Abstract
A recent prospective longitudinal neuroimaging study of 274 prodromal risk syndrome subjects revealed that those who later developed full-blown psychotic symptoms had exhibited accelerated gray matter loss and third ventricle expansion around the time of psychosis onset. Previous studies also indicate that higher levels of unusual thought content during prodromal states are a significant predictor of psychosis in clinically high-risk (CHR) youth. However, the relationship between clinical symptoms and changes in neuroanatomical structure has not been previously examined in the North American Prodrome Longitudinal Study (NAPLS) sample at the whole-brain level. In this report, we investigated whether symptom severity as measured by the Scale of Prodromal Symptoms (SOPS) predicted the accelerated gray matter decline in 274 CHR cases, including 35 who converted to psychosis. Higher levels of unusual thought content at baseline were associated with a steeper rate of gray matter loss in the prefrontal cortex bilaterally among converters. In contrast, there was no association found among nonconverters. Steeper gray matter loss seems to be unique to those (CHR) individuals with higher levels of subpsychotic predelusional symptoms that acutely worsen in the ramp-up to full-blown psychosis, and as such may reflect pathophysiological processes driving the emergence of psychosis.
Key Words: Schizophrenia, Psychosis, Prodromal risk syndrome, Magnetic resonance imaging, Scale of Prodromal Symptoms, Clinically high-risk youth
Introduction
Progressive gray matter reduction in prefrontal regions has been repeatedly observed among clinically high-risk (CHR) youths who develop full-blown psychotic illness [1,2,3,4,5,6,7,8]. As prefrontal cortical regions continue to mature via synaptic pruning and myelination throughout adolescence [9,10,11], an accelerated rate of synaptic pruning observed among individuals who develop psychosis may explain why the onset of psychosis commonly occurs during late adolescence [12,13,14,15,16]. A recent longitudinal neuroimaging study, with the largest sample of prodromal risk syndrome subjects studied to date (n = 274), revealed that individuals who converted to psychosis showed a steeper rate of gray matter loss in the prefrontal cortex and an increased rate of third ventricle expansion compared with those who did not convert and healthy controls [8]. However, the variability observed in the rate of change in cortical, subcortical, and ventricular structures, even among converters, implicates heterogeneity and individual differences in the pathophysiology of psychosis onset. A question of major importance is whether initial levels of prodromal symptom severity predict a steeper rate of cortical thinning, consistent with the theoretical view that increasing clinical symptom severity during the prodromal state is a consequence of increasing disruption in synaptic activity and functional connectivity in the brain, of which accelerated gray matter loss may be an indicator.
CHR patients are composed of help-seeking individuals with attenuated psychotic symptoms (i.e. psychosis-like symptoms of recent onset that are subpsychotic in intensity, duration, and level of insight) [17]. CHR individuals are considered to be at an imminent risk for psychosis: about 20-25% of them convert to psychosis within 1 year and 30-35% within 2 years [18,19,20]. Among many established instruments for assessing those at risk, the Scale of Prodromal Symptoms (SOPS) is an operationalized instrument developed and validated to quantitatively assess the severity of prodromal symptoms for psychosis [21,22,23]. In analyses of psychosis predictors in the first North American Prodrome Longitudinal Study (NAPLS) sample, 2 of the 19 items in the SOPS (i.e. P1 - unusual thought content/unusual ideas; P2 - severity of suspiciousness/persecutory ideas) were most independently predictive of conversion to full psychosis [19]. In particular, higher levels of unusual thought content (such as magical thinking and odd beliefs), rated by P1 symptom criteria in the SOPS, appeared to consistently offer the best prediction among the clusters of symptom ratings in multiple independent samples [19,24,25,26].
As a follow-up to the recently published NAPLS longitudinal neuroimaging study [8], we further investigated the relationship between the SOPS P1 symptom severity and neuroanatomical changes in CHR cases in the same NAPLS neuroimaging dataset. We hypothesized that the rate of gray matter reduction in the prefrontal cortex and expansion of the third ventricle would be greater among converters with higher ratings of unusual thought content as measured by SOPS P1 at baseline (BL) assessment.
Methods
Subjects
The study protocol and consent form were reviewed and approved by the Institutional Review Boards at each of the eight data collection sites (UCLA, Emory University, Beth Israel Deaconess Medical Center, Zucker Hillside Hospital, University of North Carolina, UCSD, University of Calgary, and Yale Unversity). The Structured Interview for Prodromal Syndromes (SIPS) [21] and the Structured Clinical Interview were used for axis I DSM-IV disorders [27] at each assessment. High reliability standards intraclass correlation coefficient (ICC) = 0.92-0.96] for interviewers were met [28]. CHR cases were defined as those who met SIPS/SOPS criteria for a psychosis risk syndrome [21], excluding cases who had ever met DSM-IV criteria for a psychotic disorder. Control participants who met criteria for a psychotic disorder, had a first-degree relative with a current or past psychotic disorder, or met CHR criteria were excluded. Also, general exclusion criteria included substance dependence, neurological disorder, or full-scale IQ <70.
The subjects with MRI structural data included in the analysis are the same cases that were utilized in our previous report, without any additional subjects [8]. Briefly, those with MRI scans were assessed at BL and at the 12-month follow-up (FU). For converters, MRI scans were performed at the point of psychosis conversion. Artifacts in one or both scans led to the exclusion of 14 additional subjects (2 converters, 10 nonconverters, and 2 controls). In total, 35 CHR cases who converted to psychosis, 239 CHR cases who did not convert, and 135 healthy comparison subjects had usable data and were included. The demographic characteristics of the three groups are shown in table 1.
Table 1.
Demographic characteristics, P1 symptom severity, and mean rates of change by group
| Characteristic | Converters (n = 35) | Nonconverters (n = 239) | Controls (n = 135) | Statistic | Post hoc Tukey's test |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 25 (71) | 146 (61) | 73 (54) | χ2 = 3.9, p = 0.13 | |
| Female | 10 (29) | 93 (39) | 62 (46) | ||
| Race | |||||
| Caucasian | 19 (54) | 134 (56) | 73 (54) | χ2 = 0.1, p = 0.92 | |
| Non-Caucasian | 16 (46) | 105 (44) | 62 (46) | ||
| Ethnicity | |||||
| Latino | 6 (17) | 47 (20) | 20 (15) | χ2 = 1.4, p = 0.49 | |
| Non-Latino | 29 (83) | 192 (80) | 115 (85) | ||
| Age at BL, years | 18.8 ± 3.8 | 19.7 ± 4.2 | 20.5 ± 4.6 | F = 2.7, p = 0.06 | |
| Parental education, years | 6.7 ± 1.4 | 6.2 ± 1.4 | 6.8 ± 1.4 | F = 7.7, p = 0.0005 | 2<1, 3 |
| Income level | 4.7 ± 1.9 | 4.7 ± 1.9 | 4.6 ± 1.8 | F = 0.1, p = 0.89 | |
| Interscan interval, years | 0.9 ± 0.5 | 1.0 ± 0.3 | 1.1 ± 0.4 | F = 7.1, p = 0.001 | 1<2, 3 |
| Duration, yearsa | 3.3 ± 3.1 | 2.5 ± 3.0 | – | F = 2.5, p = 0.12 | |
| Unusual thought content (P1) | |||||
| At BL | 2.2 ± 0.9 | 1.5 ± 1.0 | 0.01 ± 0.1 | F = 183.4, p < 0.0001 | 3<2<1 |
| At FU | 3.3 ± 1.2 | 0.7 ± 0.9 | 0.01 ± 0.1 | F = 284.5, p < 0.0001 | 3<2<1 |
| Suspiciousness (P2) | |||||
| At BL | 1.2 ± 1.1 | 1.1 ± 1.0 | 0 | F = 83.1, p < 0.0001 | 3<1, 2 |
| At FU | 2.2 ± 1.6 | 0.6 ± 0.9 | 0.01 ± 0.1 | F = 110.0, p < 0.0001 | 3<2<1 |
Values are presented as n (%) or means ± SD.
Information on the age at symptom onset was available for 35 converters and for 208 nonconverters.
MRI Scans
Five of the data collection sites used Siemens scanners and three sites used GE scanners, all at 3 T. All sites using Siemens scanners employed a 12-channel head coil and all sites using GE scanners employed an 8-channel head coil. Sequence parameters were optimized for each scanner manufacturer, software version, and coil configuration according to the Alzheimer's Disease Neuroimaging Initiative (ADNI) protocol (http://adni.loni.ucla.edu/research/protocols/mri-protocols/). Scans were acquired in the sagittal plane with a 1 × 1 mm in-plane resolution and a 1.2-mm slice thickness. Siemens scanners used an MPRAGE sequence with a 256 (axial) × 240 (sagittal) × 176 (coronal) mm field of view, TR/TE/TI = 2,300/2.91/900 ms, and a 9-degree flip angle, while GE scanners used an IR-SPGR sequence with a 26-cm field of view, TR/TE/TI = 7.0/minimum full/400 ms, and an 8-degree flip angle.
Image Processing
Identical image processing steps were utilized as in our previous report [8]. All MR images were processed using FreeSurfer version 5.2 (http://surfer.nmr.mgh.harvard.edu/). Surface-based cortical reconstruction was performed to extract thickness measures by calculating the shortest distance from each point on the gray/white boundary to the pial surface at each vertex, along with subcortical volumetric segmentation [29,30,31,32,33]. The subcortical segmentation procedure assigns a neuroanatomical label to each voxel of the MRI volume using a probabilistic atlas and a Bayesian classification rule [31]. The reconstructed BL and FU scans were further processed using FreeSurfer's longitudinal stream to extract change in thickness and volume estimates [34]. This processing stream initializes each time point scan by utilizing an unbiased within-subject template space and average images [35], created by robust, inverse consistent registration [36], which has been shown to significantly increase statistical power for detecting subtle changes over time [34].
Quality assurance checks were performed to verify all steps in the processing stream. Briefly, this process included visually inspecting the automated reconstruction for skull strip errors, intensity normalization failures, segmentation errors, white and pial surface misplacements, and topological defects. Nearly all scans passed a quality control assessment. A total of 14 subjects, distributed proportionally across the groups, were excluded from the analyses due to unrecoverable MRI artifacts. Control points were utilized to improve surface inaccuracies.
Scale of Prodromal Symptoms
Study participants were evaluated using the SIPS [22] for the presence of one or more CHR syndromes: attenuated subthreshold psychotic symptoms; brief intermittent psychotic symptoms, and substantial functional decline combined with a first-degree relative with a psychotic disorder. The SIPS was administered at initial assessment and at approximately 12 months of FU or at conversion. Based on the SIPS, the severity of symptoms was determined with the SOPS as follows: 0 = absent; 1 = questionably present; 2 = mild; 3 = moderate; 4 = moderately severe; 5 = severe but not psychotic, and 6 = severe and psychotic. The raters achieved high interrater reliability (κ > 0.80), as previously described [19].
Because the ratings ‘1 = questionably present’ and ‘2 = mild’ are considered subprodromal in quality, intensity, and/or frequency, differential gray matter changes among cases with scores of 0, 1, and 2, are not expected. Therefore, we grouped these categories together and rescored them all to 0. Then, syndromal level symptoms such as ‘moderate’ were scored ‘1’, ‘moderately severe’ was scored ‘2’, ‘severe but not psychotic’ was scored ‘3’, and ‘severe and psychotic’ was scored ‘4’ (table 2). This approach yielded a better distribution as there were only 2 converters with ratings in the subprodromal level at BL. Conversion to psychosis was determined by the SIPS criteria that are designed to operationalize the threshold of delusional ideation or hallucination severity required for a DSM-IV psychotic disorder diagnosis [37], as described in detail in prior studies [19,38].
Table 2.
Detailed description of the rescaled SOPS P1 and P2 symptoms
| Description | Severity rating |
||||
|---|---|---|---|---|---|
| 0 = absent/questionably present/mild | 1 = moderate | 2 = moderately severe | 3 = severe but not psychotic | 4 = psychotic/extreme | |
| P1: Unusual thought content | |||||
| Perplexity and delusional mood. Mind tricks, such as the sense that something odd is going on or puzzlement and confusion about what is real or imaginary. The familiar feels strange, confusing, ominous, threatening, or has special meaning. Sense that the self, others, or the world has changed. Changes in the perception of time. Déjà vu experience. Nonpersecutory ideas of reference. Overvalued beliefs. Preoccupation with unusually valued ideas. Magical thinking that influences behavior and is inconsistent with subculture norms. | Absent = no unusual thought content. | Unanticipated mental events/nonpersecutory ideas of reference/mind tricks/magical thinking that are not easily dismissed and may be irritating or worrisome. A sense that these experiences or compelling new beliefs are becoming meaningful because they will not go away. | Notion that experiences may be coming from outside the self or that ideas/beliefs may be real, but skepticism remains intact. Does not usually affect functioning. | Belief in reality of mind tricks/mental events/external control/magical thinking is compelling, but doubt can be induced by contrary evidence and others’ opinions. May affect functioning. | Delusional conviction (with no doubt) at least intermittently. |
| Unusual ideas about the body, guilt, nihilism, jealousy, and religion. | Questionably present = the person experiences mind tricks that are puzzling. Sense that something is different. Mild = overly interested in fantasy life. Unusually valued ideas/beliefs. Some superstitions beyond what might be expected by the average person but within cultural norms. | Usually interferes with thinking, social relations, or behavior. | |||
| P2: Suspiciousness/persecutory ideas | |||||
| Persecutory ideas of reference. Suspiciousness or paranoid thinking. Presents a guarded or even openly distrustful attitude that may reflect delusional conviction and intrude of the interview and/or behavior. | Absent = no suspiciousness. Questionably present = the person experiences wariness. Mild = doubts about safety. Hypervigilance without a clear source of danger. | Notions that people are hostile, untrustworthy, and/or harbor ill will easily. Sense that hypervigilance may be necessary. Mistrustful. Recurrent (unfounded or exaggerated) sense that people are thinking or saying negative things about the person. May appear mistrustful with the interviewer. | Clear or compelling thoughts of being watched or singled out. Sense that people intend harm. Beliefs easily dismissed. Presentation may appear guarded. Reluctant or irritable in response to questioning. | Loosely organized beliefs about danger or hostile intention. Skepticism and perspective can be elicited with nonconfirming evidence or opinion. Behavior is affected to some degree. Guarded presentation may interfere with ability to gather information in the interview. | Delusional paranoid conviction (with no doubt) at least intermittently. Likely to affect functioning. |
For the purposes of the present analysis, our focus was on SOPS item P1, indexing unusual thought content, which was the single best predictor of psychosis in NAPLS 1 [19] and also in other independent cohort studies [24,25,26]. For exploratory purposes, we also examined P2 - severity of suspiciousness/persecutory ideas, which also contributed to psychosis prediction (table 2) [19].
Statistical Analysis
Imaging measures were first transformed to annualized rates of change (ARCH) in each cortical voxel and each subcortical and ventricular region of interest (ROI), where ROIARCH = [(ROIFU - ROIBL)/ROIBL]/interval, and where interval is the time between BL and FU scans in years. Relationships between SOPS P1 and annualized gray matter percent change were tested vertex-wise, bilaterally, across the cortical surface. Monte Carlo simulations, one of the features implemented for multiple comparison correction in FreeSurfer, were used across the surface and synthesized with a cluster-forming threshold of p < 0.05 (two-sided) [39,40]. To provide a perspective on effects that might be below the statistical threshold, the rescaled P1 ratings were mapped onto the cortical surface with and without multiple comparison corrections.
The annualized rate of change of the third ventricle volume, extracted by FreeSurfer, was chosen as one of the dependent variables, as a significant expansion of this ventricular structure had been observed among converters relative to healthy controls and nonconverters in our previous study [8].
Neither age (p > 0.156) nor sex (p > 0.425) was significantly related to the annualized rate of percent change in the cortical thickness measures and the third ventricle. In addition, age and sex were not significant predictors of change in prefrontal or global cortical thickness within each group [8]. A test-retest reliability study with 8 travelling subjects showed high reliability in overall neuroanatomical measurements [8,41]. FreeSurfer-derived measures of cortical thickness and subcortical volume in the ROI that we utilized in the secondary analyses showed excellent reliability: superior frontal gyrus (left ICC = 0.908, right ICC = 0.921), rostral middle frontal gyrus (left ICC = 0.842, right ICC = 0.872), and third ventricle (ICC = 0.870). More information on the scanner reliability study can be found elsewhere [8,41].
Results
Demographic Characteristics
As shown in table 1, there were no significant differences between the diagnostic groups in age, sex, race, or socioeconomic status as measured by income. The converters showed greater BL P1 severity ratings than the nonconverters and healthy controls. In contrast, BL P2 ratings were not significantly different between converters and nonconverters among CHR individuals only. Consistent with previously reported findings [19,24,25,26], logistic regression analysis revealed that unusual thought content is a significant predictor of conversion among CHR individuals. For each 1-unit increase in P1 score, the odds ratio of being a converter increases by 2.35 (p < 0.0001). The DSM-IV psychotic disorder diagnosis after the conversion was known for 33 out of the 35 subjects who converted: schizophrenia (n = 8), schizophreniform disorder (n = 9), schizoaffective disorder (n = 2), bipolar disorder with psychotic features (n = 3), brief psychotic disorder (n = 1), and psychosis not otherwise specified (n = 10).
Whole-Brain Analyses
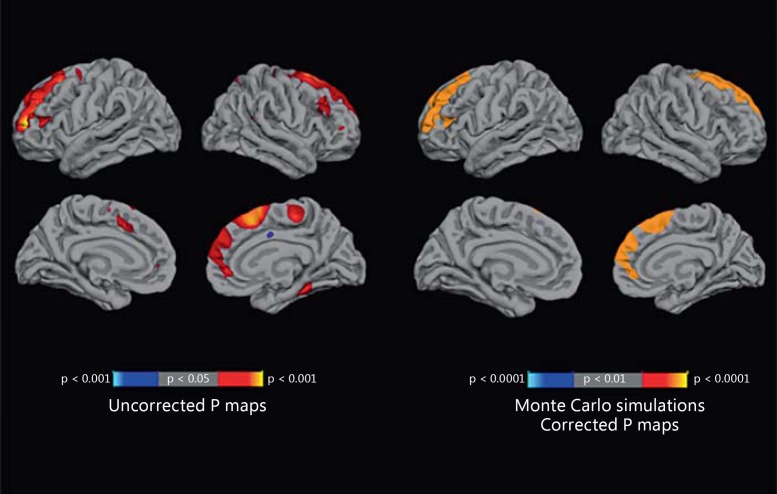
There were no significant differences between converters, nonconverters, and healthy controls in terms of cortical thickness or subcortical or third ventricular volume at BL [8], nor did BL thickness measures vary by SOPS P1 ratings among converters. P1-rescored values at BL were mapped onto the cortical surface maps representing the percent change in cortical thickness (fig. 1). Clusters of voxels covering the entire rostral middle frontal region (cluster size = 6,828.98 mm2, clusterwise p < 0.0001, corrected) in the left hemisphere and the superior frontal region (cluster size = 3,452.78 mm2, clusterwise p < 0.0001, corrected) in the right hemisphere survived the whole-brain analysis with cluster-based multiple correction, which showed significant negative correlations with P1 scores at BL. We also display the uncorrected statistical significance maps of the relationship between the annualized rate of change in cortical thickness and SOPS P1 scores, with a nominal p < 0.05 threshold. In the uncorrected maps, in addition to the regions previously identified using cluster-based correction for multiple comparisons, higher P1 ratings at BL predicted steeper rates of gray matter reductions in the right fusiform gyri and in the right paracentral region. The same set of analyses was done on nonconverters and healthy controls; in these groups, no association was found between the clinical symptom ratings and gray matter changes.
Fig. 1.
Statistical maps showing a significant association between P1 and the annualized rate of percent change at each vertex across the surface within the converters (n = 35). The statistical tests were performed using general linear models at each vertex. On the left, the statistical maps are displayed with a nominal p < 0.05 threshold. On the right, the maps are corrected for multiple comparisons by Monte Carlo simulations. Clusters of voxels covering the rostral middle frontal region (clusterwise p < 0.0001, corrected) in the left hemisphere and the superior frontal region (clusterwise p < 0.0001, corrected) in the right hemisphere were negatively correlated (warmer colors) with P1 scores at BL.
For exploratory purposes, we performed the same set of cluster-based whole-brain analyses using the P2 (suspiciousness) severity rating, which was also previously identified as a contributing predictor of psychosis conversion in the NAPLS 1 sample [19]. However, the P2 rating did not show significant associations with the rate of change in gray matter thickness. In addition, we mapped P1 ratings to the rate of change in cortical area and volume using unconstrained vertex maps with a nominal p < 0.05 threshold, but none of the peaks observed survived multiple comparison correction.
Conversion Status and P1 Interaction
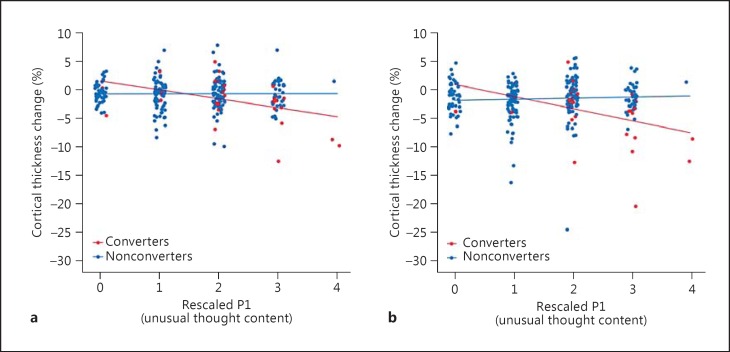
A multiple regression model estimating an effect of conversion status (converters vs. nonconverters), unusual thought content (P1 rating), and their interaction term on the annualized rate of percent thickness change in the right superior frontal cortex was performed. This model [F(3, 274) = 8.05, p < 0.0001] revealed a strong interaction effect (t = 3.34, p < 0.001), where converters with higher unusual content showed a steeper gray matter decline, whereas nonconverters showed no relationship. The same interaction effect was also present in the left rostral middle frontal cortex [F(3, 274) = 4.21, p < 0.001; P1 × conversion interaction term: t = −3.06, p < 0.01]. The interaction plots for both regions are shown in figure 2.
Fig. 2.
Scatterplot for the association between the rescaled SOPS P1 rating (unusual thought content) and the annualized rate of percent change in prefrontal cortical thickness among CHR youth moderated by conversion status. The interactive effects of conversion status (converters vs. nonconverters) are shown as well as the symptom rating on the cortical thickness change. a The ROI is the left rostral middle frontal cortex. b The ROI is the right superior frontal cortex. Both ROIs revealed significant P1 score and group status interaction (p < 0.01).
Third Ventricle
The rate of change in third ventricle volume was significantly associated with P1 ratings (r = 0.35, two-tailed p = 0.0289), with higher BL ratings on P1 being associated with a greater rate of third ventricle volume expansion.
P1 Severity and Duration
Interestingly, in our previous report, shorter symptom duration was also associated with the steeper gray matter decline and third ventricle expansion among converters [8]. Since both the duration of prodromal symptoms and P1 scores are significant predictors of the steeper gray matter decline and third ventricle expansion among converters, we performed a set of statistical tests that included both the duration of the prodromal state and P1 scores at the BL assessment (within converters) in a set of multiple regression models predicting the rate of change in the regions identified from the whole-brain analysis (i.e. the left rostral middle frontal region and the right superior frontal region). The chosen ROI measures were extracted using the Desikan atlas parcellations [42]. The P1 scores at BL remained nominally significant (p < 0.05 for the right superior frontal gyrus, left rostral middle frontal gyrus, and third ventricle) even after controlling for the duration of prodromal symptoms (table 3), suggesting an independent contribution of the symptom severity in predicting the rate of gray matter decline.
Table 3.
Regression models predicting the annualized rate of change with P1 severity and duration of prodromal symptoms as predictors among converters (n = 35)
| Predictors | B | SE | t | p (two-tailed) |
|---|---|---|---|---|
| Annualized rate of change in the right superior frontal gyrus | ||||
| SOPS P1 score | –0.022085 | 0.008129 | –2.149 | 0.010 |
| Duration | 0.004879 | 0.002270 | 2.717 | 0.039 |
| Annualized rate of change in the left rostral middle frontal gyrus | ||||
| SOPS P1 score | –0.022939 | 0.010449 | –2.195 | 0.035 |
| Duration | 0.001043 | 0.002918 | 0.357 | 0.723 |
| Annualized rate of change in the third ventricle | ||||
| SOPS P1 score | 0.050742 | 0.023274 | 2.324 | 0.026 |
| Duration | –0.016300 | 0.006499 | –1.615 | 0.115 |
Discussion
Higher levels of unusual thought content and a differential rate of prefrontal gray matter reduction have been previously shown to be significant predictors of psychosis in CHR cases [2,8,19], but the relationship of these two predictors has not been previously examined in the NAPLS sample at the atlas level. Theoretically, progressive tissue loss in the prefrontal cortex is an indicator of an increasing disruption in integrated synaptic activity and interregional functional connectivity, which are thought to drive the expression of psychotic symptoms. If this is the case, those at risk who show higher levels of positive symptoms at BL should show steeper rates of tissue loss as their symptoms cross the threshold into the psychotic range of severity. Support for this theoretical view was observed in that more severe unusual ideas and beliefs (i.e. mind tricks, external control, magical thinking, etc.) at BL predicted a steeper rate of gray matter loss in the prefrontal cortex bilaterally among the CHR cases progressing to full psychosis. Unusual thought content had previously been shown to be a significant risk predictor of transition to full psychosis in a number of independent datasets [19,24,25,26]. According to the SIPS assessment, a prodromal risk syndrome is present when attenuated psychosis-like symptoms have had their onset or worsened in the previous year. Therefore, we can infer that individuals who are rated >1 at the BL assessment are experiencing symptoms that have persisted and intensified since the time of prodromal symptom onset. Since there were no significant differences in cortical thickness, subcortical volume, and ventricular size at BL between converters, nonconverters, and healthy controls [8], and because the neuroanatomical measures at BL did not vary significantly by the initial P1 ratings, the relationships observed in this study suggest a dynamic course of changes in brain structure around the time of psychosis onset.
Interestingly, a significant interaction between the group status and the level of unusual thought content was observed when predicting the rate of change in the prefrontal cortex. When all CHR cases were included in the model, an association between the attenuated positive symptom rating and the neuroanatomical rate of change was observed only among converters. This suggests that an accelerated rate of prefrontal gray matter loss is relevant particularly to CHR individuals whose attenuated positive symptoms acutely worsen over time in association with psychosis onset. It may be that high levels of persistent and intensifying predelusional symptoms are dynamically associated with a reduction in cortical scaffolding that underlies synaptic connectivity and integration of information.
In contrast, the rate of cortical thinning did not differ from that in healthy controls among nonconverters who were characterized by attenuated symptoms that remit over time or remain constant at a subthreshold level. There is likely to be heterogeneity in the causes underlying unusual thought content in the population. It may be that stress or other mechanisms are contributing to the emergence of subpsychotic symptoms among nonconverters or that the 1-year MRI rescan window may not have captured gray matter changes in this group. For example, aberrant developmental cortical changes may be in progress at a slower rate or may not have occurred yet.
While levels of unusual thought content at BL predicted the rate of thinning in the prefrontal cortex, there was no association between the rate of neuroanatomical change and the levels of these symptoms at FU. This is due to the reduced variation in the clinical ratings assessed at conversion (82% of the cases were rated ±3 on a rescaled P1 rating at conversion). Further, the positive symptom severity rating and the rate of change in gray matter and third ventricle volume were not correlated among nonconverting participants. This is consistent with our previous finding that nonconverters and healthy controls showed no significant group differences in the neuroanatomical developmental trajectories. Taken together, these results indicate that steeper gray matter loss seems to be unique to those individuals with higher levels of persisting and intensifying subpsychotic positive symptoms around the time of psychosis onset. Our findings thus support the development of strategies for the early intervention and care for individuals with high ratings of positive symptoms to either prevent, reduce, or at least delay the disease-promoting processes in the brain.
It is interesting that the correlation between the clinical severity ratings and the cortical changes occurred bilaterally. In our previous report [8], the group differences in the rate of change in cortical thickness in converters versus nonconverters and healthy controls had also shown a bilateral distribution in the prefrontal regions when not correcting for multiple comparisons. However, only the clusters of signals in the right hemisphere had remained significant after applying a conservative vertex-wise correction for multiple comparisons [8,43]. In this follow-up report on the same sample, we have found that the severity of unusual thought content at BL is associated with bilateral reductions in the prefrontal regions using an anatomically unconstrained vertex-wise method with cluster-based correction for multiple comparisons [39,40]. This pattern is consistent with the view that the progressive tissue loss is occurring bilaterally but is more pronounced in the right hemisphere. Prior studies also reported that the differential changes associated with the conversion to psychosis occur in both hemispheres but with more robust significance on the right when more stringent p value thresholds were applied [1,2,6,8].
It is worth noting that the steeper rate of gray matter loss in the prefrontal cortex is associated with the P1 symptom ratings (i.e. unusual thought content/unusual ideas) but not with P2 - severity of suspiciousness/persecutory. This may indicate that the emergence of unusual thought content is specifically tied to developmental disturbances in the superior frontal and rostral middle frontal (i.e. dorsolateral prefrontal) regions, and other constellations of psychosis symptomatology may be explained as a secondary phenomenon or hinged on a different pathophysiology. One possible mechanism for explaining the emergence and persistence of delusional beliefs and unusual thought content is based on dysfunctions in belief formation processes, or aberrant prediction error signaling, in which the right prefrontal cortex plays a key role [44,45]. In addition, it is well known that these prefrontal regions are the neural correlates of top-down regulation and working memory, and functional imaging studies have consistently implicated neural deficits among schizophrenia patients when performing behavioral tasks that require various forms of cognitive control and working memory [46,47].
The rate of expansion in the third ventricle was significantly associated with BL SOPS P1. Enlarged ventricles have been consistently reported across the literature on schizophrenia [48,49,50]. The associations of positive symptom severity with the rate of change in both cortical and ventricular structures suggest that the biological underpinnings of the positive symptoms are not limited to the prefrontal cortex, and studies should further examine the disruption of neural circuits at a system level.
Cortical thinning during late adolescence is thought to reflect a pruning and rewiring process via experience-dependent synapse elimination [9]. Hence, a certain degree of thinning in the cortex is expected to be a sign of healthy development. However, it is unclear whether the gray matter decline we observed is an overshoot of a normal pruning process or due to a progressive process specific to schizophrenia per se. Also, based on the design of this study, we cannot conclude whether the progressive tissue loss precedes the onset of full psychosis. As conversions to full psychosis seem to occur in less than 1 year for most of the participants from BL assessments, future studies are encouraged to conduct MRI scans with multiple time points (2- to 3-month intervals) to elucidate whether more precise brain trajectories could predict change in clinical symptoms.
Disclosure Statement
The authors have no conflicts of interest to declare in relation to the subject of this study. T.D.C. reports that he is a consultant to the Los Angeles County Department of Mental Health on the implementation of early detection and intervention programs for youth at risk for psychosis.
Acknowledgements
The authors thank the following persons for assistance with subject scheduling and/or scan acquisition: Angielette Andaya, Nurit Hirsh, and Jamie Zinberg (UCLA); Richard Juelich (BIDMC - Harvard); M. Louis Lauzon, J. Stowkowy, and C. Marshall (Calgary); Jason Nunag and Daniel Roman (UCSD), and Nicole Popp Santamauro and Hedy Sarofin (Yale).
This work was supported by a collaborative U01 award from the National Institute of Mental Health at the National Institutes of Health (MH081902 to T.D.C.; MH081857 to B.C.; MH081988 to E.W.; MH081928 to L.J.S.; MH082004 to D.P.; MH082022 to K.C.; MH081984 to J.A., and MH082022 to S.W.W.) and NIMH P50 MH066286 and Staglin Music Festival for Mental Health (to C.E.B.), NIMH P50 MH080272, and the Commonwealth of Massachusetts (SCDMH82101008006) to L.J.S.
References
- 1.Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- 2.Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Wood SJ, Yung AR, Phillips LJ, Soulsby B, McGorry PD, et al. Insular cortex gray matter changes in individuals at ultra-high-risk of developing psychosis. Schizophr Res. 2009;111:94–102. doi: 10.1016/j.schres.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- 5.Ziermans TB, Schothorst PF, Schnack HG, Koolschijn PCMP, Kahn RS, van Engeland H, et al. Progressive structural brain changes during development of psychosis. Schizophr Bull. 2012;38:519–530. doi: 10.1093/schbul/sbq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgwardt SJ, McGuire PK, Aston J, Gschwandtner U, Pflüger MO, Stieglitz R-D, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–114. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Walter A, Studerus E, Smieskova R, Kuster P, Aston J, Lang UE, et al. Hippocampal volume in subjects at high risk of psychosis: a longitudinal MRI study. Schizophr Res. 2012;142:217–222. doi: 10.1016/j.schres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuroimaging study of youth at elevated clinical risk. Biol Psychiatry. 2014:1–10. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 10.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faludi G, Mirnics K. Synaptic changes in the brain of subjects with schizophrenia. Int J Dev Neurosci. 2011;29:305–309. doi: 10.1016/j.ijdevneu.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 1983;17:319–334. doi: 10.1016/0022-3956(82)90038-3. [DOI] [PubMed] [Google Scholar]
- 14.Glausier JR, Lewis DA. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keshavan MS, Anderson S, Pettegrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. J Psychiatr Res. 1994;28:239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 16.McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry. 2000;57:637–648. doi: 10.1001/archpsyc.57.7.637. [DOI] [PubMed] [Google Scholar]
- 17.Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rössler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Bonoldi I, Yung AR, Borgwardt S, Kempton MJ, Valmaggia L, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 19.Cannon TD, Cadenhead K, Cornblatt B, Woods SW, Addington J, Walker E, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch Gen Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods SW, Addington J, Cadenhead KS, Cannon TD, Cornblatt BA, Heinssen R, et al. Validity of the prodromal risk syndrome for first psychosis: findings from the North American Prodrome Longitudinal Study. Schizophr Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGlashan T, Walsh B, Woods S. The Psychosis-Risk Syndrome: Handbook for Diagnosis and Follow-Up. Oxford: Oxford University Press; 2010. [Google Scholar]
- 22.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the Structured Interview for Prodromal Syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 23.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–715. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 24.Nelson B, Yuen HP, Wood SJ, Lin A, Spiliotacopoulos D, Bruxner A, et al. Long-term follow-up of a group at ultra high risk (‘prodromal’) for psychosis. JAMA Psychiatry. 2013;70:793. doi: 10.1001/jamapsychiatry.2013.1270. [DOI] [PubMed] [Google Scholar]
- 25.Mason O, Startup M, Halpin S, Schall U, Conrad A, Carr V. Risk factors for transition to first episode psychosis among individuals with ‘at-risk mental states’. Schizophr Res. 2004;71:227–237. doi: 10.1016/j.schres.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Thompson A, Nelson B, Yung A. Predictive validity of clinical variables in the ‘at risk’ for psychosis population: international comparison with results from the North American Prodrome Longitudinal Study. Schizophr Res. 2011;126:51–57. doi: 10.1016/j.schres.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders. Patient Edition. New York: New York State Psychiatric Institute; 1995. [Google Scholar]
- 28.Addington J, Cadenhead KS, Cornblatt BA, Mathalon DH, McGlashan TH, Perkins DO, et al. North American Prodrome Longitudinal Study (NAPLS 2): overview and recruitment. Schizophr Res. 2012;142:77–82. doi: 10.1016/j.schres.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 32.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 34.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61:1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57:19–21. doi: 10.1016/j.neuroimage.2011.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuter M, Rosas HD, Fischl B. Highly accurate inverse consistent registration: a robust approach. Neuroimage. 2010;53:1181–1196. doi: 10.1016/j.neuroimage.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 38.Seidman LJ, Giuliano AJ, Meyer EC, Addington J, Cadenhead KS, Cannon TD, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67:578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33:1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayasaka S. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. doi: 10.1016/j.neuroimage.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Cannon TD, Sun F, McEwen SJ, Papademetris X, He G, van Erp TGM, et al. Reliability of neuroanatomical measurements in a multisite longitudinal study of youth at risk for psychosis. Hum Brain Mapp. 2013;35:2424–2434. doi: 10.1002/hbm.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 43.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 44.Corlett PR, Krystal JH, Taylor JR, Fletcher PC. Why do delusions persist? Front Hum Neurosci. 2009;3:12. doi: 10.3389/neuro.09.012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corlett PR, Honey GD, Fletcher PC. From prediction error to psychosis: ketamine as a pharmacological model of delusions. J Psychopharmacol. 2007;21:238–252. doi: 10.1177/0269881107077716. [DOI] [PubMed] [Google Scholar]
- 46.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, et al. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnstone E, Frith CD, Crow TJ, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet. 1976;308:924–926. doi: 10.1016/s0140-6736(76)90890-4. [DOI] [PubMed] [Google Scholar]
- 49.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 50.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]