Abstract
Although it is well known that the hepatocellular carcinoma (HCC) is an ominous complication in patients with liver cirrhosis, there has been no approved drug to prevent the development of HCC to date. We previously reported that the combined treatment of vitamin K2 (VK) and angiotensin-converting enzyme inhibitor (ACE-I) significantly suppressed the experimental hepatocarcinogenesis. A 66-year-old Japanese woman with hepatitis C virus (HCV)-related liver cirrhosis developed a dysplastic nodule in the liver detected by enhanced computed tomography along with elevation of the tumor markers, namely, alpha-fetoprotein (AFP) and lectin-reactive demarcation (AFP-L3), suggesting the presence of latent HCC. After oral administration of VK and ACE-I, the serum levels of both AFP and AFP-L3 gradually decreased without any marked alteration of the serum aminotransferase activity. After one-year treatment, not only the serum levels of AFP and AFP-L3 returned to the normal ranges, but also the dysplastic nodule disappeared. Since both VK and ACE-I are widely used without serious side effects, this combined regimen may become a new strategy for chemoprevention against HCC.
Keywords: Vitamin K2, ACE inhibitor, Hepatocellular carcinoma, VEGF, Angiogenesis
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common malignancies in the world, and its prognosis is still poor[1]. It is well known that HCC commonly develops in the patients with liver cirrhosis. Since the high-risk of HCC seems to be clearer as compared with other types of cancers, it is likely that a primary chemopreventive agent would be beneficial in improving the prognosis of HCC patients. However, there has been no approved chemopreventive agent against HCC to date. We previously reported that vitamin K2 (VK) and angiotensin-converting enzyme inhibitor (ACE-I) at clinically comparable low doses exerted a chemopreventive effect against hepatocarcinogenesis in rats[2]. It is well known that the serum level of alpha-fetoprotein (AFP) is a very useful marker for HCC[1]. AFP has a microheterogeneity due to structural variations in its sugar chain[3]. AFP-L3, an isoform of AFP, is reactive with lectin culinaris agglutinin and is known to suggest the presence of latent HCC cells in the cirrhotic liver, and the serum AFP-3 level is reported to be a useful biomarker in the chemoprevention of HCC[4,5]. We report herein a patient with hepatitis C virus (HCV)-related liver cirrhosis in whom the combined treatment of VK and ACE-I dissipated a dysplastic nodule along with suppression of the serum levels of AFP and AFP-L3.
CASE REPORT
In December 2002, a 66-year-old Japanese woman with HCV-related liver cirrhosis was diagnosed as having a dysplastic nodule or early HCC by enhanced computed tomography (CE-CT). Until that time, she had undergone regular imaging examinations such as CE-CT every three months since liver cirrhosis is known to be a high-risk factor for HCC development. In the arterial phase of CT, no obvious lesions could be observed (Figure 1A) whereas a low-density lesion measuring 12-mm in diameter appeared in the equilibrium phase of CT (Figure 1B). These patterns of CE-CT indicated the transient phase of a high-grade dysplastic nodule in early HCC[6]. Since we could not detect the nodule by ultrasonography, needle biopsy was not performed. Before detection of the nodule, the serum levels of AFP and AFP-L3 gradually increased. At the time of detection of the nodule, the serum levels of both AFP and AFP-L3 were significantly high (135.6 ng/mL and 14.2%, respectively). This patient also had mild hypertension and osteoporosis. We, thus, started to treat her with ACE-I (perindopril: 4 mg/d) and VK (menatetrenone: 45 mg/d) after receiving her informed consent. After administration of ACE-I and VK, the serum levels of both AFP and AFP-L3 gradually decreased. After one-year treatment, the serum levels of both AFP and AFP-L3 returned to the normal ranges. During this period, the serum level of aminotransferase (ALT) was not significantly altered, suggesting that the suppressive effect of this combined regimen on these tumor markers was not due to reduction of necroinflammation in the liver (Figure 2). After 15-mo treatment, the hepatic nodule disappeared in both the arterial and equilibrium phases of the CE-CT study (Figure 3A and B). The administration of VK and ACE-I was continued, and neither AFP nor AFP-L3 levels increased again. This combined treatment has been continued, and no HCC has developed until now.
Figure 1.
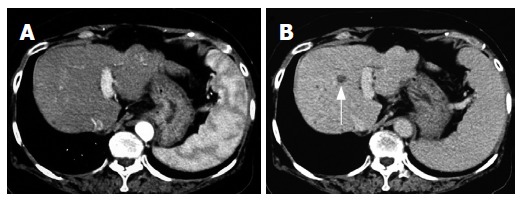
Enhanced computed-tomography (CT) scans in the arterial phase (A) and equilibrium phase (B) before administration of ACE-I in combination with VK. In the arterial phase, no obvious lesion could be detected whereas a low-density area (arrow) was noticed in the equilibrium phase.
Figure 2.
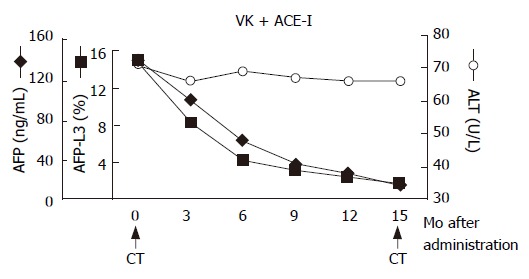
Schema of clinical course. After administration of ACE-I and VK, the serum levels of both AFP and AFP-L3 gradually decreased. After one-year treatment, the serum levels of both AFP and AFP-L3 returned to the normal ranges. The arrows indicate the time points of CT examination before and after administration of this combined regimen.
Figure 3.
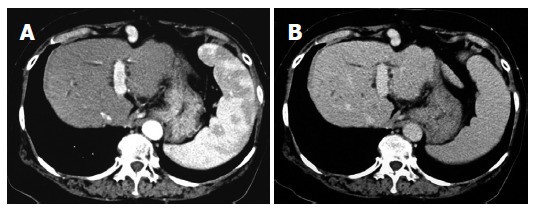
Enhanced computed-tomography (CT) scans in the arterial phase (A) and equilibrium phase (B) after administration of ACE-I in combination with VK. After a 15-mo administration, the hepatic nodule disappeared in both the arterial phase and equilibrium phase.
DISCUSSION
We report herein a patient with HCV-related liver cirrhosis in whom the combined treatment with VK and ACE-I significantly suppressed the tumor markers of HCC, i.e., AFP and AFP-L3, along with elimination of the dysplastic nodule. HCC is one of the most common malignancies in the world with an estimated incidence of more than one million new cases per year[1,7]. One of the reasons for the poor prognosis of HCC is the high rate of recurrence. It has been shown that this high recurrence rate, even after curative therapy, is due to intrahepatic metastasis or multicentric development of each respective neoplasm clone. Since the high-risk of HCC development seems to be clearer as compared with other types of tumors, it is likely that a primary or secondary chemopreventive agent would be beneficial in improving the prognosis of HCC patients.
Angiogenesis is a complex and critical process essential to support the growth of solid tumors.[8,9] It has been recently reported that angiogenesis could also be induced at the early stages of tumor formation and carcinogesis[10-13]. Recent studies have demonstrated that angiogenesis has already begun at a very early stage when the tumor consists of just 100-300 cells.[10] A recent study on the endothelial cell markers in the dysplastic lesions of the liver has suggested that in HCC, alterations in the hepatic microcirculation already occur at a very early stage of liver carcinogenesis[14]. We previously proved that angiogenesis played a pivotal role in the murine hepatocarcinogenesis[15]. Currently, several newly developed anti-angiogenic agents are being investigated around the world. Although some of these agents are still under clinical trials at limited institutes, no agents are widely available at this time in clinical practice[16]. Under the concept of anti-angiogenesis therapy, long-term administration is required to examine the compound toxicity. A potential alternative strategy may be the use of drugs with anti-angiogenic activity, which are available in an oral formulation and are currently used for treatment of different diseases. Some of the clinically available compounds, such as thalidomide and penicillamine, have been shown to possess anti-angiogenic activity, and are currently used in the clinical trials[16]. Long-term administration, however, of these agents sometimes leads to severe side effects, such as bone marrow suppression. Because long-term administration is required and the drug metabolism is usually hypoactive in the patients with liver cirrhosis, safety-proved agents would be preferable for chemoprevention against HCC. We previously reported that perindopril (PE), an ACE-I, possessed a strong anti-angiogenic activity, and that it inhibited the experimental HCC growth at clinically comparable low doses[17]. ACE-I is widely used in clinical practice without serious side effects, and the safety of its long-term administration to the patients with cirrhosis has already been proven. Moreover, VK is also safely used for treatment of osteoporosis clinically. A recent Japanese trial on patients with HCC undergoing radical therapy showed that the VK treatment significantly decreased the recurrence rate of HCC and achieved a significant improvement in the overall survival[18]. Furthermore, a recent study has revealed that VK exerted a suppressive effect against the development of HCC in women with viral hepatic cirrhosis[19]. We have recently reported that VK also showed an anti-angiogenic activity, and that the combined treatment of VK and ACE-I at low doses showed a marked suppressive effect against development of the liver enzyme-altered preneoplastic lesions in rats via angiogenesis suppression[2]. In this case, we observed that the combined treatment with VK and ACE-I significantly suppressed the serum levels of AFP and AFP-L3. This combined regimen also ameliorated the dysplastic nodule in the liver. ALP-L3 is known to be a marker of the latent HCC cells[4]. It is possible that elimination of the dysplastic nodule and decrease of the serum AFL3 level with this combined regimen may, at least partly, be mediated via inhibition of the neovascularization in the cirrhotic liver. In this case, we did not find any histological evidence of the nodules. This is the main negative point of the final diagnosis in our case. Accordingly, this report should be taken with caution, and a large-scale randomized clinical control study is required in the future.
In conclusion, herein we report a patient with HCV-related cirrhosis in whom the combined treatment with VK and ACE-I significantly attenuated the serum levels of AFP and AFP-L3 along with the elimination of the dysplastic nodule. Since both agents are widely used in clinical practice without serious side effects, this combined treatment may represent a potential new strategy for chemoprevention against HCC in the future.
Footnotes
S- Editor Liu Y L- Editor Ma JY E- Editor Liu Y
References
- 1.Befeler AS, Di Bisceglie AM. Hepatocellular carcinoma: diagnosis and treatment. Gastroenterology. 2002;122:1609–1619. doi: 10.1053/gast.2002.33411. [DOI] [PubMed] [Google Scholar]
- 2.Yoshiji H, Kuriyama S, Noguchi R, Yoshii J, Ikenaka Y, Yanase K, Namisaki T, Kitade M, Yamazaki M, Masaki T, et al. Combination of vitamin K2 and the angiotensin-converting enzyme inhibitor, perindopril, attenuates the liver enzyme-altered preneoplastic lesions in rats via angiogenesis suppression. J Hepatol. 2005;42:687–693. doi: 10.1016/j.jhep.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Aoyagi Y, Suzuki Y, Isemura M, Nomoto M, Sekine C, Igarashi K, Ichida F. The fucosylation index of alpha-fetoprotein and its usefulness in the early diagnosis of hepatocellular carcinoma. Cancer. 1988;61:769–774. doi: 10.1002/1097-0142(19880215)61:4<769::aid-cncr2820610422>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Moriwaki H, Yasuda I, Shiratori Y, Uematsu T, Okuno M, Muto Y. Deletion of serum lectin-reactive alpha-fetoprotein by acyclic retinoid: a potent biomarker in the chemoprevention of second primary hepatoma. Clin Cancer Res. 1997;3:727–731. [PubMed] [Google Scholar]
- 5.Shiraki K, Takase K, Tameda Y, Hamada M, Kosaka Y, Nakano T. A clinical study of lectin-reactive alpha-fetoprotein as an early indicator of hepatocellular carcinoma in the follow-up of cirrhotic patients. Hepatology. 1995;22:802–807. [PubMed] [Google Scholar]
- 6.Bhosale P, Szklaruk J, Silverman PM. Current staging of hepatocellular carcinoma: imaging implications. Cancer Imaging. 2006;6:83–94. doi: 10.1102/1470-7330.2006.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer DF, Sorrell MF. Hepatocellular carcinoma. Lancet. 1999;353:1253–1257. doi: 10.1016/S0140-6736(98)09148-X. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 10.Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G, Song S, Meyer-Morse N, Bergsland E, Hanahan D. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111:1287–1295. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandvold KA, Neiman P, Ruddell A. Angiogenesis is an early event in the generation of myc-induced lymphomas. Oncogene. 2000;19:2780–2785. doi: 10.1038/sj.onc.1203589. [DOI] [PubMed] [Google Scholar]
- 14.Frachon S, Gouysse G, Dumortier J, Couvelard A, Nejjari M, Mion F, Berger F, Paliard P, Boillot O, Scoazec JY. Endothelial cell marker expression in dysplastic lesions of the liver: an immunohistochemical study. J Hepatol. 2001;34:850–857. doi: 10.1016/s0168-8278(01)00049-6. [DOI] [PubMed] [Google Scholar]
- 15.Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Hicklin DJ, Wu Y, Yanase K, Namisaki T, Kitade M, et al. Halting the interaction between vascular endothelial growth factor and its receptors attenuates liver carcinogenesis in mice. Hepatology. 2004;39:1517–1524. doi: 10.1002/hep.20218. [DOI] [PubMed] [Google Scholar]
- 16.Kerbel RS. Clinical trials of antiangiogenic drugs: opportunities, problems, and assessment of initial results. J Clin Oncol. 2001;19:45S–51S. [PubMed] [Google Scholar]
- 17.Yoshiji H, Kuriyama S, Kawata M, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H. The angiotensin-I-converting enzyme inhibitor perindopril suppresses tumor growth and angiogenesis: possible role of the vascular endothelial growth factor. Clin Cancer Res. 2001;7:1073–1078. [PubMed] [Google Scholar]
- 18.Mizuta T, Ozaki I, Eguchi Y, Yasutake T, Kawazoe S, Fujimoto K, Yamamoto K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106:867–872. doi: 10.1002/cncr.21667. [DOI] [PubMed] [Google Scholar]
- 19.Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358–361. doi: 10.1001/jama.292.3.358. [DOI] [PubMed] [Google Scholar]


