Abstract
Ketogenic diet has been shown to be efficacious in some epileptic encephalopathies but rarely reported as being useful in children with Ohtahara syndrome. This could possibly be attributed to the rarity of the disease and associated short survival period. We report on a 5-year-old child with Ohtahara syndrome, whose seizures failed to improve with all known medications, continued to show persistent suppression-burst pattern on the electroencephalography (EEG) and had substantial reduction in seizure frequency for one year post-initiation of ketogenic diet. He has not had a single visit to the emergency room because of seizures in the last one year, and more importantly, there has been a clear improvement noted in his level of interaction and temperament. Patients with Ohtahara syndrome invariably have medically intractable seizures and catastrophic neurodevelopmental outcome. Ketogenic diet is a treatment modality that might be worth considering even in this group of patients.
Abbreviations: EEG, Electroencephalography/electroencephalogram; G tube, Gastric tube; ER, Emergency room; Q-EEG, Quantitative electroencephalogram
Keywords: Ohtahara syndrome, Ketogenic diet, Persistence of suppression-burst, Epileptic encephalopathies
1. Introduction
Ohtahara syndrome is a devastating infantile epileptic encephalopathy, which has an onset within the first few weeks of life, is associated with predominantly tonic seizures, and shows suppression-burst background on EEG [1]. It is one of the epileptic encephalopathies that are characterized by frequent and medically intractable seizures, along with a significant burden of interictal epileptiform activity, which may contribute to a progressive and often catastrophic neurodevelopmental outcome [2]. These disorders are classified based on their electroclinical characteristic (i.e., age at onset, seizure type and EEG pattern). Seizures of patients with epileptic encephalopathies are often resistant to antiepileptic drug therapies, but may respond to a ketogenic diet. The ketogenic diet is a high fat, low carbohydrate, and restricted protein diet that has been shown to be particularly useful in some epileptic encephalopathies such as West syndrome, infantile spasms [3], Dravet syndrome [4], [5], and Lennox–Gastaut syndrome [6]. However, its efficacy in Ohtahara syndrome has rarely been reported, and to the best of our knowledge, there is only one isolated case report [7]. We report a case with Ohtahara syndrome that showed remarkable and sustained response for close to one year post-initiation of ketogenic diet.
2. Case
Our patient is a five-year-old child, full term product of a normal spontaneous vaginal delivery. He was hypotonic at birth and had no seizure risk factors. Seizure onset was at 2 weeks of age and was initially attributed to reflux. He then began to have overt seizures, characterized by generalized tonic stiffening, which occurred multiple times per day. His seizure semiology was predominantly “generalized tonic” in nature, occurring approximately 20–30 times daily, sometimes up to 50. An initial EEG showed suppression-burst background and several tonic seizures characterized by an electrodecremental response. A full metabolic work-up, genetic studies (ARX and CDKL5), and magnetic resonance imaging of the brain were all normal, and he was presumptively diagnosed with Ohtahara syndrome based on the electroclinical characteristics. Seven subsequent EEGs were performed over a period of four years, and all showed predominantly suppression-burst background with several tonic seizures.
His further development was severely impaired and complicated by numerous medical issues such as severe sleep apnea with frequent oxygen desaturations and recurrent aspiration pneumonia requiring tracheostomy. Goals of care were addressed at each visit, and all medical decisions were made by his mother. At baseline, he focused but did not follow, was able to cry and smile to communicate, did not roll or hold objects or sit, and was in a diaper and completely gastric (G) tube fed. He required frequent hospitalizations due to recurrent infections and worsening seizures, which were refractory to every known antiepileptic drug regimen including a course of steroids and trial with pyridoxine. A recent EEG in early 2014 showed a suppression-burst background, which was more prominent in sleep and characterized by the absence of sleep structures (Fig. 1, Fig. 2). A small portion (approximately 10%) of his waking background comprised of spike and slow wave discharges. In addition, in the epilepsy monitoring unit, we captured 19 seizures in a 12-hour study, all characterized by generalized tonic stiffening and an electrodecremental response on EEG.
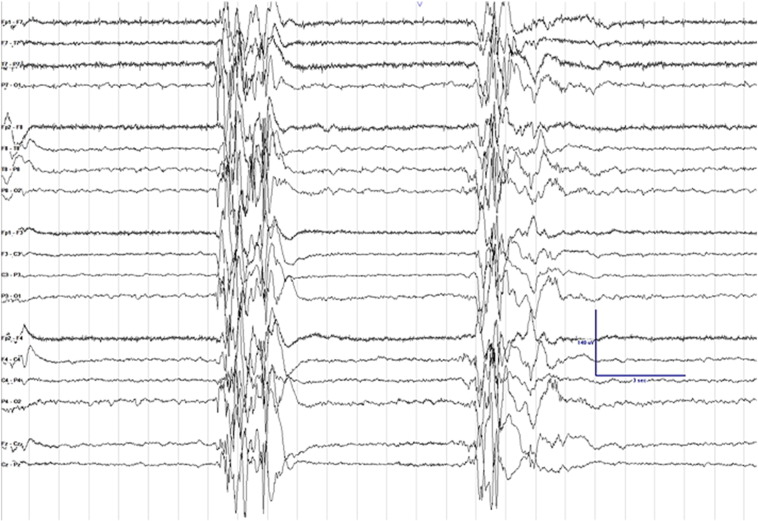
Fig. 1.
Electroencephalogram (EEG) clip showing suppression-burst background. Sensitivity is set at 7 μV, low frequency filter at 1 Hz, high frequency filter at 70 Hz, and notch filter is turned on.
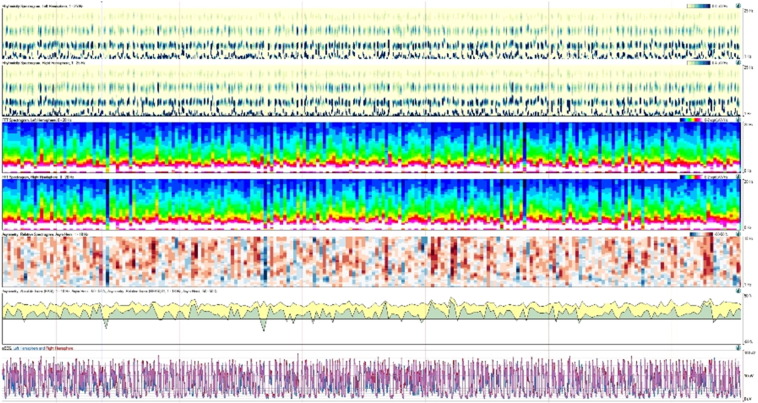
Fig. 2.
30-minute clip of quantitative electroencephalogram (Q-EEG) showing suppression-burst pattern, best appreciated on rhythmicity spectrogram at the top and amplitude integrated EEG on the bottom portion of the tracing.
Given his lack of response to medication trials, ketogenic diet was initiated in February 2014 with a goal ratio of 4:1 (ratio of grams of fat to grams of protein plus carbohydrate). He tolerated the diet well, ketosis was achieved on day four, and he was discharged home. His mother reported a remarkable improvement in seizure frequency on subsequent follow-up via office visits or phone calls. One month post-diet initiation, he was having 0–3 seizures per day, which was a significant improvement from 20–30 seizures per day. This improvement was sustained over the next nine months. He has not had a single visit to the emergency room (ER) in the last one year, which is a significant improvement from multiple seizure-related visits to the ER the previous year. This improvement was confirmed via a repeat admission to our epilepsy-monitoring unit in February 2015. His EEG background during this admission was unchanged when compared to his prior study a year ago, but we captured only 2 brief tonic seizures, lasting for approximately 10 s each, in a 24-hour period of monitoring.
3. Discussion
Ohtahara syndrome is one form of early infantile epileptic encephalopathy with medically intractable seizures and progressive neurodevelopmental deterioration. First described by Ohtahara et al. in 1976, it is an electroclinical syndrome characterized by tonic spasms and a suppression-burst pattern on EEG [1]. Electroencephalography of most patients with Ohtahara syndrome transitions into hypsarrhythmia at approximately 3 to 6 months of age, and later into diffuse slow spike waves [1]. Most survivors of Ohtahara syndrome show an EEG with focal or multifocal spikes, and suppression-burst pattern rarely persists beyond an age of one year [8]. To our knowledge, there is an isolated case report describing the persistence of suppression-burst pattern in a five-year-old girl with Ohtahara syndrome [9] and one reported case of an infant with Ohtahara syndrome who responded to ketogenic diet, but the degree and duration of response is not known [7].
We describe a patient with Ohtahara syndrome who has survived to the age of five years, and who continues to show suppression-burst pattern on EEG, with sustained improvement in seizure frequency for one year post-initiation of ketogenic diet. Increased prominence of suppression-burst pattern with sleep and a small portion of his waking background comprising of spike and slow wave discharges might suggest transition into West syndrome or Lennox–Gastaut syndrome. However, we did not find any sleep structures or hypsarrhythmia during the waking state, a large portion of his EEG continues to show a suppression-burst pattern in awake or asleep states, and he almost exclusively has tonic seizures, which is commonly reported in patients with Ohtahara syndrome. Patients with this syndrome invariably have intractable seizures and are developmentally devastated. After initiating ketogenic diet in our patient, not only did his seizure frequency improve significantly, but there was also a clear improvement noted in his level of interaction and temperament. This could arguably have been a spontaneous remission in the course of his disease, and the only way to answer that question would be to stop the diet (to which he has responded so well) and observe any worsening of his seizure frequency. This step was considered unethical, so we did not attempt it.
Given the low incidence rate of Ohtahara syndrome, the strength of evidence might never be sufficient enough to recommend any treatment modality. In addition, determining what constitutes “reasonable quality of life” can be an endless ethical debate in this subset of patients, but any intervention that can control seizures and make them more interactive is probably worth trying.
Acknowledgments
Author contributions
AS analyzed all patient data and was a major contributor in writing the manuscript. IN was involved with checking the accuracy of the ketogenic diet regimen. CC helped gather patient data over the years and was responsible for obtaining consent from patient's family. RHM is the primary caretaker, and has reviewed and revised the article ensuring accuracy of intellectual content. All authors approved the final version to be published.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
A written consent for publication was obtained from the child's parents.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ohtahara S., Yamatogi Y. Epileptic encephalopathies in early infancy with suppression-burst. J Clin Neurophysiol. 2003;20:398–407. doi: 10.1097/00004691-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Nabbout R., Dulac O. Epileptic encephalopathies: a brief overview. J Clin Neurophysiol. 2003;20:393–397. doi: 10.1097/00004691-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Hong A.M., Turner Z., Hamdy R.F., Kossoff E.H. Infantile spasms treated with the ketogenic diet: prospective single center experience in 104 consecutive infants. Epilepsia. 2010;51:1403–1407. doi: 10.1111/j.1528-1167.2010.02586.x. [DOI] [PubMed] [Google Scholar]
- 4.Caraballo R.H., Cers'osimo R.O., Sakr D. Ketogenic diet in patients with Dravet syndrome. Epilepsia. 2005;46:1539–1544. doi: 10.1111/j.1528-1167.2005.05705.x. [DOI] [PubMed] [Google Scholar]
- 5.Kang H.C., Kim Y.J., Kim D.W., Kim HD. Efficacy and safety of the ketogenic diet for intractable childhood epilepsy: Korean multicentric experience. Epilepsia. 2005;46:272–279. doi: 10.1111/j.0013-9580.2005.48504.x. [DOI] [PubMed] [Google Scholar]
- 6.Lemmon M.E., Terao N.N., Ng Y.T. Efficacy of the ketogenic diet in Lennox–Gastaut syndrome: a retrospective review of one institution's experience and summary of the literature. Dev Med Child Neurol. 2012;54:464–468. doi: 10.1111/j.1469-8749.2012.04233.x. [DOI] [PubMed] [Google Scholar]
- 7.Ishii M., Shimono M., Senju A., Kusuhara K, Shiota N. The ketogenic diet as an effective treatment for Ohtahara syndrome. No To Hattatsu. 2011;43:47–50. [PubMed] [Google Scholar]
- 8.Ohtahara S., Ohtsuka Y., Kobayashi K. Lennox–Gastaut syndrome: a new vista. Psychiatry Clin Neurosci. 1995;49:S179–S183. doi: 10.1111/j.1440-1819.1995.tb02168.x. (Suppl.) [DOI] [PubMed] [Google Scholar]
- 9.Saneto R.P., de Menezes M.S. Persistence of suppression-bursts in a patient with Ohtahara syndrome. J Child Neurol. 2007;22:631–634. doi: 10.1177/0883073807303220. [DOI] [PubMed] [Google Scholar]