Abstract
Anti-NMDA receptor encephalitis (ANRE) has been previously reported as a rare cause of nonconvulsive status epilepticus (NCSE). Vagus nerve stimulation (VNS) is generally considered as a palliative treatment for patients with drug-resistant partial-onset epilepsy. Here, we report a case of refractory NCSE that was terminated after vagus nerve stimulator implantation. To our knowledge, similar cases have not been reported previously.
Keywords: Anti-NMDA encephalitis, Refractory SE, VNS
1. Introduction
Anti-NMDA receptor encephalitis (ANRE) is a recently recognized form of paraneoplastic encephalitis and characterized by dyskinesias, psychosis, and seizures secondary to antibodies to the NR1–NR2B heteromer of the NMDA receptor [1], [2]. Seizures are present in approximately three-fourths of all patients [3], [4], with a few reports of convulsive status epilepticus (SE) in adults. However, ANRE, as a cause of generalized NCSE, is extremely rare, with 2 cases having been reported so far in the literature [5], [6]. Status epilepticus secondary to ANRE is thought to respond less favorably than SE caused by any other etiologies [7]. Vagus nerve stimulation (VNS) is generally considered as a palliative treatment for patients with drug-resistant partial-onset epilepsy [8]. However, its role in the management of refractory SE has been previously reported in few case reports both in adults and in children and in a very few case series [9], [10], [11]. Here, we report a case of refractory NCSE, secondary to ANRE that was terminated after vagus nerve stimulator implantation.
2. Case report
A previously healthy 46-year-old right-handed man was admitted to the psychiatry ward after a one-week history of mild fever, behavioral changes, headaches, and reported facial twitches. His initial CT of the brain was negative. His neurological status continues to deteriorate, with reported episodes of lip smacking and facial twitches. Therefore, he was transferred to the medical ward. The neurology team was consulted, and a lumbar puncture (LP) was suggested. The cerebrospinal fluid (CSF) analysis showed elevated white count of 521 per microliter, 98% lymphocytes, elevated protein of 51/dL, and a normal glucose level. Polymerase chain reaction for the following viruses and bacteria were negative: herpes simplex virus I and II, varizella zoster virus, human herpes virus 6, cytomegalovirus, Epstein–Barr virus, enterovirus, mycoplasma pneumonia, and TB bacillus. There were no oligoclonal bands. The Gram stain was negative. A diagnosis of encephalitis was made, and he was started on acyclovir, ceftriaxone, and vancomycin. His initial EEG showed intermitted slowing of delta range over both frontocentral head regions with faster frequency superimposed representing extreme delta brush (Fig. 1). He was loaded with phenytoin, and a brain MRI was requested. The MRI of the brain with and without contrast was reported as normal. However, his neurological status has worsened, and he started to desaturate. He was transferred to the ICU where the neurology team was called to reevaluate him. Continuous EEG recording (CEEG) was advised, and it showed a rhythmical slowing over both frontocentral head regions with a clear evolution in voltage, frequency, and field consistent with nonconvulsive status epilepticus (NCSE) (Fig. 2). This evolution of the EEG pattern became more apparent when the EEG was compressed (Fig. 3). He was loaded with midazolam which was titrated up to burst suppression. Two days later, he continued to have facial twitches, and his CEEG was still showing electrical SE pattern. Levetiracetam was loaded and continued on a titrating dose of up to 2000 mg twice per day and on phenytoin with a titrating dose of 350 mg/day, with close drug level monitoring, followed in 2 days by a loading dose of valproic acid. Four days later, he continued to have episodes of facial twitches and lip smacking, and CEEG was still showing the previously reported pattern. The epilepsy team was consulted and they advised loading with propofol which was titrated up to 50 mg/h. However, every attempt to decrease the propofol would result in worsening facial twitches and, in a number of occasions, breakthrough tonic–clonic seizures. A diagnosis of ARNE was suspected based on clinical features and EEG pattern. A repeat MRI of the brain with and without contrast remained normal. Anti-NMDA receptor in the serum was requested, and an extensive search for a tumor was recommended but did not reveal any pathology. While waiting for the results of anti-NMDA receptor antibodies, IVIG was given over a 5-day course, along with a pulse of steroids. He was loaded with pentobarbital titrated up to burst suppression. However, breakthrough electrical SE was still apparent every time the level of suppression is reduced, and midazolam was restarted and titrated up to 12 mg/h. Over several weeks, his neurological status had not changed. His repeat LP showed white count of 24 per microliter and 90% lymphocytes. He continued on both propofol and midazolam while pentobarbital was discontinued. Topiramate was added and titrated over 2 days to a dose of 200 mg twice per day. Still, his CEEG was showing SE pattern, and he continued to have facial twitches. He also continued on a combination of high doses of levetiracetam 4 g/day, topiramate 400 mg/day, phenytoin 400 mg/day, valproic acid 3000 mg/day, clonazepam 5 mg/8 h, and phenobarbital 200 mg/day. A week later, his neurological status had not changed, and every attempt to reduce his anesthetics would result in recurrent clinical and electrical seizures. While in the ICU, his dose of phenobarbital was increased up to 150 mg twice per day, and he continued on propofol and midazolam. Intravenous magnesium sulfate was also tried but without success. He was also tried on IV ketamine, but his neurological status remained the same, and he continued to show the SE pattern when the level of sedation is reduced. The result of Anti-NMDA receptor antibodies came back positive and a repeat course of IVIG was recommended over a 4-day duration, 2 weeks after the first IVIG course was completed. However, his neurological status has not changed, and he remained in NCSE for 110 days, and a decision was made, after extensive discussions with the patient's family, to try VNS. A vagus nerve stimulator was implanted and interrogated to an output current of 2.5 mA over 4 days. His duty cycle was set at 30 s on and 5 min off. After 1 week of vagus nerve stimulator implantation, midazolam was weaned off successfully over 3 days and followed 4 days later by a weaning off propofol without any clinical or electrical seizure recurrences. His CEEG showed no evidence of electrical SE pattern. His neurological status continued to improve in the succeeding months with no reported clinical seizures. The dosages of antiepileptic drugs (AEDs) were gradually reduced one at a time. He was discharged from the hospital after 8 months of rehabilitation on no AEDs with mild–moderate cognitive impairment.
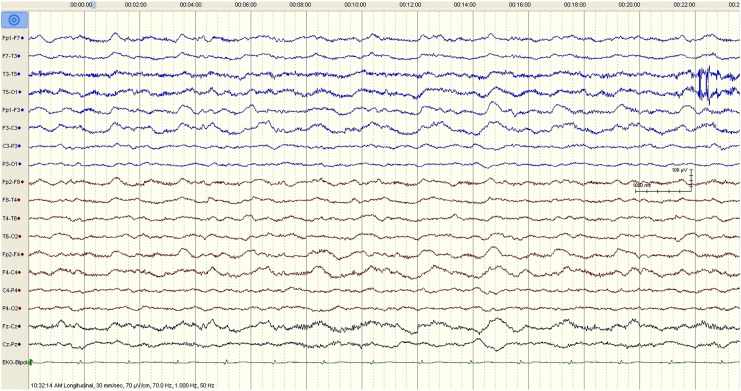
Fig. 1.
The initial EEG tracing demonstrates generalized rhythmic and semirhythmic delta frequency activity at 1 Hz with superimposed, frontally predominant bursts of rhythmic beta frequency activity. High pass filter 1 Hz; low pass filter 70 Hz; notch filter off representing extreme delta brush.
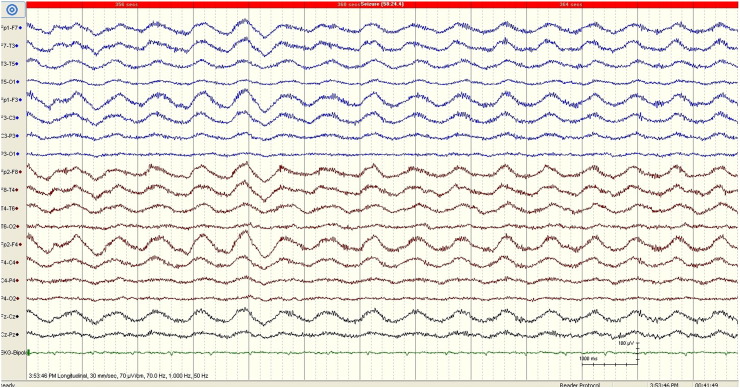
Fig. 2.
EEG obtained 4 days after admission showing generalized rhythmic delta activity. The evolution of the EEG pattern is suggestive of NCES. The calibration mark represents 1 s and 100 μV.
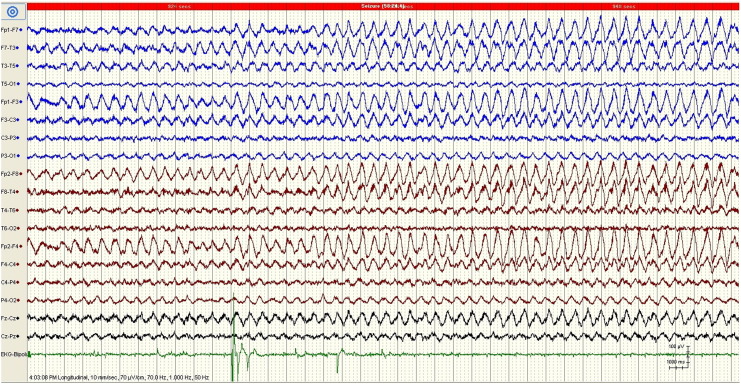
Fig. 3.
EEG from one 30-second epoch including the 10-second period represented in Fig. 2 demonstrating evolution of the ictal pattern over time. The calibration mark represents 1 s and 100 μV.
3. Discussion
Our case is typical in many respects of the ANRE syndrome, with a prodrome of headaches, fever, and confusion that progressed rapidly to prolonged NCSE. Notably, however, male cases of ANRE are quite rare. A recently reported case series attempted to differentiate male ANRE from female ANRE and suggested that male patients, similar to our case, present with early seizures, whereas behavioral changes are more common as an early manifestation in female patients [12].
Seizures are a common feature of ANRE [2], [13], [14] and occur in more than two-thirds of patients (76/100) in a recently published large series [1]. A few refractory cases of SE as a result of ANRE have been reported; however, their details were not specified. Thus, the occurrence of refractory SE seems to be an uncommon presentation of ANRE. In general, prolonged NCSE carries a poor prognosis, with a mortality rate of 56% [15]. In our case, the initial EEG pattern of extreme delta brush is highly suggestive of ANRE [16]. The rhythmic slow waves of cyclically evolving activity in frequency and spatial distribution, support the diagnosis of NCSE. Furthermore, this pattern's response to high-dose midazolam and its recurrences after reduction of the dosage further support that diagnosis.
There are 2 reported cases in the literature of NCSE in adults with anti-NMDA receptor encephalitis, including a 35 year old with refractory generalized NCSE and underlying ovarian teratoma [5] and a 19 year old with generalized NCSE and mediastinal teratoma [6]. Nonconvulsive status epilepticus in both cases has improved after tumor resection. Other cases of ANRE may respond to IV immunoglobulin, cyclophosphamide, or rituximab. Rituximab is not available in our institution, and cyclophosphamide was not tried because the patient's family was afraid of its toxic side effects. However, our patient's neurological status was refractory to the 2 courses of IVIG therapy and to a high-dose methylprednisolone. Furthermore, the lack of association with a tumor etiology in our case, unlike females with this syndrome, is similar to previously reported studies describing a low frequency of paraneoplastic etiology in the male population with this syndrome, accounting for 3% to 15% of cases [12], [17].
Nonconvulsive status epilepticus in our patient was highly resistant to AEDs and anesthetic agents, and responded only after the implantation of a vagus nerve stimulator. To our knowledge, this is the first reported case of refractory NCSE as result of ANRE that responded to VNS. Vagus nerve stimulation has been found to be effective to terminate SE in several case reports. More recently, a well-described study of eight patients with recurrent SE in Spain reported a significant improvement of seizures after VNS, with four of the eight patients remaining free of new episodes of SE after implantation, and in two additional patients, the frequency decreased by > 75% [18].
It is not clear why VNS was successful in acutely terminating SE in our patient, when the other agents that are typically used to terminate SE have failed. The precise mechanism of action of VNS as a neuromodulatory treatment for epilepsy is still unknown, but its efficacy seems mainly based on an incremental effect [19]. On the other hand, early animal experiments described an abortive effect of VNS in acute seizure models [20]. As a treatment modality for both chronic and acute epilepsy, several recent clinical and functional neuroimaging studies have demonstrated that VNS induces an increase of cerebral blood flow (CBF) in several subcortical regions, mainly the thalamus, the hypothalamus, the insula, and the cerebellum [21], [22], [23]. It is likely that the alteration of transsynaptic neurotransmission related to the bilateral thalamic CBF modification results in the inhibition of the corticothalamic relays, through a synaptic depolarization shift. Recently, it has been suggested that a possible role of the vagus nerve is controlling and modulating inflammatory responses. Patients subjected to 6 months of VNS therapy for refractory epilepsy showed significant lower levels in IL-8 induction by lipopolysaccharide-stimulated peripheral blood mononuclear cells [24]. This altered cytokine response after long-term VNS could be implied in the efficacy of this therapy to reduce the frequency of seizures and SE, based on the hypothesis that brain inflammation plays a role in the mechanisms of hyperexcitability occurring during repetitive or prolonged seizures [25].
Finally, the natural course of the patient's illness cannot be excluded as the cause of remission. However, refractory SE is rarely reported to improve spontaneously. It is also possible that the response to VNS might be as a result of delayed response from immunotherapy. This possibility seems very unlikely given the long lag between our patient's second dose of IVIG and his clinical and electrical improvement. In most cases of ANRE, the response to immunotherapy occurs within weeks and, very rarely, after several months of treatment, and in most cases following resection of the culprit tumors. It may also be hypothesized that in our case, VNS may have exerted its acute seizure effect through a direct or indirect effect on the NMDA receptor modulations in various brain lesions including the hippocampus and forebrain that are known to be rich in NMDA. Interestingly, an animal model of absence seizures shows increased expression of NMDA receptors in the thalamus, and drugs that block NMDA in the thalamus were shown to have antiseizure effect in that model [26]. It is likely that VNS, through its modulating effect in the thalamus, modulates these receptors; hence, this may explain its acute seizure effects.
4. Conclusions
This case illustrates the need to consider anti-NMDA receptor encephalitis as a cause of refractory status epilepticus should associated clinical features suggestive of this syndrome be present. Additionally, our paper suggests that adult male patients who present with seizures, normal MRI, and no clear etiology should be tested for NMDA receptor antibodies to avoid any delay in treatment initiation. Continuous EEG monitoring is crucial to the diagnosis of NCSE and to monitor the effect of various treatment modalities on ictal EEG recordings. We propose that vagus nerve stimulator implantation should be considered in cases of NCSE, and possibly, for convulsive SE that is resistant to standard pharmacotherapy. Future studies are needed to determine the efficacy of VNS in this setting and to optimize the timing of device placement and stimulation parameters for similar cases.
Abbreviations
- AEDs
antiepileptic drugs
- ANRE
anti-NMDA receptor encephalitis
- CEEG
continuous EEG recording
- CBF
cerebral blood flow
- CSF
cerebrospinal fluid analysis
- NCSE
nonconvulsive status epilepticus
- VNS
vagus nerve stimulation
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dalmau J., Gleichman A.J., Hughes E.G. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J., Tuzun E., Wu H. Paraneoplastic anti-N-methyl-d-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Florance N.R., Davis R.L., Lam C. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis in children and adolescents. Ann Neurol. 2009;66:11–18. doi: 10.1002/ana.21756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Irani S.R., Bera K., Waters P. N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667. doi: 10.1093/brain/awq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson N., Henry C., Fessler A.J. Anti-NMDA receptor encephalitis causing prolonged nonconvulsive status epilepticus. Neurology. 2010;75:1480–1482. doi: 10.1212/WNL.0b013e3181f8831a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirpatrick M.P., Clarke C.D., Sonmezturk H.H. Rhythmic delta activity represents a form of nonconvulsive status epilepticus in anti-NMDA receptor antibody encephalitis. Epilepsy Behav. 2011;20:392–394. doi: 10.1016/j.yebeh.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Titulaer M.J., McCracken L., Gabilondo I. Treatment and prognostic factors for long-term outcome in patients with anti-N-Methyl-d-Aspartate (NMDA) receptor encephalitis: a cohort study. Lancet Neurol. 2013;12(2):157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Menachem E., French J.A. VNS therapy versus the latest antiepileptic drug. Epileptic Disord. 2005;7(Suppl. 1):S22–S26. [PubMed] [Google Scholar]
- 9.Winston K.R., Levisohn P., Miller B.R., Freeman J. Vagal nerve stimulation for status epilepticus. Pediatr Neurosurg. 2001;34:190–192. doi: 10.1159/000056018. [DOI] [PubMed] [Google Scholar]
- 10.Patwardhan R.V., Dellabadia J., Jr., Rashidi M., Grier L., Nanda A. Control of refractory status epilepticus precipitated by anticonvulsant withdrawal using left vagal nerve stimulation: a case report. Surg Neurol. 2005;64:170–173. doi: 10.1016/j.surneu.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 11.Carreño M., García-Álvarez D., Maestro I. Malignant autosomal dominant frontal lobe epilepsy with repeated episodes of status epilepticus: successful treatment with vagal nerve stimulation. Epileptic Disord. 2010;12:155–158. doi: 10.1684/epd.2010.0307. [DOI] [PubMed] [Google Scholar]
- 12.Viaccoz A, Desestret V, Ducray F. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology. 2014;82:556–563. doi: 10.1212/WNL.0000000000000126. [DOI] [PubMed] [Google Scholar]
- 13.Sansing L.H., Tüzün E., Ko M.W., Baccon J., Lynch D.R., Dalmau J. A patient with encephalitis associated with NMDA receptor antibodies. Nat Clin Pract Neurol. 2007;3:291–296. doi: 10.1038/ncpneuro0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitaliani R., Mason W., Ances B., Zwerdling T., Jiang Z., Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604. doi: 10.1002/ana.20614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drislane F.W., Blum A.S., Lopez M.R. Duration of refractory status epilepticus and outcome: loss of prognostic utility after several hours. Epilepsia. 2009;50:1566–1571. doi: 10.1111/j.1528-1167.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmitt S.E., Pargeon K., Frechette E.S., Hirsch L.J., Dalmau J., Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalmau J., Lancaster E., Martinez-Hernandez M. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–71. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sierra-Marcosa A., Maestrob I., Rodrı'guez-Osorioc X. Successful outcome of episodes of status epilepticus after vagus nerve stimulation: a multicenter study. Eur J Neurol. 2012;19:1219–1223. doi: 10.1111/j.1468-1331.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- 19.DeGiorgio F.M., Schachter S.C., Handforth A. Prospective long-term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia. 2000;41(9):1195–1200. doi: 10.1111/j.1528-1157.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 20.Zabara J. Inhibition of experimental seizures in canines by repetitive vagal stimulation. Epilepsia. 1992;33:1005–1012. doi: 10.1111/j.1528-1157.1992.tb01751.x. [DOI] [PubMed] [Google Scholar]
- 21.Vonck K., Boon P., Van Laere Acute single photon emission computed tomographic study of vagus nerve stimulation in refractory epilepsy. Epilepsia. 2000;41:601–609. doi: 10.1111/j.1528-1157.2000.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 22.Henry T.R., Bakay R.A., Pennell P.B., Epstein C.M., Votaw J.R. Brain blood-flow alterations induced by therapeutic vagus nerve stimulation in partial epilepsy. II. Prolonged effects at high and low levels of stimulation. Epilepsia. 2004;45:1064–1070. doi: 10.1111/j.0013-9580.2004.03104.x. [DOI] [PubMed] [Google Scholar]
- 23.Sucholeiki R., Alsaadi T., Morris G.L., Biswal B., Ulmer J., Mueller W. fMRI in patients with vagus nerve stimulation. Seizure. 2002;11(3):157–162. doi: 10.1053/seiz.2001.0601. [DOI] [PubMed] [Google Scholar]
- 24.De Herdt V., Bogaert S., Bracke K.R. Effects of vagus nerve stimulation on pro- and anti-inflammatory cytokine induction in patients with refractory epilepsy. J Neuroimmunol. 2009;214:104–108. doi: 10.1016/j.jneuroim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Vezzani A., Balosso S., Aronica E., Ravizza T. Basic mechanisms of status epilepticus due to infection and inflammation. Epilepsia. 2009;50(Suppl. 12):56–57. doi: 10.1111/j.1528-1167.2009.02370.x. [DOI] [PubMed] [Google Scholar]
- 26.Koerner C., Danober L., Boehrer A., Marescaux C., Vergnes M. Thalamic NMDA transmission in a genetic model of absence epilepsy in rats. Epilepsy Res. 1996;25:11–19. doi: 10.1016/0920-1211(96)00015-0. [DOI] [PubMed] [Google Scholar]