Abstract
Nontuberculous mycobacteria (NTM) are a large group of environmental organisms with worldwide distribution, but only a relatively few are known to be pathogenic. Chronic, debilitating lung disease is the most common manifestation of NTM infection, which is often refractory to treatment. The incidence and prevalence of NTM lung disease are increasing in the United States and in many parts of the world. Hence, a more complete understanding of NTM pathogenesis will provide the foundation to develop innovative approaches to treat this recalcitrant disease. Herein, we demonstrate that several species of NTM show broad resistance to the antimicrobial peptide, cathelicidin (LL-37). Resistance to LL-37 was not significantly different between M. avium that contain serovar-specific glycopeptidolipid (GPL, M. avium ssGPL) and M. avium that do not (M. avium ΔssGPL). Similarly, M. abscessus containing non-specific GPL (M. abscessus nsGPL(+)) or lacking nsGPL (M. abscessus nsGPL(-)) remained equally resistant to LL-37. These findings would support the notion that GPL are not the components responsible for NTM resistance to LL-37. Unexpectedly, the growth of M. abscessus nsGPL(-) increased with LL-37 or scrambled LL-37 peptide in a dose-dependent fashion. We also discovered that LL-37 exposed to NTM had reduced antimicrobial activity, and initial work indicates that this is likely due to inactivation of LL-37 by lipid component(s) of the NTM cell envelope. We conclude that pathogenic NTM resist and inactivate LL-37. The mechanism by which NTM circumvent the antimicrobial activity of LL-37 remains to be determined.
Introduction
Nontuberculous mycobacteria (NTM) are aquaphilic and geophilic environmental organisms frequently recovered from potable and natural water sources, plumbing systems, and soil [1–3]. The majority of the more than 169 catalogued NTM species are not known to be pathogenic, but several, including Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium abscessus, Mycobacterium kansasii, Mycobacterium xenopi, and Mycobacterium malmoense are clinically significant and of growing concern for several reasons [4]. First, the incidence and prevalence of NTM lung disease are on the rise [5–9]. Second, NTM pulmonary infections are expensive to treat, ranking second only to Legionnaires’ disease for inpatient treatment costs attributed to water-associated diseases [10]. Third, while the prevailing notion is that NTM are not contagious [11, 12], person-to-person transmission has been proposed [13]. Fourth, NTM lung diseases are often resistant to treatment despite the use of lengthy, multi-drug regimens [14].
The surface of most bacteria including the cell wall of NTM is negatively charged, allowing cationic antimicrobial peptides to bind and serve as first line, innate immune defenses against invading pathogens [15, 16]. The antimicrobial peptide cathelicidin (LL-37), binds, inserts, and forms pores in the negatively charged membrane of a variety of Gram-negative and Gram-positive bacteria [17–20]. LL-37 has also been shown to kill Mycobacterium tuberculosis (Mtb) [18, 21]. Despite its broad-spectrum of activity, LL-37 did not inhibit the growth of M. avium hominissuis [22, 23].
In the current study, we sought to examine whether other clinically relevant pathogenic species of NTM including M. avium Chester and M. intracellulare are resistant to LL-37. Since NTM cell envelope components have been implicated to play a role in immune evasion, they may also contribute to resistance against LL-37 [22]. Among the various cell wall components of NTM that have been characterized, glycopeptidolipids (GPL) are lipid molecules linked to the pathogenesis of NTM infections [24–30]. More specifically, GPL are unique to NTM and contribute to colony morphology, sliding motility, and biofilm formation [31–35]. NTM such as M. avium produce polar serovar-specific GPL (ssGPL) comprised of unique serovar-specific oligosaccharides covalently bound to a basic tripeptide amino acid alcohol core, fatty acid, methylated rhammose, and 6-deoxytalose [36–38]. Other NTM such as M. abscessus do not produce ssGPL, but rather express non-polar, diglycosylated non-specific GPL (nsGPL) [32]. Thus, we also investigated whether resistance to LL-37 in M. avium and M. abscessus strains is dependent on GPL. Finally, we determined whether the antimicrobial activity of LL-37 is compromised after incubation with NTM.
Materials and Methods
Bacteria
Salmonella enteriditis Uganda and Salmonella non-typhi Nairobi were kind gifts from Dr. Edward Janoff (University of Colorado Anschutz Medical Campus, Aurora, Colorado). M. avium Chester was obtained from ATCC (#700737) and M. intracellulare 9141 was kindly provided by Dr. Leonid Heifits (National Jewish Health) [39].
The original characterization of M. avium 920A6 serovar 8 wildtype and the allelic exchange mutagenesis of the rhamnosyltransferase (rtfA) gene to generate a null strain lacking ssGPL have been previously reported [40]. In this study, we refer to M. avium serovar 8 wildtype as M. avium ssGPL (containing ssGPL) and the ssGPL null strain as M. avium ΔssGPL (lacking ssGPL).
M. abscessus produce only nsGPL [32]. M. abscessus variants with nsGPL (M. abscessus nsGPL(+)) on the cell surface are referred to as the smooth morphotype whereas those lacking nsGPL (M. abscessus nsGPL(-)) are known as rough morphotype. M. abscessus smooth was obtained from ATCC (#19977) and M. abscessus rough was a clinical isolate cultured from a patient with cystic fibrosis (CF31) (Dr. Lindsay Caverly, National Jewish Health, Denver, Colorado).
Bacterial cultures
Laboratory strains of Escherichia coli and Salmonella species were cultured in Luria Bertani (LB) broth to obtain stock cultures. All NTM strains were cultured in 7H9 broth with ADC enrichment supplement to obtain stock cultures. Mtb H37Rv was handled under BSL3 safety conditions as previously described [41].
Synthetic bioactive LL-37
The synthetic human LL-37 peptide (NH2-LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES-COOH) and scrambled LL-37 (NH2—GLKLRFEFSKIKGEFLKTPEVRFRDIKLKDNRISVQR—COOH) were synthesized by the Peptide Core, University of Colorado Anschutz Medical Campus. The peptides were purified by preparative reverse-phase high performance liquid chromatography (HPLC), verified by analytical reversed-phase HPLC, and molecular mass determined by electrospray mass spectrometry. Purity for both LL-37 and scrambled LL-37 peptide was >98%. The lyophilized peptides were stored at -20C and resuspended in 0.1% trifluoroacetic acid (final pH 2.0) before use.
Antibacterial assays and bacterial detection
The antibacterial assay culture medium for LL-37 was previously optimized (RPMI-1640 supplemented with sodium bicarbonate pH 7.3; diluted 1:4 in distilled water) [21] and referred henceforth as the “LL-37 medium.” In each kill assay, 2x105 to 2x106 bacteria per 250 μl LL-37 medium (pH 7.0) were incubated with various concentrations of LL-37. The tubes were vigorously vortexed, rotated, and incubated at 37°C up to 96 hours. Killing efficacy was analyzed by performing serial dilutions of bacterial-peptide cocktail. Each dilution was plated in duplicate onto agar plates that were incubated at 37°C. E. coli and Salmonella species were inoculated on LB plates and incubated overnight, while rapidly-growing (M. abscessus) and slow-growing mycobacteria (M. avium and M. intracellulare) were incubated for 3–5 days or 10–14 days, respectively on 7H10 agar plates. Colony forming units (CFU) were manually scored. A minimum of three or more independent experiments were completed for all antibacterial assays.
GPL isolation and thin layer chromatography
The presence of GPL from M. avium ssGPL and the absence of GPL from the M. avium ΔssGPL mutant were previously confirmed by thin layer chromatography (TLC) [40]. M. abscessus nsGPL(+) and M. abscessus nsGPL(-) variants were grown in separate 25 ml 7H9 broth cultures and GPL extracted using previously described methods [42, 43]. Lipids were saponified by incubation at 37°C for 20 minutes in 0.2M NaOH/methanol and neutralized with glacial acetic acid (0.1M final concentration). The samples were centrifuged at 20,000 x g for 10 minutes, evaporated using N2, and resuspended in chloroform:methanol (2:1). A portion of the clarified organic phase was spotted onto silica plates for analysis by TLC using a chloroform:methanol:H2O (100:14:0.8) solvent system. Each plate was dipped in 0.1% orcinol/5%H2SO4 and placed under dry heat (100°C for 20 minutes) for visualization of lipids.
Fraction purification from M. abscessus and M. intracellulare
Total lipid fractions were extracted from cell pastes of M. abscessus nsGPL(+) and M. intracellulare (11.4 g and 9.5 g, respectively). Briefly, pulses from a microfluidizer, intermittent cycles of sonication, and three to four cycles of bead beating (Zironia/Silica beads 11079101z BioSpec) were used to lyse the bacteria. Beads and insoluble debris were removed by centrifugation for five minutes at 3000 x g at 4°C. A portion of the resultant whole cell lysate (WCL) was reserved, with the remaining WCL further separated to generate cell wall (CW), cell membrane (CM), and cytosol fractions by established methods [44, 45]. Lipids of each subcellular fraction were obtained by sequential extraction with chloroform:methanol (2:1) as described previously [45]. Finally, the insoluble cell wall (ICW) fraction was obtained by exhaustive extraction of delipidated CW with SDS to remove proteins followed by harvest of ICW via centrifugation [46]. Each fraction was aliquoted into pre-weighed vials, lyophilized under N2 air, and re-weighed to determine component mass.
E. coli bioassays as a readout for LL-37 antimicrobial activity
To test whether LL-37 exposed to NTM- or NTM-derived fractions is inactivated, E. coli growth was monitored by two different bioassays. In the first bioassay, NTM cultures incubated with LL-37 for 96 hours were centrifuged at 6,000 x g, which pellets the bacteria, and leaves any residual LL-37 in the supernatant. Next, the culture supernatant was transferred into new microfuge tubes to which 2x105 E. coli was inoculated and incubated for four hours. After incubation, serial dilutions were performed and inoculated onto duplicate LB agar plates, incubated for four hours, and E. coli CFU enumerated. In the second bioassay, 10 μg of each NTM-derived lipid fraction was resuspended in DMSO to which 250 μl of LL-37 medium was added. The final concentration of DMSO in each sample was <0.1%. Next, 25μg/ml of fresh LL-37 was added to each NTM fraction and pre-incubated at 37°C for four hours. Subsequently, 2x105 E. coli was inoculated into each of the samples, incubated for an additional 16 hours, and swabbed onto LB agar plates to assess bacterial growth.
Statistical analyses
The data were analyzed with GraphPad using paired t-tests or two-way ANOVA to determine statistical significance. Values with p<0.05 were considered statistically significant. Data are expressed as means ± S.E.M. for three or more independent determinations for each experimental point.
Results
Synthetic LL-37 possesses broad-spectrum antibacterial activity
To validate the antibacterial activity of the synthesized LL-37, LL-37 at concentrations of 0–25 μg/mL was added to E. coli cultures and CFU quantified. Incubation with 25 μg/ml LL-37 effectively killed E. coli after four hours (Fig 1A and 1B), consistent with previous reports [47, 48]. LL-37 at 1–50 μg/ml also demonstrated antimicrobial activity against various species of Salmonella including a non-pathogenic laboratory isolate (Fig 2A) and two pathogenic clinical isolates (Fig 2B and 2C). Similar to a previous report [21], ten μg/ml of LL-37 incubated with Mtb reduced viability, but the reduction was not statistically significant (Fig 2D). Overall, synthetic LL-37 has antimicrobial activity against a variety of bacteria.
Fig 1. E. coli is susceptible to LL-37.
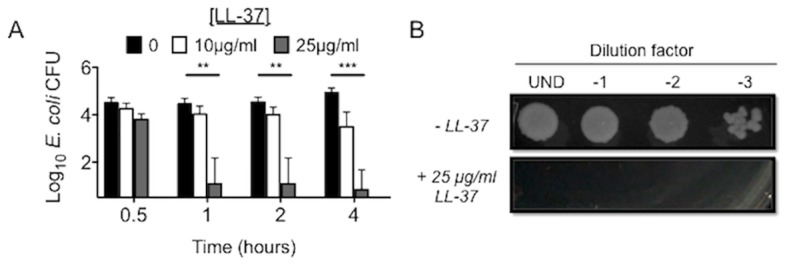
(A) Log10 CFU of E. coli after 4 hours of incubation with 0, 10, or 25 μg/ml of LL-37. **p< 0.001; ***p<0.0001. Data are the mean ± SEM of 6 independent experiments. (B) Images were taken of each serial dilution on LB agar from E. coli cultures incubated for 4 hours in the absence or presence of 25 μg/ml of LL-37.
Fig 2. LL-37 demonstrates broad-spectrum antimicrobial activity.

Log10 CFU after 1–8 hours of incubation of a (A) laboratory isolate of Salmonella enteriditis or clinical isolates of (B) Salmonella enteriditis (Uganda) or (C) Salmonella non-typhi (Nairobi) with 0–50 μg/ml of LL-37. *p< 0.01; **p< 0.001; ***p<0.0001. Data are the mean ± SEM of 3–6 independent experiments. (D) Mtb H37Rv were incubated with 10 μg/ml LL-37 and the percent change in CFU calculated after 96 hours incubation. n = 3 independent experiments.
M. avium Chester and M. intracellulare are resistant to LL-37
To evaluate the effectiveness of LL-37 against NTM species, M. avium Chester and M. intracellulare were separately incubated with 10, 125, 250, and 500 μg/ml LL-37 for up to 96 hours and CFU determined. In contrast to the aforementioned findings, there was no significant change in the viability of M. avium Chester or M. intracellulare at all LL-37 concentrations and time points tested (Fig 3A and 3B). We also found no significant change in viability of 48-hour log phase cultures incubated with up to 250 μg/ml of LL-37 (data not shown).
Fig 3. Pathogenic M. avium and M. intracellulare are resistant to LL-37.
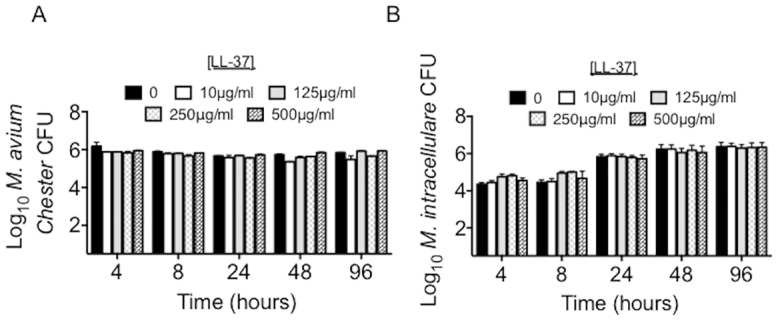
Growth curves of (A) M. avium Chester (p = 0.28) and (B) M. intracellulare (p = 0.89) after incubation with 0, 10, 125, 250, or 500 μg/ml of LL-37. Data are the mean ± SEM of 3–6 independent experiments.
M. avium ssGPL and M. avium ΔssGPL serovar 8 are equally resistant to LL-37
To investigate whether ssGPL contribute to the resistance of M. avium to LL-37, M. avium ssGPL serovar 8 and M. avium ΔssGPL were incubated with increasing concentrations of LL-37 and CFU quantified at various times. As shown in Fig 4, both M. avium ssGPL and M. avium ΔssGPL were resistant to LL-37 at all concentrations and time points examined. In contrast, gentamicin significantly reduced the growth of both variants after 48 and 96 hours.
Fig 4. ssGPL does not contribute to the resistance of M. avium to LL-37.
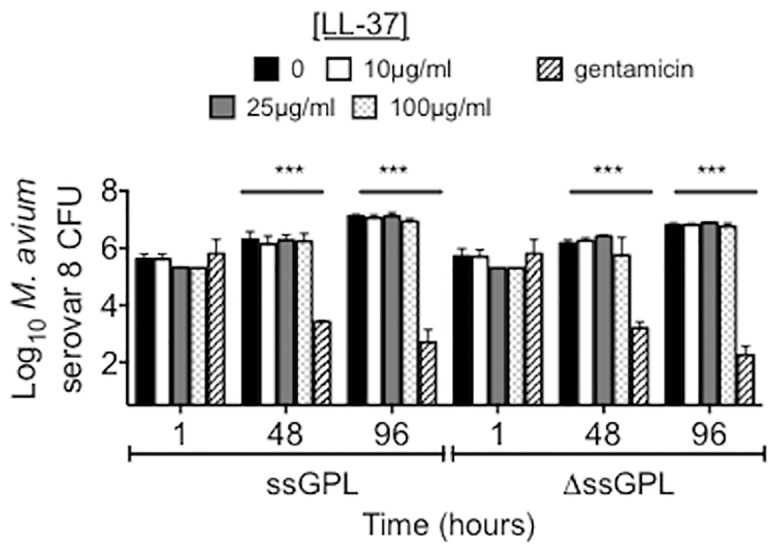
CFU determination of M. avium ssGPL and M. avium ΔssGPL serovar 8 after incubation with 0, 10, 25, and 100 μg/ml LL-37 and 20 μg/ml gentamicin. ***p<0.0001. Data are the mean ± SEM of 3 independent experiments.
Both M. abscessus nsGPL(+) and nsGPL(-) variants are equally resistant to LL-37
We also incubated a strain of M. abscessus that contains nsGPL (M. abscessus nsGPL(+)) with various concentrations of LL-37 for up to 96 hours. We found that M. abscessus nsGPL(+) was completely resistant to LL-37 at all concentrations and time points tested (Fig 5A). To determine whether the nsGPL present on the wildtype M. abscessus is responsible for this resistance, the growth of a M. abscessus variant devoid of nsGPL (M. abscessus nsGPL(-)) was tested against LL-37. After 48 and 96 hours of incubation in the absence of LL-37, the growth of M. abscessus nsGPL(-) was significantly slower than M. abscessus nsGPL(+) (Fig 5B compared to 5A, black bars). In the presence of LL-37, neither M. abscessus strain was killed by LL-37. On the contrary, M. abscessus nsGPL(-) was not only resistant to LL-37, but unexpectedly showed significant increases in growth when incubated with increasing concentrations of LL-37 compared to the untreated cultures (Fig 5B). Thin layer chromatography confirmed the presence and absence of GPL in M. abscessus nsGPL(+) and M. abscessus nsGPL(-), respectively (Fig 5C).
Fig 5. nsGPL do not mediate the resistance of M. abscessus to LL-37.
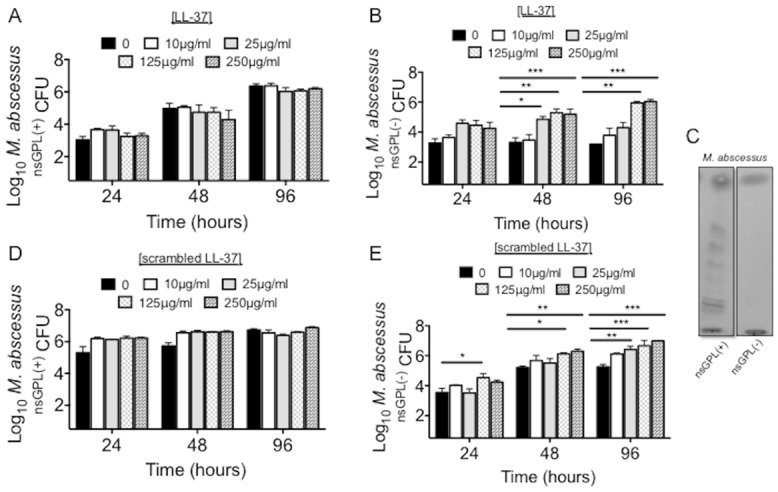
(A-B) CFU determination of M. abscessus nsGPL(+) in the presence of the indicated concentrations of native LL-37 (p = 0.69). Data are the mean ± SEM of 4 independent experiments. (B) CFU determination of M. abscessus nsGPL(-) in the presence of the indicated concentrations of native LL-37. Data are the mean ± SEM of 4 independent experiments. *p<0.01; **p< 0.001; ***p<0.0001. (C) Thin-layer chromatography demonstrates the presence and absence of GPL in M. abscessus nsGPL(+) and M. abscessus nsGPL(-), respectively. (D-E) CFU determination of M. abscessus nsGPL(+) and M. abscessus nsGPL(-), respectively in the presence of the indicated concentrations of scrambled LL-37 peptide. Data are the mean ± SEM of 3 independent experiments. *p<0.01; **p< 0.001; ***p<0.0001.
To examine whether the enhanced growth of the M. abscessus nsGPL(-) cultured with LL-37 was dependent on the primary structure of LL-37, M. abscessus nsGPL(-) and M. abscessus nsGPL(+) strains were incubated for 24, 48, and 96 hours with varying concentrations of an LL-37 peptide in which the amino acid sequence are randomly scrambled and CFU determined. Similar to that seen following incubation with LL-37, M. abscessus nsGPL(+) viability was not affected by scrambled LL-37 at any concentration or time point tested (Fig 5D) whereas M. abscessus nsGPL(-) growth also increased after incubation with scrambled LL-37 (Fig 5E).
LL-37 exposed to NTM or NTM-derived fractions loses antibacterial activity
Since we did not observe killing of NTM by LL-37, we investigated the possibility that NTM-exposed LL-37 may have become inactivated. In a bioassay, E. coli was added to culture supernatants of M. intracellulare, M. avium Chester, M. abscessus, and Mtb originally incubated with 500 μg/ml LL-37 for 96 hours. E. coli cultured in medium alone showed viable cells, which were killed in the presence of LL-37 (Fig 6A). However, when E. coli was incubated with LL-37-containing NTM culture supernatants, no significant decrease in viable E. coli was observed (Fig 6A); in contrast, no viable E. coli was recovered after incubation with LL-37-containing Mtb culture supernatant (Fig 6A).
Fig 6. Loss of LL-37 activity after exposure to NTM or NTM-derived lipids.
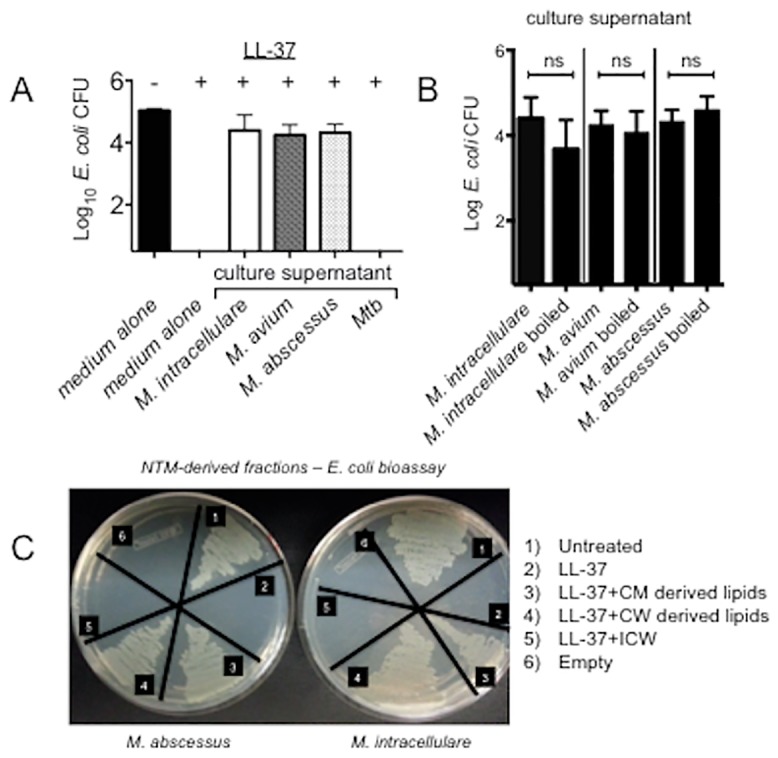
E. coli bioassays were used to evaluate LL-37 activity. (A) LL-37 remaining in NTM (but not Mtb) culture supernatant no longer kills E. coli. (B) E. coli survives in untreated or boiled NTM culture supernatants to which fresh LL-37 was added. (C) E. coli survival following incubation with M. abscessus or M. intracellulare derived cell fractions. CM = cell membrane, CW = cell wall, ICW = insoluble cell wall fraction.
To determine if the NTM component(s) that inactivates LL-37 is heat labile, culture supernatants from M. intracellulare, M. avium Chester, and M. abscessus incubated for 96-hours without the addition of LL-37 were boiled and cooled to room temperature prior to the addition of E. coli and fresh LL-37 peptide. These bioassays demonstrated that E. coli remained viable and replicated well despite incubation with LL-37 in the presence of boiled NTM culture supernatant (Fig 6B).
Based on our findings thus far, it appears that a secreted, or surface exposed, heat-stable component of the NTM cell envelope inactivates LL-37. To begin to dissect which component(s) of NTM inactivates LL-37, large-scale preparations of M. abscessus and M. intracellulare were prepared and chloroform:methanol was used to separate the total lipids of the CM and CW fractions from the glycans and proteins. Fresh LL-37 (25 μg/ml) and E. coli were added to the total lipid fractions of the CM/CW or the ICW fraction per unit of dry weight to test the ability of each fraction to inactivate LL-37. E. coli was killed when incubated with LL-37 alone and LL-37 with ICW; however, viable E. coli was recovered after incubation with LL-37 plus either M. abscessus or M. intracellulare CM or CW total lipid fractions (Fig 6C). As a control, E. coli was incubated with just the buffer solutions used to resuspend each of the M. abscessus and M. intracellulare fractions (vehicle control); in all cases, E. coli replicated in the absence of LL-37, but were killed in the presence of LL-37 in these NTM-naïve solutions (data not shown).
Discussion
We discovered that LL-37, despite being highly active against E. coli, various Salmonella species, and Mtb H37Rv, was inactive against four NTM species tested. M. avium and M. abscessus strains with genetic disruption for their respective GPLs (M. avium ΔssGPL and M. abscessus nsGPL(-)) remained resistant to LL-37. Thus, GPL is unlikely to be the NTM component that mediates resistance to LL-37. Indeed, the growth of M. abscessus nsGPL(-) unexpectedly and consistently increased when incubated with either native LL-37 or scrambled peptide. Our data also indicate that LL-37 exposed to either NTM or NTM-derived CM or CW total lipid fractions becomes inactivated. Taken together, our findings indicate that NTM are resistant to LL-37, inactivate the antimicrobial activity of LL-37, and that the NTM-derived component(s) that inactivate LL-37 are likely to be polar lipids or similarly hydrophilic component(s) of the NTM cell envelope that is extractable with an organic solvent.
The finding that many species of NTM are resistant to LL-37 is consistent with a recent report demonstrating resistance of M. avium hominissuis to LL-37 [22]. Although LL-37 was the only antimicrobial peptide tested in our studies, resistance to antimicrobial peptides may be a global defense mechanism of NTM. The differential activity of LL-37 against NTM and Mtb is interesting, but not surprising considering they contain different cell wall components; e.g., compared to lipoarabinomannan of NTM, the mannose-capped lipoarabinomannan of Mtb is distinct in structure and elicits a different host-immune response [49]. Moreover, it is well established that the surface-exposed lipids of the different mycobacterial species are highly varied in composition, location, and arrangement which may impact susceptibility or resistance to antimicrobial peptides [24]. Mtb is a strict intracellular organism and does not survive for extended periods outside of the host. Compared to Mtb, the cell envelope of NTM is likely to be considerably more impermeable to enhance survival in environments that vary greatly; e.g., natural and made-made water systems, soil, and biofilms that can vary with extremes of temperature, humidity, and nutrients [50]. A global comparative lipidomics approach, using recently established methods and mycobacterial lipid databases [51–53] will be required to identify lipids unique to NTM that possesses this activity.
Although our findings would suggest that GPLs—whether ssGPL in M. avium or nsGPL in M. abscessus—do not inactivate LL-37, there are at least 31 other serovar-specific M. avium GPL not examined in this study [36]. But based on the available evidence, it is reasonable to suspect NTM GPL do not mediate resistance to LL-37. In future studies, it would be informative to determine whether purified ssGPL from the different M. avium species including M. avium hominissuis, modulate the structure of LL-37.
The finding that the growth of the M. abscessus nsGPL(-) variant increased with increasing concentrations of LL-37 (or scrambled LL-37) was unexpected. One interpretation of these findings is that the experimental conditions alone were unfavorable for the M. abscessus nsGPL(-) variant, but adding increasing concentrations of LL-37 may have sufficiently supplemented the conditions to facilitate growth. The exact mechanisms to explain this observation are unknown, but may be due to increased activity of uptake transporters or permeases that internalize and metabolize LL-37 [54, 55]. We recognize an important limitation to these studies is that the M. abscessus nsGPL(-) and M. abscessus nsGPL(+) variants were not derived from the same parental M. abscessus strain; thus, we cannot exclude the possibility that the observed increase in the growth of the M. abscessus nsGPL(-) variant is due to attributes unique to this particular variant which may be independent of the absence of nsGPL. Isogenic M. abscessus nsGPL(-) and M. abscessus nsGPL(+) variants should be used in future studies to confirm these findings.
Bacterial evasion of host-protective LL-37 is common. In fact, there are a wide variety of evasion strategies employed by numerous bacterial species to avoid LL-37 including modification of normally anionic cell surface constituents with cationic molecules to repel LL-37, antimicrobial peptide trapping mechanisms, secretion of proteases that cleave LL-37, and downregulation of antimicrobial peptide transcription (reviewed in [50]). Our findings indicate that components of the NTM cell envelope contribute to LL-37 resistance, and, more specifically, heat-stable CM and/or CW lipids exert the anti-LL-37 activity.
In summary, the finding that clinically relevant species of NTM are uniquely resistant to and neutralize LL-37 provides a pathogenic mechanism for why it is difficult to eradicate these organisms once the infection becomes established. Elucidating the mechanism by which NTM resist and inactivate LL-37 will provide leverage in developing more unconventional approaches to therapeutics with novel mechanisms of action to slow the progression of this emerging lung disease; i.e., development of agents to counter the NTM component(s) that neutralize LL-37.
Acknowledgments
Study support provided in part by Institutional Funds and the NIH Supplement 3R01HL059785-09S1 for Dr. Sonia Flores, University of Colorado Anschutz Medical Campus Pulmonary and Critical Care Division. Jennifer R. Honda, PhD is supported by a NIH NRSA Infectious Disease Training Award (T32-AI007447-19), the Tim Gill Endowment for AIDS Research, NIH NRSA Pulmonary and Critical Care Medicine Training Award (T32 HL 7085–83), Potts Memorial and Shoot for the Cure Foundations.
Data Availability
All relevant data are within the paper.
Funding Statement
Study support was provided in part by Institutional Funds and the National Institutes of Health (NIH) Supplement 3R01HL059785-09S1 for SCF, University of Colorado Anschutz Medical Campus Pulmonary and Critical Care Division. JRH is supported by a NIH NRSA Infectious Disease Training Award (T32-AI007447-19), the Tim Gill Endowment for AIDS Research, NIH NRSA Pulmonary and Critical Care Medicine Training Award (T32 HL 7085-83), and the Potts Memorial Foundation.
References
- 1. Weiss CH, Glassroth J. Pulmonary disease caused by nontuberculous mycobacteria. Expert review of respiratory medicine. 2012;6(6):597–612; quiz 3. Epub 2012/12/14. 10.1586/ers.12.58 . [DOI] [PubMed] [Google Scholar]
- 2. Falkinham JO 3rd. Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23(3):529–51. Epub 2002/10/10. . [DOI] [PubMed] [Google Scholar]
- 3. Falkinham JO 3rd. Nontuberculous mycobacteria from household plumbing of patients with nontuberculous mycobacteria disease. Emerg Infect Dis. 2011;17(3):419–24. Epub 2011/03/12. 10.3201/eid1703.101510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Somoskovi A, Salfinger M. Nontuberculous Mycobacteria in Respiratory Infections: Advances in Diagnosis and Identification. Clin Lab Med. 2014;34(2):271–95. Epub 2014/05/27. 10.1016/j.cll.2014.03.001 . [DOI] [PubMed] [Google Scholar]
- 5. Marras TK, Chedore P, Ying AM, Jamieson F. Isolation prevalence of pulmonary non-tuberculous mycobacteria in Ontario, 1997 2003. Thorax. 2007;62(8):661–6. Epub 2007/02/22. doi: thx.2006.070797 [pii] 10.1136/thx.2006.070797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Adjemian J, Olivier KN, Seitz AE, Holland SM, Prevots DR. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185(8):881–6. Epub 2012/02/09. rccm.201111-2016OC [pii] 10.1164/rccm.201111-2016OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore JE, Kruijshaar ME, Ormerod LP, Drobniewski F, Abubakar I. Increasing reports of non-tuberculous mycobacteria in England, Wales and Northern Ireland, 1995–2006. BMC Public Health. 2010;10:612. Epub 2010/10/19. doi: 1471-2458-10-612 [pii] 10.1186/1471-2458-10-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lai CC, Tan CK, Chou CH, Hsu HL, Liao CH, Huang YT, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis. 2010;16(2):294–6. Epub 2010/02/02. 10.3201/eid1602.090675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomson RM. Changing epidemiology of pulmonary nontuberculous mycobacteria infections. Emerg Infect Dis. 2010;16(10):1576–83. Epub 2010/09/30. 10.3201/eid1610.091201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Collier SA, Stockman LJ, Hicks LA, Garrison LE, Zhou FJ, Beach MJ. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;140(11):2003–13. Epub 2012/01/12. 10.1017/S0950268811002858 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGrath EE, Blades Z, McCabe J, Jarry H, Anderson PB. Nontuberculous mycobacteria and the lung: from suspicion to treatment. Lung. 2010;188(4):269–82. Epub 2010/04/10. 10.1007/s00408-010-9240-9 . [DOI] [PubMed] [Google Scholar]
- 12. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. Epub 2007/02/06. doi: 175/4/367 [pii] 10.1164/rccm.200604-571ST . [DOI] [PubMed] [Google Scholar]
- 13. Bryant JM, Grogono DM, Greaves D, Foweraker J, Roddick I, Inns T, et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet. 2013;381(9877):1551–60. Epub 2013/04/02. doi: S0140-6736(13)60632-7 [pii] 10.1016/S0140-6736(13)60632-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith DE. Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2010;23(2):185–90. Epub 2010/01/21. 10.1097/QCO.0b013e328336ead6 . [DOI] [PubMed] [Google Scholar]
- 15. Lytle D, Frietch C, Covert T. Electrophoretic Mobility of Mycobacterium avium Complex Organisms. Appl Environ Microbiol. 2004;70(9):5667–71. Epub 2004/09/04. 10.1128/AEM.70.9.5667-5671.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agerberth B, Gudmundsson GH. Host antimicrobial defence peptides in human disease. Curr Top Microbiol Immunol. 2006;306:67–90. Epub 2006/08/17. . [DOI] [PubMed] [Google Scholar]
- 17. Sorensen O, Cowland JB, Askaa J, Borregaard N. An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Methods. 1997;206(1–2):53–9. Epub 1997/08/07. doi: S0022-1759(97)00084-7 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18. Rivas-Santiago B, Hernandez-Pando R, Carranza C, Juarez E, Contreras JL, Aguilar-Leon D, et al. Expression of cathelicidin LL-37 during Mycobacterium tuberculosis infection in human alveolar macrophages, monocytes, neutrophils, and epithelial cells. Infect Immun. 2008;76(3):935–41. Epub 2007/12/28. IAI.01218-07 [pii] 10.1128/IAI.01218-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larrick JW, Hirata M, Zhong J, Wright SC. Anti-microbial activity of human CAP18 peptides. Immunotechnology. 1995;1(1):65–72. Epub 1995/05/01. doi: 1380293395000062 [pii]. . [DOI] [PubMed] [Google Scholar]
- 20. Henzler-Wildman KA, Martinez GV, Brown MF, Ramamoorthy A. Perturbation of the hydrophobic core of lipid bilayers by the human antimicrobial peptide LL-37. Biochemistry. 2004;43(26):8459–69. Epub 2004/06/30. 10.1021/bi036284s . [DOI] [PubMed] [Google Scholar]
- 21. Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–3. Epub 2006/02/25. 1123933 [pii] 10.1126/science doi: 1123933. . [DOI] [PubMed] [Google Scholar]
- 22. Motamedi N, Danelishvili L, Bermudez LE. Genetic basis for Mycobacterium avium hominissuis resistance to host antimicrobial peptides. J Med Microbiol. 2014. Epub 2014/05/20. 10.1099/jmm.0.072744-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Motamedi N, Danelishvili L, Bermudez LE. Identification of Mycobacterium avium genes associated with resistance to host antimicrobial peptides. J Med Microbiol. 2014;63(Pt 7):923–30. Epub 2014/05/20. 10.1099/jmm.0.072744-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ortalo-Magne A, Lemassu A, Laneelle MA, Bardou F, Silve G, Gounon P, et al. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J Bacteriol. 1996;178(2):456–61. Epub 1996/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Billman-Jacobe H. Glycopeptidolipid synthesis in mycobacteria Current Science 2004;86:111–4. [Google Scholar]
- 26. Kocincova D, Singh AK, Beretti JL, Ren H, Euphrasie D, Liu J, et al. Spontaneous transposition of IS1096 or ISMsm3 leads to glycopeptidolipid overproduction and affects surface properties in Mycobacterium smegmatis. Tuberculosis (Edinb). 2008;88(5):390–8. Epub 2008/04/29. doi: S1472-9792(08)00021-8 [pii] 10.1016/j.tube.2008.02.005 . [DOI] [PubMed] [Google Scholar]
- 27. Ripoll F, Deshayes C, Pasek S, Laval F, Beretti JL, Biet F, et al. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics. 2007;8:114 Epub 2007/05/11. 10.1186/1471-2164-8-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Torrelles JB, Ellis D, Osborne T, Hoefer A, Orme IM, Chatterjee D, et al. Characterization of virulence, colony morphotype and the glycopeptidolipid of Mycobacterium avium strain 104. Tuberculosis (Edinb). 2002;82(6):293–300. Epub 2003/03/08. doi: S1472979202903732 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29. Reddy VM, Luna-Herrera J, Gangadharam PR. Pathobiological significance of colony morphology in Mycobacterium avium complex. Microb Pathog. 1996;21(2):97–109. Epub 1996/08/01. 10.1006/mpat.1996.0046 . [DOI] [PubMed] [Google Scholar]
- 30. Kansal RG, Gomez-Flores R, Mehta RT. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex. Microb Pathog. 1998;25(4):203–14. Epub 1998/11/18. doi: S0882-4010(98)90227-3 [pii] 10.1006/mpat.1998.0227 . [DOI] [PubMed] [Google Scholar]
- 31. Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006;152(Pt 6):1581–90. Epub 2006/06/01. 10.1099/mic.0.28625-0 . [DOI] [PubMed] [Google Scholar]
- 32. Schorey JS, Sweet L. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology. 2008;18(11):832–41. Epub 2008/08/30. doi: cwn076 [pii] 10.1093/glycob/cwn076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Belisle JT, Klaczkiewicz K, Brennan PJ, Jacobs WR Jr., Inamine JM. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J Biol Chem. 1993;268(14):10517–23. Epub 1993/05/15. . [PubMed] [Google Scholar]
- 34. Recht J, Kolter R. Glycopeptidolipid acetylation affects sliding motility and biofilm formation in Mycobacterium smegmatis. J Bacteriol. 2001;183(19):5718–24. Epub 2001/09/07. 10.1128/JB.183.19.5718-5724.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Freeman R, Geier H, Weigel KM, Do J, Ford TE, Cangelosi GA. Roles for cell wall glycopeptidolipid in surface adherence and planktonic dispersal of Mycobacterium avium. Appl Environ Microbiol. 2006;72(12):7554–8. Epub 2006/10/03. doi: AEM.01633-06 [pii] 10.1128/AEM.01633-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chatterjee D, Khoo KH. The surface glycopeptidolipids of mycobacteria: structures and biological properties. Cell Mol Life Sci. 2001;58(14):2018–42. Epub 2002/01/30. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Besra GS, Khoo KH, McNeil MR, Dell A, Morris HR, Brennan PJ. A new interpretation of the structure of the mycolyl-arabinogalactan complex of Mycobacterium tuberculosis as revealed through characterization of oligoglycosylalditol fragments by fast-atom bombardment mass spectrometry and 1H nuclear magnetic resonance spectroscopy. Biochemistry. 1995;34(13):4257–66. Epub 1995/04/04. . [DOI] [PubMed] [Google Scholar]
- 38. Lopez-Marin LM, Quesada D, Lakhdar-Ghazal F, Tocanne JF, Laneelle G. Interactions of mycobacterial glycopeptidolipids with membranes: influence of carbohydrate on induced alterations. Biochemistry. 1994;33(23):7056–61. Epub 1994/06/14. . [DOI] [PubMed] [Google Scholar]
- 39. Bai X, Ovrutsky AR, Kartalija M, Chmura K, Kamali A, Honda JR, et al. IL-32 expression in the airway epithelial cells of patients with Mycobacterium avium complex lung disease. Int Immunol. 2011;23(11):679–91. Epub 2011/10/29. 10.1093/intimm/dxr075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Irani VR, Lee SH, Eckstein TM, Inamine JM, Belisle JT, Maslow JN. Utilization of a ts-sacB selection system for the generation of a Mycobacterium avium serovar-8 specific glycopeptidolipid allelic exchange mutant. Ann Clin Microbiol Antimicrob. 2004;3:18 Epub 2004/10/02. doi: 10.1186/1476-0711-3-18 1476-0711-3-18 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chick JF, Chauhan NR, Bair RJ, Chauhan VR. The Lady Windermere syndrome. Intern Emerg Med. 2013;8(1):83–5. Epub 2012/10/26. 10.1007/s11739-012-0870-1 . [DOI] [PubMed] [Google Scholar]
- 42. Brennan PJ, Heifets M, Ullom BP. Thin-layer chromatography of lipid antigens as a means of identifying nontuberculous mycobacteria. J Clin Microbiol. 1982;15(3):447–55. Epub 1982/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. Mycobacterium abscessus Glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J Immunol. 2009;183(3):1997–2007. Epub 2009/07/15. doi: jimmunol.0802181 [pii] 10.4049/jimmunol.0802181 . [DOI] [PubMed] [Google Scholar]
- 44. Mawuenyega KG, Forst CV, Dobos KM, Belisle JT, Chen J, Bradbury EM, et al. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Mol Biol Cell. 2005;16(1):396–404. Epub 2004/11/05. 10.1091/mbc.E04-04-0329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wolfe LM, Mahaffey SB, Kruh NA, Dobos KM. Proteomic definition of the cell wall of Mycobacterium tuberculosis. J Proteome Res. 2010;9(11):5816–26. Epub 2010/09/10. 10.1021/pr1005873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Barnes PF, Mehra V, Hirschfield GR, Fong SJ, Abou-Zeid C, Rook GA, et al. Characterization of T cell antigens associated with the cell wall protein-peptidoglycan complex of Mycobacterium tuberculosis. J Immunol. 1989;143(8):2656–62. Epub 1989/10/15. . [PubMed] [Google Scholar]
- 47. Travis SM, Singh PK, Welsh MJ. Antimicrobial peptides and proteins in the innate defense of the airway surface. Curr Opin Immunol. 2001;13(1):89–95. Epub 2001/01/13. doi: S0952-7915(00)00187-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 48. Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173(5):2909–12. Epub 2004/08/24. doi: 173/5/2909 [pii]. . [DOI] [PubMed] [Google Scholar]
- 49. Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev. 2011;35(6):1126–57. Epub 2011/04/28. 10.1111/j.1574-6976.2011.00276.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nizet V. Antimicrobial peptide resistance mechanisms of human bacterial pathogens. Curr Issues Mol Biol. 2006;8(1):11–26. Epub 2006/02/03. . [PubMed] [Google Scholar]
- 51. Sartain MJ, Dick DL, Rithner CD, Crick DC, Belisle JT. Lipidomic analyses of Mycobacterium tuberculosis based on accurate mass measurements and the novel "Mtb LipidDB". J Lipid Res. 2011;52(5):861–72. Epub 2011/02/03. doi: jlr.M010363 [pii] 10.1194/jlr.M010363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Layre E, Sweet L, Hong S, Madigan CA, Desjardins D, Young DC, et al. A comparative lipidomics platform for chemotaxonomic analysis of Mycobacterium tuberculosis. Chem Biol. 2011;18(12):1537–49. Epub 2011/12/27. 10.1016/j.chembiol.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Layre E, Moody DB. Lipidomic profiling of model organisms and the world's major pathogens. Biochimie. 2013;95(1):109–15. Epub 2012/09/14. 10.1016/j.biochi.2012.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodrigues L, Sampaio D, Couto I, Machado D, Kern WV, Amaral L, et al. The role of efflux pumps in macrolide resistance in Mycobacterium avium complex. Int J Antimicrob Agents. 2009;34(6):529–33. Epub 2009/09/11. doi: S0924-8579(09)00365-3 [pii] 10.1016/j.ijantimicag.2009.07.010 . [DOI] [PubMed] [Google Scholar]
- 55. Schmalstieg AM, Srivastava S, Belkaya S, Deshpande D, Meek C, Leff R, et al. The antibiotic resistance arrow of time: efflux pump induction is a general first step in the evolution of mycobacterial drug resistance. Antimicrob Agents Chemother. 2012;56(9):4806–15. Epub 2012/07/04. 10.1128/AAC.05546-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.


