Abstract
AIM: The treatment of liver disease is severely limited by a shortage of donor livers. In trying to address this growing problem, hepatocellular transplantation (HTx) has received much attention as an alternative to whole organ transplant. However, the expansion of transplanted cells is at low level, and the reconstitution of functional liver tissue is limited by this cellular property. We set up an animal model to better understand cell dose effect and the kinetics of liver repopulation following HTx.
METHODS: Dipeptidyl peptidase IV (DPPIV)-deficient rats treated with retrorsine and subjected to partial hepatectomy were infused with DPPIV-positive hepatocytes. Rats were injected with varying numbers of donor hepatocytes down to 100 cells low, and liver repopulation was examined at different time points up to 20 mo long. Repopulation was assessed by computer-aided quantitative detection.
RESULTS: Transplanted hepatocytes underwent multiple rounds of proliferation and stably repopulated the injured livers after 20 mo and at all cell doses. Transplanted cells divided 14 times within the 3-mo time period following infusion, and the liver repopulation reached a plateau between 3 and 20 mo. Approximately 90% replacement occurred. Donor-derived cells also reconstituted the bile ductules of the recipients.
CONCLUSION: The ability of transplanted hepatocytes to fully reconstitute injured livers strongly supports further investigation into the clinical potential of HTx. Additionally, the observation that transplanted hepatocytes also form components of the biliary system suggests that these cells may have bi-potential property of the stem cells.
Keywords: Hepatocellular transplantation, Hepatic stem cell, Kinetics, Cell dose, Long-term repopulation, Bile ductules, Quantification, In vivo, Therapeutic potential
INTRODUCTION
In patients with end-stage liver disease, liver transplantation is the only effective treatment, but this is limited by the shortage of potential organs. Cell transplantation (Tx) has the potential to overcome this shortage as well as the high cost and morbidity associated with the whole organ transplant. Isolated hepatocellular transplantation (HTx) was investigated several decades ago as one of the earliest forms of cell transplantation[1] and has come to the stage of clinical therapies in recent years[2,3]. However, transplanted hepat-ocytes do not seem to proliferate well and reconstitution of liver function is not sufficient in the models used thus far[3,4]. Endogenous hepatocytes greatly outnumbered the transplanted cells and it is conceivable that, if both populations respond equally well to proliferative stimuli, donor cells would never achieve sufficient numbers[5]. Some models allowing for the selective proliferation of transplanted cells have made progress in addressing this problem[6-9]. Disruption of the proliferative capacity of endogenous hepatocytes followed by transplantation is an alternative strategy to achieve these goals. Retrorsine[7,10] or irradiation[9] has been used to inhibit the proliferation of endogenous hepatocytes and allow transplanted hepatocytes to fully repopulate the liver, making the clinical application of HTx more feasible. Application of the prospective therapy may become a reality if answers to the following questions are obtained. How long can donor-derived hepatocytes continue to repopulate the damaged recipient liver? What is the relationship between repopulation efficiency and cell dose of HTx? Will transplanted hepatocytes be able to repopulate other tissues in addition to the liver parenchyma? Moreover, the capability of long-term tissue repopulation[5] should also be addressed in the animal model, with a longer post-transplant period than the life span of liver cells.
We used dipeptidyl peptidase IV (DPPIV) as a genetic marker of transplanted cells in rats treated with retrorsine and subjected to partial hepatectomy (PH). Retrorsine, a DNA-alkylating agent that disrupts cell-cycle progression in hepatocytes, prevented the recipient hepatocytes from proliferating, and, following PH, only transplanted cells proliferated and repopulated the liver[7]. Using this model, we addressed the above questions and discussed the prospects of HTx in clinical application, and also the heterogeneity and the stem cell property of hepatocytes.
MATERIALS AND METHODS
Animals
Male Fischer 344 DPPIV wild-type (DPPIV+) rats used as hepatocyte donors for Tx were purchased from SLC of Japan and female Fischer 344/DuCrj DPPIV-deficient (DPPIV-) recipient rats were purchased from Charles River of Japan. Animals were maintained in daily 12-h cycles of alternating light and darkness. All studies were conducted under protocols approved by the Laboratory Animal Resource Center of University of Tsukuba, Japan.
Retrorsine treatment and partial hepatectomy
Recipients, 4-wk-old Fischer 344 rats, were treated with retrorsine similar to previous descriptions with some modifications[7,11]. Briefly, a retrorsine (Sigma) working solution was prepared in acidified saline with 1 mol/L HCl; this was neutralized with 1 N NaOH and adjusted to a final concentration of 6 mg/mL. The animals received two intraperitoneal injections with 30 mg/kg body weight 2 wk apart. Donor cells were infused either 1 mo or 2 wk following retrorsine treatment. All recipients were subjected to two-thirds surgical PH.
Cell isolation and transplantation
Parenchymal hepatocytes were isolated from normal adult rats (8-10-wk old) using a standard two-step collagenase perfusion method described by Seglen[12]. After perfusion, the cells were centrifuged at 50 g for 1 min and filtered with a nylon mesh to remove aggregated cells and residual tissue. Cell viability was between 80% and 90% as determined by trypan blue dye exclusion. Limiting dilution of the freshly isolated cells was performed from 2?06, 1?06, 1?05, 1?04, and 1?03 to 1?02. Hepatocytes in 0.2 mL Dulbecco's modified Eagle's medium were infused through the portal vein. Two to three animals were used for each time point, and experiments were repeated at least twice. After 1-3 mo, and up to 20 mo following Tx, animals were killed and the livers were sampled.
Histochemical staining
DPPIV histochemical staining was performed as described[7,13]. Sampled rat livers were embedded in optimum cutting temperature compound, frozen in liquid nitrogen and stored at -80 °C. DPPIV activity was visualized in tissues fixed with chloroform朼cetone (1:1, v/v) for 10 min, followed by incubation with glycyl-L-proline-4-methoxy-2-naphthyl-amide (Sigma) substrate for 30 min at room temperature. The counterstaining was performed with hematoxylin.
Quantitative detection
DPPIV-positive areas were captured by Photograb-2500, v1.0 (Fujifilm) through a Nikon Eclipse E800 microscope and analyzed by computer program, WinROOF v3.2 (Mitani Corp.).
To determine the number of hepatocytes per repopulated colony, we considered a colony as a large sphere and a single hepatocyte as a small sphere. If the average radius of one colony in cross-sectional analysis on slide is expressed as R, the overall volume of the colony is 2pR3√1.5. The number of hepatocytes estimated per colony sphere was calculated by volumecolony-sphere/volumehepatocyte. Cell division during Tx for a colony sphere was calculated by log10(1.5(R/r)3√1.5)/log102 (r≈10 mm, radius of one hepatocyte).
RESULTS
Kinetics of liver repopulation
The canaliculi enzyme DPPIV is highly expressed in epithelial cells (including hepatocytes) of many organs in rats except in one substrain of inbred Fischer rats. It is a stable and easily distinguishable marker for use in enzyme histochemistry in models of HTx[7,14]. After transplantation, DPPIV-positive cells constituted 14.8?.2%, 50.7?3.9% and 70.4?.0% of the total hepatocyte number at 1, 2, and, 3 mo of follow-up, respectively (Figures 1A-C). Twenty months after transplantation, a repopulation rate as high as 95% was observed, with an average rate of 89.8?.7% (Figure 1D). Histochemical analysis also clearly showed that donor cells proliferated and expanded through recipient liver tissue (Figure 2A and Table 1), and, at the latest time point, occupied nearly the full volume of the recipient liver (Figure 1D, inset). Between 3 and 20 mo, the repopulation rate increased by only 20%, suggesting that the phenomena had reached a plateau. Using a similar model, Laconi et al[15] observed a repopulation rate of 81% at 8 mo in female recipients; this is consistent with our results.
Figure 1.
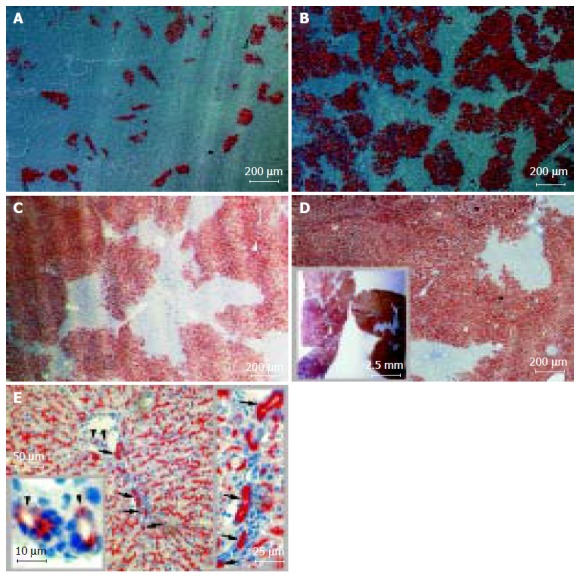
Long-term liver repopulation 1-20 mo after hepatocellular Tx and hepatic epithelial reconstitution from donor hepatocytes. Recipient DPPIV-deficient Fischer rats were treated with retrorsine and subjected to two-thirds PH. DPPIV-positive donor hepatocytes were infused through the portal vein. 2?06 donor cells per recipient in A-C and 1?06 in D and E. Cryostat sections show DPPIV-positive cells as red-brown after histochemical staining. Data presented as mean± SD from three to six individuals in two or three independent experiments. Tx: transplantation; DPPIV: dipeptidyl peptidase IV. A: 1-mo repopulation: 14.8?.2%; polarization contrast image; B: 2-mo repopulation: 50.7?3.9%; polarization contrast image; C: 3-mo repopulation: 70.4?.0% with hematoxylin counterstaining; D: 20-mo repopulation: 89.8?.7% with hematoxylin counterstaining. The inset box displays the cross-sections of hepatic lobes under stereomicroscopy and E: DPPIV-positive cells not only were expressed in parenchyma but also were found in Hering抯 canal-like structures (arrow) and interlobular bile duct-like structure (arrowhead) in recipient liver; hematoxylin counterstaining.
Figure 2.
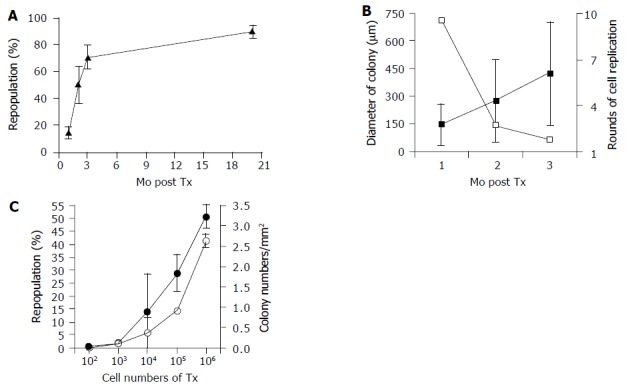
Repopulation kinetics of hepatocellular Tx. DPPIV+ donor hepatocytes were isolated and infused into DPPIV-deficient rats through the portal vein. The visualized DPPIV+ cells in recipient were processed by a computer-aided analysis. Data are presented as mean± SD from three to six individuals in two or three independent experiments. Tx: transplantation; DPPIV: dipeptidyl peptidase IV. A: Kinetics of liver repopulation. Transplanted cell numbers into 1-3 mo and 20 mo were 2?06 and 1?06, respectively. The repopulation rate increased dramatically over 3 mo and reached a plateau at 20 mo; B: Kinetics of colony size and cell replication. Transplanted cell numbers into 1-2 mo and 3 mo were 2?06 and 1?06, respectively. The estimation technique of cell replication rounds is explained in Results. The average colony size from donor hepatocytes in the 3 mo after Tx are filled squares and the estimated number of rounds of cell replication are open squares and C: Cell-dose effect on liver repopulation and colony numbers. All samples collected at 3 mo after Tx and the repopulation rate (filled circles) and colony numbers (open circles) showed a similar trend.
Table 1.
Effect of transplant cell dose and time on the efficiency of liver repopulation in vivo after HTx
| Time of Tx (mo) |
Kinetics of repopulation |
Effect of cell dose on repopulation |
|||||||
| 1 | 2 | 3 | 20 | 3 | 3 | 3 | 3 | 3 | |
| Infused cell number Post Tx | 2×106 | 2×106 | 2×106 | 1×106 | 1×106 | 1×105 | 1×104 | 1×13 | 1×102 |
| Liver repopulation (%) | 14.8±4.2 | 50.7± 13.9 | 70.4?±9.0 | 89.8± 4.7 | 51.0± 4.7 | 28.9± 7.2 | 13.8± 5.0 | 1.1± 1.2 | 0.6± 0.6 |
| Colony number/mm2 | 5.48± 1.10 | 5.1±.1.40 | NA | NA | 2.64±0.16 | 0.91±0.05 | 0.37±0.39 | 0.09±0.07 | 0.01±.01 |
| Diameter of colony (μm) | 149.4± 110.5 | 280.1±219.4 | NA | NA | 425.2± 278.8 | 376.7± 64.8 | 476.5± 54.5 | 354.5± 108.9 | 698.7± 39.2 |
| Number of animals | 6 | 6 | 3 | 4 | 3 | 3 | 4 | 4 | 4 |
| Area examined (mm2) | 365.0 | 402.0 | 66.6 | 2386.5 | 77.6 | 284.1 | 464.8 | 458.0 | 470.5 |
Donor cells were derived from DPPIV+ rats. Recipients were DPPIV-deficient rats treated with retrorsine and two-thirds PH; DPPIV+ cells were visualized by their enzymatic activity after histochemical staining of cryostat sections. The characteristics of repopulation, colony number and colony diameter were analyzed based on DPPIV+ cells. Computer-aided area analysis was used for quantitative detection of repopulation. All of the data presented as mean± SD from three to six individuals in two or three independent experiments. HTx: hepatocellular transplantation; Tx: transplantation; NA: not applicable.
The lobular architecture and overall histological appearance of the livers were normal following HTx, and hepatocyte morphology, hepatic plate structure, and the appearance of cells lining the sinuses were normal compared to untreated livers (data not shown). Despite extensive hepatocyte proliferation and the appearance of "nodules" of transplanted cells, no tumors were observed in the liver up to 20 mo after Tx. Additionally, the survival of all four recipients up to 20 mo indicates that no abnormalities incompatible with life develop following transplant.
Colony size and cell replication
Colony size analyses more clearly defined the magnitude of transplanted cell proliferation in recipient animals. Table 1 presents the characteristics and distribution of donor cells following transplantation with varied cell numbers and at different time points after Tx. DPPIV+ colonies expanded linearly between 1 and 3 mo after Tx from 150 to 425 mm in diameter (Figure 2B). The area of recipient livers occupied by donor cells similarly increased over 3 mo.
Hepatocytes are thought to be symmetric, 8 or 12-sided cells[16], but, in order to simplify calculations, we considered a hepatocyte as a sphere and assumed that one colony arose from one hepatocyte. Additionally, we made the assumption that repopulated colonies assumed a grossly spherical shape, and the number of cells occupying these colonies could be calculated by comparing the volume of one cell with the volume of a colony. Based on these assumptions and analyses, transplanted hepatocytes underwent about nine rounds of cell replication after the 1st mo and another three and two rounds in 2nd and 3rd mo, respectively. Donor cell division was high only in the 1st mo, and it dramatically decreased over the following period (Figure 2B), but replication never stopped although it slowed down to very low level.
Effect of cell dose on liver repopulation
The ability of different numbers of transplanted cells to repopulate the liver is unknown, despite the importance of this information for stem cell biology and the clinical application of HTx. As seen in Table 1 and Figure 2C, transplantation of 102-106 led to liver repopulation rates from 0.6% up to 51% 3 mo after transplant, and the repopulated colonies reached 0.01-2.64/mm2 in recipient livers. Effective repopulation occurred even in the 100-cell group. Therefore, liver repopulation for no more than 3 mo does not seem to depend on an infrequent cell fraction, but hepatocytes in general are able to form colonies following transplantation.
DPPIV-positive cholangiocytes in recipient livers|
DPPIV is expressed in both hepatocytes and bile duct epithelial cells, in a characteristic bile canalicular distribution and a diffuse cytoplasmic pattern, respectively[7,17,18]. Twenty months after transplant with one million hepatocytes, interlobular bile duct-like cells and Hering's canal-like cells also expressed the donor-derived genetic marker, DPPIV (Figure 1E). To our knowledge, this is the first report of bile duct differentiation of transplanted hepatocytes. This finding contrasts with the widely accepted view that hepatocytes are uni-potential following transplant[19].
DISCUSSION
In contrast to fully differentiated cells in adult mammalian tissues, hepatocytes possess an enormous proliferative capacity and are capable of replacing the entire liver mass under some circumstances. In the normal resting liver, the hepatocyte turnover rate at any given time is about 1 in 20 000-40 000 cells, and normal liver tissue is replaced over the course of 1 year[20]. If transplanted hepatocytes did not undergo cell division or the division did not last for a sufficient duration of time, few donor cells would have remained at the 20-mo time point. However, recipient livers were almost exclusively comprised of donor cells at 20 mo after transplant (Figure 1D, inset). The tissue renewal by donor-derived hepatocytes occurs multiple times in the recipient liver in more than 1-year long-term repopulation. The present findings demonstrate that the ‘long-term tissue repopulation’ characteristics defined for stem cells[5] are expressed by adult hepatocytes as well. The present study markedly differs from previous reports[7,14,15] in having the post-transplantation period longer than the life span of liver cells, and it also takes into account the cell-dose effect. The extremely long period of observation was necessary because it facilitated analysis of cell kinetics of long-lived cells to trace the transplanted cells and to show whether they possess the stem cell properties or not.
To differentiate and colonize, a wide range of tissues to reconstitute them in vivo is another important property of stem cells. In the present study, bi-potential capability of donor-derived cells displayed an interesting aspect of tissue reconstitution. Hepatocytes are thought to be "functional" or "committed" progenitors[19], but 20 mo after transplant, cholangiocytes (interlobular bile duct-like cells and Hering's canal-like cells) expressed the donor cell marker (Figure 1E). It indicates that the bi-potential stem cells, however, existed in donor-derived cells. We had not previously observed donor-derived cholangiocytes in other no longer than 6-mo old Tx groups. Why is the regeneration of biliary system so rare to be observed? One possible answer is that a minor proportion of donor-derived bi-potential cells spend a long time to differentiate and competitively reconstitute the system in the recipient. The low frequent appearance may predict and represent an important inherent nature of hepatic stem cells.
Hepatocytes may be capable of only one or two rounds of cell division when responding to cell loss under normal physiological conditions[21,22], but we observed at least 14 rounds of replication in the 3-mo time period following transplantation. Various physiological needs may stimulate a variety of responses from different populations of hepatocytes. It is possible that the transplanted cells were not a homogenous population of hepatocytes. This could explain the ability of the donor cells to proliferate to a greater extent than previously thought as well as the generation of cholangiocytes. Indeed, adult hepatocytes exhibit heterogeneous proliferative potential in vitro[23]. The potential complexity of the liver cells populations is outlined in Figure 3. Liver tissue may be comprised of a large number of mature hepatocytes that rapidly respond to physiological cell loss and tissue injury (population 1) for a short time, a small number of uni-potential progenitors that rapidly replicate in response to liver injury for a limited time period (population 2), and rare bi-potential stem cells capable of self-renewal that slowly respond to severe liver injury for a long time (population 3). Further studies with some other approaches, such as, flow-cytometric cell sorting and in vitro colony assay[24,25] are clearly needed to investigate this hypothesis.
Figure 3.
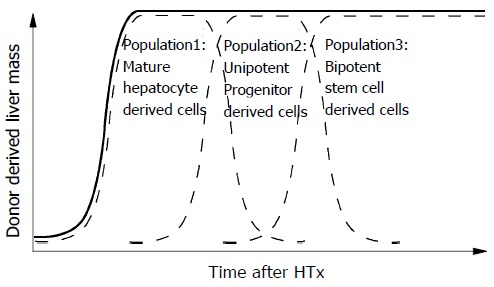
Schematic diagram of long-term repopulation after HTx. Different cell populations may be required for different phases of liver regeneration/repopulation. The solid lines express the repopulation of donor-derived cells in the liver and the broken lines are the repopulation from the possible contributors. This assumes a heterogeneous population of transplanted hepatocytes. HTx: hepatocellular transplantation.
While the biological phenomena underlying our observations are not very clear, the therapeutic potential of hepatocyte transplant is very clear. A small number of transplanted cells efficiently and stably repopulated injured rat livers. With the expansion of waiting lists for orthotopic liver transplant growing, the availability of donor cells makes HTx even more attractive. Among the potential applications of HTx, supply metabolic support in acute or chronic liver failure and definitive treatment of inherited metabolic disorders are included. At the very least, HTx could be used as a bridge therapy to prolong the lives and function of patients awaiting transplants. It is reasonable to assume that mechanism that controls regeneration may be fairly similar among various species, and the knowledge obtained from researches of liver regeneration in the animal model is applicable to the human liver. Our study may be a guide for initial investigations of HTx in human. For example, in our animal model, the cell infusion ratio of donor cells to recipient liver cells was about 1/200 (2?06/4?08), and we achieved 50% and 70% repopulation rates 2 and 3 mo after Tx. Human liver tissue is thought to weigh 1 500 g with 2.5?011 parenchymal cells. Extrapolating from our data, approximately 1.25?09 cells would be used for human HTx, and if 10% of isolated hepatocytes could be collected from a liver (2.5?010cells), a single liver could be used for 20 HTx. The low cost, high preservability and availability of sources could make HTx an extremely promising treatment for end-stage liver disease.
Taken together, the long-term repopulation potential, high replacement rate and full tissue reconstitution following HTx in our model further strengthens the therapeutic potential of this technique. Additionally, our observations raise interesting possibilities as to the composition and proliferation of hepatocyte populations as well as the hepatic stem cell nature.
ACKNOWLEDGMENTS
Authors thank Dr. Mei Gu, Dr. Pei Gan, Dr. Hirotoshi Miyoshi, Dr. Kennichi Yanagi and Dr. Chika Miyoshi for expert technical assistances on liver perfusion and surgical operation.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
Supported by the National Project for Realization of ‘regenerative Medicine’ and Grants-in-Aid (14207046, 12557096) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan and a grant from MITSUBISHI Foundation
References
- 1.Matas AJ, Sutherland DE, Steffes MW, Mauer SM, Sowe A, Simmons RL, Najarian JS. Hepatocellular transplantation for metabolic deficiencies: decrease of plasms bilirubin in Gunn rats. Science. 1976;192:892–894. doi: 10.1126/science.818706. [DOI] [PubMed] [Google Scholar]
- 2.Grossman M, Raper SE, Kozarsky K, Stein EA, Engelhardt JF, Muller D, Lupien PJ, Wilson JM. Successful ex vivo gene therapy directed to liver in a patient with familial hypercholesterolaemia. Nat Genet. 1994;6:335–341. doi: 10.1038/ng0494-335. [DOI] [PubMed] [Google Scholar]
- 3.Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 4.Grossman M, Rader DJ, Muller DW, Kolansky DM, Kozarsky K, Clark BJ, Stein EA, Lupien PJ, Brewer HB, Raper SE. A pilot study of ex vivo gene therapy for homozygous familial hypercholesterolaemia. Nat Med. 1995;1:1148–1154. doi: 10.1038/nm1195-1148. [DOI] [PubMed] [Google Scholar]
- 5.Shafritz DA, Dabeva MD. Liver stem cells and model systems for liver repopulation. J Hepatol. 2002;36:552–564. doi: 10.1016/S0168-8278(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 6.Rhim JA, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Replacement of diseased mouse liver by hepatic cell transplantation. Science. 1994;263:1149–1152. doi: 10.1126/science.8108734. [DOI] [PubMed] [Google Scholar]
- 7.Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319–329. doi: 10.1016/S0002-9440(10)65574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overturf K, Al-Dhalimy M, Tanguay R, Brantly M, Ou CN, Finegold M, Grompe M. Hepatocytes corrected by gene therapy are selected in vivo in a murine model of hereditary tyrosinaemia type I. Nat Genet. 1996;12:266–273. doi: 10.1038/ng0396-266. [DOI] [PubMed] [Google Scholar]
- 9.Guha C, Sharma A, Gupta S, Alfieri A, Gorla GR, Gagandeep S, Sokhi R, Roy-Chowdhury N, Tanaka KE, Vikram B, et al. Amelioration of radiation-induced liver damage in partially hepatectomized rats by hepatocyte transplantation. Cancer Res. 1999;59:5871–5874. [PubMed] [Google Scholar]
- 10.Gordon GJ, Coleman WB, Grisham JW. Bax-mediated apoptosis in the livers of rats after partial hepatectomy in the retrorsine model of hepatocellular injury. Hepatology. 2000;32:312–320. doi: 10.1053/jhep.2000.9144. [DOI] [PubMed] [Google Scholar]
- 11.Gordon GJ, Coleman WB, Grisham JW. Temporal analysis of hepatocyte differentiation by small hepatocyte-like progenitor cells during liver regeneration in retrorsine-exposed rats. Am J Pathol. 2000;157:771–786. doi: 10.1016/S0002-9440(10)64591-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/S0091-679X(08)61797-5. [DOI] [PubMed] [Google Scholar]
- 13.Lojda Z, Gossrau R, Schiebler TH. Peptidase. In: Enzyme Histochemistry: A laboratory manual. Berlin Springer Verlag; 1979. pp. 199–203. [DOI] [Google Scholar]
- 14.Coleman WB, McCullough KD, Esch GL, Faris RA, Hixson DC, Smith GJ, Grisham JW. Evaluation of the differentiation potential of WB-F344 rat liver epithelial stem-like cells in vivo. Differentiation to hepatocytes after transplantation into dipeptidylpeptidase-IV-deficient rat liver. Am J Pathol. 1997;151:353–359. [PMC free article] [PubMed] [Google Scholar]
- 15.Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771–777. doi: 10.1016/S0002-9440(10)64019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elias H, Sherrick JC. Morphology of the liver. New York Academic Press. 1969. pp. 5–74. [Google Scholar]
- 17.Hubbard AL, Bartles JR, Braiterman LT. Identification of rat hepatocyte plasma membrane proteins using monoclonal antibodies. J Cell Biol. 1985;100:1115–1125. doi: 10.1083/jcb.100.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walborg EF, Tsuchida S, Weeden DS, Thomas MW, Barrick A, McEntire KD, Allison JP, Hixson DC. Identification of dipeptidyl peptidase IV as a protein shared by the plasma membrane of hepatocytes and liver biomatrix. Exp Cell Res. 1985;158:509–518. doi: 10.1016/0014-4827(85)90474-4. [DOI] [PubMed] [Google Scholar]
- 19.Zheng YW, Taniguchi H. Diversity of hepatic stem cells in the fetal and adult liver. Semin Liver Dis. 2003;23:337–348. doi: 10.1055/s-2004-815557. [DOI] [PubMed] [Google Scholar]
- 20.Zimmermann A. Liver regeneration: the emergence of new pathways. Med Sci Monit. 2002;8:RA53–RA63. [PubMed] [Google Scholar]
- 21.Sell S. Heterogeneity and plasticity of hepatocyte lineage cells. Hepatology. 2001;33:738–750. doi: 10.1053/jhep.2001.21900. [DOI] [PubMed] [Google Scholar]
- 22.Magami Y, Azuma T, Inokuchi H, Kokuno S, Moriyasu F, Kawai K, Hattori T. Cell proliferation and renewal of normal hepatocytes and bile duct cells in adult mouse liver. Liver. 2002;22:419–425. doi: 10.1034/j.1600-0676.2002.01702.x. [DOI] [PubMed] [Google Scholar]
- 23.Tateno C, Takai-Kajihara K, Yamasaki C, Sato H, Yoshizato K. Heterogeneity of growth potential of adult rat hepatocytes in vitro. Hepatology. 2000;31:65–74. doi: 10.1002/hep.510310113. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32:1230–1239. doi: 10.1053/jhep.2000.20349. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki A, Zheng YW, Kaneko S, Onodera M, Fukao K, Nakauchi H, Taniguchi H. Clonal identification and characterization of self-renewing pluripotent stem cells in the developing liver. J Cell Biol. 2002;156:173–184. doi: 10.1083/jcb.200108066. [DOI] [PMC free article] [PubMed] [Google Scholar]


