the concentrations were less than 32 ug/ml of flavonoid glycoside (P < 0.05) (Fig 8E). All these biomarkers would be reduced greatly if the concentrations were more than 64 ug/ml, suggesting that high concentrations of flavonoid glycoside have toxicity toward gastric cells and inhibit the production of these molecules. All these results implied that flavonoid glycoside may show its anti-inflammatory functions by increasing the levels of IFN-gamma, IL-4 and gastrin.
Fig 8. The effects of flavonoid glycoside on gastric cells infected by H. pylori.
A, the effects of different concentrations of flavonoid glycoside on the number of gastric cells MGC803. B, the effects of different concentrations of flavonoid glycoside on protein levels of INF-gamma. C, the effects of different concentrations of flavonoid glycoside on protein levels of IL-4. D, the effects of different concentrations of flavonoid glycoside on protein levels of gastrin. E, the effects of different concentrations of flavonoid glycoside on protein levels of somatostatin. *P < 0.05 vs a control group without flavonoid glycoside.
Discussion
H. pylori infection is well known to be associated with the risk of many diseases. For example, H. pylori infection contributes to cardiovascular diseases and stroke, and Alzheimer's disease (AD) [46]. Another example, H. pylori infection is most likely to cause chronic gastritis, peptic ulcer disease and liver-related diseases[47]. H. pylori infection significantly affects the life quality of many people in the world and has become a global burden. H. pylori infection therapy often includes pharmaceutical treatment, such as clarithromycin, omeprazole and amoxicillin [48–50]. However, all the medicine has side effects which greatly limit the usage: Omeprazole treatment can cause hypergastrinemia and trophic effects in the stomach with an increase of histamine-producing enterochromaffin-like cells[51]; Combination therapy of clarithromycin and rabeprazole can increase the risk of neurotoxicity[52]; Amoxicillin therapy can also lead to severe adverse effects and death[17]. Thus, it is necessary to explore the new medicine with fewer side effects and therapeutic efficacy. Flavonoid glycoside is a kind of Chinese herbs and has been widely used for the therapy of urinary tract infection [21]. Recent work indicates that flavonoid glycoside can be used for the treatment of H. pylori infection with a few side effects (Chinese patent No. CN102824417A). Amoxicillin is often combined with other medicine for the therapy of H. pylori infection [53], so the combination therapy of flavonoid glycoside and amoxicillin is an effective way for the therapy of H. pylori infection.
To understand the effects of flavonoid glycoside and amoxicillin on a H. pylori infected disease, a mouse gastritis model was established using H. pylori. To explore the molecular mechanisms of effects of flavonoid glycoside and amoxicillin on a mouse gastritis model, IFN-gamma, IL-4, gastrin and somatostatin may be the best molecules for the purpose because of the following reasons: H. pylori infection can elevate IFN-gamma-mediated gastric inflammation [43]; IL-4 is an anti-inflammatory and Th2-type cytokine. H. pylori infection in mammals induces an immune response, which is characterized by an increase of IFN-gamma and absence of IL-4[54]; Serum levels of gastrin are higher in H. pylori-infected patients than in uninfected subjects, and H. pylori infection induces hypergastrinemia in mammals[55]; Somatostatin is a regulatory peptide, which is mainly existed in the stomach. Somatostatin is needed for IL-4-mediated resolution of H. pylori gastritis [56]. Flavonoid glycoside intervention can reduce the serum levels of INF-gamma but not IL-4(Figs 7D and 8E). However, the combination of flavonoid glycoside and amoxicillin doesn’t change the levels of INF-gamma, which cannot improve the inflammation of the tissues infected with H. pylori. The results suggested that only flavonoid glycoside therapy can control the inflammation caused by H. pylori infection.
For further mechanism, flavonoid glycoside has the potential antioxidant activity and the antioxidant functions of flavonoid glycoside have been reported[57]. Most species of Polygonum have bioactive constituents, which contribute to many medicinal properties. P. cuspidatum and P. capitatum exhibit great antioxidant properties and are a potent resource of natural bioactive antioxidants. Here, we found only flavonoid glycoside treatment repaired the gastric injury compared with those treated with the combination of flavonoid glycoside and amoxicillin. The difference may be caused by the antioxidant bioactivities of flavonoid glycoside and side effects of amoxicillin for gastric mucosa. Therefore, we explored the protect function of flavonoid glycoside for gastric mucosa. A high concentration of amoxicillin may be harmful to gastric mucosa although it can enhance the eradicate rate of H. pylori. Certainly, there are some limits for present study and some important experiments are not performed. It would be better if inflammatory responses can be measured in the gastric tissues (for the local inflammatory responses) and within the spleen of infected mice. Furthermore, the functions of many constituents of P. capitatum were not explored. All the work will be performed in the future. Flavonoid glycosides show effects on many cytokines, which plays a critical role for the therapy of H. pylori infection. Flavonoid glycoside can affect the levels of IFN-gamma, IL-4 and gastrin but not somatostatin (Fig 8), which is also the basis for further studying the mechanisms for the functions of flavonoid glycoside.
We demonstrated that the protective and anti-bacterial functions of flavonoid glycoside from P. capitatum for the therapy of H. pylori-infected diseases. The function may be associated with its protective functions of gastric mucosa, antioxidant bioactivities and regulation for the levels of IFN-gamma, IL-4, gastrin and somatostatin. All these functions can reduce the injury of gastric tissues infected by H. pylori and improve the symptoms. We therefore propose that flavonoid glycoside from P. capitatum is potential for the H. pylori infection and should be developed a new drug for H. pylori infected diseases.
Materials and Methods
Materials and Reagent
H. pylori SS1 were purchased from the Chinese Center for Disease Control (Beijing, China). The strains were cultured on selective agar (Wilkins—Chalgren agar supplemented with 5 percent of horse blood, 10.5 ug/mL vancomycin, 0.5 ug/mL cefsulodin, 1 ug/mL trimethoprim lactate, and 1 ug/mL fungizone (Biogerm, Maia, Portugal)) and incubated at 37°C under microaerobic conditions for one day. The extracts of Polygonum capitatum, flavonoid glycosides were prepared according to a previous report[21, 58] and identified by Professor Ma Lin of the Institute of Material Medicine, Chinese Academy of Chinese Medical Sciences (Beijing, China). Amoxicillin, omeprazole and clarithromycin was purchased from Xinya Co., (Shanghai, China).
Minimum inhibitory concentration (MIC) test
The determination of MICs of flavonoid glycosides for the H. pylori was examined by use of the serial dilution method as described previously [59]. Briefly, the bacteria were sub-cultured on Mueller-Hinton agar supplemented with 5 percent sheep blood for two days. A bacterial suspension with 107 CFU/ml was placed onto each flavonoid glycoside dilution agar plate. After incubation for three days, the MIC of each sample was determined. Quality control was performed with H. pylori SS1.
Lymphocyte proliferation assay
Lymphocytes were isolated from mice spleens using Amaxa Mouse T Cell Nucleofector Kit (Amaxa, Gaithersburg, USA). Lymphocyte proliferations upon the stimulation of flavonoid glycoside and controls were determined with the colorimetric Cell Counting Kit-8 (CCK-8, Beyotime, Shanghai, China). Isolated lymphocytes were plated in flat-bottom 96-well microtitre plate at a density of 5×105 cells/well, 10 μl of CCK-8 was added to each well and incubated for further 4 h, and absorbing value at 450 nm was measured to count cell proliferation. The stimulation index (SI) was calculated as the ratio of mean OD value of the wells containing flavonoid glycoside-stimulated cells to mean OD value of the wells containing cells without flavonoid glycoside stimulation. All assays were conducted in triplicates.
Model establishment
A total 100 mice C57BL/6 (6~8 weeks, male/female = 1:1, weight(20±5)g) were purchased from Experimental Animals Center of Chongqing Medical University (license No. SYXK 2007–0001). All animals were housed in cages with a 12 h light/dark cycle. The cages were kept at 23 ± 1°C with 50% relative humidity. Food and water could be available ad libitum. Animal care and handling procedures were conducted according to the International Association for Study of Pain guidelines for animals in pain research. All efforts were performed to minimize the number of animals and their suffering in the experiment. Animals were provided with sawdust bedding material and were housed under these conditions for at least 1 week prior to the experiments. Mice were fasted for 12 h before all experimental studies.
Before the infection of mice, H. pylori from plate cultures were inoculated into in Brucella broth culture medium (Becton Dickinson, Cockeysville, USA) containing 10% fetal bovine serum and were cultured for 18 h under microaerobic conditions. A total of 90 pathogen-free C57BL/6 8-week-old mice were used in compliance with guidelines and a protocol approved by the Animal Care and Use Committee of Guiyang medical college. Using a 20-gauge ballpoint metal feeding tube (Harvard Apparatus, Inc., Holliston, MA, USA), 90 mice were inoculated intragastrically with 0.1 mL of H. pylori SS1 cell suspension containing 108 colony-forming units /mL on three alternate days. Ten healthy mice were inoculated with saline solution and used as a control.
After nine days, ten mice from a model group and ten from a control group were sacrificed using cervical dislocation without anesthesia prior to the end of the experiment. Subsequently, the stomachs were isolated from the mice by cutting the tissues from the esophagus to the duodenum. The non-glandular portion of fore-stomach was removed from the glandular stomach. The glandular stomach was dissected and rinsed with PBS, and divided into three longitudinal strips, which were used for bacterial culture, RNA analysis, and histology.
Evaluation of a mouse model infected with H. pylori
Gastric tissues were homogenized via Tissue Tearor (BioSpecProducts, Bartlesville, USA). The homogenate were placed on trypticase soy agar (TSA) with different dilutions, complementing with 5 percent horse blood, 10 μg/mL nalidixic acid, 100 μg/mL vancomycin, 2 μg/mL amphotericin, and 200 μg/mL bacitracin (all antibiotics were from Sigma-Aldrich Shanghai Trading Co Ltd, Shanghai, China). After 5–7 d of culture under microaerobic conditions, H. pylori colonies were counted and the number of colony forming units per gram of tissue calculated (CFU/g). Colonies were used to test the bioactivity of urease, catalase, and oxidase. H. pylori colonies were identified using the following methods: Gram-staining, H. pylori colonies were identified using a Gram-staining kit (BD Biosciences, San Jose, USA) according to the manuscripture’s instructions; Rapid urease test, rapid urease test was performed according to a previous report[60]. A biopsy was inoculated into 1mL of 10% urea dissolved in distilled water (pH 6.8), to which two drops of one percent phenol red solution were added and incubated at 37°C for one day. A color change from yellow to pink within 1 h from the start of the test was considered a criterion for the presence of H. pylori infection; Catalase test, catalase test was conducted according to a previous report[61]. The colony grown in selective medium was placed on a slide, and bubbling following dropping 3% H2O2 was determined as positive reaction; Oxidase test, H. pylori uses disks impregnated with a reagent such as N,N,N',N'-tetramethyl-p-phenylenediamine (TMPD), which is a redox indicator[62]. The reagent can be changed from a dark-blue to maroon color when it is oxidized.
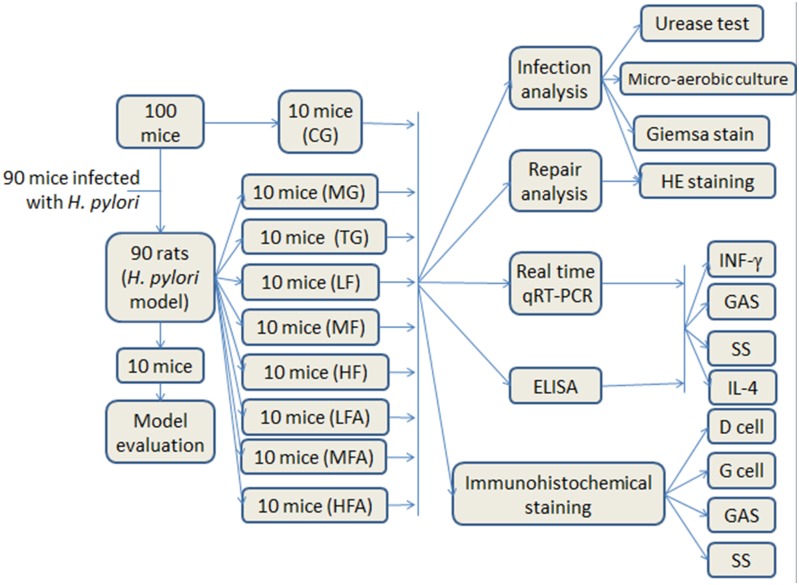
Groups
As Fig 9 showed, 90 mice were used for the establishment of mice models infected with H. pylori and 10 normal mice were used as control group (CG). From the mice models, 10 mice were used for the assessment of model and remaining mice were randomly divided into 8 groups. As Fig 9 showed, normal mice (pathogen free) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group), triple combination therapy group (TG group. According to a previous report, for an adult/50 kg, daily medicine intake is 1000 mg clarithromycin, 40 mg omeprazole, and 2000 mg amoxicillin for the eradication of H. pylori[63]. For a mouse/25 g, the daily medicine intake was 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low concentration of flavonoid glycoside group (LF group, treated with one daily dose of flavonoid glycoside at 32 ug), middle concentration of flavonoid glycoside group (MF group, treated with one daily dose of flavonoid glycoside at 64 ug), high concentration of flavonoid glycoside group (HF group, treated with one daily dose of flavonoid glycoside at 128 ug), low concentration of flavonoid glycoside and amoxicillin group (LFA group, treated with one daily dose of flavonoid glycoside at 32 ug and amoxicillin at 1 ug), middle concentration of flavonoid glycoside and amoxicillin group (MFA group, treated with one daily dose of flavonoid glycoside at 64 ug and amoxicillin at 1 ug) and high concentration of flavonoid glycoside and amoxicillin group (LFA group, treated with one daily dose of flavonoid glycoside at 128 ug and amoxicillin at 1 ug). Mice in CG and MG groups were fed with saline solution. After two weeks, all mice were sacrificed using cervical dislocation without anesthesia prior to the end of the experiment. Each sample was fixed in 10 percent neutral formalin. The remains of tissues were stored at -80°C All the protocols for mice studies were approved by the Animal Care and Use Committees of Guiyang Medical College (Guiyang, China). The therapeutic efficiency of these groups was assessed from two aspects: 1) eradication rate of H. pylori; 2) the analysis of histochemistry and histopathology.
Fig 9. The flowchart of study.
Normal mice (without H. pylori infection) were assigned as a control group (CG group) and mice models infected with H. pylori were divided into model group (MG group, only treated with saline solution), triple combination therapy group (TG group, the daily medicine intake is 0.5 ug clarithromycin, 0.02 ug omeprazole, and 1 ug amoxicillin), low/middle/high concentrations of flavonoid glycoside group (LF/MF/HF group, treated with one daily dose of flavonoid glycoside at 32/64/128 ug), low/middle/high concentrations of flavonoid glycoside and common concentration of amoxicillin group (LFA/MFA/HFA group, treated with one daily dose of Flavonoid glycoside at 32/64/128 ug and amoxicillin at 1 ug). Gastrin, GAS; Somatostatin, SS.
Eradication rate of H. pylori
The eradication rate of H. pylori in different groups was performed according to a previous report[64].
Analysis of histochemistry and histopathology
Hematoxylin and eosin (H&E) staining. A longitudinal strip from the greater curvature of the stomach was excised and placed in 10% normal buffered formalin for 24 h, embedded in paraffin and stained with for H&E staining according to a previous report[65]. Pathological scores of gastric tissues of the mice were graded according to the criteria described in a previous report [66].
Immunohistochemical staining for G and D cells. For each mouse, half of the stomach was totally processed for immunohistochemistry study to observe the mucosa from the distal esophagus to the duodenum. Two-micrometer-thick sections were cut from the buffered formalin-fixed paraffin-embedded tissue blocks and placed onto Super frost plus slides (Menzel-Gläser, Braunschweig, Germany). After baking in an oven, the sections were dewaxed and rehydrated. Endogenous peroxidase was blocked with 2% H2O2 in absolute methanol for 10 min. Monoclonal Mouse anti-Gastrin antibody (#G2020-08, Beijing Huamei Scientific, Beijing, China) and Monoclonal Mouse anti-somatostatin antibody (#MA5-17182, Thermo Fisher Scientific, Inc., Rockford, USA) were used and immunohistochemical staining for G and D cells was conducted with the strept-avidin-biotin-peroxidase complex (Byotime, Shanghai, China). Five images were randomly viewed under microscope (200×) from each anti-gastrin immunohistochemical staining section. The number and grey values of G cells were counted by a computer. The number and grey values of D cells were measured in the same way as the G cells.
Real-time qRT-PCR
RNA was isolated from the stomach using t using a RNA isolation kit (Bioteke, Beijing, China). The cDNAs were synthesized from purified RNA with Reverse Transcription Kit (Takara, Dalian, China). The mRNA levels of IFN-gamma, IL-4, Gastrin, Somatostatin and H. pylori specific gene CagA (GenBank No. AB090103.1)[42], were measured using the primers were synthesized as Table 7. For real time qRT-PCR, β-actin was used as the normalizer, and tissue from uninfected mouse stomachs served as a blank control. All cDNA samples were analyzed in triplicate, along with β-actin controls. Levels of mRNA are compared between the tissue from H. pylori-infected mice and the tissue from uninfected mice. Relative units were calculated as 2-ΔΔCt (Ct, cycle threshold) where ΔΔCt is equal to the difference between the ΔCt of the gene of interest of the experimental sample and the calibrator tissue. The ΔCt of target genes were calculated as the difference between the cycle threshold of target genes and the cycle threshold of β-actin.
Table 7. Primers used in real-time qRT-PCR.
| Genes | Primers (5' to 3') | Size(bp) |
|---|---|---|
| β-actin A0009 | GAGACCTTCAACACCCCAGC | 263 |
| β-actin A0010 | ATGTCACGCACGATTTCCC | |
| CagA F1 | ttcagtaaggtagagcaagc | 180 |
| CagA R1 | caattctttcctgatatccg | |
| GAS F1 | TGCTGGCTCTAGCTACCTTCTC | 230 |
| GAS R1 | TCCGTAGGCCTCTTCTTCTTC | |
| SS F1 | GAGCCCAACCAGACAGAGAAT | 151 |
| SS R1 | AGAAGTTCTTGCAGCCAGCTT | |
| IFN-gamma F1 | TGGCTGTTTCTGGCTGTTACT | 218 |
| IFN-gamma R1 | GATGGCCTGATTGTCTTTCAA | |
| IL-4 F1 | GTCCTCACAGCAACGAAGAAC | 241 |
| IL-4 R1 | TGATGCTCTTTAGGCTTTCCA |
PCR amplification was performed with an initial denaturation cycle at 95°C for 5 min, followed by 50 amplification cycles consisting of 95°C for 5 sec, annealing at 60°C for 10 sec, and extension at 72°C for 20 sec. After amplification, a melting step was performed, consisting of 95°C for 5 sec, cooling to 45°C for 30 sec (with a temperature transition rate of 20°C per second), and finally a slow rise in the temperature to 85°C at a rate of 0.1°C per second with a fluorescence decline.
ELISA
Serum sample was placed in each well with the same volume in Microtiter plates and ELISA was performed by using ELISA Kit for according to an instruction manual (Mouse IL-4 ELISA Kit, #RAB0300, Sigma-Aldrich Shanghai Trading Co Ltd., Shanghai, China; Mouse IFN-gamma ELISA Kit, #EM1001, Pierce Biotechnology, Inc., Chicago, USA; Mouse Gastrin ELISA Kit, # CSB-E12924m, Wuhan Hi-tech Medical Devices Park, Wuhan, China; Mouse Somatostatin Elisa Kit, #CSB-E08205m, Wuhan Hi-tech Medical Devices Park, Wuhan, China). The absorption value for Nitrophenolate 158 was measured at 405 nm using Automated ELISA analyzer (Yantai Addcare Bio-Tech Co., Ltd., Yantai, China). The series of different concentrations of IFN-gamma, IL-4, Gastrin and Somatostatin were used to plot a standard curve.
The effects of flavonoid glycosides on Gastric cells infected by H. pylori
Gastric cell line MGC803 from Shanghai Institutes for Biological Sciences, CAS (Shanghai, China). MGC803 cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS) at 37°C in 5% CO2. H. pylori were routinely grown on sheep blood agar plates in 10% CO2 at 37°C. Before infections, H. pylori was cultured for 24 h on BAP, harvested with centrifuge, and resuspended in 1 ml of brucella broth. For biochemical assays, 5 × 106 MGC803 cells were plated in 6-cm-diameter dishes with DMEM medium. The next day, the cells were washed with PBS, pH 7.0, and serum starved in serum-free DMEM for 4 h. The media were then exchanged for fresh DMEM with 2% FBS. The eukaryotic cells were then stimulated with H. pylori at a multiplicity of infection of approximately 100:1 for 1 h and cultured for one day. Subsequently, the cells were treated with a series of diluted flavonoid glycoside. After three-day culture, all the cells were washed with DMEM and lysed on ice with lysis buffer (50 mM Tris, pH 7.0, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA). Supernatants were collected via centrifugation, and evaluated for IL-4, INF-gamma, gastrin and Somatostatin by ELISA, as described before.
Statistical analysis
All data were analyzed via SPSS 20 software (Chicago, IL, USA). Histograms and the Kolmogorov—Smirnov methods were conducted to determine a normal distribution of the variables. With a normal distribution, quantitative data were presented as mean ± SD. T-test for independent means is used to test whether there is a difference between groups. P < 0.05 was regarded as statistically significant.
Acknowledgments
We are grateful to the anonymous reviewer for constructive criticism and strategic advice which greatly improved the article. The project was supported by the joint fund of Science and Technology Department of Guizhou province and Medical College of Guiyang (No. LG(2012)012 and LG(2012)054), Specialized Program Construction Project in University (No.(No.[2010]15), the Science and Technology Program of Guizhou (No. [2014]2027), The Ph.D. Programs Foundation of Affiliated hospital of Guiyang Medical College (No. 2014) and College students' innovative entrepreneurial training program of Guizhou (No.201410660014).
Data Availability
Data are available upon request due to ethical and legal restrictions. Data related to Polygonum capitatum and preparation of its extracts are available upon request to: Prof. Liyan Zhang, Guizhou Weimen Pharmaceutical Co. Ltd., Address No23, Gaoxin Road, Wudang District, Guiyang 550018, Guizhou Province, China; xzb@warmen.com. All other data are available upon request to author Fei Mo due to ethical restrictions imposed by Ethic Committee of Guiyang Medical College (Guiyang, China).
Funding Statement
The project was supported by the joint fund of Science and Technology Department of Guizhou province and Medical College of Guiyang (No. LG(2012)012 and LG(2012)054), Specialized Program Construction Project in University (No. 21 (No.[2010]15), the Science and Technology Program of Guizhou (No. [2014]2027), The Ph.D. Programs Foundation of Affiliated hospital of Guiyang Medical College (No. 2014) and College students' innovative entrepreneurial training program of Guizhou (No.201410660014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kodaman N, Pazos A, Schneider BG, Piazuelo MB, Mera R, Sobota RS, et al. Human and Helicobacter pylori coevolution shapes the risk of gastric disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(4):1455–60. 10.1073/pnas.1318093111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts D, Hopkins M, Miller S, Schafer W. Gastric MALT lymphoma in the absence of Helicobacter pylori infection presenting as an upper gastrointestinal hemorrhage. Southern medical journal. 2006;99(10):1134–6. 10.1097/01.smj.0000215746.17667.b1 . [DOI] [PubMed] [Google Scholar]
- 3. Sena Teixeira Mendes L, DA A, CW A. Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: a re-evaluation. Gut. 2014;63(9):1526–7. 10.1136/gutjnl-2014-307389 . [DOI] [PubMed] [Google Scholar]
- 4. Ono S, Kato M, Takagi K, Kodaira J, Kubota K, Matsuno Y, et al. Metachronous gastric cancer following complete remission of gastric MALT lymphoma. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2009;20(10):1748–9. 10.1093/annonc/mdp389 . [DOI] [PubMed] [Google Scholar]
- 5. Mihaljevic S, Katicic M, Karner I, Vuksic-Mihaljevic Z, Dmitrovic B, Ivandic A. The influence of Helicobacter pylori infection on gastrin and somatostatin values present in serum. Hepato-gastroenterology. 2000;47(35):1482–4 . [PubMed] [Google Scholar]
- 6. Shiota S, Murakami K, Okimoto T, Kodama M, Yamaoka Y. Serum Helicobacter pylori CagA antibody titer as a useful marker for advanced inflammation in the stomach in Japan. Journal of gastroenterology and hepatology. 2014;29(1):67–73. 10.1111/jgh.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen MY, He CY, Meng X, Yuan Y. Association of Helicobacter pylori babA2 with peptic ulcer disease and gastric cancer. World journal of gastroenterology: WJG. 2013;19(26):4242–51. 10.3748/wjg.v19.i26.4242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin HJ, Perng CL, Lo WC, Wu CW, Tseng GY, Li AF, et al. Helicobacter pylori cagA, iceA and vacA genotypes in patients with gastric cancer in Taiwan. World journal of gastroenterology: WJG. 2004;10(17):2493–7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo Q, Guo S, Zhang Y. Treatment of gastric MALT lymphoma with a focus on Helicobacter pylori eradication. International journal of hematology. 2013;97(6):735–42. 10.1007/s12185-013-1348-2 . [DOI] [PubMed] [Google Scholar]
- 10. Nishizawa T, Maekawa T, Watanabe N, Harada N, Hosoda Y, Yoshinaga M, et al. Clarithromycin Versus Metronidazole as First-line Helicobacter pylori Eradication: A Multicenter, Prospective, Randomized Controlled Study in Japan. Journal of clinical gastroenterology. 2014. 10.1097/MCG.0000000000000165 . [DOI] [PubMed] [Google Scholar]
- 11. Gong EJ, Yun SC, Jung HY, Lim H, Choi KS, Ahn JY, et al. Meta-analysis of first-line triple therapy for helicobacter pylori eradication in Korea: is it time to change? Journal of Korean medical science. 2014;29(5):704–13. 10.3346/jkms.2014.29.5.704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qureshi NN, Gallaher B, Schiller NL. Evolution of Amoxicillin Resistance of Helicobacter pylori In Vitro: Characterization of Resistance Mechanisms. Microbial drug resistance. 2014. 10.1089/mdr.2014.0019 . [DOI] [PubMed] [Google Scholar]
- 13. Tu IF, Liao JH, Yang FL, Lin NT, Chan HL, Wu SH. Lon protease affects the RdxA nitroreductase activity and metronidazole susceptibility in Helicobacter pylori. Helicobacter. 2014;19(5):356–66. 10.1111/hel.12140 . [DOI] [PubMed] [Google Scholar]
- 14. Yue JY, Yue J, Wang MY, Song WC, Gao XZ. CagA status & genetic characterization of metronidazole resistant strains of H. pylori from: A region at high risk of gastric cancer. Pakistan journal of medical sciences. 2014;30(4):804–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yilmaz O, Demiray E. Clinical role and importance of fluorescence in situ hybridization method in diagnosis of H pylori infection and determination of clarithromycin resistance in H pylori eradication therapy. World journal of gastroenterology: WJG. 2007;13(5):671–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Picoli SU, Mazzoleni LE, Fernandez H, De Bona LR, Neuhauss E, Longo L, et al. Resistance to amoxicillin, clarithromycin and ciprofloxacin of Helicobacter pylori isolated from Southern Brazil patients. Revista do Instituto de Medicina Tropical de Sao Paulo. 2014;56(3):197–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gresser U. Amoxicillin-clavulanic acid therapy may be associated with severe side effects—review of the literature. European journal of medical research. 2001;6(4):139–49 . [PubMed] [Google Scholar]
- 18. Liu P, Kong M, Yuan S, Liu J, Wang P. History and experience: a survey of traditional chinese medicine treatment for Alzheimer's disease. Evidence-based complementary and alternative medicine: eCAM. 2014;2014:642128 10.1155/2014/642128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu LW, Jia M, Salchow R, Kentsch M, Cui XJ, Deng HY, et al. Efficacy and side effects of chinese herbal medicine for menopausal symptoms: a critical review. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:568106 10.1155/2012/568106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He CY, Fu J, Ma JY, Feng R, Tan XS, Huang M, et al. Biotransformation and in vitro metabolic profile of bioactive extracts from a traditional Miao-nationality herbal medicine, Polygonum capitatum. Molecules. 2014;19(7):10291–308. 10.3390/molecules190710291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao SG, Zhang LJ, Sun F, Zhang JJ, Chen AY, Lan YY, et al. Antibacterial and anti-inflammatory effects of extracts and fractions from Polygonum capitatum. Journal of ethnopharmacology. 2011;134(3):1006–9. 10.1016/j.jep.2011.01.050 . [DOI] [PubMed] [Google Scholar]
- 22. Ni B, Rajaram MV, Lafuse WP, Landes MB, Schlesinger LS. Mycobacterium tuberculosis decreases human macrophage IFN-gamma responsiveness through miR-132 and miR-26a. Journal of immunology. 2014;193(9):4537–47. 10.4049/jimmunol.1400124 . [DOI] [PubMed] [Google Scholar]
- 23. Bi Y, Zhou J, Yang H, Wang X, Zhang X, Wang Q, et al. IL-17A produced by neutrophils protects against pneumonic plague through orchestrating IFN-gamma-activated macrophage programming. Journal of immunology. 2014;192(2):704–13. 10.4049/jimmunol.1301687 . [DOI] [PubMed] [Google Scholar]
- 24. Soo Hoo W, Jensen ER, Saadat A, Nieto D, Moss RB, Carlo DJ, et al. Vaccination with cell immunoglobulin mucin-1 antibodies and inactivated influenza enhances vaccine-specific lymphocyte proliferation, interferon-gamma production and cross-strain reactivity. Clinical and experimental immunology. 2006;145(1):123–9. 10.1111/j.1365-2249.2006.03107.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Advances in immunology. 2007;96:41–101. 10.1016/S0065-2776(07)96002-2 . [DOI] [PubMed] [Google Scholar]
- 26. Vivas JR, Regnault B, Michel V, Bussiere FI, Ave P, Huerre M, et al. Interferon gamma-signature transcript profiling and IL-23 upregulation in response to Helicobacter pylori infection. International journal of immunopathology and pharmacology. 2008;21(3):515–26 . [DOI] [PubMed] [Google Scholar]
- 27. Wagner B, Burton A, Ainsworth D. Interferon-gamma, interleukin-4 and interleukin-10 production by T helper cells reveals intact Th1 and regulatory TR1 cell activation and a delay of the Th2 cell response in equine neonates and foals. Veterinary research. 2010;41(4):47 10.1051/vetres/2010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kelly-Welch AE, Hanson EM, Boothby MR, Keegan AD. Interleukin-4 and interleukin-13 signaling connections maps. Science. 2003;300(5625):1527–8. 10.1126/science.1085458 . [DOI] [PubMed] [Google Scholar]
- 29. Salmon-Ehr V, Ramont L, Godeau G, Birembaut P, Guenounou M, Bernard P, et al. Implication of interleukin-4 in wound healing. Laboratory investigation; a journal of technical methods and pathology. 2000;80(8):1337–43 . [DOI] [PubMed] [Google Scholar]
- 30. Holden JA, Attard TJ, Laughton KM, Mansell A, O'Brien-Simpson NM, Reynolds EC. Porphyromonas gingivalis LPS weakly activates M1 and M2 polarised mouse macrophages but induces inflammatory cytokines. Infection and immunity. 2014:IAI 02325–14. 10.1128/IAI.02325-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VW, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. Journal of the American Society of Nephrology: JASN. 2010;21(6):933–42. 10.1681/ASN.2009060592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ratto GB. Pyloric antrum gastrin cell hyperplasia after jejunum or colon transposition: effects of jejunal or colonic mucosa on G cells. European surgical research Europaische chirurgische Forschung Recherches chirurgicales europeennes. 1991;23(5–6):327–32 . [DOI] [PubMed] [Google Scholar]
- 33. Sordal O, Waldum H, Nordrum IS, Boyce M, Bergh K, Munkvold B, et al. The gastrin receptor antagonist netazepide (YF476) prevents oxyntic mucosal inflammation induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2013;18(6):397–405. 10.1111/hel.12066 . [DOI] [PubMed] [Google Scholar]
- 34. Lucke C, Hoffken B, Muhlen A. Studies on the postponed growth hormone secretion following the infusion of somatostatin. Acta endocrinologica. 1976;82(3):460–6 . [PubMed] [Google Scholar]
- 35. Yamamoto S, Kaneko H, Konagaya T, Mori S, Kotera H, Hayakawa T, et al. Interactions among gastric somatostatin, interleukin-8 and mucosal inflammation in Helicobacter pylori-positive peptic ulcer patients. Helicobacter. 2001;6(2):136–45 . [DOI] [PubMed] [Google Scholar]
- 36. Datta BK, Datta SK, Chowdhury MM, Khan TH, Kundu JK, Rashid MA, et al. Analgesic, antiinflammatory and CNS depressant activities of sesquiterpenes and a flavonoid glycoside from Polygonum viscosum. Die Pharmazie. 2004;59(3):222–5 . [DOI] [PubMed] [Google Scholar]
- 37. Kline EK. Bergey's Manual of Determinative Bacteriology, 6th edition. Am J Public Health N. 1948;38(12):1700-. . [Google Scholar]
- 38. Nisha KJ, Nandakumar K, Shenoy KT, Janam P. Periodontal disease and Helicobacter pylori infection: a community-based study using serology and rapid urease test. Journal of investigative and clinical dentistry. 2014. 10.1111/jicd.12122 . [DOI] [PubMed] [Google Scholar]
- 39. Slesak G, Douangdala P, Inthalad S, Silisouk J, Vongsouvath M, Sengduangphachanh A, et al. Fatal Chromobacterium violaceum septicaemia in northern Laos, a modified oxidase test and post-mortem forensic family G6PD analysis. Annals of clinical microbiology and antimicrobials. 2009;8:24 10.1186/1476-0711-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mohammadi M, Kashani SS, Garoosi YT, Tazehkand SJ. In vivo measurement of Helicobacter pylori infection. Methods in molecular biology. 2012;921:239–56. 10.1007/978-1-62703-005-2_26 . [DOI] [PubMed] [Google Scholar]
- 41. Cover TL. Perspectives on methodology for in vitro culture of Helicobacter pylori. Methods in molecular biology. 2012;921:11–5. 10.1007/978-1-62703-005-2_3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Siavoshi F, Salmanian AH, Akbari F, Malekzadeh R, Massarrat S. Detection of Helicobacter pylori-specific genes in the oral yeast. Helicobacter. 2005;10(4):318–22. 10.1111/j.1523-5378.2005.00319.x . [DOI] [PubMed] [Google Scholar]
- 43. Wang YC, Chen CL, Sheu BS, Yang YJ, Tseng PC, Hsieh CY, et al. Helicobacter pylori Infection Activates Src Homology-2 Domain-Containing Phosphatase 2 To Suppress IFN-gamma Signaling. Journal of immunology. 2014;193(8):4149–58. 10.4049/jimmunol.1400594 . [DOI] [PubMed] [Google Scholar]
- 44. Orsini B, Ottanelli B, Amedei A, Surrenti E, Capanni M, Del Prete G, et al. Helicobacter pylori cag pathogenicity island is associated with reduced expression of interleukin-4 (IL-4) mRNA and modulation of the IL-4delta2 mRNA isoform in human gastric mucosa. Infection and immunity. 2003;71(11):6664–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zavros Y, Paterson A, Lambert J, Shulkes A. Expression of progastrin-derived peptides and somatostatin in fundus and antrum of nonulcer dyspepsia subjects with and without Helicobacter pylori infection. Digestive diseases and sciences. 2000;45(10):2058–64 . [DOI] [PubMed] [Google Scholar]
- 46. Kountouras J, Gavalas E, Polyzos SA, Deretzi G, Kouklakis G, Grigoriadis S, et al. Association between Helicobacter pylori burden and Alzheimer's disease. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2014;21(12):e100 10.1111/ene.12563 . [DOI] [PubMed] [Google Scholar]
- 47. Siddique I, Al-Qabandi A, Al-Ali J, Alazmi W, Memon A, Mustafa AS, et al. Association between Helicobacter pylori genotypes and severity of chronic gastritis, peptic ulcer disease and gastric mucosal interleukin-8 levels: Evidence from a study in the Middle East. Gut pathogens. 2014;6(1):41 10.1186/s13099-014-0041-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yoon KH, Park SW, Lee SW, Kim BJ, Kim JG. Clarithromycin-based standard triple therapy can still be effective for Helicobacter pylori eradication in some parts of the Korea. Journal of Korean medical science. 2014;29(9):1240–6. 10.3346/jkms.2014.29.9.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Peng YC, Huang LR, Shyu CL, Cheng CC, Ho SP. Interaction of omeprazole and Helicobacter pylori-induced nuclear factor-kappaB activation and mediators in gastric epithelial cells. Journal of the Chinese Medical Association: JCMA. 2014. 10.1016/j.jcma.2014.07.006 . [DOI] [PubMed] [Google Scholar]
- 50. Lim HC, Lee YJ, An B, Lee SW, Lee YC, Moon BS. Rifabutin-based High-dose Proton-pump Inhibitor and Amoxicillin Triple Regimen as the Rescue treatment for Helicobacter pylori. Helicobacter. 2014. 10.1111/hel.12147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hakanson R, Axelson J, Tielemans Y, Johansson AG, Willems G, Sundler F. Unilateral vagal denervation suppresses omeprazole-induced trophic effects on the denervated side of the rat stomach. Scandinavian journal of gastroenterology. 1992;27(1):65–70 . [DOI] [PubMed] [Google Scholar]
- 52. Feng Z, Huang J, Xu Y, Zhang M, Hu S. Dissociative disorder induced by clarithromycin combined with rabeprazole in a patient with gastritis. The Journal of international medical research. 2013;41(1):239–43. 10.1177/0300060513475384 . [DOI] [PubMed] [Google Scholar]
- 53. Chen MC, Lei WY, Lin JS, Yi CH, Wu DC, Hu CT. Levofloxacin-amoxicillin/clavulanate-rabeprazole versus a standard seven-day triple therapy for eradication of Helicobacter pylori infection. BioMed research international. 2014;2014:158520 10.1155/2014/158520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36(3):341–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology. 2005;128(5):1229–42 . [DOI] [PubMed] [Google Scholar]
- 56. Kao JY, Pierzchala A, Rathinavelu S, Zavros Y, Tessier A, Merchant JL. Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori. Regulatory peptides. 2006;134(1):23–9. 10.1016/j.regpep.2005.11.002 . [DOI] [PubMed] [Google Scholar]
- 57. Taiwo BJ, Igbeneghu OA. Antioxidant and antibacterial activities of flavonoid glycosides from ficus exasperata vahl-holl (moraceae) leaves. African journal of traditional, complementary, and alternative medicines: AJTCAM / African Networks on Ethnomedicines. 2014;11(3):97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liao SG, Zhang LJ, Sun F, Wang Z, He X, Wang AM, et al. Identification and characterisation of phenolics in Polygonum capitatum by ultrahigh-performance liquid chromatography with photodiode array detection and tandem mass spectrometry. Phytochemical analysis: PCA. 2013;24(6):556–68. 10.1002/pca.2432 . [DOI] [PubMed] [Google Scholar]
- 59. Kim JM, Kim JS, Jung HC, Kim N, Kim YJ, Song IS. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrobial agents and chemotherapy. 2004;48(12):4843–7. 10.1128/AAC.48.12.4843-4847.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel SK, Mishra GN, Pratap CB, Jain AK, Nath G. Helicobacter pylori is not eradicated after triple therapy: a nested PCR based study. BioMed research international. 2014;2014:483136 10.1155/2014/483136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang YH, Park D, Yang G, Lee SH, Bae DK, Kyung J, et al. Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Laboratory animal research. 2012;28(1):55–60. 10.5625/lar.2012.28.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tsukita S, Koyanagi S, Nagata K, Koizuka H, Akashi H, Shimoyama T, et al. Characterization of a cb-type cytochrome c oxidase from Helicobacter pylori. Journal of biochemistry. 1999;125(1):194–201 . [DOI] [PubMed] [Google Scholar]
- 63. Chen S, Chen Z, Bei L. Omeprazole, clarithromycin and amoxicillin therapy for Helicobacter pylori infection. Zhonghua nei ke za zhi. 1996;35(12):799–802 . [PubMed] [Google Scholar]
- 64. Park CS, Lee SM, Park CH, Koh HR, Jun CH, Park SY, et al. Pretreatment Antimicrobial Susceptibility-Guided Vs. Clarithromycin-Based Triple Therapy for Helicobacter pylori Eradication in a Region With High Rates of Multiple Drug Resistance. The American journal of gastroenterology. 2014;109(10):1595–602. 10.1038/ajg.2014.222 . [DOI] [PubMed] [Google Scholar]
- 65. Irani S, Monsef Esfahani A, Bidari Zerehpoush F. Detection of Helicobacter pylori in Oral Lesions. Journal of dental research, dental clinics, dental prospects. 2013;7(4):230–7. 10.5681/joddd.2013.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer cell. 2008;14(5):408–19. 10.1016/j.ccr.2008.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request due to ethical and legal restrictions. Data related to Polygonum capitatum and preparation of its extracts are available upon request to: Prof. Liyan Zhang, Guizhou Weimen Pharmaceutical Co. Ltd., Address No23, Gaoxin Road, Wudang District, Guiyang 550018, Guizhou Province, China; xzb@warmen.com. All other data are available upon request to author Fei Mo due to ethical restrictions imposed by Ethic Committee of Guiyang Medical College (Guiyang, China).