Abstract
Once a memory has formed, it is thought to undergo a gradual transition within the brain from short- to long-term storage. This putative process, however, also poses a unique problem to the memory system in that the same learned items must also be retrieved across broadly varying time scales. Here, we find that neurons in the ventrolateral prefrontal cortex (VLPFC) of monkeys, an area interconnected with both temporal and frontal associative neocortical regions, signaled the need to alter between retrieval of memories formed at different times. These signals were most closely related to the time interval between initial learning and later retrieval, and did not correlate with task switch demands, novelty, or behavioral response. Consistent with these physiological findings, focal inactivation of the VLPFC led to a marked degradation in retrieval performance. These findings suggest that the VLPFC plays a necessary regulatory role in retrieving memories over different temporal scales.
We must often acquire and retrieve memories over widely varying temporal scales. Once an item is learned, for example, it can be retrieved minutes to hours later if rehearsed (Eichenbaum 2000, 2004; Kluwe et al. 2003; Remondes and Schuman 2004; Foster and Wilson 2006). Many memories, however, can also be accurately retrieved days to months (sometimes years) without intervening rehearsal (Stickgold et al. 2002; Walker and Stickgold 2004; Inostroza and Born 2013). Our ability to dynamically retrieve memories across these wide-ranging temporal scales is essential to many common behaviors. For example, when reading a scientific book, we must often go back-and-forth between recalling parts of the book read minutes-to-hours earlier to recalling parts read months before in order to place such information in context. Similarly, we must often go back-and-forth between recalling the names of people we recently met and those of colleagues who are well known. This ability, therefore, allows us to dynamically access both memories that had been acquired long ago as well as those that have been recently formed (Eichenbaum 2000, 2004; Hoffman and McNaughton 2002; Stickgold et al. 2002; Dudai 2004; Maviel et al. 2004; Remondes and Schuman 2004; Walker and Stickgold 2004; Takehara-Nishiuchi and McNaughton 2008).
Here, we aimed to understand this process by obtaining single-neuronal recordings and delivering event-related microstimulation in the ventrolateral prefrontal cortex (VLPFC) of Rhesus macaques (Fig. 1A, left). We focused on this area because it is known to possess broad reciprocal connections (Gerbella et al. 2010) with both temporal and associative neocortical regions believed to play respective roles in short- and long-term memory storage (Bontempi et al. 1999; Eichenbaum 2000, 2004; Kluwe et al. 2003; Dudai 2004; Maviel et al. 2004; Remondes and Schuman 2004; Foster and Wilson 2006; Shema et al. 2007). Moreover, imaging studies have demonstrated robust activation in the VLPFC when subjects retrieve previously learned images (McIntosh 1999; Badre et al. 2005). Finally, ablative studies that disconnect the VLPFC from bottom-up sensory input suggest that this and surrounding areas are likely involved in the top-down control of memory retrieval (Hasegawa et al. 1998; Tomita et al. 1999).
Figure 1.

Task design. (A) On the left, schematic illustration depicting the location of Brodmann area 45 of the VLPFC and connectivity with temporal and frontal cortical regions. On the right, anatomic locations of the recording and stimulation sites (interaural line referenced). The locations of the individual electrode arrays are shown in gray and white, respectively, for the two monkeys. Red and green circles indicate the array sites used of stimulation. (B) Sequence of events over a single illustrative trial. Here, the center dot represents the eye fixation point, and the two circles on each end are the targets of saccade. Therefore, on image presentation, the two images are first displayed. Then after a delay, two targets corresponding to the remembered location of the two images are given. (C) A sample sequence of trials presented in successive order (only the image presentation periods are shown). The letters indicate whether the image-pairs were consolidated or nonconsolidated (see text). Each image-pair consisted of a pair of images (i.e., C2–C2′), with the number indicating the identity of the images pairing and apostrophe indicating the rewarded of the two images (shown randomly on the left versus right on each successive trial).
Toward this goal, primates were trained to perform a multitemporal memory task that involved the successive, interleaved presentation of distinct image-pairs which were originally learned at different times. Here, the monkeys performed item discrimination in which they had to recall which of two distinct items (e.g., apple or orange) was associated with receipt of reward based on prior training (Schusterman 1962; Rygula et al. 2010; Asaad and Eskandar 2011; van Wingerden et al. 2012). This task was specifically used because it allowed the monkeys to learn and recall multiple unique image-pairs per recording session, and to transition between multiple image-pairs per day. Finally, to further allow us to differentiate between memory- and task-related responses (e.g., “task-switch” effect or novelty response), we examined responses of neurons when the monkeys; (1) retrieved image-pairs that were and were not rehearsed and (2) retrieved image-pairs that had been recently versus distantly learned (see further details below).
Results
Task overview
Two adult male Rhesus monkeys (Macacca mulatta) performed a task that required them to learn and later retrieve multiple, distinct image-pairs (Fig. 1B). Before performing the main recall task, the primates learned sets each consisting of four image-pairs using a distributed tapering training protocol whereby each image-pair was trained, by interleaved repetition (Materials and Methods) (Kahana and Howard 2005). For separate sessions and days, four image-pairs were initially learned 1 h before the retrieval task (NC) whereas the other four image-pairs were learned 3 mo before the retrieval task (C). Here, the monkeys were made to retrieve the four previously trained NC image-pairs and four previously trained C image-pairs in successive interleaved sets (i.e., eight distinct image-pairs and 16 images per retrieval session), therefore, allowing us to distinguish between memory-related changes in activity from changes simply associated with image identity or novelty.
However, to further distinguish between memory-related changes in activity from possible differences in behavioral demand or “task-switch” response (Kerns et al. 2004; Williams et al. 2004; Liston et al. 2006; Sheth et al. 2012), we also evaluated image-pairs that were learned 1 d prior to retrieval but that were also not rehearsed for at least 24 h (MC). Based on behavioral criteria described previously (Eichenbaum 2000, 2004; Hoffman and McNaughton 2002; Stickgold et al. 2002; Dudai 2004; Maviel et al. 2004; Remondes and Schuman 2004; Walker and Stickgold 2004; Takehara-Nishiuchi and McNaughton 2008), consolidated image-pairs were defined as those in which retrieval performance remained either intact or enhanced at least 24 h following initial training without rehearsal. All NC, MC, and C image-pairs underwent the same standardized training. As described in further detail in Materials and Methods, the primates concurrently learned groups of four image-pairs over a total of 372 trials. For each set of image-pairs, we confirmed that the monkeys successfully learned all four image-pairs to high accuracy by demonstrating a >95% correct performance.
During the main recall task used for neuronal recordings, the monkeys were made to retrieve four previously trained NC image-pairs and four previously trained C image-pairs in successive interleaved sets (i.e., eight distinct image-pairs and 16 images per retrieval session). The serial position of the individual image-pairs were randomly shuffled on each set, and were given in sequential fashion such that no two image-pairs would repeat in any eight consecutive trials (e.g., C3, C1, C2, C4, NC2, NC3, NC1, NC4; Fig. 1C). Images always appeared in the same pairings and were not interchanged, and each corresponding transition between image-pairs always differed (e.g., C4–NC2 versus C2–NC1, etc.). To further limit potential expectancy of such transitions, we also randomly added three C or NC trials in half of sets but reversed the locations of the images from those shown four trials before (see also further analyses below) (Wheatley et al. 2005).
Retrieval performance
The monkeys were trained to near optimal performance in order to ensure consistency across image-pairs. Focusing first on the NC and C image-pairs, the monkeys performed a total of 2120 C and 2098 NC trials across seven retrieval sessions, of which 396 and 390 trials represented NC–C or C–NC transitions, respectively. As mentioned above, both C and NC image-pairs were well-trained prior to proceeding to the main task. Consistently, behavioral performances during retrieval were 98.4% and 98.7% for C and NC image-pairs, respectively, and were statistically similar (χ2 test, P = 0.47). Performance for the first C trial following the NC–C transition was 99.5% and performance for the first NC trial following the C–NC transitions was 99.0%, both of which were similar to performances of the other nontransitional C–C and NC–NC trials (χ2 test 2 × 4 contingency, P = 0.51; Fig. 2A, gray). Reaction times were 204 ± 43 msec for the first trial following NC–C transitions and 204 ± 41 msec for the first trial following C–NC transitions (mean ± SEM; t-test, P = 0.97). Similarly, reaction times were 209 ± 42 msec for all other NC–NC trials and 204 ± 43 msec for all other C–C trials (t-test, P = 0.86; Fig. 2B, black).
Figure 2.
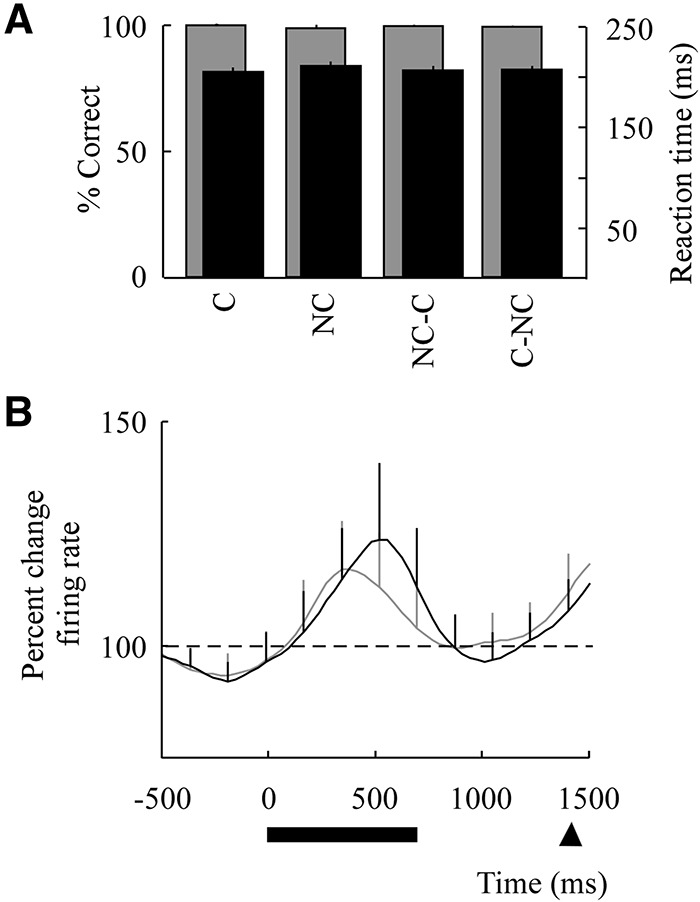
Behavioral performances and population response. (A) Mean behavioral performances based on percent correct and saccade reaction times. Performances are aligned to the 1+ trial following all C–NC and NC–C transitions versus all other C and NC trials. Percent correct is in gray and reaction time is in black ±SEM. (B) Mean population response aligned to trial onset for all C (gray line) and all NC (black line) image-pairs (normalized to baseline fixation; see Materials and Methods). The horizontal solid line indicates the time of image presentation and the arrow indicates the earliest go cue presentation. Error bars indicate SEM.
Neural responses in the VLPFC during dynamic retrieval
We recorded from 157 well-isolated single neurons from the VLPFC (Fig. 1A, right), of which 37 demonstrated a significant firing rate modulation during the image presentation period compared with baseline (fixation period; two-tailed t-test, P < 0.05). While many cells also responded to image selection and reward, we focused here only on cells that responded to the image presentation period; as in prior reports (Asaad et al. 1998; Rainer et al. 1998; Smith et al. 2004). Peak activity for these cells appeared 500 msec after image presentation and shortly before the working memory delay period. Of the cells that demonstrated a change in firing activity, population responses were statistically similar when comparing C to NC trials (t-test, P = 0.25; Fig. 2B).
Next, we examined neural responses based on the serial position of the retrieved image-pairs to each NC–C and C–NC transition. This was done by aligning all C trials to the NC–C transition and all NC trials to the C–NC transition, and then determining their serial order (i.e., 1+, 2+, 3+ … trials after the transition). While no significant difference was found in the population response between C and NC image-pairs, responses were significantly higher on the 1+ NC trial following the C–NC transition when compared with the 4+ NC trial (two-tailed t-test, Bonferroni corrected, P = 0.0071; Fig. 3A,C). Responses on the 1+ NC trial were also higher than responses during the previous C trial (t-test, P = 0.045) and responses on 1+ NC trial were higher than the 1+ C trial (t-test, P = 0.012). Results were similar across the two primates. Neurons in the first primate demonstrated a 50% ± 36% increase in firing activity when comparing the 1+ NC to 4+ NC trials following the C–NC transition (t-test, P = 0.084) and neurons in the second primate demonstrated a 48% ± 15% increase in firing activity (t-test, P = 0.033). Individually, 28 cells demonstrated an increase in activity when comparing the 1+ to 4+ NC trial positions following the C–NC transition and 9 cells demonstrated a decrease, with 64% of cells demonstrating a significant change (t-test, P < 0.05; Fig. 4A).
Figure 3.
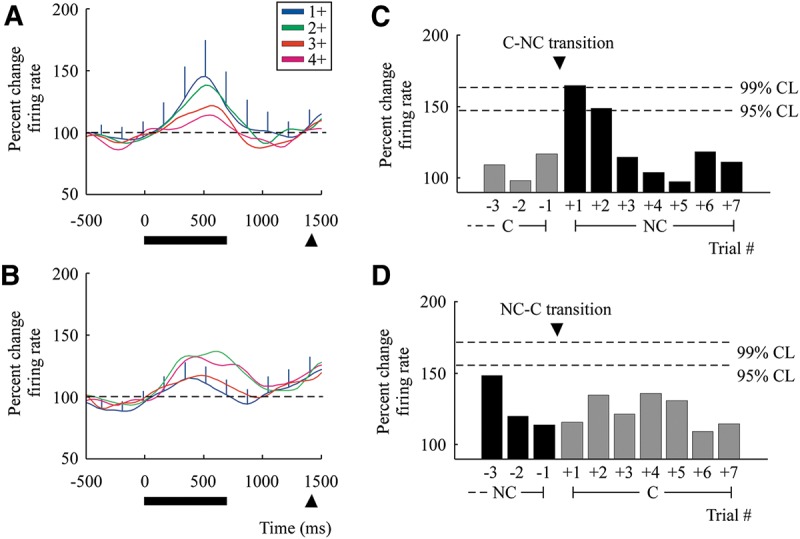
Neural responses when transitioning between consolidated and nonconsolidated image-pairs during retrieval. Mean responses for the population aligned to the C–NC transitions in A,C and to the NC–C transitions in B,D. (A) Mean responses are given based on the serial order of NC trials in relation to the C–NC transitions (e.g., 1+ indicates the first NC trial following a C trial). Note that SEM is only given for the 1+ trial following the C–NC and NC–C transitions to aid with clarity (see also Fig. 7 for SEM on all population curves). (B) Mean responses are given based on the serial order of C trials in relation to the NC–C transitions. (C) Differences in population responses across individual trials and their 95% and 99% confidence bounds following C–NC transitions. Here, +1 represents the first NC trial following the C–NC transition, with trials +5 to +7 representing the three trial positions added on half of the sets. Also, all trials in the graph are aligned to transitions that are staggered by four or eight trials. Therefore, trial −3 on the NC–C transition figure corresponds to trial +2 following half of C–NC transitions on the previous block. (D) Differences in population responses across individual trials following NC–C transitions.
Figure 4.

Single cell responses. (A) Peri-stimulus histograms (above) and rasters (below) for a single cell following C–NC transitions. The firing rate of this cell is higher on the 1+ NC trial (blue) immediately following a C–NC transition compared with the 4+ trial (magenta). (B) Firing rate of the same cell (shown above) is lower on the 1+ C trial (blue) immediately following a NC–C transition compared to the 4+ trial (magenta).
Neuronal responses in the VLPFC differentiated between C–NC and NC–C transitions. Unlike C–NC transitions, neural activity following NC–C transitions demonstrated a slight but nonsignificant decrease in activity (t-test, P = 0.030; Fig. 3B,D). Individually, 21 cells demonstrated a significant increase in activity following the NC–C transition and 16 cells demonstrated a decrease, with 32% of cells demonstrating a significant change (t-test, P < 0.05; Fig. 4B). However, there was no significant difference when comparing the 1+ C trial to the preceding NC trial (t-test, P = 0.54).
Neural responses during dynamic retrieval did not reflect memory-independent task-switching
Differences in the time interval between learning and retrieval could have produced changes in neuronal responses due to task-set switching rather than memory-related factors. To examine whether such differences in temporal novelty or task-set switching contributed to the observed neuronal responses (Williams et al. 2004; Liston et al. 2006; Sheth et al. 2012), we additionally examined responses of MC image-pairs. Like NC image-pairs, MC image-pairs were learned approximately three before the monkeys learned C image-pairs (i.e., MC image-pairs were learned 1 d before NC image-pairs and C image-pairs were learned 3 mo before NC image-pairs). However, unlike NC image-pairs, they were retrieved with no rehearsal for at least 24 h (Bontempi et al. 1999; Stickgold et al. 2002; Dudai 2004; Maviel et al. 2004; Walker and Stickgold 2004; Wiltgen et al. 2004; Shema et al. 2007). Consistently, we identified retrieval performance on MC trials at 98.6% over 1425 trials. This was similarly true when considering only the first 10 trials of each session (98.4%). As expected, performance on MC trials was slightly lower than performances of the C image-pairs (χ2 test, P = 0.022). Sixty cells were recorded during these trials (t-test, P < 0.05; MC image-pair and NC image-pair retrieval sessions were recorded separately).
We observe that neural activity on the 1+ NC trial following C–NC transitions was significantly higher than neural activity on the 1+ MC trial following C–MC transition (unpaired t-test, P = 0.0013). There were also significantly more cells that demonstrated a change in firing rate on the 1+ to 4+ trial following the C–NC transition compared with C–MC transitions (62% versus 11%, respectively; χ2 test, P = 0.002). With regard to the MC image-pairs themselves, there was little difference in activity when comparing the 1+ MC trial to the 4+ MC trial position (t-test, P = 0.42) or the 1+ MC trial to the prior C trial position (t-test, P = 0.76). In contrast, when comparing MC–NC transitions, we did observe a significant difference in response. Sixty-eight cells were recorded, of which 21 demonstrated a change in activity during image presentation compared with baseline. Similar to the C–NC transitions, we find an enhancement in activity on the 1+ NC trial compared with the 4+ NC trial when following the MC–NC transition (t-test; P = 0.0010). These data, therefore, provided strong support that neuronal responses in the VLPFC during dynamic retrieval were not being modulated by covert differences in behavior or simple task-specific events. This conclusion is also supported by the fact that all task requirements and variables such as image location, motor response, reward, and timings were kept identical across trial types.
Neural responses do not reflect a difference in expectancy and behavioral response
No two image-pairs repeated in any successive eight trials and the length of trial repetitions were randomly varied during the task. Nonetheless, to examine whether an expectancy of the transition between C and NC image-pairs may have influenced neural activity, we looked at sets in which three additional trials were randomly added to the block sequence. We then evaluated activity between the 4+ and 5+ C trial following the NC–C transitions (i.e., when transition to an NC image-pair would be expected to occur on half of trials). During these trials, however, we found no increase in activity between the 4+ and 5+ C trials (t-test, P = 0.38) or between the 4+ C trial and all subsequent C trial positions (P = 0.43). Behaviorally, there was also no difference in retrieval performance (χ2 test, P = 0.63) or reaction times (t-test, P = 0.97) at the beginning versus end of individual C sets.
We also examined whether VLPFC neurons may concurrently signal simpler changes in motor-related behavior (Williams et al. 2004). When the monkeys altered saccade directions on successive trials (e.g., left–left–left–right or vice versa), there was little difference in neural activity between the 1+ trial following the switch and the same-positioned trial in which no switch occurred (t-test, P = 0.74). There was also no difference in activity between the 1+ trial following the switch and the preceding trial (t-test, P = 0.82).
Memory-specific effect of VLPFC stimulation on retrieval performance
Given the above findings, we hypothesized that transiently disrupting VLPFC activity by event-related microstimulation (Fig. 1A, right) may lead to a change in retrieval performance when transitioning between C and NC image-pairs. Brief series of electrical pulses were delivered to the VLPFC on half of 925 random retrieval trials (1000 msec triggered at image presentation; 100 μA, 200 μsec biphasic pulse durations with cathodal phase leading; see Materials and Methods). We observed that stimulation did not differentially affect the animals’ behavior when considering retrieval performances across all C and NC trials outside the point of C–NC or NC–C transition. Performance for NC image-pairs was 96.7% with stimulation (i.e., ON stimulation) and 96.5% without stimulation (i.e., OFF stimulation). Performances for C image-pairs was 95.1% with ON stimulation and 96.3% with OFF stimulation (χ2 test 2 × 4 contingency, P = 0.91).
In contrast, when comparing ON to OFF stimulation for all 1+ NC trials following the C–NC transitions, stimulation led to a marked reduction in retrieval performance from 92.0% to 61.9% (χ2 test, P = 0.005; Fig. 5A). Moreover, the effect of ON stimulation on the 1+ NC trial following the C–NC transition was markedly significant when compared to all other ON stimulation NC trials (χ2 test, P = 0.0001). A smaller but nonsignificant reduction in performance was noted on the 2+ NC trial following the C–NC transition (χ2 test, P = 0.25).
Figure 5.

Effect of VLPFC stimulation on retrieval performance. (A) Retrieval performances following C–NC transitions are given as a percentage of correct/incorrect trials. Here, +1 represents the first NC trial following the C–NC transition. Black bars indicate performances on OFF stimulation trials (i.e., nonstimulated trials) and gray bars indicate performances during ON stimulation trials. (B) Behavioral performances in relation to the NC–C transitions. Error bars indicate SEM. (*) P = 0.005, (**) P = 0.0001.
No reduction in performances was found when comparing the 1+ ON to OFF stimulation trial following the NC–C transitions (χ2 test, P = 0.62; Fig. 5B). There was also no difference in retrieval performances on the 1+ MC trial following C–MC transitions when comparing ON to OFF stimulation trials (1051 trials; χ2 test, P = 0.55). Finally, there was no difference in performance between the 1+ trial following the C–MC transition when compared with other ON stimulation trials (χ2 test, P = 0.68). Therefore, transient disruption of VLPFC activity led to a specific degradation in retrieval performance when specifically altering between items originally learned at different times.
Evaluating the effect of VLPFC stimulation on motor responses
We investigated whether changes in retrieval performance may be associated with a simpler behavioral effect. VLPFC stimulation led to a reduction in retrieval performance during C–NC transitions but did not affect performance on other C, NC, or MC trials, making it unlikely that stimulation produced a nonmemory-related response (e.g., by disrupting saccade or affecting concentration). Nonetheless, we examined this possibility more directly by comparing the monkey's reaction times, eye fixation position, and saccade responses on all ON and OFF stimulation trials. We find that mean horizontal and vertical eye positions remained the same when comparing ON and OFF stimulation trials (during the 1000-msec time-window in which stimulation was given; t-test, P = 0.48 and P = 0.53, respectively; Fig. 6). There was also no difference in mean saccade reaction time when comparing ON versus OFF stimulation trials (t-test, P = 0.83). With regard to the monkeys’ response selections, stimulation did not lead to a higher likelihood of selecting the right versus left targets (χ2 test, P = 0.97), and did not affect the animal's ability to transition between alternate saccade directions on successive trials (e.g., left–left–left–right; χ2 test, P = 0.45). Therefore, consistent with our physiological findings, VLPFC stimulation did not affect other unrelated behavioral processes such as attention or motor selection.
Figure 6.

Eye position with and without stimulation. Each dot indicates the mean eye position during an individual trial (0–1000 msec from image onset) across a sample session. ON stimulation trials are shown in gray and OFF stimulation trials are shown in black. The monkeys fixated within a 2° window (discretized here from −10 to 10 in each axis).
Discussion
Despite ongoing progress in our understanding of how memories are encoded and stored over time (Eichenbaum 2000, 2004; Hoffman and McNaughton 2002; Stickgold et al. 2002; Dudai 2004; Maviel et al. 2004; Remondes and Schuman 2004; Walker and Stickgold 2004; Takehara-Nishiuchi and McNaughton 2008), the neural basis by which we dynamically retrieve memories over varying temporal scales has remained far less well understood. In this study, we find that, while neurons in Brodmann area 45 of the VLPFC displayed little differential response between C and NC image-pairs overall, they demonstrated a selective enhancement of response during C–NC transitions. The VLPFC, however, displayed a significantly smaller differential response following C–MC transitions suggesting that these neuronal signals did not reflect a simple task-switch response or novelty effect. Similarly, VLPFC neurons did not reflect a difference in image expectancy and behavioral response. Finally, focal disruption of VLPFC activity by stimulation led to a selective reduction in recall performance when the monkeys transitioned from recalling C to NC image-pairs, but demonstrated little influence when the monkeys were retrieving NC image-pairs at other time points during the trial (Fig. 7).
Figure 7.

Mean population responses following C–NC and NC–C transitions. Here, SEM is shown separately for all serial trial positions (same convention as Fig. 3A).
Taken together, these findings support the proposed role of prefrontal neurons in dynamically allocating cognitive resources based on task conditions (Tomita et al. 1999; Miller and Cohen 2001; Koechlin et al. 2003; Miyashita 2004; Sheth et al. 2012) as well as the known connectivity of the VLPFC with other associative areas (Bontempi et al. 1999; Eichenbaum 2000; Kluwe et al. 2003; Maviel et al. 2004; Miyashita 2004; Gerbella et al. 2010). Here, however, the resource in question is that of memory and is based on whether the need arises to access memories that had initially formed at different times. Therefore, when image-pairs are successively retrieved from the same “memory store,” the VLPFC remained relatively quiescent. However, when the animals alternated between retrieving image-pairs held in different memory stores, neural response in the VLPFC changed.
The VLPFC, however, did not play an equal role in transition to and from short-term memory. For example, NC–C transitions were associated with a slight and nonsignificant decrease in activity. Similarly, VLPFC stimulation led either to a diminishment or no change in retrieval performance following NC–C transitions suggesting that this area played a role in the transition from long- to short-term memory retrieval. This therefore suggested that the VLPFC plays a selective role in the transition to retrieval from short-term memory. Alternatively, it may be that areas other than the VLPFC are responsible for signaling such transition or simply that spiking in the VLPFC is too low to identify an effect. In particular, most phasic, well-isolated prefrontal neurons fire at low baseline rates and, therefore, identifying drops in rate require many more trials than increases in rate (Asaad et al. 1998; Sheth et al. 2012).
Findings from the present study may have relevance to certain memory processes such as reconsolidation and read-retrieval-review strategies commonly used in many school systems. For example, reconsolidation involves the disruption of long-term memories by their presumptive return to a labile state akin to that present shortly after initial learning (Walker et al. 2003; Schiller et al. 2010), whereas read-retrieval-review strategies rely on the constant back-and-forth between retrieving well-established memories and retrieving information that has been only recently acquired (Karpicke et al. 2009; McDaniel et al. 2009). Based on the above stimulation experiments, it may be possible to selectively disrupt this function with potential application to reconsolidation-based approaches for diseases such as addiction or post-traumatic stress disorder (O'Brien et al. 1990; Schiller et al. 2010). These findings, therefore, provide an important new avenue for modulating memories based on the timing of their initial formation.
Materials and Methods
Trial structure
All procedures were approved by the Massachusetts General Hospital institutional review board and were conducted under IACUC-approved guidelines. Two adult male Rhesus monkeys performed a task in which they were required to saccade to the rewarded of two images randomly displayed on the left and right of a screen (Fig. 1B). The image-pairs were stimulus–reward image-pairs and, therefore, required the monkeys to determine which image per pair was associated with receipt of reward. At the onset of each trial, the monkeys maintain fixation on a central point for 700 msec (baseline fixation). Following this, two images were displayed for 700 msec, with the locations of the images being given randomly on the left versus right of the screen (image presentation). The images were then erased, and a blank screen would be shown for another variable delay of 700–900 msec (delay period). Finally, a central go cue (central green circle) would appear indicating that the monkeys could saccade to one of the two previously displayed images. Saccade to only one of the two images led the monkey to receive reward (which image was associated with reward was based on prior trial-and-error training; see below). The task was run using a customized software package written in MATLAB (MathWorks, Inc., Natick, MA) that provided millisecond temporal precision of all task events (Asaad and Eskandar 2008).
Image-pair training
Image-pairs were learned at different times before being retrieved. Based on prior definition and behavioral performance metrics memory (Eichenbaum 2000, 2004; Hoffman and McNaughton 2002; Stickgold et al. 2002; Dudai 2004; Maviel et al. 2004; Remondes and Schuman 2004; Walker and Stickgold 2004; Takehara-Nishiuchi and McNaughton 2008), we identified image-pairs that were learned 1 h prior to retrieval as not having begun consolidation (NC), image-pairs that were learned 3 mo before as having begun consolidation (C) and, finally, image-pairs that were learned 24 h prior to retrieval as having begun consolidation (MC; see precise timelines below).
In order to ensure that the monkeys learned all C, NC, and MC image-pairs to similar high performance, all were trained using the same interleaved tapering training protocol (Kahana and Howard 2005). The primates learned, by trial-and-error, 4 distinct image-pairs for each of the C, NC, and MC image-pair types. Initial training for all C, NC, and MC image-pairs was made in identical fashion. Following is an example of how an NC image-pair (e.g., NC1–NC1′ image pair) would have been trained together within a set of three other NC image-pairs (e.g., NC2–NC2′, NC3–NC3′, and NC4–NC4′) on a given session and day (here, the number indicates the specific identity of the image pair and the apostrophe indicates the rewarded image; Fig. 1C).
At the beginning of the day, the image-pair would be repeatedly presented over 40 consecutive trials, with the locations of the images being randomly displayed on the right and left side of the screen per trial. The monkey would have to learn, by trial-and-error, which one of the two images was rewarded by making saccades to their respective locations. After training on that image pair for 40 trials, the monkeys would then be given another completely different image pair (i.e., of the three remaining pairs) and have to similarly learn which image per pairing was associated with reward. In total, all four image-pairs would be trained over 40 × 4 trials. Next, the same four image-pairs would be presented again, in a tapering fashion, over 10 × 8, 2 × 16, and 1 × 100 consecutive trials. For example, for the 10 × 8 trial set, the monkeys would perform the same image-pair 10 times in a row before moving on to a different image-pair, and then later repeat again for a total of two repetitions per 10 × 8 set. The sequence in which each of the four image-pairs was given within the interleaved tapering protocol was made randomly in order to encourage the monkeys to learn all four image-pairs (per C, NC, or MC set) to equal performance. Therefore, in sum, the primates learned the four image-pairs over a total of 372 trials. For each set of image-pairs, we confirmed that the monkeys successfully learned each of the four image-pairs to high accuracy by demonstrating a >95% correct performance per image-pair over the last 100 trials.
All C, MC, and NC image-pairs were trained in the same fashion (above). The principal difference between them is the time interval between initial training and subsequent retrieval. C image-pairs were retrieved ∼3 mo after initial training, MC image-pairs were retrieved 1 d after initial training, and NC image-pairs were retrieved ∼1 h after the end of training. We confirmed >95% performance for C and MC image-pairs by rehearsing the image-pairs over 100 trials the days prior to the recording/main retrieval task. If the monkeys did not recollect all four image-pairs with >95% accuracy, they did not proceed to the main session. We excluded any sessions in which the C image-pairs were not correctly remembered. The monkeys did not successfully retrieve two sets of image-pairs (5.1% of all trained image-pairs) and did not proceed to the main retrieval task for these sets.
Main retrieval task
Once the monkeys learned the different image-pairs, they proceeded to performing the main retrieval task. The animals either performed successive sets of …C–NC–C–NC… image-pairs or …C–MC–C–MC… image-pairs. The sequences in which the four NC, four MC and four C image-pairs were presented in each successive set were given randomly such that the sequence in one set may be …NC2–NC3–NC1–NC4… whereas, in another, it may be …NC1–NC4–NC3–NC2...Similarly, the transition between individual image-pairs could be …C3–NC2… in one pair of trials whereas, in another, it may be …C1–NC4… The locations of the rewarded images (i.e., left versus right) were also given randomly on each trial (Fig. 1C).
This, therefore, allowed neural activity to be examined in direct relation to the transition between image-pairs separately from the identities of the individual image-pairs themselves (as C1 versus C2, etc.), their particular sequence within sets or the animal's upcoming saccade direction. Finally, on half of sets, we randomly added three trials in order to limit ability of the animals to expect these transitions. Here, three of the four preceding C, NC, or MC image-pairs were repeated but the locations of images themselves on the left and right of the screen were switched (e.g., C1–C1′ versus C1′–C1). Importantly, the task requirements were always the same—in essence, to correctly retrieve the displayed image-pair, with the difference being the time in which the image-pairs were originally learned. This is in contrast to studies in which the task requirements, rules and/or sensorimotor contingencies differ between conditions (Williams et al. 2004; Johnston et al. 2007; Sheth et al. 2012).
Single-unit isolation and recordings
Multiple silicone multielectrode arrays (NeuroNexus Technologies Inc.) were surgically implanted in each monkey (Nicolelis 2008). A craniotomy was placed over Brodmann area 45 with three to four arrays being implanted in each monkey (Fig. 1A, right). The array was advanced ∼2 mm into the cortex with the electrode contacts spanning the bottom 1.6 mm of each shank. Confirmation of electrode positions was done in both monkeys by direct visual inspection of the sulci and gyral pattern through the craniotomy (i.e., with respect to the inferior bank of the principal sulcus and anterior bank of the inferior arcuate sulcus).
Recordings began 2 wk following surgical recovery. A Plexon multichannel acquisition processor was used to amplify and band-pass filter the neuronal signals (150 Hz—8 kHz; 1 pole low-cut and 3 pole high-cut with 1000× gain; Plexon Inc.). Shielded cabling carried the signals from the electrode array to a set of six 16-channel amplifiers. Neural signals were then digitized at 40 kHz and processed to extract action potentials by the Plexon workstation. Classification of the waveforms was performed using template matching and principle component analysis based on waveform parameters. Only single, well-isolated units with identifiable waveform shapes and adequate refractory periods were used. When an individual electrode recorded more than one neuron, a high degree of isolation was required in order to include each as a single-unit (P < 0.01, multivariate ANOVA across the 1+ two principal components). We did not include multiunit activity.
Electrical stimulation protocol
During stimulation trials, the monkeys performed the same task as before including the same training protocol and interleaved retrieval task design. During retrieval, however, a brief series of electrical stimulation pulses were given at the onset of half of trials. These trials were selected randomly irrespective of the image-pair type (i.e., C versus NC) or transition type (i.e., C–NC versus NC–C). Each stimulus run, per trial, lasted for 1000 msec and was triggered at the onset of image presentation (and prior to the go cue). Stimulation parameters were 100 μA and 200 Hz biphasic pulses, with cathodal phase leading. Average impedance at the time of the stimulation experiments was 100–500 kΩ. Here, all 32 electrode contacts were simultaneously stimulated per array (Fig. 1A, right).
Statistical analysis
Behavioral performance was defined as total number of correct versus incorrect trials performed by the animals. Reaction time was defined as the time it took the animals to saccade to the circular targets after go cue presentation. Differences in behavioral performance, based on percent of correct trials, were evaluated by χ2 analysis based on 2 × 2 and 2 × 4 contingency tables (P < 0.05). Differences in reaction times were evaluated by a two-tailed Student's t-test (P < 0.05).
Peri-stimulus histograms and rasters were constructed for all single units. A cell was considered to be modulated by the task if their firing rate during image presentation was significantly different from baseline (two-tailed Student's t-test, P < 0.05). Activity during the small proportion of incorrect trials was excluded. To further evaluate activity for all modulated cells in the population, firing rates for each neuron were normalized by dividing the firing rate during the image presentation period by that of the baseline fixation period (i.e., prior to image presentation). For all main analyses, differences in neuronal responses were analyzed over a 500-msec interval centered at 500 msec from the time of image presentation. We assessed differences in activity between image-pair types (e.g., C versus NC) and differences in activity based on their serial trial position (i.e., the 1+ versus 4+ trial following a C–NC transition) with a two-tailed Student's t-test (Bonferroni corrected for comparison across four periods, P < 0.0125). Determining differences in activity during the transition (e.g., before versus after the C–NC transition itself) were done by single trial comparison using a two-tailed Student's t-test without correction (P < 0.05). All appropriate values were expressed with their standard error of the mean (±SEM). In order to quantify the confidence limits (CL) for differences in activity across the population, we performed a bootstrap analysis. The bootstrap is essentially a Monte Carlo stimulation where no parametric assumptions are made about the underlying population that generated the sample. Here, we randomly sampled 100 times, with replacement, from all trial that were not 1+ NC in the session for a total of 4.2 × 106 trial permutations. From this, we estimated the distribution of firing rates for all trials (i.e., the bootstrap replicates). By sorting the firing rates from this randomly sampled distribution, going from lowest to highest, we then determined the confidence limits (bootstrap test, 99% and 95% confidence bounds; Ho: 50%) (Davidson and Hinkley 1997).
Acknowledgments
This study was funded by the NIH 5R01-HD059852, the White House Presidential Early Career Award for Scientists and Engineers, the Whitehall Foundation and the NREF. We thank K. Haroush, W. Assad, J. Asaad, and S. Patel for their insightful discussion and critical review of the manuscript as well as M. Powers for her contribution to animal care.
Footnotes
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.036806.114.
References
- Asaad WF, Eskandar EN 2008. Achieving behavioral control with millisecond resolution in a high-level programming environment. J Neurosci Methods 173: 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Eskandar EN 2011. Encoding of both positive and negative reward prediction errors by neurons of the primate lateral prefrontal cortex and caudate nucleus. J Neurosci 31: 17772–17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK 1998. Neural activity in the primate prefrontal cortex during associative learning. Neuron 21: 1399–1407. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD 2005. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron 47: 907–918. [DOI] [PubMed] [Google Scholar]
- Bontempi B, Laurent-Demir C, Destrade C, Jaffard R 1999. Time-dependent reorganization of brain circuitry underlying long-term memory storage. Nature 400: 671–675. [DOI] [PubMed] [Google Scholar]
- Davidson AC, Hinkley DV 1997. Bootstrap methods and their applications, Cambridge University Press, Cambridge. [Google Scholar]
- Dudai Y 2004. The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55: 51–86. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H 2000. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci 1: 41–50. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H 2004. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron 44: 109–120. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA 2006. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440: 680–683. [DOI] [PubMed] [Google Scholar]
- Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G 2010. Cortical connections of the macaque caudal ventrolateral prefrontal areas 45A and 45B. Cereb Cortex 20: 141–168. [DOI] [PubMed] [Google Scholar]
- Hasegawa I, Fukushima T, Ihara T, Miyashita Y 1998. Callosal window between prefrontal cortices: cognitive interaction to retrieve long-term memory. Science 281: 814–818. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, McNaughton BL 2002. Coordinated reactivation of distributed memory traces in primate neocortex. Science 297: 2070–2073. [DOI] [PubMed] [Google Scholar]
- Inostroza M, Born J 2013. Sleep for preserving and transforming episodic memory. Annu Rev Neurosci 36: 79–102. [DOI] [PubMed] [Google Scholar]
- Johnston K, Levin HM, Koval MJ, Everling S 2007. Top-down control-signal dynamics in anterior cingulate and prefrontal cortex neurons following task switching. Neuron 53: 453–462. [DOI] [PubMed] [Google Scholar]
- Kahana MJ, Howard MW 2005. Spacing and lag effects in free recall of pure lists. Psychon Bull Rev 12: 159–164. [DOI] [PubMed] [Google Scholar]
- Karpicke JD, Butler AC, Roediger HL III 2009. Metacognitive strategies in student learning: do students practise retrieval when they study on their own? Memory 17: 471–479. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW III, Cho RY, Stenger VA, Carter CS 2004. Anterior cingulate conflict monitoring and adjustments in control. Science 303: 1023–1026. [DOI] [PubMed] [Google Scholar]
- Kluwe RH, Lüer G, Rosler F 2003. Principles of learning and memory. Birkhauser, Basel. [Google Scholar]
- Koechlin E, Ody C, Kouneiher F 2003. The architecture of cognitive control in the human prefrontal cortex. Science 302: 1181–1185. [DOI] [PubMed] [Google Scholar]
- Liston C, Matalon S, Hare TA, Davidson MC, Casey BJ 2006. Anterior cingulate and posterior parietal cortices are sensitive to dissociable forms of conflict in a task-switching paradigm. Neuron 50: 643–653. [DOI] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B 2004. Sites of neocortical reorganization critical for remote spatial memory. Science 305: 96–99. [DOI] [PubMed] [Google Scholar]
- McDaniel MA, Howard DC, Einstein GO 2009. The read-recite-review study strategy: effective and portable. Psychol Sci 20: 516–522. [DOI] [PubMed] [Google Scholar]
- McIntosh AR 1999. Mapping cognition to the brain through neural interactions. Memory 7: 523–548. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24: 167–202. [DOI] [PubMed] [Google Scholar]
- Miyashita Y 2004. Cognitive memory: cellular and network machineries and their top-down control. Science 306: 435–440. [DOI] [PubMed] [Google Scholar]
- Nicolelis MAL 2008. Methods for neural ensemble recordings, 2nd ed. CRC Press, Boca Raton, FL: Frontiers in Neuroscience. [PubMed] [Google Scholar]
- O'Brien CP, Childress AR, McLellan T, Ehrman R 1990. Integrating systemic cue exposure with standard treatment in recovering drug dependent patients. Addict Behav 15: 355–365. [DOI] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK 1998. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393: 577–579. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM 2004. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature 431: 699–703. [DOI] [PubMed] [Google Scholar]
- Rygula R, Walker SC, Clarke HF, Robbins TW, Roberts AC 2010. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. J Neurosci 30: 14552–14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA 2010. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature 463: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusterman RJ 1962. Transfer effects of successive discrimination-reversal training in chimpanzees. Science 137: 422–423. [DOI] [PubMed] [Google Scholar]
- Shema R, Sacktor TC, Dudai Y 2007. Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317: 951–953. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN 2012. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488: 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Frank LM, Wirth S, Yanike M, Hu D, Kubota Y, Graybiel AM, Suzuki WA, Brown EN 2004. Dynamic analysis of learning in behavioral experiments. J Neurosci 24: 447–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Fosse R, Walker MP 2002. Linking brain and behavior in sleep-dependent learning and memory consolidation. Proc Natl Acad Sci 99: 16519–16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara-Nishiuchi K, McNaughton BL 2008. Spontaneous changes of neocortical code for associative memory during consolidation. Science 322: 960–963. [DOI] [PubMed] [Google Scholar]
- Tomita H, Ohbayashi M, Nakahara K, Hasegawa I, Miyashita Y 1999. Top-down signal from prefrontal cortex in executive control of memory retrieval. Nature 401: 699–703. [DOI] [PubMed] [Google Scholar]
- van Wingerden M, Vinck M, Tijms V, Ferreira IR, Jonker AJ, Pennartz CM 2012. NMDA receptors control cue-outcome selectivity and plasticity of orbitofrontal firing patterns during associative stimulus-reward learning. Neuron 76: 813–825. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R 2004. Sleep-dependent learning and memory consolidation. Neuron 44: 121–133. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R 2003. Dissociable stages of human memory consolidation and reconsolidation. Nature 425: 616–620. [DOI] [PubMed] [Google Scholar]
- Wheatley T, Weisberg J, Beauchamp MS, Martin A 2005. Automatic priming of semantically related words reduces activity in the fusiform gyrus. J Cogn Neurosci 17: 1871–1885. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN 2004. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nat Neurosci 7: 1370–1375. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Brown RA, Talton LE, Silva AJ 2004. New circuits for old memories: the role of the neocortex in consolidation. Neuron 44: 101–108. [DOI] [PubMed] [Google Scholar]


