Abstract
Background
A simple emergency risk prediction tool should be developed for clinicians to quickly identify the prognosis of patients with acute aortic dissection.
Methods
We enrolled 280 patients with acute aortic dissection admitted to emergency department between May 2010 and February 2013. Multivariate logistic regression analysis was performed to identify independent predictors of in-hospital death.
Results
The in-hospital mortality of our patients with acute aortic dissection was 32.5%, in-hospital deaths with surgery less than the survived (34.1% VS 54.5%). Multivariate analysis identified that age (≥65 years old), Type A, blood pressure (mean systolic blood pressure ≤ 90 mmHg), neutrophil percentage (≥ 80%) and serum D-dimer (≥ 5.0 mg/L) were significant predictors of death. With the simple emergency risk prediction tool, scores of all in-hospital deaths were ≥ 3, whereas almost all of the survivors (97.9%) had scores < 15. A score of 10 offered the best threshold value, with the highest sensitivity (81.3%) and specificity (86.8%).
Conclusions
The in-hospital mortality rate of patients with acute aortic dissection is high and can be predicted. Early surgery would be beneficial for in-hospital survive. This tool should be available for clinicians in the emergency department to quickly identify the prognosis of patients with acute aortic dissection.
Keywords: Acute aortic dissection, Prediction, Risk factors
Introduction
Acute aortic dissection (AAD) is a life-threatening condition that manifests in various ways. The misdiagnosis rate is high, and the main cause of death is rupture of the dissection. Before 2004, the incidence of aortic dissection was 5-30 per million people, and approximately 3% suffered sudden death. The one-week-mortality is as high as 60%-70%. In recent years, the AAD-related morbidity has trended upward, which is detrimental to the health of patients (1–3).
Although many studies have described prediction tools for AAD, they only focused on mortality and relative risk factors without using scores (4–9). In this study, we examined patients with AAD admitted to emergency department over the past 3 years and tried to build a simple and sensitive emergency risk prediction tool with certain allocated scores, which can rapidly and noninvasively identify the death of patients with all types of AAD.
Materials and Methods
Study Population
We enrolled all patients with AAD admitted to Emergency Department of the second Xiangya Hospital of Central South University between May1, 2010 and February 26, 2013. AAD was defined according to the 2010 AHA Guidelines for the Diagnosis and Management of Patients with Thoracic AAD (3). The diagnosis was made on computed tomography arteriography.
Of the 280 patients admitted to emergency department with AAD, 91 (32.5%) patients died while in the hospital and 189 (67.5%) survived. To minimize bias, we excluded patients who were re-admitted, patients with incomplete data and traumatic dissection. Our study mainly focused on the in-hospital prognosis of patients with AAD, without follow-up data (Fig. 1).
Fig. 1.
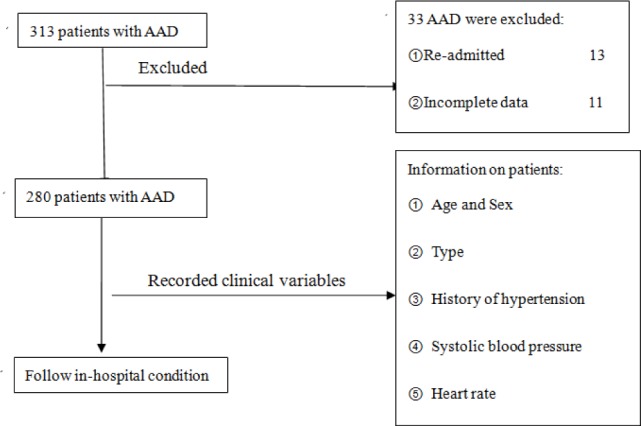
Flowchart of enrolling patients
Data Collection
All data used in the model were come from emergency department, noted by researchers in separate table, which ensured the reliability and validity of the model. Clinical variables were recorded on a standard form that included information on patient age, sex, history of hypertension, mean systolic blood pressure (the mean value of every 1 minute systolic blood pressure in 5 minute), heart rate, laboratory examination (routine blood, serum D-dimer, liver and kidney function, blood glucose), electro-cardiogram, and the type of AAD. All data come from the first admitted to emergency department. And, follow the in-hospital treatment (Type A was treated by ascending aorta replacement in cardiothoracic surgery; Type B was treated by en-dovascular repairing in vascular surgery).
Statistical Analysis
We compared patients who died in-hospital to those who survived. Missing data were not defaulted to negative, and denominators reflect only cases reported. Summary statistics between the 2 groups are presented as frequencies and percentages for categorical variables and mean ± standard deviation for continuous variables. Univariate associations with death among clinical variables were obtained using a χ2 test, 2-sided Fisher exact test, and Student t test.
Predictive Modeling
A logistic regression modeling for in-hospital death, with the likelihood ratio test used for model selection and ward statistics to assess the significance of coefficients, was performed. Initial mod-eling used variables marginally suggestive of an unadjusted association with in-hospital death (P<0.20). Variables were reviewed for clinical sig-nificance before testing. A Hosmer-Lemeshow test and ward statistic were used for the final model selection. SPSS 17.0 was used for all analyses and P<0.05 was considered statistically sig-nificant.
Development of a Simple Emergency Risk Prediction Tool
The variables found to be significantly associated with in-hospital death in the regression model were assigned a point equal to their coefficients in the fitted model (P<0.05). Each patient would have a sum of this numerical point (Score), and we evaluated the sensitivity and specificity of every score to predict the in-hospital death according to known patient prognosis. The score in an individual patient could then be used to predict his or her in-hospital death. A risk prediction tool would have a threshold value with appropriate sensitivity and specificity in predicting in-hospital death for AAD patients.
Results
Overall Clinical Characteristics of our hospital Patients
Of the final 280 patients with AAD admitted to our hospital, a majority of patients were male (74.6%) and 123 patients had type A AAD (50.4%). Nearly 82.9% of patients had a history of hypertension and 23.6% had tachycardia. The mean age was 58.4 ± 13.1 years. Patients who were ≥65 years were more likely to die in the hospital. Half of the patients underwent surgery for repair of their AAD. In-hospital death occurred in surgically treated patients far less than those treated medically (34.1 VS 65.9%, P<0.01), and, 54.5% of surviving patients underwent surgical treatment. A small number of AAD patients had associated liver and renal dysfunction, abnormal blood glucose levels and abnormal electrocardiograms (Table 1).
Table 1:
Clinical characteristics of all patients with AD
| Variable | Overall (n=280) |
Died (n=91) |
Survived (n=189) |
P |
|---|---|---|---|---|
| Age, mean ± SD, y | 58.4±13.1 | 59.3±11.6 | 58.1±15.3 | >0.05 |
| Age ≥65 y (%) | 83(29.6) | 38(41.8) | 49(26.0) | <0.05 |
| Male (%) | 209(74.6) | 76(83.5) | 136(72.0) | >0.05 |
| Type A (%) | 141(50.4) | 63(69.2) | 85(45.0) | <0.01 |
| Hypertension (%) | 232(82.9) | 74(81.3) | 157(83.1) | >0.05 |
| Mean Systolic Blood Pressure ≤ 90 mmHg (%) | 22(7.9) | 21(23.1) | 6(3.2) | <0.01 |
| Mean Systolic Blood Pressure > 140 mmHg (%) | 123(43.9) | 30(33.0) | 89(47.1) | >0.05 |
| Abnormal AST or ALT (%)* | 48(17.1) | 13(14.3) | 34(18.0) | >0.05 |
| Abnormal BUN or Cr (%) * | 80(32.8) | 16(29.1) | 64(33.9) | >0.05 |
| Abnormal Blood Glucose (%) * | 47(16.8) | 20(19.8) | 30(15.9) | >0.05 |
| White blood cells, ≥12×109/L (%) | 88(31.4) | 40(43.9) | 53(28.0) | <0.05 |
| Neutrophil percentage(%) | 81.3±24.9 | 88.7±22.4 | 77.4±26.5 | <0.01 |
| Neutrophil percentage ≥ 80% (%) | 148(52.9) | 70 (76.9) | 87(46.0) | <0.01 |
| ECG: left ventricular hypertrophy (%) | 72(25.7) | 17(18.7) | 53(28.0) | >0.05 |
| In-hospital Heart Rate ≥ 100 bpm (%) | 66(23.6) | 23(25.3) | 43(22.8) | <0.05 |
| Surgery(%) | 140(50.0) | 31(34.1) | 103(54.5) | <0.01 |
* Normal figure: AST(0-40 mmol/L), ALT(0-40 IU/L), BUN(3.2-7.1 mmol/L), Cr(53-106 mmol/L), Blood Glucose(3.9-6.1 mmol/L). The abnormal figure is beyond the normal figure above.
Univariate Predictors of In-Hospital Death for All Patients with AAD
Compared to patients who survived, patients who died in the hospital were much more likely to have a low mean systolic blood pressure (23.1%), high white blood cell count (43.9%) and neutrophil percentage (76.9%). The mean neutrophil percentage was significantly higher (88.7% ± 22.4% versus 77.4% ± 26.5%) in patients who died in the hospital. Analysis of the types of AAD showed that type A AAD patients were more likely to die in the hospital versus those with type B AAD. On the other hand, there was no difference in sex, mean age, history of hypertension, high in-hospital blood pressure, liver and renal function, blood glucose levels, left ventricular hypertrophy and heart rate for the patients who died versus those who survived (Table 1).
Serum D-dimer was an Independent Predictor of In-Hospital Death for All Patients with AAD
All but 2 patients with AAD had a serum D-dimer > 0.1 mg/L and 213 patients had a serum D-dimer > 2.0 mg/L (76.1%). Serum D-dimer for patients who died in the hospital (12.3 ± 2.5 mg/L) was significantly higher than patients who survived (2.4 ± 1.1 mg/L). Serum D-dimer for all patients who died in the hospital was > 2.2 mg/L; 76 of these patients had a D-dimer ≥ 5.0 (83.5%). When the serum D-dimer was ≥ 10.0 mg/L, the sensitivity was 61.5% and specificity was 84.1% in predicting in-hospital death for all AAD patients (Table 2).
Table 2:
Serum D-dimer in predicting in-hospital death for all patients with AD
| Serum D-dimer Value(mg/L) | Sensitivity (%) |
Specificity (%) |
P |
|---|---|---|---|
| ≥2.0 | 90.1 | 18.0 | >0.05 |
| ≥5.0 | 83.5 | 58.2 | <0.01 |
| ≥10.0 | 61.5 | 84.1 | <0.01 |
| ≥20.0 | 22.0 | 95.8 | <0.01 |
Predictive Model for In-Hospital Death
Through significant testing of all variables, independent predictors of in-hospital death were discovered and are shown in Table 3. The deviance probability value was 0.00, Logistic model was verified when the area under ROC curve was equal to 0.79, and Hosmer-Lemeshow statistic of the model involved all seven variables suggesting good model discrimination from a perfect fit (χ2=29.65, df =6, P<0.01).
Table 3:
Risk factors in the prediction model
| Overall Model Variables | Parameter Coefficient |
Sb | Wald | P | Death OR (95% CI) |
|---|---|---|---|---|---|
| Age (≥65 y) | 0.81 | 0.39 | 4.24 | 0.04 | 2.24(1.04-4.85) |
| Type A | 1.03 | 0.51 | 4.13 | 0.04 | 2.79(1.23-6.14) |
| Mean systolic blood pressure (≤ 90 mmHg) | 1.47 | 0.62 | 5.59 | 0.02 | 4.35(1.99-8.80) |
| White blood cell(≥12×109/L) | 1.01 | 0.56 | 3.22 | 0.07 | 2.75(1.04-7.75) |
| Neutrophil percentage(≥ 80%) | 0.88 | 0.46 | 3.77 | 0.02 | 2.32(0.99-5.92) |
| Serum D-dimer (mg/L) | 0.92 | 0.39 | 5.50 | 0.00 | 2.21(1.16-5.40) |
| Surgery | -1.22 | 0.59 | 4.26 | 0.01 | 3.25(1.28-7.15) |
| Constant | -0.98 | 0.41 | 7.62 | 0.02 |
Sb: Standard error of regressions. Hosmer-Lemeshow statistic of the regression model: (χ2=29.65, df =6, P<0.01).
Variables points and the Simple Emergency Risk Prediction Tool
The risk variables and corresponding assigned points are listed in Table 4. Every corresponding assigned point was according to the OR value (Round to the nearest whole number) of each variable. Mean systolic blood pressure was given the highest points 4 and age, Neutrophil percentage was given the lowest points 2. Serum D-dimer was divided into 3 grades and each grade allocated 2 points. The total scores for each patient with AAD are included in the simple emergency risk prediction tool (Table 5). The scores for all in-hospital deaths were > 3, and almost all of the survivors (97.9%) had scores < 15. A score of 10 was the best threshold value for the prediction tool, with the highest sensitivity (81.3%) and specificity (86.8%).
Table 4:
Points assigned in the predictive model
| Risk Factors | Allocated Points | Instruction |
|---|---|---|
| Age | 2 | ≥65 years |
| Type A | 3 | Type A aortic dissection |
| Mean systolic blood Pressure | 4 | systolic blood pressure≤90 mmHg |
| Neutrophil percentage | 2 | Neutrophil percentage≥80% |
| Serum D-dimer | 2 | *Add 2 points per upgrade |
*Certain grade of D-dimer: 5.0~10.0 mg/L, 10.0~20.0 mg/L,>20.0 mg/L
Table 5:
The simple emergency risk prediction tool
| Scores | Sensitivity (%) |
Specificity (%) |
P |
|---|---|---|---|
| ≥3 | 100.0 | 48.1 | <0.01 |
| ≥7 | 92.3 | 65.1 | <0.01 |
| ≥8 | 91.2 | 70.9 | <0.01 |
| ≥10 | 81.3 | 86.8 | <0.01 |
| ≥12 | 72.5 | 93.1 | <0.01 |
| ≥15 | 52.7 | 97.9 | <0.01 |
Discussion
Model Predictions for Patients with AAD
In our study, the in-hospital death with AAD occurred 32.5% in patients, which is similar than that calculated in the recent meta-analysis (38%) (10). Von Kodolitsch (11) and Tang (12) separately designed simple early warning tools, which can somewhat improve the early diagnosis of AAD. However, most studies built prediction tools only for type A or B AAD. Furthermore, the tools did not utilize scores to identify the prognosis of patients with AAD (4–9). Our simple emergency risk prediction tool resolves these shortcomings and should help instruct clinicians when counseling patients and their families about a realistic decision for treatment. Furthermore, the prediction tool should be useful in evaluating the effects of new diagnostic and treatment methods for patients with AAD.
Clinical Variables Associated With High In-Hospital Mortality Rates
Our study identified several clinical variables that were predictors of death in patients with AAD (Table 1 and 2). Although the mean age of dead patients with AAD (59.3 years) was similar to the mean age of all patients (58.1 years), when we stratified patients into age groups, those patients ≥65 years had a higher risk of in-hospital mortality on the chi-square test and the logistic regression analysis (P<0.05). Similarly, according to the worldwide meta-analysis, death more often occurs in old and the highest mortality occurs at the age of more than 80 years old (13). In our country, the patients above 80 years seldom come to see doctor. Our study showed that type A AAD was associated with worse outcomes (P<0.01), which is consistent with previous studies (5–8). Type A AAD usually be a manifestation of more sudden, severe, and extensive tearing and thus may have independently predicted greater risk of in-hospital death in our study. The heart rate is usually used by physicians to estimate the severity of illness (3). However, in our study the heart rate did not correlate with the prognosis of AAD. The role of heart rate control in patients with AAD requires further study. Some other variables, such as hepatic function, renal function and blood glucose levels, were not significantly associated with in-hospital mortality in our study.
Previous publications showed that many AAD patients had hypertension and concluded that blood-pressure-control should be a critical step in the management of these patients (3). However, our study finds that there is no correlation between the risk of death and hypertension. Nevertheless, mean systolic blood pressure (≤ 90 mmHg) played an important role in our study, which is similar to the study by Santini (5). In our clinical study, most of the AAD patients who suffered from shock often died in three days even after surgery. There are many probable reasons for the development of hypotension in AAD patients. It is often believed that most AAD patients develop hypotension when the hematoma breaks in to hollow spaces, such as the thoracic cavity and abdominal cavity. In some special types of AAD, dissection can occur at the beginning of peripheral arteries and produce limited hypotension in one limb. Some other types can involve the aortic valve or the coronary artery, which leads to a low cardiac output. In elderly patients, persistent and severe pain inhibits the vascular center and causes changes in resistance vessels and capacitance vessels during diastole. In this situation, the cardiac output and the blood pressure decrease. When patients suffer from shock, inadequate organ perfusion and malignant arrhythmias (when they influence the heart) can definitely confer a high mortality rate.
Recent studies have shown that an inflammatory mechanism plays an important role in the degeneration and reduction of smooth muscle cells in the media of aortic vessels, which is closely related to the prognosis of patients with AAD (14–16). The neutrophil percentage is a key factor in the chain of inflammatory reactions. The neutrophil percentage could reflect the severity of the inflammatory reaction. In this study, we found that the neutrophil percentage was not only an inflammatory marker but also predicted prognosis for patients with AAD.
It has been illustrated that serum D-dimer was vital to the rapid diagnosis of AAD. Some researchers treated D-dimer as an independent diagnostic factor that greatly improved the diagnosis of AAD (12, 17–19). The thrombosis of the artery wall in a dissected aorta would lead to the release of tissue factors that activate the fibrinolysis system. The serum D-dimer, as a degradation product of cross-linked fibrin, increased accordingly (17). A high level ofserum D-dimer may be a manifestation of more severe and extensive tearing and may independently predict a greater risk of death in our study. But in some meta-analysis (20), the D-dimer plays an inessential role in the prognosis and how serum D-dimer predicts AAD-related mortality should be studied further. In our study, we found that combined with multiple factors, serum D-dimer has the excellent effect on the prognosis. On the other hand, the patient, who got a high score, evaluated only by D-dimer, wasn’t dead. So we do not suggest only using it as the prediction tool. Early surgery of the AAD would be beneficial for in-hospital survive in our study, we found that surgery patients survived more and surgery was an independently protective factor of AAD in the logistic model. Early surgery may not only help prevent further extensive tearing but also is the only option that will provide a reasonable chance of survival to these individuals with otherwise dismal outcome.
Limitations of our study
First, this study sample is too small to fully reflect all patients with AAD. We measured a small number of risk factors. Furthermore, our end-points are restricted to the in-hospital period. We did not conduct any follow-ups, so we could not obtain any information on out-of-hospital mortality related to AAD or chronic AAD. More prospective, randomized controlled trial is needed to analyze the predictors of mortality for both acute and chronic AAD.
Conclusions
Our study demonstrates that the in-hospital mortality rate of patients with AAD is high and can be predicted. Early surgery would be beneficial for in-hospital survive. According to these risk factors, a simple emergency risk prediction tool was developed. This simple emergency risk prediction tool is available for clinicians in the emergency department to quickly identify the prognosis of patients with AAD. Based on this tool, a bedside mortality risk evaluation system can be created in the near future for predicting the prognosis of patients with AAD.
Ethical considerations
Ethical issues (Including plagiarism, Informed Consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc) have been completely observed by the authors.
Acknowledgements
The authors declare that there is no conflict of interest.
Reference
- 1.Ramanath VS, Oh JK, Sundt TM 3rd, Eagle KA(2009). Acute Aortic Syndromes and Thoracic Aortic Aneurysm. Mayo Clin Proc, 84(5): 465–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clouse WD, Hallett JW Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ 3rd (2004). Acute AD: population based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc, 79: 176–180. [DOI] [PubMed] [Google Scholar]
- 3.Hiratzka LF, Bakris GL, Beckman JAet al. (2010). 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic AD. Circulation, 121: e266–269. [DOI] [PubMed] [Google Scholar]
- 4.Goossens D, Schepens M, Hamerlijnck R, Hart-man M, Suttorp MJ, Koomen E, Vermeulen F (1998). Predictors of hospital mortality in type A AD: a retrospective analysis of 148 consecutive surgical patients. Cardiovasc Surg, 6(1): 76–80. [DOI] [PubMed] [Google Scholar]
- 5.Santini F, Montalbano G, Casali G, Messina A, Iafrancesco M, Luciani GB, Rossi A, Mazzucco A (2007). Clinical presentation is the main predictor of in-hospital death for patients with acute type A AD admitted for surgical treatment: a 25 years experience. Int J Cardiol, 115(3): 305–311. [DOI] [PubMed] [Google Scholar]
- 6.Aalberts JJ, Boonstra PW, van den Berg MP, Waterbolk TW (2009). In-hospital mortality and three-year survival after repaired acute type A AD. Neth Heart J, 17(6): 226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apaydin AZ, Buket S, Posacioglu H, Islamoglu F, Calkavur T, Yagdi T, Ozbaran M, Yuksel M (2002). Perioperative risk factors for mortality in patients with acute type A aortic dissection. Ann Thorac Surg, 74(6): 2034–2039. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, Maroto LC, Cooper JV, Smith DE, Armstrong WF, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators (2002). Predicting death in patients with acute type a aortic dissection. Circulation, 105(2): 200–206. [DOI] [PubMed] [Google Scholar]
- 9.Kallenbach K, Oelze T, Salcher R, Hagl C, Karck M, Leyh RG, Haverich A (2004). Evolving strategies for treatment of acute aortic dissection type A. Circulation, 110(11 Suppl 1): II243–249. [DOI] [PubMed] [Google Scholar]
- 10.Jonker FH, Schlosser FJ, Indes JE, Sumpio BE, Botta DM, Moll FL, Muhs BE (2010). Management of type A aortic dissections: a meta-analysis of the literature. Ann Thorac Surg, 89(6): 2061–2066. [DOI] [PubMed] [Google Scholar]
- 11.Van Kodolitseh Y,Schwartz AG,Nienaber CA (2000). Clinical prediction of acute AD. Arch Intern Med, 160: 2977–2982. [DOI] [PubMed] [Google Scholar]
- 12.Tang M, Liu QM, Zhou SH, Chai XP (2010). Analysis on an early diagnosis grading model for acute aortic dissection. Chinese J Cardiol, 38(5): 425–428. [PubMed] [Google Scholar]
- 13.Biancari F, Vasques F, Benenati V, Juvonen T (2011). Contemporary results after surgical repair of type A aortic dissection in patients aged 80 years and older: a systematic review and meta-analysis. Eur J Cardiothorac Surg, 40(5): 1058–1063. [DOI] [PubMed] [Google Scholar]
- 14.Li JJ, Zhu CG, Yu B, Liu YX, Yu MY (2007). The role of inflammation in coronary artery calcification. Ageing Res Rev, 6(4): 263–270. [DOI] [PubMed] [Google Scholar]
- 15.del Porto F, Proietta M, Tritapepe L, Miraldi F, Koverech A, Cardelli P, Tabacco F, de Santis V, Vecchione A, Mitterhofer AP, Nofroni I, Amodeo R, Trappolini M, Aliberti G (2010). Inflammation and immune response in acute aortic dissection. Ann Med, 42(8): 622–629. [DOI] [PubMed] [Google Scholar]
- 16.Lindholt JS, Shi GP (2006). Chronic inflammation, immune response, and infection in abdominal aortic aneurysms. Eur J Vasc Endovasc Surg, 31(5): 453–463. [DOI] [PubMed] [Google Scholar]
- 17.Yu M, Nardella A, Pechet L (2000). Screening tests of disseminated intra-vascular coagulation:guidelines for rapid and specific laboratory diagnosis. Crit Care Med, 28(6): 1777–1780. [DOI] [PubMed] [Google Scholar]
- 18.Eggebrecht H, Naber CK, Bruch C, Kröger K, von Birgelen C, Schmermund A, Wichert M, Bartel T, Mann K, Erbel R (2004). Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol, 44(4): 804–809. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland A, Escano J, Coon TP (2008). D-dimer as the sole screening test for acute AD: a review of the literature. Ann Emerg Med, 52(4): 339–343. [DOI] [PubMed] [Google Scholar]
- 20.Shimony A, Filion KB, Mottillo S, Dourian T, Eisenberg MJ (2011). Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol, 107(8): 1227–1234. [DOI] [PubMed] [Google Scholar]


