Abstract
Background:
Instability of the knee joint, after anterior cruciate ligament (ACL) injury, is contraindication to osteochondral defect repair. This prospective study is to investigate the role of combined autologous chondrocyte implantation (ACI) with ACL reconstruction.
Materials and Methods:
Three independent groups of patients with previous ACL injuries undergoing ACI were identified and prospectively followed up. The first group had ACI in combination with ACL reconstruction (combined group); the 2nd group consisted of individuals who had an ACI procedure having had a previously successful ACL reconstruction (ACL first group); and the third group included patients who had an ACI procedure to a clinically stable knee with documented nonreconstructed ACL disruption (No ACL group). Their outcomes were assessed using the modified cincinnati rating system, the Bentley functional (BF) rating system (BF) and a visual analog scale (VAS).
Results:
At a mean followup of 64.24 months for the ACL first group, 63 months for combined group and 78.33 months for the No ACL group; 60% of ACL first patients, 72.73% of combined group and 83.33% of the No ACL group felt their outcome was better following surgery. There was no significant difference demonstrated in BF and VAS between the combined and ACL first groups. Results revealed a significant affect of osteochondral defect size on outcome measures.
Conclusion:
The study confirms that ACI in combination with ACL reconstruction is a viable option with similar outcomes as those patients who have had the procedures staged.
Keywords: Anterior cruciate ligament, autologous chondrocyte, chondrocyte, knee, osteochondral defect
Keywords: Anterior cruciate ligament, chondrocytes, autologous, knee
INTRODUCTION
Anterior cruciate ligament (ACL) injuries commonly affect the athletic patient, resulting in instability and impaired function. Articular cartilage injury to the knee may occur with ACL injuries in the acute setting, with incidence of 16–46%,1,2 and in chronic ACL deficient knees.3 The presence of articular cartilage damage can impact on the outcome from ACL reconstruction, with the suggestion that it is a predictor of failure.4,5 Inadequately managed osteochondral defects predispose individuals to pain and early onset osteoarthritis of the joint.6,7
Patients with ACL deficient knees have an altered region of load bearing on the articular surface due to a shift in the tibiofemoral biomechanics.8 The transmitted axial load through the articular cartilage and subchondral bone can culminate in the formation of chondral lesions.9,10,11 At present, instability of the knee joint, after ACL injury, is a contraindication to osteochondral lesion repair.
Areas of articular cartilage damaged will repair without intervention, forming fibrocartilage rather than the original hyaline cartilage, with its inherent inferior mechanical properties.12 Reported management options for osteochondral defects include physiotherapy, fibrocartilage forming (marrow stimulating) techniques such as debridement and curettage; bone drilling; abrasion chondroplasty; and microfracture. Articular cartilage autografting (such as mosaicplasty), and autologous chondrocyte implantation (ACI) are techniques aimed at hyaline cartilage repair.12,13,14,15,16 Bentley et al.17 have reported favorable outcomes of ACI over mosaicplasty for the repair of osteochondral lesions. Evolution of ACI techniques has led to the use of an inert porcine type I/III collagen membrane sutured to the chondral defect (ACI-C), with the cultured chondrocyte cells injected below the seal.17,18,19,20,21 With matrix assisted autologous chondrocyte implantation (MACI), a porcine type I/III collagen bilayer is seeded with the cultured chondrocyte cells, which is then glued to the chondral defect using a fibrin sealant.22,23
This prospective study is to investigate the affect of correction of instability (ACL injury) on outcome of ACI procedures and to evaluate the results of a combined ACL reconstruction with ACI procedure.
MATERIALS AND METHODS
Three independent groups of patients with previous ACL injuries who underwent ACI-C or MACI at our institution were identified and prospectively followed up. The first group had ACI in combination with ACL reconstruction (combined group); the 2nd group had an ACI procedure having previously had a successful ACL reconstruction (ACL first group); and the third group included had an ACI procedure for a chondral or osteochondral lesion to a clinically stable knee with documented ACL injury in the past, but had no reconstruction (no ACL group).
Combined group
Twenty two patients (14 male and eight female) who had combined ACL reconstruction with ACI, with an average age at the time of surgery of 32.2 years (range 20–45 years). Hamstring autograft15 or bone-patella tendon-bone autograft (BPB) (seven) was used for the ACL reconstruction and MACI (19) or ACI-C (three) for ACI. Previous surgical procedures to these knees included medial menisectomy;10 lateral menisectomy (three); screw fixation for osteochondral fragments (two); drilling of chondral lesions (two); previous ACL reconstruction (two) and osteochondral allograft to a chondral lesion (one). The mean area of defect was 300 mm2 (range 225–600 mm2) involving the medial femoral condyle,16 lateral femoral condyle (three), patella (two) and with one patient having no documentation of site or defect size. Followup was for an average of 63 months (range 8–112 months).
Anterior cruciate ligament first group
Twenty five patients (20 males and 5 females) who had previous ACL reconstruction using hamstring autograft,16 BPB (six) and synthetic graft (three) that underwent ACI for a chondral lesion. The average time from ACL reconstruction to the 2nd stage of ACI was 74.04 months (range 12–156 months). Mean age at time of 2nd stage procedure was 35.94 years (range 27–48 years), with 14 patients undergoing MACI and 11 had ACI-C. One patient had no documentation for the site and size of chondral defect. The average area of defect was 300 mm2 (range 212–465 mm2) involving the medial femoral condyle,15 trochlea (four), patella (three), lateral femoral condyle (one) and multi site defect (one). Previous surgical procedures in the knees included medial menisectomy (eight); lateral menisectomy (three); drilling of chondral defect (three); microfracture (two); ACI (two) and open reduction with internal fixation of medial femoral condyle fracture (one). Followup was an average of 64.24 months (range 11–102 months).
No anterior cruciate ligament group
Twelve patients (10 male and two female) who underwent an ACI procedure to a knee that was felt to be stable, although there had been a previous nonreconstructed ACL injury. Eight of these patients had partial disruption and four of these had no laxity of the ACL. These patients presented with pain however their knee was felt stable on clinical examination and assessed further at first stage arthroscopy.
The Lachman test was utilized to assess the ACL in all 12, with three knees being graded as 0, seven as 1 and two as 2. Previous surgical procedures to these knees included; medial menisectomy (four); drilling to chondral defect (two); open reduction and internal fixation for proximal tibia and fibula fracture (one); lateral soft tissue release (one) and osteochondral allograft to a chondral lesion (one). The average age at time of 2nd stage procedure was 33.45 years (range 20–47 years), with seven patients undergoing MACI and five having ACI-C. The mean defect size was 225 mm2 (range 150–500 mm2), involving the medial femoral condyle (five), trochlea (three), lateral femoral condyle (two), patella (one) and multi site defects (one). There was one patient for whom we had no documentation for the size of the defect. Followup was for an average of 78.33 months (range 12–139 months).
There was another subset (complete rupture) within this group that was investigated separately; these patients were clinically stable although there was documented evidence of complete ACL rupture. There were four patients (three male and one female) with a mean age of 37.45 years, with two undergoing ACI-C and two having a MACI procedure. Three patients had a Lachman grade of 1+ and one was assessed as grade 2+, with average defect size of 524 mm2 (range 300–660 mm2) and the medial femoral condyle and patella were affected equally.
The diagnosis and treatment plan was formulated following clinical review, plain radiographs, MRI and arthroscopy. The Kellegren–Lawrence grading system was used24 to assess degenerative changes on plain radiographs and the MRI findings were used to confirm this assessment. Prior to surgery the patients completed the modified Cincinnati (MC) rating system questionnaire,25 the Meister et al. functional rating (BF) system26 and a visual analog scale (VAS).
Operative procedure
Each patient underwent a two stage procedure; an initial arthroscopy to assess the chondral lesion for suitability for ACI and if suitable a biopsy of nonweight bearing articular cartilage was harvested from the trochlea margin and sent for cell culturing. All the patients in the ACL first and No ACL group had stable knees on examination under anaesthesia. Postoperative management was routine for arthroscopy.
The 2nd stage was performed 4–6 weeks later, after satisfactory cell culture, in all patients. In patients in the combined group, the ACL reconstruction was performed prior to ACI. The ACI procedure was performed through either a medial or lateral arthrotomy incision, depending on site of lesion and the defect prepared before implantation. With ACI-C a template sized piece of porcine type I/III collagen membrane was sutured (using 6/0 vicryl) to the margins of the defect and a watertight seal ensured with the use of fibrin glue to the margins. This seal was checked by injecting a saline solution below the membrane, looking for leaks, prior to the cultured cells being injected below the membrane, and the final suture and glue applied to the membrane cartilage junction.19 With MACI the chondrocyte cell seeded type I/III collagen bilayer membrane was cut to size to fit the defect and secured with fibrin glue.22,27 The wound was closed in layers using nonabsorbable sutures, with wool and crepe pressure bandaging applied.
Rehabilitation
The patients in the ACL first and No ACL groups were placed in plaster-of-Paris backslab for 24 h, with rest and elevation advice, as well as foot and ankle exercise encouragement. After 24 h full weight bearing was allowed. The knee was placed in a lightweight cylinder cast in full extension at 48 h, which was removed on the 10th day postoperatively. The patients were progressively mobilized with daily physiotherapy for 2 weeks, after which more strenuous, low-impact activities were permitted. By 6 months light jogging was allowed and strenuous sports at 12 months if the knee was painless.
For patients undergoing the combined procedures, no range of movement exercises were permitted and the patients were placed in a hinged knee brace at 48 h postoperatively, for up to 4 weeks. From 28 h, they were progressively mobilized with regular physiotherapy to work on strength, core stability, proprioception and mobility. Closed channel active range of movement exercises were commenced after 1-week as symptoms allowed, but no open kinetic quadriceps work was started until 12 weeks. From 6 weeks controlled active range of movement, strengthening of muscles stabilizing the knee and core stability work was started. At 12 weeks, a full range of movement was achieved and exercises progressed from static to dynamic as tolerated. By 9 months patients returned to sport-specific activities, such as low-impact jogging in the gym and swimming and return to normal sports were achieved after 1-year.
Patients were reviewed at 6 weeks, 6 months, 1-year and then annually to assess them clinically. At these times, they were also asked to complete the MC questionnaire, BF and VAS score systems for postoperative evaluation. The patients also graded their outcome as better, same or worse.
RESULTS
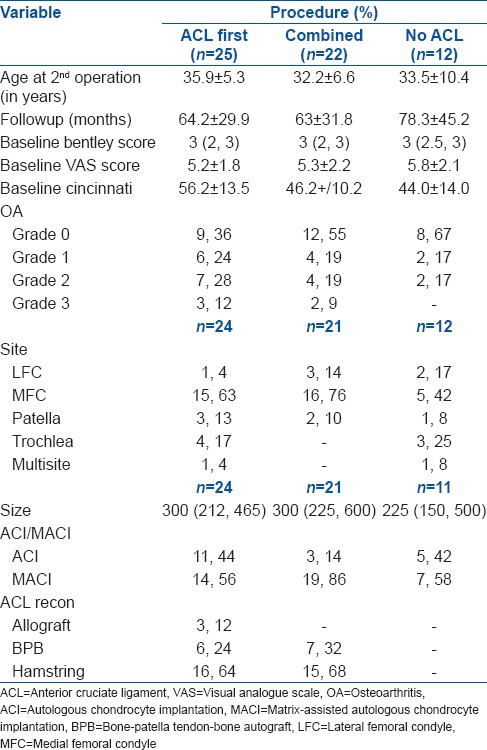
Patients in the three groups had varying baseline characteristics and scores for BF, VAS and MC preoperatively [Table 1]. Analysis was adjusted for these known variables.
Table 1.
Clinical details of three groups
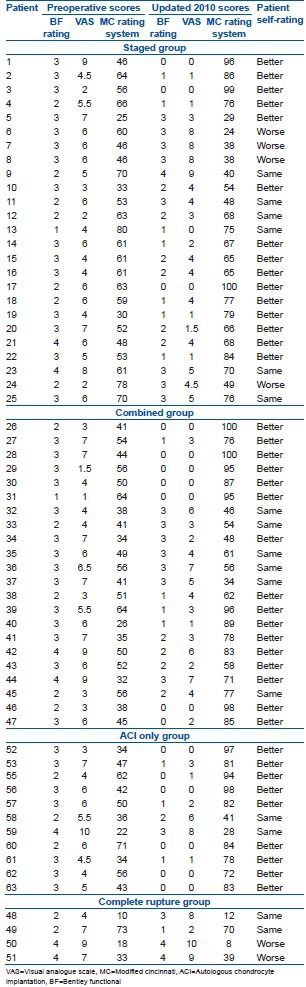
At final followup, 16 (72.73%) patients in the combined group reported their outcome as better, with six patients (27.27%) reporting the symptoms as the same as the preoperative level. All the knees in this group were found to be clinically stable on examination at final followup. In the ACL first group the outcome was better in 15 cases (60%), the same in six cases (24%) and worse in four (16%). The 12 patients in the No ACL group reported the outcome as better in 10 instances (83.33%) and the same in two patients (16.67%). This is in contrast to the complete rupture group; with 2 (50%) reporting a worse outcome and 2 (50%) reporting the outcome to be the same [Table 2].
Table 2.
Functional scores and subjective outcome
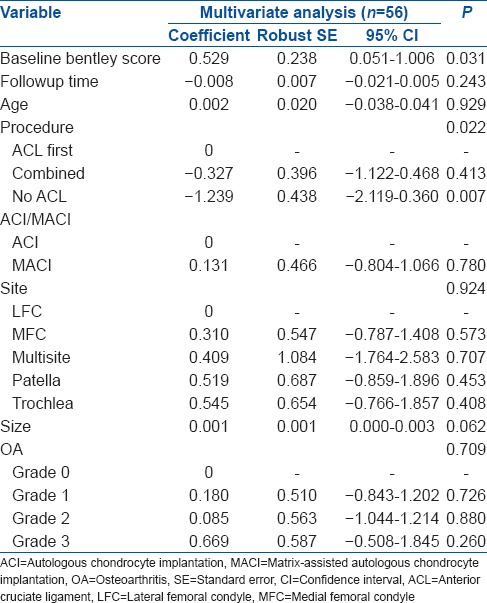
The mean change in BF from baseline was largest for the No ACL group [Figure 1]. There was a significant association between the procedure and BF (P = 0.004) [Table 3]. In comparison to the ACL first group, the No ACL group had significantly lower postoperative BF (P = 0.001), while comparison with the combined group was not significantly different (P = 0.251). Multiple linear regression analysis of procedure demonstrated similar association [Table 4].
Figure 1.
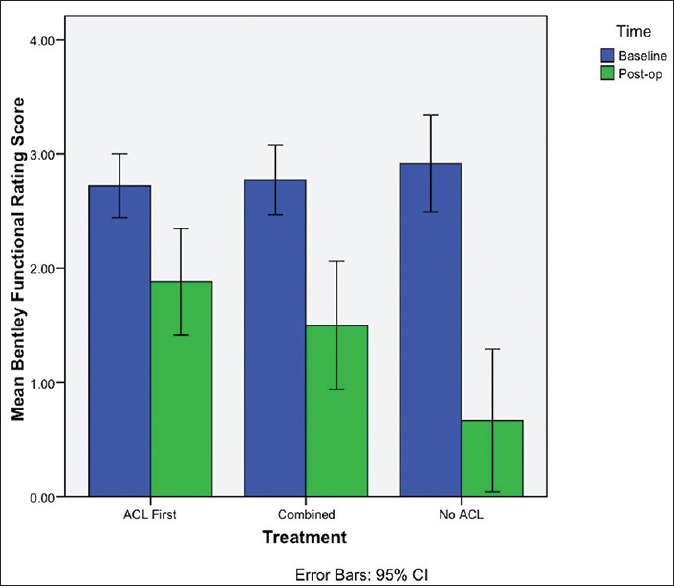
Bar diagram demonstrating mean change in Bentley functional score
Table 3.
Linear regression model
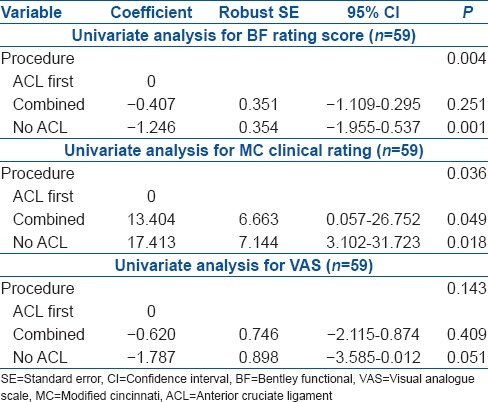
Table 4.
Multiple linear regression analysis of procedure, with the postoperative Bentley score as the dependent variable
When considering the MC score, the largest change from baseline was observed in the No ACL group, while the least change was in the ACL first group [Figure 2]. A significant association between the procedure and MC score was observed (P = 0.036), with the No ACL group having significantly higher postoperative MC scores (P = 0.018) compared with the ACL first group [Table 3]. Patients in the combined group also had significantly better MC in comparison with the ACL first group (P = 0.049). With multiple linear regression analysis, comparison of the postoperative MC in the combined and ACL first groups were found not to be significant (P = 0.113) [Table 5].
Figure 2.
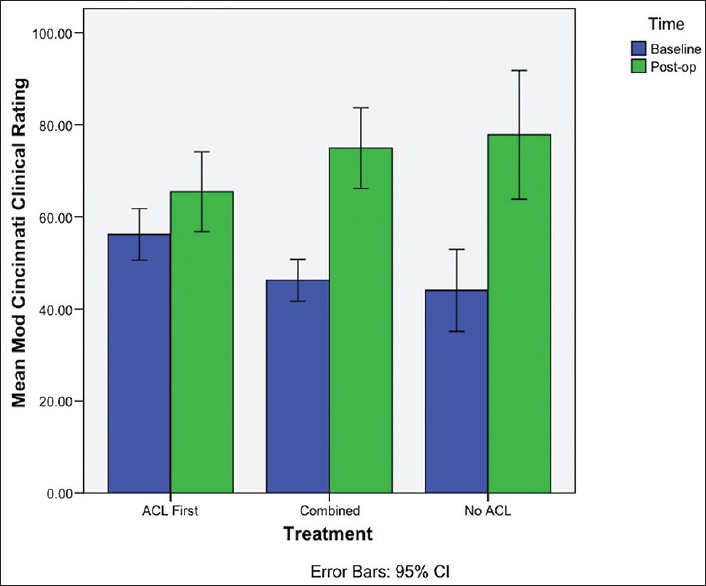
Bar diagram demonstrating mean change in modified Cincinnati score
Table 5.
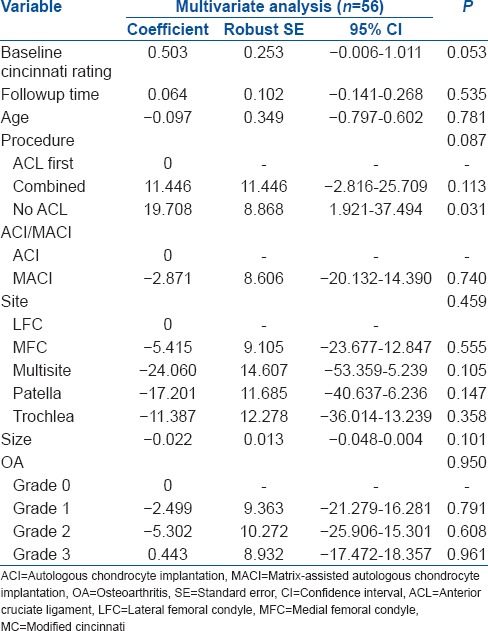
Multiple linear regression analysis of procedure, with postoperative MC rating score as the dependent variable
The ACL first group also demonstrated the smallest mean change in VAS from baseline, with the largest change seen in the No ACL group [Figure 3]. The No ACL group had marginally significant lower VAS than the ACL first patients (P = 0.051) [Table 3]. Comparing the combined group with the ACL first group failed to show a significant difference in VAS (P = 0.409). This was confirmed by multiple linear regression analysis of procedure [Table 6].
Figure 3.
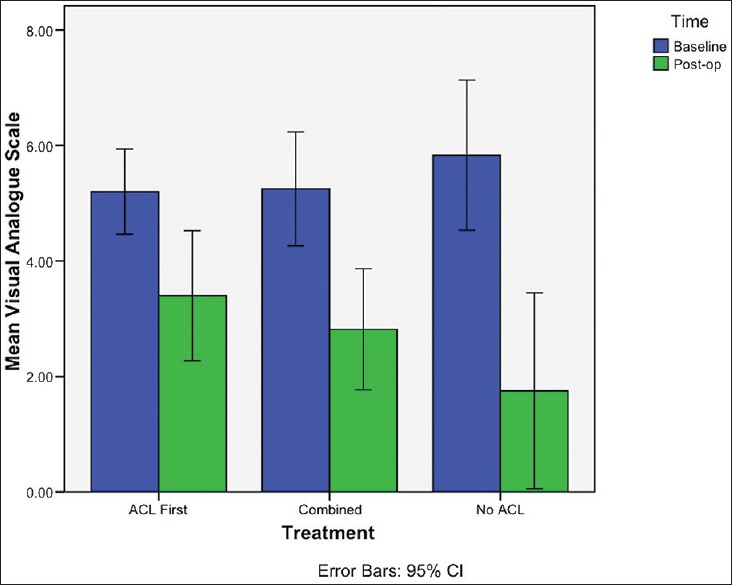
Bar diagram demonstrating mean change in visual analogue scale
Table 6.
Multiple linear regression analysis of procedure, with postoperative VAS as the dependent variable
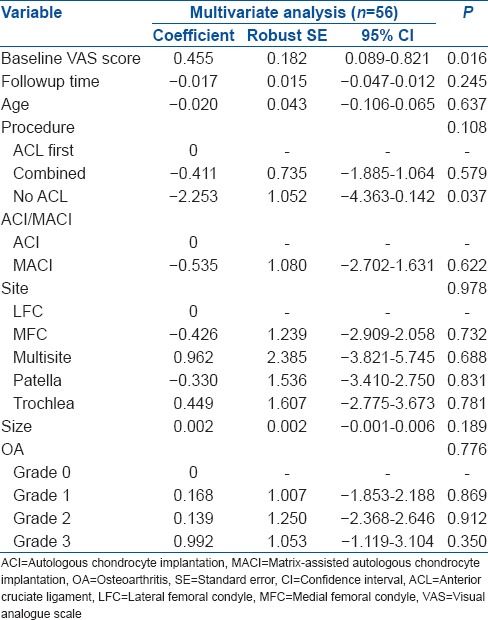
In the combined group, there was no significant difference in clinical outcome scores, nor mean change in outcome scores, between individuals treated with ACI-C and those treated with MACI. Multiple linear regression analysis demonstrated that whether ACI-C or MACI had been performed had no significant affect on BF (P = 0.197), and neither was the use of hamstring or BPB graft (P = 0.088), as well as reporting the significance of OA grade (P = 0.038), followup time (P = 0.025) and size of defect (P < 0.001) in the combined group [Table 7].
Table 7.
Multiple linear regression analysis was conducted to derive the independent effects of ACI versus MACI and hamstring versus BPB on the outcome scores while additionally controlling for the other measured determinants of outcome in the combined group
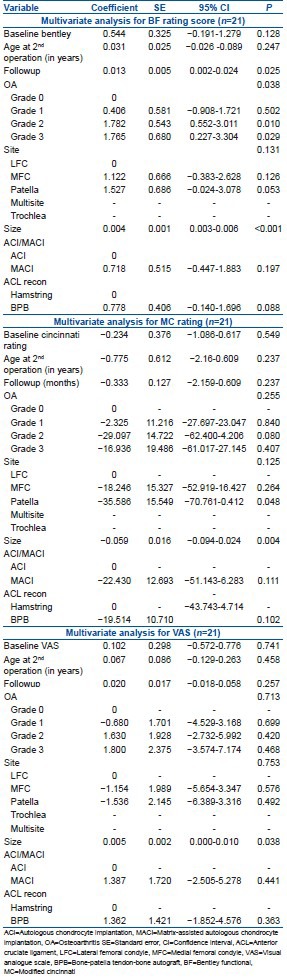
The size of defect (P = 0.004) and patella site (P = 0.048) was found to significantly affect MC [Table 7], and using multiple linear regression analysis, the size of the defect (P = 0.038) was found to significantly affect the VAS in the combined group [Table 7].
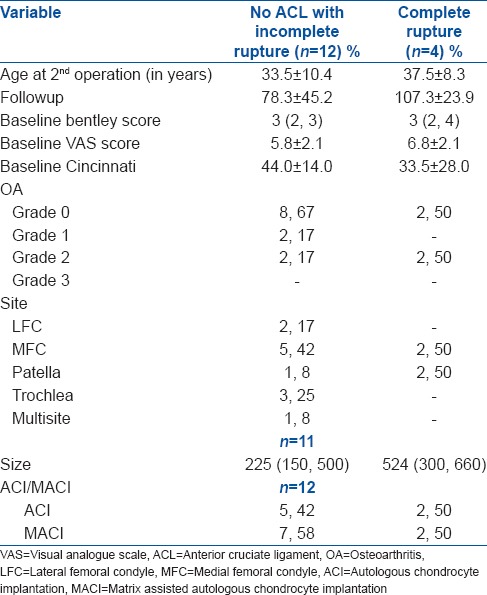
In the No ACL group, the baseline characteristics of the twelve patients against the four with documented evidence of complete ACL rupture (complete rupture group) [Table 8]. On average, there was a much bigger change in BF, MC and VAS outcomes for the cases that did not have complete ACL ruptures, in comparison to those who had complete ACL ruptures. Independent t-tests confirmed a significant difference in the mean change in BF (P = 0.002), MC (P = 0.006) and VAS score (P = 0.012) by rupture status.
Table 8.
Baseline summary data table for no ACL group and complete rupture group
DISCUSSION
An ACL injury results in functional instability that can lead to osteoarthritis.10,27,28 ACI is contraindicated in those patients with instability from ACL injury, due to potential damage to the repair tissue from shearing forces and damage from abnormal biomechanical stresses across the knee joint.8,29 ACL reconstruction should be performed if ACL rupture is clinically evident to provide stability followed by any osteochondral defect can be addressed.
In our series, the patients who underwent combined ACI with ACL reconstruction reported their outcome to be better in 72.73% and the same in 27.27%. The patients who were ACL deficient, but thought to be clinically stable and underwent ACI only reported the outcome to be worse in 50%. There were four patients in the ACL first group that felt their outcome was worse. This can be multifactorial. An explanation may be the number of previous surgeries performed; as these patients had the most procedures prior to referral to our institution.
The role of combined repair of osteochondral defects and ACL reconstruction has been reported using osteochondral autograft,9,10,30,31,32 autologous periosteum transplantation1 and ACI.12,21 The use of osteochondral transfer and periosteum transplantation for the repair of osteochondral defects has raised concern regarding the long term stability of the repair tissue and its integration with surrounding articular cartilage.15,31
The use of ACI in osteochondral defect repair has produced encouraging clinical outcomes;18,20,21,33 however, the use of a periosteum cover is associated with periosteal hypertrophy and donor site morbidity among after complications. The use of the MACI technique avoids these complications, as well as providing greater stability22,34 and reduces operative time, as suturing of the membrane is not usually required. Good outcomes have been reported with the use of the MACI technique for osteochondral defect repair.22,34,35
Peterson et al.21 demonstrated encouraging results with the use of ACI, with a periosteum cover, in combination with ACL reconstruction. Amin et al.,12 reported good to excellent results in eight patients in a nine patient series, using ACI-C or MACI, in combination with ACL reconstruction (both hamstring and bone-patellar tendon-bone autograft).
As separate procedures, ACL reconstruction and ACI are costly to health care providers as well as the patient, as they must undergo a long rehabilitation period with restriction in function.29 Thus the benefit of performing the procedures in combination applies to both the patient and health care provider. Further work is required to determine whether this procedure is effective in preventing osteoarthritis in the joint, as well as to define specific factors that impact on outcome and thus can be used to select ideal patients as well as predict outcome in the future.
CONCLUSION
The similar outcomes of the combined and ACL first groups suggest that in specific indications the combined procedure will produce good to excellent outcomes with reduced cost and impact on the individuals. The poor outcome in the complete rupture group emphasize that ACL deficient knees, whether clinically stable or not, are a contraindication to osteochondral defect repair. The outcome of the remaining patients in the ACI only group suggest that it is safe to perform ACI in those patients with a clinically stable knee after partial ACL injury. The results have been encouraging in this study and demonstrate that ACI in combination with ACL reconstruction is a possible option in the management of the young and active patient who wishes to return to their preinjury activity level. The role of this procedure in acute ACL rupture requires further investigation.
Footnotes
Source of Support: This study was funded by our institution.
Conflict of Interest: None.
REFERENCES
- 1.Alfredson H, Thorsen K, Lorentzon R. Treatment of tear of the anterior cruciate ligament combined with localised deep cartilage defects in the knee with ligament reconstruction and autologous periosteum transplantation. Knee Surg Sports Traumatol Arthrosc. 1999;7:69–74. doi: 10.1007/s001670050124. [DOI] [PubMed] [Google Scholar]
- 2.Brophy RH, Zeltser D, Wright RW, Flanigan D. Anterior cruciate ligament reconstruction and concomitant articular cartilage injury: Incidence and treatment. Arthroscopy. 2010;26:112–20. doi: 10.1016/j.arthro.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Shelbourne KD, Jari S, Gray T. Outcome of untreated traumatic articular cartilage defects of the knee: A natural history study. J Bone Joint Surg Am. 2003;85-A(Suppl 2):8–16. doi: 10.2106/00004623-200300002-00002. [DOI] [PubMed] [Google Scholar]
- 4.Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five-to fifteen-year evaluations. Am J Sports Med. 2000;28:446–52. doi: 10.1177/03635465000280040201. [DOI] [PubMed] [Google Scholar]
- 5.Takeda T, Matsumoto H, Fujikawa K. Influence of secondary damage to menisci and articular cartilage on return to sports after anterior cruciate ligament reconstruction. J Orthop Sci. 1997;2:215–21. [Google Scholar]
- 6.Jomha NM, Borton DC, Clingeleffer AJ, Pinczewski LA. Long term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res. 1999;358:188–93. [PubMed] [Google Scholar]
- 7.Küllmer K, Letsch R, Turowski B. Which factors influence the progression of degenerative osteoarthritis after ACL surgery? Knee Surg Sports Traumatol Arthrosc. 1994;2:80–4. doi: 10.1007/BF01476477. [DOI] [PubMed] [Google Scholar]
- 8.Nishimori M, Deie M, Adachi N, Kanaya A, Nakamae A, Motoyama M, et al. Articular cartilage injury of the posterior lateral tibial plateau associated with acute anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2008;16:270–4. doi: 10.1007/s00167-007-0458-x. [DOI] [PubMed] [Google Scholar]
- 9.Klinger HM, Baums MH, Otte S, Steckel H. Anterior cruciate reconstruction combined with autologous osteochondral transplantation. Knee Surg Sports Traumatol Arthrosc. 2003;11:366–71. doi: 10.1007/s00167-003-0422-3. [DOI] [PubMed] [Google Scholar]
- 10.Murrell GA, Maddali S, Horovitz L, Oakley SP, Warren RF. The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med. 2001;29:9–14. doi: 10.1177/03635465010290012001. [DOI] [PubMed] [Google Scholar]
- 11.Speer KP, Spritzer CE, Bassett FH, 3rd, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20:382–9. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 12.Amin AA, Bartlett W, Gooding CR, Sood M, Skinner JA, Carrington RW, et al. The use of autologous chondrocyte implantation following and combined with anterior cruciate ligament reconstruction. Int Orthop. 2006;30:48–53. doi: 10.1007/s00264-005-0025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czitrom AA, Langer F, McKee N, Gross AE. Bone and cartilage allotransplantation. A review of 14 years of research and clinical studies. Clin Orthop Relat Res. 1986;208:141–5. [PubMed] [Google Scholar]
- 14.Hangody L, Kish G, Kárpáti Z, Szerb I, Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262–7. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 15.Hangody L, Ráthonyi GK, Duska Z, Vásárhelyi G, Füles P, Módis L. Autologous osteochondral mosaicplasty. Surgical technique. J Bone Joint Surg Am. 2004;86-A(Suppl 1):65–72. [PubMed] [Google Scholar]
- 16.Tomford WW, Springfield DS, Mankin HJ. Fresh and frozen articular cartilage allografts. Orthopedics. 1992;15:1183–8. doi: 10.3928/0147-7447-19921001-09. [DOI] [PubMed] [Google Scholar]
- 17.Bentley G, Biant LC, Carrington RW, Akmal M, Goldberg A, Williams AM, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223–30. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 18.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 19.Haddo O, Mahroof S, Higgs D, David L, Pringle J, Bayliss M, et al. The use of chondrogide membrane in autologous chondrocyte implantation. Knee. 2004;11:51–5. doi: 10.1016/S0968-0160(03)00041-3. [DOI] [PubMed] [Google Scholar]
- 20.Minas T. Chondrocyte implantation in the repair of chondral lesions of the knee: Economics and quality of life. Am J Orthop (Belle Mead NJ) 1998;27:739–44. [PubMed] [Google Scholar]
- 21.Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two-to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;374:212–34. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett W, Skinner JA, Gooding CR, Carrington RW, Flanagan AM, Briggs TW, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: A prospective, randomised study. J Bone Joint Surg Br. 2005;87:640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 23.Cherubino P, Grassi FA, Bulgheroni P, Ronga M. Autologous chondrocyte implantation using a bilayer collagen membrane: A preliminary report. J Orthop Surg (Hong Kong) 2003;11:10–5. doi: 10.1177/230949900301100104. [DOI] [PubMed] [Google Scholar]
- 24.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noyes FR, Mooar LA, Barber SD. The assessment of work-related activities and limitations in knee disorders. Am J Sports Med. 1991;19:178–88. doi: 10.1177/036354659101900214. [DOI] [PubMed] [Google Scholar]
- 26.Meister K, Cobb A, Bentley G. Treatment of painful articular cartilage defects of the patella by carbon-fibre implants. J Bone Joint Surg Br. 1998;80:965–70. doi: 10.1302/0301-620x.80b6.8194. [DOI] [PubMed] [Google Scholar]
- 27.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22:632–44. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 28.Hangody L, Karpati Z. A new surgical treatment of localized cartilaginous defects of the knee. Hung J Orthop Traumatol. 1994;37:237–42. [Google Scholar]
- 29.Levy AS, Meier SW. Approach to cartilage injury in the anterior cruciate ligament-deficient knee. Orthop Clin North Am. 2003;34:149–67. doi: 10.1016/s0030-5898(02)00065-2. [DOI] [PubMed] [Google Scholar]
- 30.Gaweda K, Walawski J, Weglowski R, Patyra M. Rehabilitation after one-stage anterior cruciate reconstruction and osteochondral grafting. Int Orthop. 2006;30:185–9. doi: 10.1007/s00264-005-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinert AF, Ghivizzani SC, Rethwilm A, Tuan RS, Evans CH, Nöth U. Major biological obstacles for persistent cell-based regeneration of articular cartilage. Arthritis Res Ther. 2007;9:213. doi: 10.1186/ar2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsusue Y, Yamamuro T, Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318–21. doi: 10.1016/s0749-8063(05)80428-1. [DOI] [PubMed] [Google Scholar]
- 33.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: A long term followup. Am J Sports Med. 2010;38:1117–24. doi: 10.1177/0363546509357915. [DOI] [PubMed] [Google Scholar]
- 34.Bachmann G, Basad E, Lommel D, Steinmeyer J. MRI in the followup of matrix-supported autologous chondrocyte transplantation (MACI) and microfracture. Radiologe. 2004;44:773–82. doi: 10.1007/s00117-004-1084-y. [DOI] [PubMed] [Google Scholar]
- 35.Ronga M, Grassi FA, Bulgheroni P. Arthroscopic autologous chondrocyte implantation for the treatment of a chondral defect in the tibial plateau of the knee. Arthroscopy. 2004;20:79–84. doi: 10.1016/j.arthro.2003.11.012. [DOI] [PubMed] [Google Scholar]


