Abstract
Background
Video-assisted thoracic surgery (VATS) is routinely performed with general anesthesia and double-lumen endotracheal intubation, but this technique may stress an elderly patient’s functional reserve. We chose to study the safety and efficacy of non-intubated VATS, utilizing local anesthesia, sedation, and spontaneous ventilation in the elderly.
Methods
The medical records of all patients aged 80 years and older who underwent VATS under local anesthesia and sedation during the time period 6/1/2002 to 6/1/2010 at Geisinger Health System (Pennsylvania, USA) and 10/1/2011 to 12/31/2014 at Sinai Hospital (Maryland, USA) were retrospectively reviewed. Unsuccessful attempts at this technique were eligible for inclusion but there were none. No patient was excluded based on comorbidity.
Results
A total of 96 patients ranging in age from 80 to 104 years underwent 102 non-intubated VATS procedures: pleural biopsy/effusion drainage with or without talc 73, drainage of empyema 17, evacuate hemothorax 4, pericardial window 3, lung biopsy 2, treat chylothorax 2, treat pneumothorax 1. No patient required intubation or conversion to thoracotomy. No patient required a subsequent procedure or biopsy. Complications occurred in three patients (3.1% morbidity): cerebrovascular accident, pulmonary embolism, prolonged air leak. One 94-year-old patient died from overanticoagulation and two 84-year-old patients died of their advanced lung cancers (3.1% morbidity).
Conclusions
Non-intubated VATS utilizing local anesthesia and sedation in the elderly is well tolerated and safe for a number of indications.
Keywords: Empyema, geriatric surgery, geriatric anesthesia, pleural effusion, thoracoscopy, video-assisted thoracic surgery (VATS)
Authors’ introduction:
Figure 1 is a picture of Dr. Mark R. Katlic, the Chairman of Department of Surgery in Sinai Hospital of Baltimore, USA. Figure 2 is a picture of Dr. Matthew A. Facktor from Geisinger Health System in Danville, USA.
Figure 1.

Dr. Mark R. Katlic.
Figure 2.

Dr. Matthew A. Facktor.
Introduction
One of the principles of geriatric surgery (1) is: the elderly handle stress satisfactorily but handle severe stress poorly because of lack of organ system reserve. For example, the elderly can return to normal function after stressful operations (such as colectomy and hepatectomy) but after the most stressful operations (such as Whipple pancreaticoduodenectomy) it will take much longer (2). Fortner and Lincer (3) found that the increased number of deaths among elderly patients undergoing hepatic resection for liver cancer were nearly all in the extended-resection group (i.e., extended right hepatectomy or trisegmentectomy), among whom 60% of deaths were due to hepatic insufficiency.
There is some evidence that stressing functional reserve even less will be beneficial. This can be done by performing lesser cancer operations without compromising survival (such as pulmonary wedge resection or segmentectomy rather than lobectomy) or by performing identical operations in a less stressful way [such as video-assisted thoracic surgery (VATS) lobectomy or laparoscopic colectomy].
Jaklitsch et al. (4) found decreased mortality, length of hospital stay, and postoperative delirium after 307 video-assisted procedures in patients aged 65-90 compared to those factors associated with open thoracotomy. Video-assisted pulmonary lobectomy in half of a group of elderly lung cancer patients resulted in fewer complications (P=0.04) and decreased length of stay (P<0.001) compared to the half who underwent open (thoracotomy) lobectomy (5). Patel (6) reported shorter hospitalization and similar late outcomes following endovascular thoracic aortic procedures in patients greater than 75 years, compared to open procedures.
Even these “lesser” procedures noted above require general anesthesia and endotracheal intubation, but some operations do not; and there are risks to such anesthesia.
Encouraged by the results treating pleural disease with VATS under local anesthesia—and success creating an unplanned pericardial window in a patient undergoing such surgery for a malignant pleural effusion—we broadened our indications for this technique (7). We believed that it would prove to be safe and particularly valuable for octogenarians, nonagenarians, and centenarians.
Methods
The medical records of all patients aged 80 years or older who underwent VATS utilizing local anesthesia and sedation at the Geisinger Health System (Pennsylvania, USA) between 6/1/2002 and 6/1/2010 and Sinai Hospital (Maryland, USA) between 10/1/2011 and 12/31/2014 were retrospectively reviewed. The authors or residents under their direct supervision performed all procedures. Unsuccessful attempts at this technique were eligible for inclusion but there were none. No patient was excluded based on comorbidity. The Geisinger Health System Institutional Research Review Board (Protocol 2005-0166) and the Sinai Hospital Institutional Review Board (Protocol 1915) approved this research. Eighteen of these patients were part of a preliminary study (115 patients, ages 21-88 years) published in 2006 (7). Sixty-three patients were included in a more recent general paper discussing the technique (353 patients in our study time period, ages 21-100 years) (8).
Technique
Selection criteria
Patients were not selected for this technique if any of the following pertained: hemodynamic instability, patient already intubated and ventilated, anticipated need for extensive decortication, solitary pulmonary nodule, or pericardial effusion without coexisting large pleural effusion. All other patients with large unilateral pleural effusion, empyema, or diffuse lung disease were offered local anesthesia and sedation (Table 1). No patient was excluded based on comorbidity. No patient who met the criteria underwent general anesthesia or endotracheal intubation.
Table 1. Patient selection.
| General anesthesia |
| Hemodynamic instability |
| Patient already intubated/ventilated |
| Empyema, decortication anticipated |
| Solitary pulmonary nodule |
| Pericardial effusion |
| Local anesthesia/sedation |
| Hemodynamic stability |
| Large unilateral pleural effusion |
| Empyema |
| Diffuse lung disease, multiple nodules |
| Pericardial effusion with coexisting pleural effusion |
| Chronic hemothorax |
General
Patients were sedated with an individualized combination of midazolam, fentanyl, and propofol; a continuous infusion of propofol has been effective (starting at about 120 mg/kg/min and increasing as needed). Supplemental oxygen was administered via face mask; and oxygen saturation, electrocardiogram, and blood pressure were monitored. End-tidal carbon dioxide could be monitored via a catheter tucked into an oral airway. Flexible bronchoscopy was carried out when indicated, then the patient was turned into full lateral position. Local anesthesia (1% xylocaine, 10 to 30 mL depending on number of incisions) was infiltrated into skin, then 1-3 2-cm incisions were made. Optimally, intercostal muscle and pleura were infiltrated under direct vision or palpation through the skin incision.
Contingency plans for intubation or conversion to thoracotomy (never used) included immediate placement of a chest tube through one incision and occlusive dressings to others, followed by turning the patient supine for intubation. Alternatively, a laryngeal mask airway could be placed with the patient in lateral position depending upon circumstances.
Elective patients were discharged the same or next day, usually with a small drainage container (Atrium Mini-Express®, Atrium Medical Corporation, Hudson, New Hampshire, USA) attached to the chest tube or, in some cases, with a PleurX® catheter (CareFusion Corporation, San Diego, CA, USA). The chest tube was removed in the office as appropriate.
Pleural disease
One port was employed, with cup biopsy forceps and possible talc insufflation catheter passed along the outside wall of the short trocar (Endopath®, Ethicon). When necessary, e.g., for multiloculated empyema, a second site without trocar allowed introduction of other instruments in order to disrupt adhesions.
Lung biopsy
Three incisions allowed introduction of telescope via trocar, grasping ring forceps, and endoscopic stapling device. Finger palpation was performed as needed. Pleural adhesions could be divided bluntly or with scissors or cautery. Typically, two or three wedge biopsies were performed with targeted areas of the lung identified from preoperative computed tomographic (CT) scans.
Pericardial window
If a pleural effusion co-existed, and the lung was thereby “accustomed” to being collapsed, two sites would suffice, with grasper being passed alongside the telescope and an anterior site for #15 scalpel blade then endoscopic scissors. If necessary a third anterior-superior site allowed the lung to be further retracted superiorly with a grasper or blunt instrument.
Results
Ninety-six patients ranged in age from 80 to 104 years (mean 84, median 84). There were 51 men and 45 women. American Society of Anesthesiologists Physical Status Class was as follows: 1 (none), 2 (5 patients), 3 (52 patients), 4 (45 patients).
Diagnoses (Table 2) included malignant pleural effusion 36, benign pleural effusion 34, empyema 17, chronic hemothorax 4, malignant pericardial effusion 3, mesothelioma 3, usual interstitial pneumonitis 2, chylothorax 2, pneumothorax 1.
Table 2. Diagnoses.
| Diseases | Number |
|---|---|
| Malignant pleural effusion | 36 |
| Lung cancer | 17 |
| Breast cancer | 8 |
| Mesothelioma | 5 |
| Other, 1 each (laryngeal, prostate, colon, prostate, anaplastic carcinoma, stomach) | 6 |
| Benign pleural effusion | 34 |
| Chronic pleuritis | 30 |
| Chronic pleuritis, radiotherapy | 3 |
| Ascites | 1 |
| Empyema | 17 |
| Chronic hemothorax | 4 |
| Pericardial effusion (all metastatic lung cancer) | 3 |
| Mesothelioma | 3 |
| Lung disease (both usual interstitial pneumonitis) | 2 |
| Chylothorax | 2 |
| Pneumothorax | 1 |
| Total | 102 |
Operations (Table 3) included drainage of pleural effusion/pleural biopsy 73 (63 with talc insufflation, 10 without talc), drainage of empyema 17, evacuate hemothorax 4, pericardial window 3, lung biopsy 2, treat chylothorax (drainage and localized pleurodesis along course of thoracic duct) 2, treat pneumothorax 1. Overall mean operating time was 25 min (range, 9-173 min), with types of cases as follows: drain effusion/pleural biopsy 24 min (range, 10-68 min), drain empyema 24 min (range, 9-173 min), drain hemothorax 27 min (range, 13-40 min), pericardial window 25 min (range, 17-34 min), lung biopsy 33 min (mean, 31-34 min), treat chylothorax 37-48 min, treat pneumothorax 17 min.
Table 3. Operations.
| Operations | Number |
|---|---|
| Drain effusion/pleural biopsy | 73 |
| With talc insufflation | 63 |
| Without talc insufflation | 10 |
| Drain empyema | 17 |
| Evacuate hemothorax | 4 |
| Pericardial window | 3 |
| Lung biopsy | 2 |
| Treat chylothorax | 2 |
| Treat pneumothorax | 1 |
| Total | 102 |
No patient required intraoperative intubation or epidural or nerve block analgesia. No patient required conversion to thoracotomy. Diagnosis was achieved, without need for additional procedure, in all cases of biopsy. No subsequent procedure was required for empyema. No patient had awareness or memory of the operation. Oxygen saturation was maintained at preoperative levels or greater, generally greater than 90%. There were three complications (3.1% morbidity): an 84-year-old man suffered a non-fatal cerebrovascular accident following drainage of malignant pericardial and bilateral pleural effusions; an 89-year-old woman developed a pulmonary embolus and atrial fibrillation a week following decortication for chronic hemothorax; an 86-year-old man manifested an air leak for 8 days following a procedure for pneumothorax. There were three deaths (3.1% morbidity): a 94-year-old woman died from bleeding (and her desire for no further therapy) 5 days following drainage of malignant pericardial effusion, when she becomes over anticoagulated; two 84-year-old men died of their advanced lung cancers on postoperative days 1 and 6 following procedures for malignant pleural effusions.
Discussion
General anesthesia and endotracheal intubation are needed for many types of VATS lobectomy or thymectomy for example. For the procedures in our present study, however, neither general anesthesia nor intubation is necessary, and there are risks to each in the elderly. In addition, many of these procedures are performed for palliation in patients with advanced malignancy, making risk minimization even more salutary.
Deep anesthesia—itself necessary for double-lumen endotracheal intubation—carries hemodynamic consequences and slower recovery. The nonagenarian, even without advanced malignancy or empyema, cannot maintain cardiac output if venous tone and cardiac filling are compromised. In most cases muscle paralysis is required. There also is more potential for drying of the airway.
Rarely discussed, but reported every year in the surgical literature, is the potential for endotracheal tube trauma to the trachea, esophagus, or hypopharynx. In 2005 Gómez-Caro Andrés (9) reviewed 90 cases of iatrogenic tracheobronchial injuries from seven series. Conti (10) in 2006 discussed 30 consecutive cases over a 12-year period. Schneider (11) in 2007 reported 29 cases from a single institution over a 10-year period. Miñambres (12) found 182 reported cases of post-intubation tracheal rupture over 40 years, with a mortality of 22% and significant morbidity. The elderly patient may tolerate the stress of the original surgery but not the added stress of iatrogenic trauma, due to their lack of reserve. An unknown author has written that “the elderly tolerate operations but not complications”.
It is beyond the scope of this report to argue for the effectiveness of VATS for drainage of empyema (13), the effectiveness of talc insufflation or PleurX® for pleurodesis (14) or the reliability of pleural biopsy [as opposed to pleural fluid cytology (15)] in suspected malignancy; the authors advocate all of these. Tube thoracostomy and talc pleurodesis at bedside is an alternative to our technique for patients with simple pleural effusions. Endotracheal intubation and spontaneous ventilation under deep sedation is another alternative, though our experience shows that this is unnecessary. Our goal was to show that these procedures can be safely performed under local anesthesia/sedation in the elderly, with spontaneous ventilation and without intubation.
Our “oldest old” tolerate VATS utilizing local anesthesia, sedation, and spontaneous ventilation. The obligatory unilateral pneumothorax is a concept that frightens even experienced surgeons but it should not. Many patients have had one lung partially collapsed already from effusion or empyema. In addition, the ipsilateral lung receives both less ventilation and less perfusion with the patient in lateral position, resulting in less physiologic shunt than might be expected. Even patients with empyema and bilateral pneumonia or pericardial and pleural effusions (Figure 3) tolerated their procedure. One tedious but successful decortication lasted 173 min. One centenarian, as many younger patients, was discharged home the same day of the procedure with his chest tube; the other went home with her chest tube the day following surgery.
Figure 3.
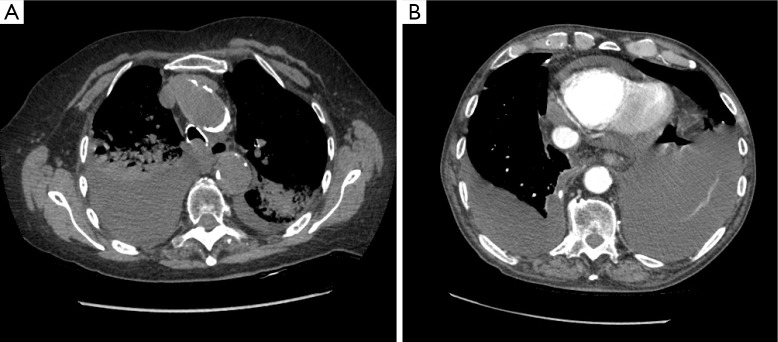
(A) Right empyema and bilateral pneumonia; (B) pericardial effusion and bilateral pleural effusions.
It may be argued that the line separating deep sedation and general anesthesia is indistinct. Whatever our technique is called, it is safe and effective in the elderly, who tolerate the obligatory pneumothorax, spontaneous ventilation, and local analgesia.
Ironically, thoracoscopy under local anesthesia is integral to the early history of VATS (16,17), but was supplanted for decades by the siren call of general anesthesia. Our present technique is made possible by sedating drugs, attentive local anesthesia, and careful manipulation of instruments. These cases require no special skills and are routinely performed by our residents under the authors’ guidance. Our anesthesiology staff has come to prefer this approach and express disappointment when we request general anesthesia for a more complicated case.
In conclusion, less stressful procedures which accomplish the same goal are particularly valuable for the elderly due to their lack of reserve. Non-intubated VATS utilizing local anesthesia and sedation is well-tolerated, safe, and valuable in octogenarians, nonagenarians, and centenarians.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Katlic M. Principles of Geriatric Surgery. In: Rosenthal R, Zenilman M, Katlic M. eds. Principles and Practice of Geriatric Surgery 2nd Edition. New York: Springer, 2011:235-51. [Google Scholar]
- 2.Lawrence VA, Hazuda HP, Cornell JE, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg 2004;199:762-72. [DOI] [PubMed] [Google Scholar]
- 3.Fortner JG, Lincer RM. Hepatic resection in the elderly. Ann Surg 1990;211:141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaklitsch MT, DeCamp MM, Jr, Liptay MJ, et al. Video-assisted thoracic surgery in the elderly. A review of 307 cases. Chest 1996;110:751-8. [DOI] [PubMed] [Google Scholar]
- 5.Cattaneo SM, Park BJ, Wilton AS, et al. Use of video-assisted thoracic surgery for lobectomy in the elderly results in fewer complications. Ann Thorac Surg 2008;85:231-5; discussion 235-6. [DOI] [PubMed] [Google Scholar]
- 6.Patel HJ, Williams DM, Upchurch GR, Jr, et al. A comparison of open and endovascular descending thoracic aortic repair in patients older than 75 years of age. Ann Thorac Surg 2008;85:1597-603; discussion 1603-4. [DOI] [PubMed] [Google Scholar]
- 7.Katlic MR. Video-assisted thoracic surgery utilizing local anesthesia and sedation. Eur J Cardiothorac Surg 2006;30:529-32. [DOI] [PubMed] [Google Scholar]
- 8.Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [DOI] [PubMed] [Google Scholar]
- 9.Gómez-Caro Andrés A, Moradiellos Díez FJ, Ausín Herrero P, et al. Successful conservative management in iatrogenic tracheobronchial injury. Ann Thorac Surg 2005;79:1872-8. [DOI] [PubMed] [Google Scholar]
- 10.Conti M, Pougeoise M, Wurtz A, et al. Management of postintubation tracheobronchial ruptures. Chest 2006;130:412-8. [DOI] [PubMed] [Google Scholar]
- 11.Schneider T, Storz K, Dienemann H, et al. Management of iatrogenic tracheobronchial injuries: a retrospective analysis of 29 cases. Ann Thorac Surg 2007;83:1960-4. [DOI] [PubMed] [Google Scholar]
- 12.Miñambres E, Burón J, Ballesteros MA, et al. Tracheal rupture after endotracheal intubation: a literature systematic review. Eur J Cardiothorac Surg 2009;35:1056-62. [DOI] [PubMed] [Google Scholar]
- 13.Wurnig PN, Wittmer V, Pridun NS, et al. Video-assisted thoracic surgery for pleural empyema. Ann Thorac Surg 2006;81:309-13. [DOI] [PubMed] [Google Scholar]
- 14.Tan C, Treasure T, Browne J, et al. Appropriateness of VATS and bedside thoracostomy talc pleurodesis as judged by a panel using the RAND/UCLA appropriateness method (RAM). Interact Cardiovasc Thorac Surg 2006;5:311-6. [DOI] [PubMed] [Google Scholar]
- 15.Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002;346:1971-7. [DOI] [PubMed] [Google Scholar]
- 16.Jacobaeus HC. The practical importance of thoracoscopy in surgery of the chest. Surg Gynecol Obstet 1922;34:289-96. [Google Scholar]
- 17.Bethune N.Pleural poudrage. A new technique for the deliberate production of pleural adhesion as a preliminary to lobectomy. J Thorac Surg 1935;4:251-61. [Google Scholar]


