Abstract
One challenge constraining the use of microalgae in the food and biofuels industry is growth and lipid accumulation. Microalgae with high growth characteristics are more likely to originate from the local environment. However, to be commercially effective, in addition to high growth microalgae must also have high lipid productivities and contain the desired fatty acids for their intended use. We isolated microalgae from intertidal locations in South East Queensland, Australia with adverse or fluctuating conditions, as these may harbor more opportunistic strains with high lipid accumulation potential. Screening was based on a standard protocol using growth rate and lipid accumulation as well as prioritizing fatty acid profiles suitable for biodiesel or nutraceuticals. Using these criteria, an initial selection of over 50 local microalgae strains from brackish and sea water was reduced to 16 strains considered suitable for further investigation. Among these 16 strains, the ones most likely to be effective for biodiesel feedstock were Nitzschia sp. CP3a, Tetraselmis sp. M8, Cymbella sp. CP2b, and Cylindrotheca closterium SI1c, reaching growth rates of up to 0.53 day−1 and lipid productivities of 5.62 μg mL−1day−1. Omega-3 fatty acids were found in some strains such as Nitzschia sp. CP2a, Nitzschia sp. CP3a and Cylindrotheca closterium SI1c. These strains have potential for further research as commercial food supplements.
Keywords: biodiesel, diatom, fatty acids, microalgae, omega-3 fatty acids
Introduction
Microalgae grow in most natural environments, typically aquatic and marine systems, but they are also found in soil, ice, rock pools or in volcanic water that can have extreme environmental fluctuations (Duong et al., 2012). Microalgae suffering from adverse or fluctuating conditions often have the ability to accumulate higher contents of biochemical products for survival, such as lipid, starch, protein or carotenoid contents (Lim et al., 2012). Microalgae can produce a variety of lipids that can be used by the food and biofuel industry (Huerlimann et al., 2010). For the production of biodiesel, fatty acids with a chain length of 14–18 carbons are preferable. Saturated fatty acids C14, C16, and C18 and unsaturated fatty acids such as C16:1, C16:2, C18:1, and C18:2 are the most important for producing good biodiesel quality (Schenk et al., 2008). This is because the other unsaturated fatty acids with 3 or 4 double bonds have reduced stability in storage (Knothe, 2006; Chisti, 2007).
The components of fatty acids, including saturated and unsaturated fatty acids produced by microalgae, differ for different species and strains (Renaud et al., 1994; Salama et al., 2013). The accumulation of fatty acid components can be controlled by environmental conditions such as temperature, nutrient availability and salinity (Renaud et al., 2002; Huerlimann et al., 2010). Alternatively, the necessary fatty acids can be developed using a chemical process. For example, unsaturated fatty acids from microalgae, especially fatty acids with 3 or 4 double-bonds, can be easily hydrogenated under partial catalysts (Jang et al., 2005), but it is preferable to identify and propagate microalgae that do not require secondary treatment to modify their fatty acid profile. Growth temperature is a major factor that dramatically influences lipid accumulation in general or lipid composition in particular. For instance, a higher growth temperature can stimulate lipid accumulation in microalgae or a lower growth temperature can lead to increased saturated fatty acid composition (Renaud et al., 2002).
The screening of microalgae is not limited to those suitable for biodiesel production, because microalgae also offer multiple bio-products that can be used in a variety of industry (Schenk et al., 2008; Huerlimann et al., 2010). For instance, unsaturated fatty acids containing more than three double bonds, including omega-3 fatty acids, are not preferable for biodiesel but they can be useful as nutritious food or feed supplement. These compounds currently have a high value and could compete with fish oil in terms of productivity and environmental sustainability (Adarme-Vega et al., 2014).
Although microalgae are known to produce considerable amounts of lipids this it is not true for all microalgal strains. The purpose of our research was to screen microalgal isolates collected from brackish and sea water and identify those with the highest growth and lipid production with desirable fatty acid composition. As lipid accumulation capacity of algae changes when subjected to adverse external factors, such as nutrient supply and environmental conditions (Huerlimann et al., 2010; Chen et al., 2011), the research focused on microalgae from sites that have known adverse or fluctuating environmental conditions (e.g., intertidal rock pools; Underwood, 1984). After isolating pure algal cultures, growth and lipid production were compared to establish which strains are most stable and productive and most likely to be effective as a feedstock source for biofuel production. In addition, strains which produce fatty acids suitable for the health food industry were identified.
Materials and methods
Microalgae samples were collected from a variety of sites in the lower reaches of the Brisbane River, Stradbroke Island, and Sunshine Coast in South East Queensland—Australia. The samples represent different environmental conditions of tidal brackish river water (50 cm depth), rock pools and beaches. Two species from the Australian National Algae Culture Collection from the Commonwealth Scientific and Industrial Research Organisation (CSIRO) were used to compare growth and lipid production with the strains that were isolated from the field (Table 1). The microalgae were intentionally gathered from different environments to provide a diverse taxonomy, stored in a cold box and transferred to the laboratory for analysis.
Table 1.
Sources and accessions of microalgae used in this study.
| Species | GPS coordinates | Species origin and collection time | Site status | Genbank accession number |
|---|---|---|---|---|
| Achnanthes sp. BR22.5_3 | 27°29′29.00S 153°00′48.00E | Brisbane River (11.40 am 22/05/2012) | Brackish water | KF 360813 |
| Bacillariophyta sp. B3 | 26°48′12.11S 153°08′50.86E | Bullcock Beach (3 pm 12/06/2011) | Tidal rock pool | KF 360815 |
| Bacillariophyta sp. SI1a | 27°26′14.23S 153°30′51.08E | Stradbroke Island (3 pm 07/07/2011) | Brackish rook pool | KF 360816 |
| Chlorella sp. BR2 | – | Brisbane River Lim et al., 2012 | Brackish water | – |
| Cylindrotheca closterium SI1c | 27°26′14.23S 153°30′51.08E | Stradbroke Island (3 pm 07/07/2011) | Brackish rock pool | KF 360818 |
| Cymbella cistuliformis CP2c | 24°59′19.96S 153°21′04.74E | Frazer Island, Champagne Pools (10 am 1/4/2012) | Tidal rock pool | KF 360819 |
| Cymbella sp. CP2b | 24°59′19.96S 153°21′04.74E | Frazer Island, Champagne Pools (10 am 1/4/2012) | Tidal rock pool | KF 360820 |
| Dunaliella tertiolecta | – | CSIRO Tasmania (CS-175/8) | – | – |
| Navicula sp. BR22.52 | 27°29′29.00S 153°00′48.00E | Brisbane River (11.40 am 22/05/2012) | Brackish water | KF 360822 |
| Navicula sp. CP6a | 24°59′19.96S 153°21′04.74E | Frazer Island, Champagne Pools (10 am 1/4/2012) | Tidal rock pool | KF 360823 |
| Navicula sp. SI2d | 27°25′33.82S 153°31′45.70E | Stradbroke Island (3 pm 07/07/2011) | Tidal rock pool | KF 360824 |
| Nitzschia sp. CP2a | 24°59′19.96S 153°21′04.74E | Frazer Island, Champagne Pools (10 am 1/4/2012) | Tidal rock pool | KF 360825 |
| Nitzschia sp. CP3a | 24°59′19.96S 153°21′04.74E | Frazer Island, Champagne Pools (10 am 1/4/2012) | Tidal rock pool | KF 360826 |
| Phaeodactylum tricornutum | – | CSIRO Tasmania (CS-29/8) | – | – |
| Tetraselmis sp. M8 | – | Rock Pool Maroochydore, Lim et al., 2012 | Tidal rock pool | JQ 423158 |
| Thalassiosira rotula SI2a | 27°25′33.82S 153°31′45.70E | Stradbroke Island (3 pm 07/07/2011) | Tidal rock pool | KF 360828 |
Isolation and preliminary screening
Microalgae were cultured in f/2 medium at 25 ± 1°C with a 12/12 h light/dark photoperiod at a light intensity of 120 μmol photons m−2s−1 from fluorescent lights (Osram L36W/840) and constant bubbling conditions (LP-100 air pump, Shen-Zhen Xingrisheng Industrial Co. Ltd) in a temperature-controlled environment room. Single cells were isolated by a micropipette on an inverted microscope and grown in 96 wells plates before transferring to 100 mL flasks for pure cultivation as described previously (Duong et al., 2012). The isolation procedure was conducted with sterilized equipment and in the laminar flow. Single cells were isolated by the micromanipulation method and the pure culture was checked regularly for the presence of contaminating algae or high abundance of bacteria during inoculation and growth experiments. It should be mentioned that the cultures in this study are not axenic. While it can be excluded that they contain other microalgae or protists, they still contain associated bacteria. This was considered desirable as microalgae-associated bacteria often provide improved growth (Amin et al., 2012). Algal strains were then provisionally screened based on their ability for rapid growth and lipid fluorescence using Nile red staining as described by Lim et al. (2012).
Classification by DNA sequencing
Microalgal biomass was collected at the late exponential phase of cultivation for DNA extraction. DNA extraction was performed using a phenol:chloroform method. After extraction, genomic DNA within the 18S rRNA region was amplified on a PCR machine by using the following primers: Forward 5′-GCGGTAATTCCAGCTCCAATAGC-3′ and Reverse 5′-GACCATACTCCCCCCGGAACC-3′. The process followed was developed by Lim et al. (2012). PCR templates were then purified by using a Wizard SV Gel PCR Clean-Up System (Promega). For sequencing preparation, 5 μL of a 25 ng μL−1 PCR product were combined with 1 μL of a 10 μM solution of each of the above primers. The reaction was topped up to 12 μL with Millipore water in a 1.5 mL tube and sent to the Australian Genome Research Facility (AGRF) at The University of Queensland for sequencing and analysis. The DNA sequence data was compared with Genbank entries for classification.
Standard protocol for growth experiments
After obtaining pure cultures, all isolated strains were grown in f/2 medium following a cultivation protocol that used bubbling for aeration and mixing. The standard protocol can be briefly described as follows: All strains were inoculated with 5 mL from a recently grown master culture and cultured in 150 mL f/2 medium until the end of the exponential growth phase was reached (less than 10% cell density increase/day) before starting the standard growth experiment. This culture was then used as the inoculum at a ratio of 1/10 for 300 mL f/2 medium in 400 mL bottles that were connected to a bubbling system and exposed to 12/12 h light/dark photoperiod at a light intensity of 120 μmol photons m−2s−1. Cells were counted daily by using a hemocytometer. Nitrate concentrations were monitored daily until the nutrient levels reached zero (below detection). Nitrate was determined by using a colorimetric assay (API test kit) and measured on a spectrophotometer at a wavelength 545 nm. Growth rates were calculated by the following equation (Levasseur et al., 1993)
where N1 and N2 equal cell counts at time 1 (t1) and time 2 (t2), respectively. Doubling time was also calculated once the specific growth rate was known.
Microalgae were cultured for another 3 days after the nitrate concentration in the medium became undetectable to induce lipid biosynthesis.
Fatty acid methyl esters (FAME) analysis
Samples for FAME analysis were collected when lipid accumulation reached its peak, after 3 days of starvation. A total of 4 mL of microalgal culture was collected and centrifuged at 8000 × g for 5 min. Biomass was collected and dried by a vacuum pump for 30 min. Lipids in the microalgal pellet were hydrolyzed and methyl-esterified in 300 μL of a 2% H2SO4 and methanol solution for 2 h at 80°C. Prior to the reaction, 50 μg of heneicosanoic acid (Sigma, USA) was added as internal standard. After the esterification step, 300 μL of 0.9% (w/v) NaCl solution and 300 μL of hexane were added and mixed for 20 s. To separate the phase, samples were centrifuged at 16,000 × g for 3 min. A total of 1 μL of hexane layer was injected into an Agilent 6890 gas chromatograph (GC) coupled to a 5975 MSD mass spectrometer (MS). The running conditions were described by Agilent's RTL DBWax method (Brown, 1991).
Results
Growth of selected microalgal strains
Out of 50 microalgal strains that were isolated, 16 were shortlisted based on their rapid growth and high lipid fluorescence following Nile red staining (Figure 1 shows an example). Strains were then compared in standard growth and lipid accumulation assays to determine the most suitable strains as feedstock for biodiesel and/or nutraceuticals. The growth of microalgal strains in the collection is presented in two groups for easier comparison: diatoms and green microalgae. The fastest growing strain Thalassiosira rotula SI2a grew 5.3 times faster than the slowest growing strain Bacillariophyta sp. B3 (Table 2). Among the diatomaceous species, Thalassiosira rotula SI2a's cell density was 2.47 × 106 cells mL−1 and this strain exhibited the highest growth rate and lowest doubling time, achieving 0.64 day−1 and 1.09 days, respectively. The cell density of Phaeodactylum tricornutum reached 6.97 × 106 cells mL−1 and grew more after the standard assay's completion. The growth rate and doubling time of this strain was 0.49 day−1 and 1.42 days, respectively. Cylindrotheca closterium SI1c's growth reached a peak of 1.89 × 106 cells mL−1. Bacillariophyta sp. SI1a, Bacillariophyta sp. B3, and Navicula sp. SI2d grew slowly and reached peaks of 3.18 × 105 cells mL−1, 4.5 x 104 cells mL−1 and 1.18 × 105 cells mL−1, respectively.
Figure 1.
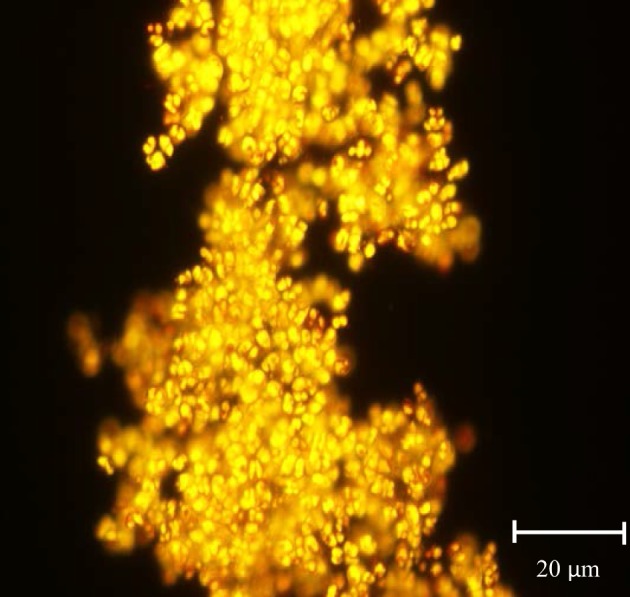
Cymbella sp. CP2b after Nile red staining under fluorescence microscopy. Yellow droplets show lipid containing triacylglycerides and orange droplets show autofluorescence from chlorophyll. Cymbella sp. CP2b displayed fast growth rates and one of the highest lipid productivities (4.51 μg mL−1 day−1).
Table 2.
Growth characteristics of the isolated strains in f/2 medium during the duration of the standard assay.
| Species | Mean growth rate (μ day−1) | Doubling time (days) | Maximum cell density (x104 cells mL−1) |
|---|---|---|---|
| Achnanthes sp. BR22.5_3 | 0.13 | 5.48 | 28.50 |
| Bacillariophyta sp. B3 | 0.12 | 5.96 | 6.17 |
| Bacillariophyta sp. SI1a | 0.32 | 2.16 | 31.83 |
| Chlorella sp. BR2 | 0.45 | 1.54 | 245.83 |
| Cylindrotheca closterium SI1c | 0.53 | 1.31 | 189.00 |
| Cymbella cistuliformis CP2c | 0.26 | 2.65 | 28.67 |
| Cymbella sp. CP2b | 0.53 | 1.31 | 103.17 |
| Dunaliella tertiolecta | 0.46 | 1.51 | 266.83 |
| Navicula sp. BR22.52 | 0.34 | 2.05 | 24.50 |
| Navicula sp. CP6a | 0.39 | 1.80 | 42.33 |
| Navicula sp. SI2d | 0.29 | 2.40 | 11.83 |
| Nitzschia sp. CP2a | 0.22 | 3.08 | 298.33 |
| Nitzschia sp. CP3a | 0.17 | 3.99 | 123.83 |
| Phaeodactylum tricornutum | 0.49 | 1.42 | 696.67 |
| Tetraselmis sp. M8 | 0.43 | 1.60 | 262.17 |
| Thalassiosira rotula SI2a | 0.64 | 1.09 | 247.00 |
Shown are mean values from three separately-grown cultures each.
Dunaliella tertiolecta, Chlorella sp. BR2 and Tetraselmis sp. M8 were the fastest growing green algal strains. Cell density of three strains increased gradually and peaked after 7 to 9 days. The cell density peaks for Chlorella sp. BR2, Tetraselmis sp. M8, and Dunaliella tertiolecta were 2.46 × 106 cells mL−1, 2.62 × 106 cells mL−1, and 2.67 × 106 cells mL−1, respectively. These strains had similar growth rates and doubling times (Table 2).
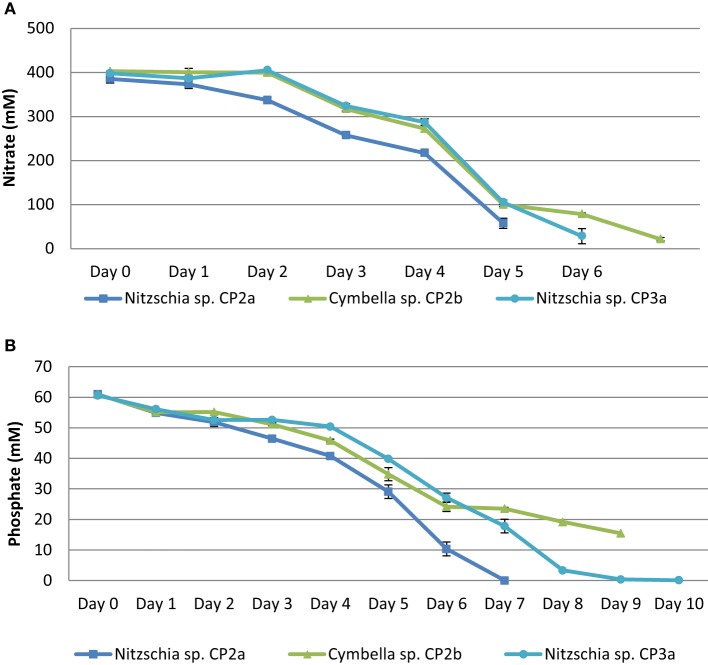
Nitrogen and phosphorous concentrations changed during the cultivation of the strains. Nitrate concentration in the medium decreased dramatically from day 3 to day 5 and nitrogen depletion occurred from day 5 to day 7 of the experiment. The concentration of phosphate decreased gradually over the experimental period. A depletion of phosphate occurred from day 7 to day 10. Nitzschia sp. CP2a and Nitzschia sp. CP3a consumed most nutrients among the strains tested (Figure 2).
Figure 2.
Nitrate (A) and phosphate (B) depletion in growth medium (daily uptake) of Cymbella sp. CP2b, Nitzschia sp. CP2a, and Nitzschia sp. CP3a. Shown are mean values ± SEs from three separately-grown cultures each.
Lipid contents in microalgae collection
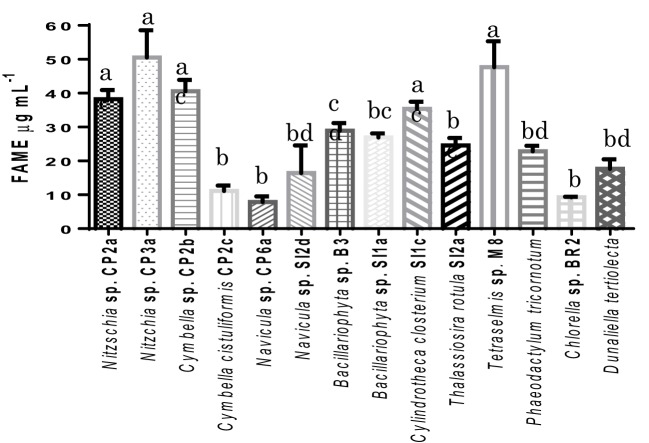
The result from GC/MS showed that all selected microalgal strains can produce fatty acids. Eighteen different fatty acids were detected, however not all strains produced all 18 fatty acids. There was no detection of some unsaturated fatty acid with 3 or 4 double bonds such as hexadecatrienoic acid, hexadecatetraenoic acid, and stearidonic acid in Nitzschia sp. CP2a, Cymbella sp. CP2b, Cymbella cistuliformis CP2c, Nitzschia sp. CP3a, and Navicula sp. CP6a. Total FAMEs ranged from 9.27 to 50.53 μg mL−1. The highest FAME content was achieved for Nitzchia sp. CP3a and the lowest was measured for Chlorella sp. BR2 (Figure 3). Palmitic acid and stearic acid were dominant among the total fatty acids. The percentage of palmitic acid ranged from 19.83 to 37.65% in most of the strains, except for Bacillariophyta sp. SI1a, Cylindrotheca closterium SI1c, Thalassiosira rotula SI2a, and Navicula sp. SI2d. Stearic acid that accumulated in the strains ranged from 14.81 to 57.73%, except for Nitzschia sp. CP2a, Cymbella sp. CP2b, and Nitzschia sp. CP3a.
Figure 3.
Total fatty acid methyl esters (FAMEs) contents of the isolated microalgal strains, expressed in μg mL−1. Shown are mean values ± SEs from three separately-grown cultures. Different letters above bars indicate statistically significant differences (p < 0.05; Two-Way ANOVA, Tukey's HSD test using GraphPad Prism 6.0).
Interestingly, omega-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) were found in most of the strains. The highest EPA percentage of total FAMEs reached 30.85% for Phaeodactylum tricornotum, 14.49% for Nitzschia sp. CP3a, and 12.46% for Nitzschia sp. CP2a. DHA reached 1.11 and 1.36% for Nitzschia sp. CP3a and Nitzschia sp. CP2a, respectively. On average, saturated fatty acids were the major FAMEs in most strains; the highest values were found in Thalassiosira rotula SI2a (62.18%), Bacillariophyta sp. B3 (71.9%) and Navicula sp. CP6a (71.61%). However, total unsaturated fatty acids were higher than total saturated fatty acids in strains such as Dunaliella tertiolecta (62.08%), Cylindrotheca closterium SI1c (61.65%), and Nitzschia sp. CP3a (60.47%).
Discussion
All strains in the study were collected from estuary, coastal waters and rock pools where environmental conditions change frequently, especially changes of temperature, salinity, light exposure, and nutrients. The changes require microalgae to adapt and/or produce more biochemical products to ensure their survival (Lim et al., 2012). Lipid is one of the biochemical products that microalgae can produce along with starch under nutrient deprived conditions in the light (Schenk et al., 2008). Lipid productivities were highest for Nitzschia sp. CP3a (5.62 μg mL−1 day−1), Tetraselmis sp. M8 (5.29 μg mL−1 day−1), Cymbella sp. CP2b (4.51 μg mL−1 day−1), and Cylindrotheca closterium SI1c (3.93 μg mL−1 day−1). The value for Tetraselmis sp. M8 was higher than the lipid productivity previously reported for this strain when grown under laboratory conditions (2.1 μg mL−1 day−1; Lim et al., 2012). It is interesting to note that all of these strains have been isolated from coastal rock pools subjected to tides. Their high lipid productivities suggest that these microalgae may be more opportunistic than others from more stable environments (e.g., stationary lakes or open ocean). A future study comparing the growth rates and lipid productivities of microalgae isolated from stable and unstable environments may further substantiate this notion. High growth rates of these microalgae lead to rapid production of biomass while their high lipid accumulation capability is likely to assist in higher survival rates during adverse conditions. Interestingly, divergent from this trend is Nitzschia sp. CP3a which despite its high lipid productivity had a very slow growth rate. Further studies should be conducted to test the survival rates of these strains and if these correlate with their cellular lipid and/or starch contents.
Diatoms can survive in harsh environments (Seckbach, 2007) and usually produce more lipids in harsher environments (Lim et al., 2012). For this research, the difference of environmental conditions between natural habitats and the laboratory may have created a harsh environment that impacted on growth and chemical accumulation of microalgae tested. Many of the strains in this study adapted to their new environment and grew well under laboratory conditions. For instance, Cymbella sp. is a diatom that usually requires multi nutrients and minerals from natural sources (Tarapchak et al., 1983; Takeda, 1998). However, this strain grew well in f/2 medium and displayed one of the best growth rates (Table 2). The indoor growth experiments were set up for conditions similar to the natural habitats (temperature, nutrients, light). However, the indoor conditions are not identical to the natural conditions and the adaptability of the selected microalgae to the laboratory condition is a useful indicator of microalgae's ability to adapt to different external conditions which also will be encountered when grown at large scale for commercial purposes.
In addition, nutrient availability also affects diatom growth. For example, silica is the main components of the diatom cell wall. Changing nutrient availability may directly influence the structure of silica composition in the cell wall and affect diatom growth (Tarapchak et al., 1983). Thus optimizing nutrient availability can also substantially affect growth rates and biomass production. For example, increasing of nutrients or nitrogen leads to an increase of growth rate (Converti et al., 2009; Chen et al., 2011; Borowitzka and Moheimani, 2013). However, to enable a direct side-by-side comparison, a standard assay with unoptimized parameters was used in the current study. Clearly, higher growth rates and lipid productivities are achievable after careful cultivation optimization. For example, this has been achieved for Tetraselmis sp. M8 (Sharma et al., 2014). A comparison of growth rates among the fastest growth strains between the study and other publications has been undertaken. It showed that the growth of the strains in the present study was similar or faster to those in other studies for Chlorella sp. BR2, Tetraselmis sp. M8 (24.5 and 18.6% increase, respectively, compared to the study by Lim et al., 2012) or slightly lower for Thalassiosira rotula (23.4% reduction compared to the study by Doan et al., 2011). The difference may be due to a difference of strains used and growth conditions, such as air and nutrient supply, temperature, culture systems and photosynthesis periods (Renaud et al., 2002; Converti et al., 2009).
Total FAMEs results from GC/MS analysis showed that all 14 microalgal strains tested were lipid producers under the standard conditions used (Table 3). Nitzschia sp. CP3a was the highest lipid producer, followed by Tetraselmis sp. M8, Cymbella sp. CP2b, Nitzschia sp. CP2a and Cylindrotheca closterium SI1c. The lipid content produced by Tetraselmis sp. M8 was similar to the results of Lim et al. (2012). The proportion of saturated fatty acids reached more than 50% for most of the diatom strains and was dominated by myristic acid, palmitic acid, and stearic acid. These fatty acids are the main desirable components for biodiesel production (Schenk et al., 2008). Based on the above properties, we conclude that the two diatoms, Cymbella sp. CP2b and Cylindrotheca closterium SI1c, are potential strains that can be used to develop microalgal biofuel production.
Table 3.
Fatty acid composition in percentage of total fatty acid methyl esters (FAMEs) of different microalgal strains collected in South East Queensland, Australia after cultivation to nitrate depletion and an additional 3 days of starvation.
| Fatty acids | Bacillariophyta sp. B3 | Bacillariophyta sp. SI1a | Cylindrotheca closterium SI1c | Thalassiosira rotula SI2a | Navicula sp. SI2d | Tetraselmis sp. M8 | Phaeodactylum tricornotum | Chlorella sp. BR2 | Dunaliella tertiolecta | Nitzschia sp. CP2a | Cymbella sp. CP2b | Cymbella cistuliformis CP2c | Nitzschia sp. CP3a | Navicula sp. CP6a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lauric (C12:0) | 1.12 | 1.64 | 1.59 | 2.17 | 2.18 | 0.95 | 0.13 | 2.05 | 0.27 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Myristic (C14:0) | 21.32 | 0.74 | 0.63 | 0.66 | 0.81 | 0.93 | 8.74 | 1.65 | 0.47 | 5.96 | 9.56 | 6.73 | 5.33 | 4.88 |
| Palmitic (C16:0) | 23.64 | 0.88 | 0.23 | 1.27 | 1.12 | 28.33 | 29.31 | 31.68 | 19.87 | 30.10 | 37.65 | 30.22 | 28.82 | 37.04 |
| Palmitoleic (C16:1) | 0.45 | 1.43 | 4.66 | 1.19 | 1.37 | 1.79 | 0.21 | 4.71 | 0.35 | 31.57 | 38.26 | 21.31 | 27.28 | 15.75 |
| Hexadecadienoic (C16:2) | 5.08 | 13.72 | 8.97 | 3.18 | 6.56 | 14.59 | 0.43 | 1.42 | 1.44 | 2.80 | 1.60 | 2.49 | 3.33 | 1.63 |
| Hexadecatrienoic (C16:3) | 4.80 | 23.29 | 23.28 | 2.32 | 6.92 | 18.27 | 5.59 | 8.84 | 4.22 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Hexadecatetraenoic (C16:4) | 0.72 | 1.85 | 1.77 | 0.93 | 2.06 | 0.25 | 0.01 | 0.04 | 23.30 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Stearic (C18:0) | 25.15 | 39.13 | 35.21 | 57.73 | 52.32 | 19.79 | 14.81 | 29.28 | 16.65 | 5.71 | 6.56 | 20.39 | 5.25 | 28.74 |
| Oleic (C18:1) | 3.57 | 5.57 | 10.11 | 19.24 | 18.07 | 8.08 | 7.13 | 11.86 | 0.82 | 1.97 | 1.18 | 2.37 | 2.92 | 2.07 |
| Linoleic (C18:2) | 1.48 | 0.21 | 0.17 | 0.09 | 0.05 | 0.24 | 1.76 | 6.12 | 1.85 | 0.80 | 0.28 | 1.21 | 1.33 | 0.38 |
| Linolenic (C18:3) | 5.64 | 6.90 | 10.83 | 9.95 | 4.23 | 3.41 | 0.34 | 0.39 | 29.98 | 1.79 | 0.09 | 0.61 | 1.16 | 0.26 |
| Stearidonic (C18:4) | 0.10 | 0.15 | 0.20 | 0.10 | 0.15 | 0.14 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Arachidic (C20:0) | 0.52 | 0.18 | 0.42 | 0.17 | 0.11 | 0.26 | 0.60 | 1.28 | 0.57 | 0.19 | 0.20 | 0.77 | 0.13 | 0.95 |
| Arachidonic (C20:4) | 0.12 | 3.20 | 0.67 | 0.04 | 0.13 | 1.54 | 0.00 | 0.04 | 0.00 | 5.28 | 0.68 | 4.80 | 8.85 | 1.87 |
| Eicosapentaenoic (EPA) (C20:5) | 5.71 | 0.28 | 0.38 | 0.21 | 3.44 | 0.28 | 30.85 | 0.08 | 0.12 | 12.46 | 3.94 | 8.75 | 14.49 | 6.16 |
| Behenic (C22:0) | 0.15 | 0.40 | 0.27 | 0.18 | 0.19 | 0.34 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Docosatetraenoic (C22:4) | 0.26 | 0.25 | 0.29 | 0.32 | 0.18 | 0.44 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Docosahexaenoic (DHA)(C22:6) | 0.17 | 0.17 | 0.34 | 0.26 | 0.09 | 0.36 | 0.00 | 0.00 | 0.01 | 1.36 | 0.00 | 0.34 | 1.10 | 0.28 |
| Saturated fatty acids (%) | 71.90 | 42.96 | 38.35 | 62.18 | 56.73 | 50.60 | 53.59 | 65.93 | 37.82 | 41.96 | 53.97 | 58.11 | 39.53 | 71.61 |
| Unsaturated fatty acids (%) | 28.10 | 57.04 | 61.65 | 37.82 | 43.27 | 49.40 | 46.31 | 33.64 | 62.08 | 58.04 | 46.03 | 41.89 | 60.47 | 28.39 |
| Total fatty acids (μg mL−1) | 28.91 | 26.91 | 35.36 | 24.56 | 16.41 | 47.65 | 22.85 | 9.27 | 17.69 | 38.21 | 40.58 | 11.13 | 50.53 | 7.90 |
| Lipid productivity (μg mL−1 day−1) | 3.21 | 2.99 | 3.93 | 2.73 | 1.82 | 5.29 | 2.54 | 1.03 | 1.97 | 4.25 | 4.51 | 1.24 | 5.62 | 0.88 |
Shown are mean values from three separately-grown cultures each.
Author contributions
All authors designed the work, wrote the manuscript and approve the final version of the manuscript. VD acquired the material. VD, ST carried out the experiments, analyzed and interpreted the data. ST, PS critically revised the work and the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to Meat & Livestock Australia, the Australian Research Council, The University of Queensland and the Endeavour scholarship program of the Australian Government for financial support. We wish to thank Dorothee Hahne from Metabolomics Australia for technical assistance and Prof Dennis Poppi, Dr Stuart McLennan, Dr Simon Quigley, Dr Faruq Ahmed and Paul Rodman for useful discussions.
References
- Adarme-Vega T. C., Thomas-Hall S. R., Schenk P. M. (2014). Towards sustainable sources for omega-3 fatty acids production. Curr. Opin. Biotechnol. 28, 14–18. 10.1016/j.copbio.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Amin S. A., Parker M. S., Armbrust E. V. (2012). Interactions between diatoms and bacteria. Microbiol. Mol. Biol. Rev. 76, 667–684. 10.1128/MMBR.00007-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka M., Moheimani N. (2013). Sustainable biofuels from algae. Mitigat. Adaptat. Strateg. Glob. Change 18, 13–25 10.1007/s11027-010-9271-9 [DOI] [Google Scholar]
- Brown M. R. (1991). The amino acid and sugar composition of sixteen species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 145, 79–99 10.1016/0022-0981(91)90007-J [DOI] [Google Scholar]
- Chen M., Tang H., Ma H., Holland T. C., Ng K. Y. S., Salley S. O. (2011). Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresour. Technol. 102, 1649–1655. 10.1016/j.biortech.2010.09.062 [DOI] [PubMed] [Google Scholar]
- Chisti Y. (2007). Biodiesel from microalgae. Biotechnol. Adv. 25, 294–306. 10.1016/j.biotechadv.2007.02.001 [DOI] [PubMed] [Google Scholar]
- Converti A., Casazza A. A., Ortiz E. Y., Perego P., Del Borghi M. (2009). Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. 48, 1146–1151 10.1016/j.cep.2009.03.006 [DOI] [Google Scholar]
- Doan T. T. Y., Sivaloganathan B., Obbard J. P. (2011). Screening of marine microalgae for biodiesel feedstock. Biomass Bioenerg. 35, 2534–2544 10.1016/j.biombioe.2011.02.021 [DOI] [Google Scholar]
- Duong V. T., Li Y., Nowak E., Schenk P. M. (2012). Microalgae isolation and selection for prospective biodiesel production. Energies 5, 1835–1849 10.3390/en5061835 [DOI] [Google Scholar]
- Huerlimann R., de Nys R., Heimann K. (2010). Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 107, 245–257. 10.1002/bit.22809 [DOI] [PubMed] [Google Scholar]
- Jang E. S., Jung M. Y., Min D. B. (2005). Hydrogenation for low trans and high conjugated fatty acids. Compr. Rev. Food Sci. Food Saf. 4, 22–30 10.1111/j.1541-4337.2005.tb00069.x [DOI] [PubMed] [Google Scholar]
- Knothe G. (2006). Analyzing biodiesel: standards and other methods. J. Am. Oil Chem. Soc. 83, 823–833. 10.1007/s11746-006-5033-y22316704 [DOI] [Google Scholar]
- Levasseur M., Thompson P. A., Harrison P. J. (1993). Physiological acclimation of marine phytoplankton to different nitrogen sources. J. Phycol. 29, 587–595 10.1111/j.0022-3646.1993.00587.x [DOI] [Google Scholar]
- Lim D. K. Y., Garg S., Timmins M., Zhang E. S. B., Thomas-Hall S. R., Schuhmann H., et al. (2012). Isolation and evaluation of oil producing microalgae from subtropical coastal and brackish waters. PLoS ONE 7:e40751. 10.1371/journal.pone.0040751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud S. M., Parry D. L., Thinh L.-V. (1994). Microalgae for use in tropical aquaculture I: gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia. J. Appl. Phycol. 6, 337–345 10.1007/BF02181948 [DOI] [Google Scholar]
- Renaud S. M., Thinh L.-V., Lambrinidis G., Parry D. L. (2002). Effect of temperature on growth, chemical composition and fatty acid composition of tropical Australian microalgae grown in batch cultures. Aquaculture 211, 195–214 10.1016/S0044-8486(01)00875-4 [DOI] [Google Scholar]
- Salama E.-S., Kim H.-C., Abou-Shanab R. I., Ji M.-K., Oh Y.-K., Kim S.-H., et al. (2013). Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioprocess Biosyst. Eng. 36, 827–833. 10.1007/s00449-013-0919-1 [DOI] [PubMed] [Google Scholar]
- Schenk P. M., Thomas-Hall S. R., Stephens E., Marx U., Mussgnug J., Posten C., et al. (2008). Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenerg. Res. 1, 20–43. 10.1007/s12155-008-9008-821570833 [DOI] [Google Scholar]
- Seckbach J. (2007). Algae and Cyanobacteria in Extreme Environments, Vol. 11 Springer, Dordrecht: Springer Science and Business Media. [Google Scholar]
- Sharma K., Li Y., Schenk P. M. (2014). UV-C-mediated lipid induction and settling, a step change towards economical microalgal biodiesel production. Green Chem. 16, 3539–3548 10.1039/C4GC00552J [DOI] [Google Scholar]
- Takeda S. (1998). Influence of iron availability on nutrient consumption ratio of diatoms in oceanic waters. Nature 393, 774–777 10.1038/31674 [DOI] [Google Scholar]
- Tarapchak S. J., Slavens D. R., Quigley M. A., Tarapchak J. S. (1983). Silicon contamination in diatom nutrient enrichment experiments. Can. J. Fish. Aquat. Sci. 40, 657–664 10.1139/f83-088 [DOI] [Google Scholar]
- Underwood A. J. (1984). The vertical distribution and seasonal abundance of intertidal microalgae on a rocky shore in New South Wales. J. Exp. Mar. Biol. Ecol. 78, 199–220 10.1016/0022-0981(84)90159-X [DOI] [Google Scholar]