Abstract
Domoic acid (DA), the cause of Amnesic Shellfish Poisoning, is a naturally occurring marine biotoxin that is usually produced by the microscopic algae Pseudo-nitzschia. As is the case for other types of toxic algae, Pseudo-nitzschia outbreaks are becoming more frequent. Acute high-dose symptomology in humans includes vomiting, cramping, coma and death as well as neurological effects such as hallucinations, confusion and memory loss. Experimental studies and medical reports have collectively shown that DA exposure primarily affects the hippocampal regions of the brain and is associated with seizures and the disruption of cognitive processes. The neurobehavioral signature of DA is unique in that it includes transient and permanent changes in memory function that resemble human antegrade amnesia. Experimental studies with adult nonhuman primates have established that DA is a dose-dependent emetic that produces clinical and neuropathological changes consistent with excitotoxicity. Behavioral evaluations of treated rodents have shown that hyperactivity and stereotypical scratching are the first functional markers of toxicity. Mid-dose treatment is associated with memory impairment and behavioral hyperreactivity, suggesting changes in arousal and/or emotionality. At higher doses, DA treatment results in frank neurotoxicity that is characterized by seizures, status epilepticus and death in treated animals. The route of DA exposure is important and influences the severity of effects; intraperitoneal and intravenous treatments produce classic signs of poisoning at significantly lower doses than oral exposure. While developmental studies are few, DA readily crosses the placenta and enters the fetal brain. Domoic acid is not associated with congenital dysmorphia but is linked to persistent changes in motor behavior and cognition in exposed offspring. Comparative research suggests that functional losses associated with DA can be persistent and injuries to the CNS can be progressive. Long-term studies will be necessary to accurately track the expression of DA-related injury, in health and behavior, over the life-span.
The sea is everything. It covers seven tenths of the terrestrial globe. Its breath is pure and healthy. It is an immense desert, where man is never lonely, for he feels life stirring on all sides. ~Jules Verne, 20,000 Leagues Under the Sea (1870)
1. Introduction
Over 70% of the earth's surface is covered with ocean waters. From the earliest days of recorded history, human civilizations have depended on marine waters for food, commerce, recreation and travel. The ethereal qualities of ocean water have served as a wellspring of inspiration for ancient and modern artists. Stories and songs from indigenous people on the coasts of North America often include reference to the power of the ocean and the integral role that it plays in the spiritual and physical health of tribal members. As human dependence on the oceans grows for everything from new biopharmaceutical agents to healthy sources of protein, there is increasing recognition of the important relationship between the health of the ocean waters and human health, including the growth and development of infants and children [24]. However, growing demand for fish and shellfish coupled with widespread contamination and development in coastal areas, is challenging the biological stability of the world's oceans. The role that ocean waters plays in global health is increasingly recognized as an important public health issue and scientific studies will be necessary to understand the fragile ecological balance that exists between land-dwelling and marine populations.
1.1 Linking Public Health and Harmful Algal Blooms
Health concerns stemming from the ocean generally revolve around exposure to substances in the marine ecosystem such as environmental chemicals, metals, and biotoxins. Marine biotoxins can cause serious illness and examples of human illness from poisoned fish can be found as early as 800 BC in Homer's Odyssey. In fact, ocean-borne illnesses in contemporary society are still primarily caused by the consumption of contaminated fish or shellfish. In researching the relationship between ocean health and human illness, marine scientists have identified the phenomenon of harmful algal blooms (HABs) as an important source of marine biotoxins [14]. Marine waters teem with microscopic species of algae that serve as the basis for all ocean life. Most species of algae are harmless but there are a few dozen that produce potent toxins, capable of serious and sometimes devastating health effects in seabirds, marine mammals and humans (http://www.cdc.gov/hab/about.htm). On a global level, over 60,000 people are poisoned by HABs each year and these marine-based illnesses often involve changes in neurological functioning [17, 66]. Harmful algal blooms have been linked to human death and illness, mass killings of fish, marine mammals and seabirds and adverse changes in key marine habitats [51].
In recognition of the increasing danger that HABs pose to all coasts, estuaries and inland waterways, the United States Congress enacted the Harmful Algal Bloom and Hypoxia Amendments Act of 2004. This Act provided federal authority to investigate the ecological, economic and health risks of HABs and the nation's ability to predict and respond to outbreaks of exposure. Worldwide, the frequency and intensity of HABs appears to be increasing and toxic strains of algae are spreading across geographic regions [14, 29].
The marine food chain includes thousands of species of microscopic algae (diatoms) and among these are a small number of algae that produce naturally-occurring marine toxins. When these toxic diatoms are filtered from the water by feeding zooplankton, shellfish and fish, accumulation of the toxin occurs in the muscle and viscera [15]. There is a range of human and animal health effects associated with the consumption of shellfish contaminated with toxic diatoms [6, 17] and five clinical poisoning syndromes linked to HABs have been identified; Amnesic Shellfish Poisoning, Ciguatera Fish Poisoning, Diarrhetic Shellfish Poisoning, Neurotoxic Shellfish Poisoning and Paralytic Shellfish Poisoning. Within these five syndromes, Amnesic Shellfish Poisoning is unique in that its neurotoxic signature includes transient and permanent changes in memory functioning.
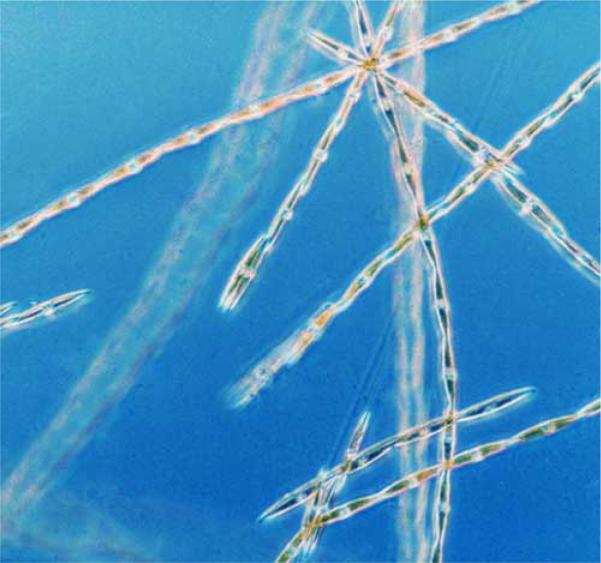
Domoic Acid (DA), the cause of Amnesic Shellfish Poisoning, is a naturally occurring marine biotoxin that is usually, but not always, produced by the microscopic algae Pseudo-nitzschia (see figure 1) [58]. Unlike “red tides” from the phytoplankton Karenia brevis that signal toxic blooms, no distinctive color characterizes the Pseudo-nitzschia blooms that produce DA.
Figure 1.
Blooms of the algae Pseudo-nitzschia produce Domoic Acid which causes Amnesic Shellfish Poisoning.
The lack of observable changes in DA contaminated waters makes it difficult to determine when blooms are occurring. According to the Seafood Network Information Center at the University of California at Davis, unsafe levels of DA have been found in the muscle tissue of oysters, mussels, razor clams and the viscera (not muscle) of scallops, sardines, anchovies, crab and lobster. As is the case for other types of toxic algae blooms, Pseudo-nitzschia outbreaks are becoming more frequent. Maps of HAB events developed at the National Office for Harmful Algal Blooms at the Woods Hole Oceanographic Institute reveal that prior to 1972, there was no evidence of DA-producing Pseudo-nitzschia blooms on the coasts of the U.S. However, from 1972 to 2006 there have been close to 20 site-specific incidents (single and recurrent) of DA toxins in seafood on the Western and Eastern seaboards (http://www.whoi.edu/redtide/page.do?pid=14898&tid=542&cid=47899&c=3). Contamination of the western coast of the U.S has been reported in the literature by Walz et al [68], Wekell et al [69] and Trainer et al (1998). Environmental sampling has revealed the presence of DA-contaminated ocean water in New Zealand [44], Mexico [48], Ireland [22], and Canada [37].
Sea life as diverse as sanddabs and blue whales have tested positive for the presence of DA [26] and neurological symptoms have been reported in pelicans, cormorants, loons, grebes, sea otters, dolphins and sea lions [19,46,48]. The neurological effects of DA make it challenging for exposed animals to stay afloat and breathe in the water, driving them to beach (haul out of the water) in an effort to survive. A recent publication by Goldstein et al [18] documents the changing nature of DA-related health effects in California Sea Lions (Zalophus califorianus). In addition to the acute high-dose DA toxicosis that has been observed in the field, these animals also exhibit a separate clinical syndrome associated with chronic, lower-level DA exposure. Of particular significance is the new finding that prenatally exposed sea lion pups are at increased risk for seizures in adult life, suggesting that fetal DA exposure influences the onset of adult disease [41].
In humans, DA toxicity is characterized by gastrointestinal and neurological symptoms. The largest outbreak of DA poisoning occurred in Canada in 1987 with 153 cases of acute intoxication including 4 deaths from the consumption of contaminated mussels [37,38]. Acute symptomology included nausea, vomiting, abdominal cramping, excessive respiratory secretions, coma, and death. Neurological symptoms such as headaches, hallucinations, confusion and memory impairment were reported. Subsequent environmental sampling in Canada revealed blooms of Psuedo-nitzschia pungens in the Cardigan River where the mussels had been harvested. In the Canadian outbreak, the most severe neurological sequelae were documented in subjects who developed neurological symptoms within 48 hours, were male and older than 60 years of age. The most vulnerable patients from younger age groups were those with preexisting illnesses such as diabetes, renal disease or hypertension. In a separate published report, an elderly man developed temporal lobe epilepsy after suffering DA poisoning [8]. Initial symptoms included nausea, vomiting, confusion, and coma. Memory impairment was present following intoxication but resolved after three weeks. After one relatively symptom-free year, complex partial seizures resembling temporal lobe epilepsy developed. At autopsy, severe bilateral hippocampal sclerosis was noted. The medical profile of this patient suggests that DA intoxication at high-levels of exposure can result in acute and delayed effects on the hippocampus and the expression of clinical disease.
1.2 A historical look at a contemporary environmental problem
An abbreviated timeline associated with the discovery of DA is outlined below to provide a historical perspective on this increasingly prevalent marine biotoxin:
1958- In a search for new antiparasitic compounds, Japanese scientists discover DA in Chrondria armata, a red algae found in tropical and sub-tropical waters [54].
1961- In Capitola, CA, nonaggressive, fish-eating seabirds, primarily Sooty shearwaters, exhibit unusual and aggressive behavior such as attacking humans and crashing through glass windows. Residents were quoted as saying the birds “cried like babies”. Alfred Hitchcock used this frightening event as inspiration for his classic 1963 horror film “The Birds”. Marine scientists now largely believe that the Sooty shearwaters in Capitola were suffering from DA poisoning (www.noaa.gov/focus/pdf/daf.pdf).
1967- Takemoto et al determines the final structure and stereochemistry of DA [55].
1987- First documented episode of DA poisoning in humans occurs in Canada [37, 38]. The unusual focus of the clinical symptomology on memory impairment leads to the clinical label of Amnesic Shellfish Poisoning (ASP).
1991-1993- First documented episode of DA contamination in the United States occurs on Washington State beaches [69]. This episode prompts closure of recreational and commercial fisheries and beaches. During the same period, unusual behavior is noted in seabirds living near Monterey Bay in California [68]. The behavior of the affected birds suggests neurological involvement and necropsies reveal evidence of DA poisoning. Marine scientists note that poisoned seabirds had consumed anchovies feeding on the DA-producing diatom Pseudo-nitzschia.
1998- First documentation of large-scale marine mammal killing from DA poisoning. More than 400 sea lion (Zalophus californianus) deaths are attributed to DA exposure. Symptoms include weaving and bobbing, seizures, bulging eyes, disorientation, and ataxia (drunken movements) [46]. Stomach contents reveal the sea lions had been eating anchovies contaminated with Pseudo-nitzschia.
2005- Shellfish monitoring in Ireland reveals that 55% of King scallops are above the regulatory limit for DA and some samples provide the highest levels ever recorded (2820 microg DA/g) [22].
2007-2008- Scientists from the Santa Barbara Museum of Natural History document record high levels of DA in coastal waters and an unusual mortality event involving sea lions, dolphins and birds (particularly the endangered brown pelican) is reported [33]. Marine scientists report that fetal exposure to DA in California sea lions is associated with the development of a seizure-like disease in adulthood [41].
2. Brain Injury, Mortality and Clinical Signs in DA-Exposed Adults
Domoic acid is a water-soluble amino acid that exerts its toxicity by activating the AMPA/kainite subtype of glutamate receptors [20]. Pharmacokinetic studies have indicated that DA is well distributed in body water and primarily cleared from plasma through the kidneys [53, 60]. The half-life of DA is brief (approximately 20 minutes) and in mice, 99% of DA is cleared from serum within four hours of exposure [31,53,60]. Excretion of DA occurs almost exclusively in the feces, suggesting that DA is poorly absorbed in the gut [21]. Domoic acid-induced excitotoxicity leads to cellular and structural brain damage in exposed individuals through activation of the glutamate receptors [40]. Thought to be a chemical analogue to kainic acid, DA binds at the same receptor sites in the central nervous system, producing a pattern of brain damage that closely resembles kainic acid neurotoxicity. In fact, cross-species research has demonstrated that DA-related neuropathology in adults occurs primarily in the hippocampus, a brain region that is rich in its density of kainite receptors and essential for memory function [16,63].
Although real-world DA exposure in humans is limited to oral exposure, most of the adult animal models developed for this compound have been conducted using intravenous (IV) or intraperitoneal (IP) treatment paradigms [23]. The route of exposure influences the potency of DA; IP and IV treatments result in clinical symptomology at lower doses than oral exposure [21]. Two separate experiments with adult mice have reported death at IP doses from 2.4 to 4.0 mg DA/kg bw [21, 35]. However, adult rats treated with oral doses as high as 5.0 mg/kg/day for over two months do not develop overt signs of behavioral or hematologic toxicity [61]. Primate studies have demonstrated that a single IP treatment of 4.0 mg DA/kg bw results in extensive CNS injury, retinal damage and death [64]. Intravenous doses greater than or equal to 1.0 mg DA/kg bw result in a greater than 50% mortality rate [45]. Little is known about parameters of human exposure, but estimates of total oral dose in the Canadian DA outbreak range from 60 to 290 mg DA (about 1 to 5 mg/kg for a 60kg person) (37,38). Not surprisingly given the rapid clearance of this biotoxin, DA was not detected in samples of blood, serum and cerebrospinal fluid in these subjects approximately 48 hours after the onset of clinical symptoms.
2.1 Effects in Adults (see Table 1)
Table 1.
Neurobehavioral Effects of Domoic Acid Exposure in Adults
| IP Treatment Effects (mg/kg) (M=monkey, R=Rodent, B=Both) Doses | |||||||||||||
| 0.25 | 0.5 | 1 | 1.32 | 1.5 | 2 | 4 | 7.5 | ||||||
| Lethargy (R) | Hyperactivity (R) Minor convulsive signs (M) |
Scratching (R) Withdrawal (R) Minor convulsive signs M) |
Hyperreactivity (R) Scratching (R) Death (R) |
Impaired memory (R) | Withdrawal (R) Exploration (R) Lethargy (R) Scratching (R) Impaired memory (R) Seizures (R) |
Withdrawal (R) Exploration (R) Scratching (R) Vomit/Gag (M) Chewing/salivation (B) Rigidity of movement (B) Wet-dog shakes (R) Praying Posture (R) Vomit/Retching (M) Tremors (B) Loss of balance (B) Seizures (B) Death (B) |
Withdrawal (R) Exploration (R) Scratching (R) Chewing/salivation (R) Rigidity of movement (R) Wet-dog shakes (B) Praying Posture (R) Tremors (R) Loss of balance (R) Seizures (R) |
||||||
| IV Treatment Effects (mg/kg) (M=monkey, R=Rodent, B=Both) | |||||||||||||
| 0.025 | 0.05 | 0.2 | 0.5 | 1 | 4 | ||||||||
| Lipsmacking (M) Chewing (M) Vomit/Gag (M) |
|
Lipsmacking (M) Chewing (M) Vomit/Gag (M) Lethargy (M) |
Lipsmacking (M) Chewing (M) Vomit/Gag (M) Lethargy (M) Rolling (M) |
Chirping (M) Lipsmacking (M) Chewing (M) Vomit/Gag (M) Scratching (B) Rigidity (M) Loss of balance (M) Seizures (R) Death (R) |
Vomit/Gag (M) Scratching (M) Tremors (M) Lethargy (M) Death (M) |
Vomit/Gag (M) Scratching (M) Tremors (M) Lethargy (M) Death (M) |
|||||||
| Oral Treatment Effects (mg/kg) (M=monkey, R=Rodent, B=Both) | |||||||||||||
| 0.1 | 0.5 | 0.75 | 5 | 10 | 60-80 | ||||||||
| No effects (R) | No effects (M) | No effects (M) | Chewing (M) Lipsmacking (M) Salivation (M) Vomit/Gag (M) |
Chewing (M) Salivation (M) Vomit/Gag (M) |
Lethargy (R) Hyperactivity (R) Seizures (R) Death (R) |
||||||||
2.1.1 Human -Oral exposure
As noted above, the largest outbreak of DA poisoning occurred in Canada in 1987 with 153 cases of acute intoxication including 4 deaths from the consumption of contaminated mussels (60 to 290 mg DA) [37,38]. Autopsies of exposed adults from this poisoning episode revealed neuronal loss and necrosis concentrated primarily in the amygdala and hippocampal regions [56,57]. Less severe neuronal loss and necrosis and astrocytosis were found in the claustrum, secondary olfactory areas, septum, nucleus accumbens, dorsal medial thalamus, and the insular and subfrontal cortex. There were no effects on the brainstem motor nuclei or the spinal cord and the hippocampus was the primary site of DA-induced injury. Clinical evaluations made on 14 adult human subjects after the outbreak revealed that 12/14 had severe antegrade memory deficits with relative preservation of higher cortical functions. Affected patients were unable to remember events that occurred after DA intoxication and had difficulty recalling new information. Clinical evidence of motor or sensory motor neuronopathy or axonopathy was observed in 11 cases. In a separate report, an 84 year old male was treated at the hospital for DA poisoning [8]. Memory impairment was present but resolved after three weeks. Complex partial seizures developed after about a year and upon the subject's death, severe bilateral hippocampal sclerosis was noted at autopsy. In humans, memory function is clearly disrupted by high-level DA exposure and the hippocampus is the site of the greatest neural damage.
2.1.2 Nonhuman Primates -Intraperitoneal Treatment
In 1990, Tryphonas and colleagues treated an adult Macaca fascicularis monkey with 4.0 mg/kg IP and described initial clinical symptomology following dosing [64]. Symptoms were expressed quickly, characterized by persistent chewing, teeth grinding, gagging, and vomiting. The subject also displayed abnormal positioning of the head and body, lack of balance, rigidity of movement, and tremors. This animal developed acute pulmonary edema and died.
In an effort to develop a new animal model for temporal lobe epilepsy, adult marmosets were treated with DA injections between 0.5 mg/kg to 4.0 mg/kg IP [36]. Animals were evaluated for the presence of acutely-induced and spontaneous seizures over time. At the two highest doses (3.5 and 4.0 mg/kg), animals either did not show signs of seizures or had seizures so severe that they were fatal (status epilepticus). In contrast, animals injected with doses ranging 0.5 to 3 mg/kg developed only minor convulsive signs during a six-month post-dosing observation period. Histopathological examinations of brain regions revealed only minor neuropathologic changes. The authors conclude that DA treatment is not effective as a model of chronic temporal lobe epilepsy in marmosets because doses that do not lead to fatal status epilepticus do not produce strong, well-defined behavioral changes consistent with temporal lobe epilepsy.
2.1.3. Nonhuman Primates -Intravenous Treatment
Juvenile and adult monkeys (Macaca fascicularis) receiving IV injections of DA at one of a range of doses from 0.25 to 4.0 mg/kg showed signs of nausea (gagging, retching) and latency to illness was strongly age- and dose-dependent [45]. Adult animals were more severely impacted by DA exposure than juvenile animals. At the 1.0 mg/kg dose and above, four of seven animals became moribund, exhibiting tremors and extreme lethargy before death. Monkeys also exhibited ipsalateral scratching at the neck and/or behind their ears with a foot (also reported in rodents treated with DA). Surviving animals began eating food about six hours after dosing and were normal in gait and behavior for the next seven days, the end of the observation period. Histopathological examination found degenerating nerves and axons as well as two distinct kinds of hippocampal lesions, the limited Type A in the CA2 stratum lucidum at lower dose levels (observed at 0.5 to 1.0 mg/kg) and the more widespread Type B lesion affecting CA4, CA3, CA2, CA1 and the subiculum subfields of the hippocampus (observed at 1.0 mg/kg and above). Using a degeneration specific histochemical technique, investigators reexamined brain slices from this cohort of animals [47]. While hippocampal changes were observed at 0.5 mg/kg, frank neuronal degeneration was only observed at 1.0 mg/kg and above. Degenerating neuronal bodies were found within the hippocampus and other limbic regions including the entorhinal cortex, the subiculum, the piriform cortex, the lateral septum and the dorsal thalamus; patterns similar to those reported after acute high-dose DA poisoning in humans [56,57]. Overall, the results from this study underscore the significance of hippocampal and limbic damage in DA neurotoxicity and emphasize that the expression of poisoning, in both behavior and neuropathology, is dose dependent.
Tryphonas and colleagues treated adult Macaca fascicularis monkeys with 0.025 to 0.5 mg/kg IV DA to evaluate initial clinical symptomology following dosing [64]. Transient signs of illness were exhibited at 0.025 mg/kg in the form of brief gagging, empty mastications and vomiting. At 0.05 and 0.2 mg/kg doses, treatment effects also included lipsmacking, lethargy and rolling. At the highest dose, 0.5 mg/kg, all aforementioned signs were observed plus persistent chirping, loss of balance, rigidity and abnormal body positioning. The duration of overt symptomology was strongly dose dependent and all animals fully recovered within three hours. At necropsy, brain lesions were detected in the area postrema, hypothalamus, hippocampus and inner retina only at the 0.5 mg/kg dose. This study also included one monkey who was administered a 4.0 mg/kg IP dose (see section 2.2.1). When data across exposure routes were compared, retinal damage was documented at 0.5 mg/kg IV and 4 mg/kg IP. Overall, the results from this study suggest that at doses of 0.025 to 0.2 mg/kg IV, DA acts as potent emetic in nonhuman primates. At doses of 0.5 mg/kg IV and 4 mg/kg IP, lesions in the central nervous system are observed and symptoms of clinical illness are intensified.
2.1.4. Nonhuman Primates -Oral Exposure
To more closely parallel human exposure scenarios, investigators treated adult macaque monkeys (Macaca fascicularis) with single oral doses of DA from 0.5 to 10 mg/kg [65]. The monkeys were observed for periods ranging from four to 44 days. Control animals and those treated with the 0.5 dose were unaffected. In monkeys treated with 5 mg/kg DA and above, symptoms such as salivation, retching, and vomiting were observed. Onset of clinical signs generally occurred between one and three hours after exposure and the duration of illness ranged from seven to 96 hours. Licking and smacking of the lips as well as empty mastications were observed in some treated animals. Lesions in the hippocampus and cerebral cortex were observed in all treatment groups. Affected neurons were shrunken, hyperchromatic and/or eosinophilic, angular or triangular in shape and surrounded by clear space. In this study, emesis was the primary effect of DA although treatment-related changes in the brain were documented in asymptomatic animals (0.5 mg DA/kg dose). In a separate study, macaque monkeys (Macaca fascicularis) were orally gavaged with doses of 0.5 mg/kg for 15 days and then at 0.75 mg/kg for another 15 days [62]. Animals were clinically monitored for body weight and food and water consumption during the treatment period and no DA-related differences were found. At necropsy, hematology values, serum chemistry, and light microscopy of all major organs (including brain and retina) in treated animals were indistinguishable from controls. At the dose of 0.5 mg/kg, chronic oral exposure did not result in overt signs of toxicity.
There have been no systematic studies of neurobehavioral changes in DA-treated nonhuman primates beyond the initial descriptions of overt poisoning (e.g. gagging, vomiting) and recovery.
2.1.5 Rodents -Intraperitoneal Treatment
A look at the neurobehavioral effects of DA exposure in adult rodents after IP exposure reveals a constellation of behaviors that include stereotypic scratching, wet-dog shakes, freezing, staring, and seizures. Tryphonas and colleagues [63] treated adult female rats with a single IP dose of 0.0, 1.0, 2.0, 4.0, or 7.5 mg/kg DA and observed them for a period of up to 24 hours. There were no clinical signs in the 1.0 mg/kg animals while 75% of animals in the 2.0 mg/kg displayed minor behavioral signs such as withdrawal and scratching. Behaviors consistent with advanced DA neurotoxicity such as wet dog shakes, praying posture (anterior part of body raised with front paws clasped), generalized tremor, and loss of postural control were observed in all rats given 4.0 or 7.5 mg/kg. Illness in these animals culminated in status epilepticus. Histological examination of the brain in severely affected rats revealed selective encephalopathy marked by neuronal degeneration and vacuolation of the neuropil in the limbic (hippocampus, amygdala) and the olfactory systems. Similar behavioral effects have been reported in adult rats treated after a single IP treatment of 2.25 mg/kg [3].
The dose dependent neurobehavioral effects associated with DA were further explored by Peng and Ramsdell [35] who treated adult mice with a single 0.25 to 4.0 mg/kg IP injection. Serum levels of DA were measured 60 minutes after injection. Serum DA levels increased from 0.034 to 0.98 microg/ml as the experimental dose increased from 0.25 to 4.0 mg/kg. The first behavioral indicator of excitotoxicity was hyperactivity at 0.5 mg/kg (0.076 microg/ml serum DA). Stereotypic scratching, a classic sign of DA toxicity, was observed at 1.0 mg/kg (0.25 microg/ml serum DA). Doses of 2.0 mg/kg and higher resulted in seizures and convulsions (0.54 microg/ml serum DA). Results from this well crafted study indicate that serum levels of DA are related to behavioral symptomology. In addition, brain c-fos, a well-recognized biomarker for the neuroexcitatory effects of DA, was expressed in the hippocampus at doses as low as 0.5 mg/kg.
In humans, the hallmark of DA-induced neurotoxicity is the rather dramatic disruption of memory processing. The rodent model has been used extensively in defining the effects of IP treatment on spatial learning and memory. Rats were examined using a comprehensive neurobehavioral test battery after IP DA injections of 0, 0.22, 0.65, or 1.32 mg/kg to explore the behavioral effects of DA in the relative absence of measurable brain injury [50]. Animals were tested on cognitive measures of passive avoidance, auditory startle, and conditioned avoidance. Clinical symptomology related to toxicity (stereotypic scratching) was observed in 63% of the animals from the 1.32 mg/kg dose. Approximately 25% of the animals receiving 1.32 mg/kg DA died or were euthanized after becoming moribund. Three days after treatment, surviving animals in this dose group showed treatment-related changes on the auditory startle test. This effect was limited to exaggerated startle responding as measured by mean-response amplitude changes and did not include changes in habituation, suggesting the presence of behavioral hyperreactivity that is distinct from memory impairment. Across experimental groups (0.22, 0.65 and 1.32 mg/kg), performance on the other two measures of avoidance learning was not distinguishable from controls.
In another study, adult mice receiving a single IP dose of 2.0 mg/kg DA showed marked deficits in the acquisition of the place task on the Morris water maze [39]. When searching for the underwater platform on this task, treated mice had significantly longer escape latencies than controls. Similar results were obtained in a separate study that also utilized the Morris water maze [25]. Adult rats treated with a single IP dose of 1.5 or 3.0 mg/kg exhibited significant learning deficits while animals treated with 0.75 mg/kg performed as well as controls. With the addition of a visual cue trial (a black flag was positioned over the goal platform to eliminate the learning and memory components), investigators were able to rule out sensory and motor deficits in the treated animals unable to solve the task.
In a study to evaluate single versus repeated doses of DA on learning and memory, adult mice were treated with single or four IP injections of 1.0 or 2.0 mg/kg over a seven day period. Animals were evaluated on a spatial delayed matching-to-sample test in a water maze context [9]. Animals given a single injection of 2.0 mg/kg DA performed more poorly than controls on “nonalternation” test days; sessions in which the correct response was the same as the preceding day. This finding suggests that treated animals had difficulty recalling information after a 24 hour delay period and were exhibiting behavioral signs consistent with human antegrade amnesia. Animals given multiple injections displayed initial greater symptomatic toxicity but after recovery, did not show greater cognitive impairment than subjects treated with a single injection. The data strongly support a deficit in spatial working memory that is similar in form to anterograde amnesia after treatment with a single IP injection of DA as low as 2.0 mg/kg.
2.1.6 Rodents - Intravenous and Direct Brain Treatment
The hippocampus is the brain area most affected in rodents treated with systemic IV injections of DA; a result consistent with neuropathology reports in other animals and humans [46]. To fully characterize the fundamental neurotoxicity of this compound, investigators have studied direct injections of DA into the brain. In a study examining both IV and direct brain injections, adult rats treated with DA (0.5 to 1.0 mg/kg IV or 0.04 to 0.08 μg intraventricularly) displayed hippocampal seizure discharges, scratching, shaking, clonic-tonic convulsions and all animals ultimately died [34]. Rats in the IV treatment group died more quickly than those in the intraventricular group by 2 to 5 days. Pretreatment with diazepam (5 mg/kg, ip) prevented convulsions and death in intraventricularly treated animals at 0.04 μg DA but not at the 0.08 μg dose or in the IV treated animals. Based on these data, a dose of 0.04 μg DA/kg administered intraventricularly (and diazepam, ip) was selected to evaluate learning and memory in treated animals. Results showed severe learning impairment on a radial arm maze, suggesting deficits in working memory. In subjects that were able to ultimately solve the maze, performance deficits were documented on relearning the same task. The memory-based deficits observed in these animals is consistent with human antegrade amnesia.
2.1.7 Rodents -Oral Exposure
There have been only two studies of oral exposure in the rodent animal models. In the first study, adult rats were administered a single oral dose of DA from mussel extracts at 60 to 80 mg/kg (65). Behavioral alterations were varied between individuals such that onset of symptoms and severity were widely distributed. In general, initial hypoactivity and withdrawal were followed closely by empty mastications, salivation, minor seizures and hyperactivity. One rat in the 80 mg/kg group developed status epilepticus and died. All remaining subjects fully recovered. Lesions in the central nervous system (primarily in the olfactory cortex and hippocampus) were present in all animals in the 80 mg/kg group. There were no lesions in the 60 or 70 mg/kg groups. In the second study, adult rats were treated with 0.1 or 5 mg/kg/day DA for 64 days. Animals showed no clinical abnormalities in behavior and blood chemistry and all histopathology results were negative [61]. Given that the route of exposure in human and wildlife populations is oral, there is a need for more studies of this nature to fully understand the health risks associated with the ingestion of DA-contaminated shellfish in human populations.
3. Effects on Fetal and Postnatal Development in Mammalian Species
While the laboratory data on developmental exposure are limited to rodent models, research findings from these studies suggest a dramatic fetal sensitivity to DA. Domoic acid does not produce structural malformations in exposed neonates so, in this sense, it does not act as a classic teratogen. Developmental exposure has, however, been associated with hippocampal damage, seizure disorders and persistent changes in behavior that have been documented across multiple routes of treatment. Like adults, there is a continuum of clinical symptomology that is closely linked with dose.
3.1 Studies of Prenatal Exposure (see Table 2)
Table 2.
Neurobehavioral Effects of Developmental Exposure to Domoic Acid
| IP Treatment Effects (mg/kg) (Rodent data) Doses | ||||
| 0.05 | 0.1 | 0.2 | 2 | |
| Hyperactivity | Scratching | Scratching Seizures | Death | |
| IV Treatment Effects (mg/kg) (Rodent data) | ||||
| 0.6 | ||||
| EEG abnormalities | ||||
| Subcutaneous Treatment Effects (mg/kg) (Rodent data) | ||||
| 0.05 | 0.1 | 0.33 | 0.5 | 1.2 |
| Lethargy | Death | Paralysis Tremor Death | Paralysis Tremor Death | Changes in cognition and motor behavior |
| Subcutaneous Treatment (μg/kg) (Rodent data) | ||||
| 20 | ||||
| Facilitative effects on early CNS development and adult learning Memory impairment Emotionality Hyperreactivity Behavioral seizures | ||||
3.1.1. Rodents -Intravenous Treatments
Studies examining the consequences of developmental DA exposure on fetal kinetics, brain morphology and physiology are few. Using a single IV dose of DA (0.6 or 1.6 mg/kg/body weight) on either Gestational Day (GD) 13 (time of maximal hippocampal cell proliferation) or GD 20 (last day gestation), investigators measured DA levels in maternal plasma and brain, fetal brain and amniotic fluid [32]. Results showed that that at both doses, DA crosses the placenta, accumulates in amniotic fluid, and enters the fetal brain. At GD13, partitioning of DA in the maternal and fetal brain was similar and suggested that the fetal brain is exposed to roughly the same dose as the mother. At GD20, the concentration of DA in the fetal brain was approximately half that found in the maternal brain.
To investigate DA effects on offspring brain morphology and chemistry, pregnant mice were treated with a subconvulsive dose of DA on GD13 (0.6 mg/kg IV injection) [10]. At birth, there was no evidence of congenital anomalies and there were no significant differences between control and treated animals in litter size, birth weight and brain weight. Although no seizure activity was observed in the pups, abnormalities were noted in the basal EEG records of treated pups across the first 30 days of life. In-utero exposure was associated with several significant changes in hippocampal neuroanatomy and physiology. Injury was observed in the CA3 and dentate gyrus regions in animals examined at birth. Additional effects such as damage to the CA4 area of the hippocampus and decreased brain GABA/increased glutamate levels were noted, however, when animals were examined at 30 days of age. These findings demonstrate that prenatal exposure to DA is associated with significant and progressive hippocampal injury at levels that do not result in clinical seizures.
3.1.2. Rodents -Subcutaneous Treatment
Levin and colleagues studied in utero DA exposure in rats and found subtle but persistent changes in the neurobehavioral development of treated pups. Pregnant animals were injected with 0, 0.3, 0.6, or 1.2 mg/kg subcutaneous (sc) on GD13 [27]. There were no adverse effects on maternal weight, litter size, birth weight or offspring growth. Beginning at approximately 4 weeks of age and ending on week 13, the offspring of treated dams were evaluated for cognitive and motor effects using a T-maze, a Figure-8 locomotor task and a radial arm maze. A linear trend indicating a slowing of response latencies across doses was observed on the T-maze. Treated pups at the 1.2 mg/kg dose showed a significant slowing of response latencies but overall learning performance was comparable to controls. On the Figure 8 locomotor task, activity was influenced by exposure such that the rate of locomotor habituation was increased in the 1.2 mg/kg group. Treated animals showed higher initial activity followed by a greater decline during the test session. In the radial arm maze, the species-typical difference in spatial learning that favors male rats was attenuated in the highest dose group (1.2 mg/kg). This finding suggests that the DA effects on the hippocampus may be sex related resulting in a disruption of the expression of normal sex-related differences in spatial memory performance. When challenged with the amnestic drug scopolamine, DA-treated animals at the 1.2 mg/kg dose were more adversely affected than controls on the radial arm maze task. A linear trend indicating decreased performance across doses was also observed. Overall results from this study suggest that subconvulsive maternal doses of DA administered during critical stages of fetal neurogenesis (GD13) can result in subtle but persistent changes in motor and cognitive functioning.
3.2 Studies of Postnatal Exposure (see Table 2)
3.2.1 Rodents -Intraperitoneal Treatment
The effects of DA on postnatal development were studied in neonatal rat pups treated with a single IP injection of DA (0.05 to 2.0 mg/kg) on postnatal day 2, 5 or 10 [70]. Treated animals exhibited a time and dose-dependent neurotoxicity, characterized by an orderly progression of symptoms that includes hyperactivity, stereotypic scratching, convulsions and death. Domoic acid induced symptomology in neonatal rats was consistent with adult symptomology at doses as low as 0.05 mg/kg (hyperactivity). Stereotypic scratching occurred at the 0.1 and 0.2 mg/kg doses and seizures were documented at 0.2 mg/kg. These results suggest that DA exposure in neonatal rats results in clinical symptomology that is similar to adults but occurs at doses 40 times lower for onset of seizures and 80 times lower for onset of stereotypic scratching. Serum levels of DA were collected 60 minutes following the conclusion of behavioral testing and results showed that concentrations increased in a dose-dependent manner. Surprisingly, the blood levels associated with clinical symptoms in neonates were similar to the blood levels seen in affected adults. The increased sensitivity of the neonate may be partially due to higher than expected serum levels as a result of slower clearance of DA and higher levels in the brain.
3.2.2 Rodents -Subcutaneous Treatment
In a study designed to investigate the toxic effects of DA on the developing spinal cord, neonatal rats were administered a single sc dose of DA ranging from 0.1 to 0.5 mg/kg on postnatal day 7 [67]. Seizures were observed at all doses and at higher doses (> or = 0.33 mg/kg) treated animals exhibited hind limb paralysis, forelimb tremor and death. In 73% of the animals showing paralysis and/or tremor, lesions and the swelling and vacuolization of neurons were found throughout the spinal cord. The authors suggest that at higher levels of exposure, the observed behavioral effects of DA may be due to spinal cord damage rather than brain injury.
Modest, long-term neurobehavioral changes stemming from sc treatment on the first two days of postnatal life have been demonstrated by Levin and associates [28]. Neonatal rats were treated twice per day with doses from 0.025 to 0.1 mg/kg sc on postnatal days 1 and 2; a time point corresponding to the completion of neuroproliferation in most brain regions and corresponding to week 24 of human gestation. The dose of 0.1 mg/kg resulted in lethality. In adolescence, animals receiving nonlethal doses were examined on tests of exploration, locomotor activity and spatial learning and memory. Subjects in the 0.05 mg/kg group showed significant hypoactivity in a Figure-8 apparatus. This effect was seen in both males and females. In contrast, there were no effects of DA on exploration, learning and memory. Rats were also challenged with low-dose scopolamine and no adverse effects of prior DA exposure were detected. These results suggest that low-dose postnatal exposure to DA is not associated with deficits in spatial cognition but is associated with a modest depression of normal activity levels. It is worthwhile to note that hypoactivity/inertia has been identified as the first behavioral indicator of clinical symptomology in DA-treated adults [35].
Postnatal exposure studies from the Douchette laboratory, conducted primarily in the context of the glutamate system and epilepsy, have provided new information on behavioral development in pups treated with DA sc injections [1,2, 7, 11, 12, 13]. Daily low-dose treatment in newborn rats (5 or 20 microg DA/kg) from postnatal days 8 to 14 did not impact typical measures of toxicity such as weight gain, startle, ultrasonic vocalizations and maternal retrieval [11]. At the 20 microg/kg dose, eye opening occurred earlier in treated pups and performance on an olfactory conditioning task was enhanced. No effect was found on locomotor activity. These results suggest that low-dose DA treatment was not toxic but did influence, even facilitate, central nervous system maturation and neonatal learning. Additional tests on the same group of animals revealed that early DA treatment appears to impact the sexes differently [7]. As adults, subtle changes in spatial cognition and emotionality were observed in female subjects at the 20 microg/kg dose[13]. Memory interference was observed on a Morris Water maze test but only for female neonates treated with 20 microg/kg DA. Female subjects in the 20 microg/kg group also spent significantly more time in the “open” arm of an elevated plus maze. When tested on a behavioral startle procedure, male rats treated with 20 micog/kg DA showed deficits in pre-pulse inhibition (suppression of startle response) while treated females displayed a heightened startle response [1]. These findings suggest that treatment-related changes in arousal, emotionality and inhibitory processing may impact male and female subjects differently. The exaggerated startle response of females treated neonatally with DA in this study is consistent with the increased startle amplitude exhibited by both sexes after DA treatment in adulthood [50]. Abnormal behavioral reactivity may be an important behavioral marker of DA-induced neurotoxicity. Results of additional observations on these animals indicated that animals from both treatment groups exhibited a permanent, reproducible seizure syndrome (12). Termed “behavioral seizures”, the authors noted that they were strikingly reminiscent of stage 2 epileptic seizures. Long-term morphological changes including increases in mossy fiber staining and a reduction in hippocampal cell counts were observed at both treatment groups. These results highlight the irreversible effects of low-level neonatal exposure to DA on neurobehavioral and hippocampal morphology.
In the latest publication from this research team, a new cohort of animals received daily sc injections of 20 microg/kg DA sc on PND 8-14 (2). When tested on two spatial memory tasks as adults (the radial arm maze and Morris water maze), the performance of treated animals was superior to that of controls. The authors emphasize the persistent and enduring nature of these behavioral changes after DA exposure during critical periods of central nervous system development.
3.2.3 Rodents -Lactational Exposure
In the only study to address postnatal exposure through breast milk transmission, Mauscher and Ramsdell [30] demonstrated that DA is transferred to nursing pups from the milk of treated rodent dams. Milk collection in dams treated with 1 mg/kg DA IP showed that the amount of DA present in milk decreased rapidly with 99% eliminated within 2 hours. Maternal blood levels of DA decreased even more rapidly, with 99% eliminated within an hour. There was a 20% change in DA in milk from 1 to 8 hours after exposure. This compared to a >99% change in maternal blood DA concentrations over the same time-period. Approximately 8 hours after exposure, the DA concentration in milk was 4 times higher than that found in the plasma of the dam and DA remained measurable in milk after a quantifiable concentration was no longer detectable in plasma. In pups orally gavaged with 0.03 or 1.0 mg/kg DA in spiked milk, 99% of DA had cleared from the plasma in both dose groups. Given that neonatal rats have been shown to be more susceptible to DA than adults, lactational exposure may be a relevant route of exposure for human infants.
3.3 Fetal Exposure in Wild Population of California Sea Lions
Although not traditionally studied in toxicology research, the California sea lion has been a frequent victim of DA poisoning in the last decade and the subject of several important field investigations [19,46]. Developmental exposure to DA in sea lions is generally the result of maternal fish consumption, often anchovies and sardines. Anchovies and sardines can carry a high load of DA-containing diatoms which accumulate in their gut and ultimately, contaminate the marine food web that sea lions depend on. Sea lions reproduce at several well-known rookeries off the coast of Southern California and Mexico, providing an opportunity to closely study parturition and pup survival. Recent evidence suggests that in utero DA exposure in these animals may affect seizure susceptibility in later life, providing a fetal basis for a novel, neurologic disease in adulthood [41].
4. Conclusions and Future Research Directions
The scientific literature, as reviewed in this paper, clearly demonstrates that DA acts as a strong emetic and is associated with both structural and functional damage in exposed subjects. The clinical expression of DA poisoning in adults is dose-dependent, ranging from mild hyperactivity to coma and death. Experimental studies and medical reports have collectively shown that DA exposure primarily affects the hippocampal regions of the brain and is associated with seizures and the disruption of cognitive processes. Research with animals and humans has provided a reasonable understanding as to how acute, high-dose DA exposure is expressed at the level of frank toxicity but less is known about the effects of lower-dose, chronic exposure. Route of exposure strongly influences the severity of effects and the consequences of IP and IV treatment are greater than those associated with oral exposure. There is a need for studies using oral exposure methodology to more closely model real-world exposures in human and wildlife populations, particularly in sensitive subpopulations such as pregnant women. Some important generalizations can be drawn about the neurobehavioral signature of this emerging marine neurotoxin:
For adults, the neurobehavioral changes in exposed animals are consistent with reports of impaired memory function in humans
Results from multiple rodent studies [9,25,34,39] indicate memory impairment in DA-treated animals that is consistent with clinical data from human cases of DA poisoning. Both rodents and humans have difficulty remembering recently acquired information. In exposed humans, neuropsychiatric exams revealed a profound retrograde amnesia without the involvement of other cognitive abilities [56,57]. Performance on the Weschler Adult Intelligence Test was within the normal range and deficits in performance were limited to tasks that involved delayed recall of verbal and visuospatial memory. Like exposed humans, DA-treated rodents have difficulty retaining new information in memory and exhibit significant deficits in cognition on spatial navigation tasks.
Domoic Acid produces a constellation of neurobehavioral changes in exposed subjects that is dose and route dependent
Neurobehavioral changes associated with DA are dose and route dependent. Higher doses result in greater symptomology and illness. Intravenous doses as low as 0.25 mg/kg act as an emetic in monkeys, causing persistent chewing, salivation, gagging and vomiting in exposed subjects. At the 0.05 and 0.2 mg/kg dose IV, lipsmacking and hypoactivity are apparent. Classic behavioral signs of DA-induced neurotoxicity (stereotypical scratching) are seen at IV doses of 1.0 mg.kg and above but these levels are also associated with a greater than 50 % mortality rate. Doses of 3.5 and 4.0 mg.kg administered with IP injections are associated with seizures and death. Results from studies using IP injections with rodents have shown that higher doses (above 2.0 mg/kg) result in frank neurotoxicity that is characterized by seizures, status epilepticus and death in treated animals. Mid-range exposures (1.0 to 2.0 mg/kg) are associated with memory impairment that resembles human antegrade amnesia, behavioral hyperreactivity, rigidity, stereotypic scratching, wet-dog shakes and a unique praying posture. At the lower range of exposures (under 1.0 mg/kg), neurobehavioral effects of DA treatment in rodents include hypoactivity and hyperactivity. Overall, results from both animal models suggest that oral doses above 0.75 mg/kg act as an emetic and at 5.0 mg/kg, classic signs of DA toxicity are observed. Extremely high oral doses (60 to 80 mg/kg) can result in seizures and death [65].
Certain age groups are more susceptible to the neurotoxic effects of DA than others
The adverse effects of DA exposure vary by age at exposure and experimental evidence suggests that both the aging and developing brains are more vulnerable to DA-induced injury. Data from cases of human intoxication have revealed that older patients were more likely to be adversely affected by exposure [4]. In the poisoning episode that occurred in Canada, individuals greater than 60 years of age were at the greatest risk for severe memory impairment [56,57]. Studies with monkeys have also demonstrated that adult animals are more sensitive to brain injury from DA exposure than juvenile animals [45,47]. Age-related sensitivity has also been documented for very young animals in the laboratory. After parenteral postnatal exposure, observable symptomology in neonatal rats (seizure onset) occurs at doses 40 times lower than those required to produce similar results in adults [70]. Diminished blood clearance and possibly higher brain levels may play important roles in the high susceptibility of neonates to DA.
Fetal exposure is not associated with frank teratogenicity but is associated with persistent neurobehavioral effects
Experimental work with the rodent animal model clearly demonstrates that DA accumulates in amniotic fluid, crosses the placenta and enters the fetal brain [32]. An IV injection of 0.6 mg/kg on GD13 does not result in frank congenital malformations but abnormalities in electrophysiological measures are present for the first month of life [8]. Hippocampal damage in these neonates became progressively worse over time even though exposure was limited to a single dose during gestation. Levin and colleagues have demonstrated that exposure of pregnant rats to a sc dose of 1.3 mg/kg DA on GD13 is associated with long-term, persistent changes in cognitive and motor development in exposed offspring [27].
It is difficult to fully understand the long-term consequences of DA exposure because the primary site of injury is the brain. The functional loss associated with treatment-related brain injury may express itself in different ways across the lifespan. As environmental factors converge to make DA poisoning a more relevant public health threat, it will be important to address the gaps that exist in our knowledge of this compound. Foremost among these is the need for scientific studies that address the consequences of chronic, low-dose, oral exposure. While acute, high-dose studies have yielded fundamental and important insights on the neurotoxicity of this compound, long-term studies using oral treatment would more closely model real world exposures such as those occurring in coastal dwelling populations. Additional studies of the pharmacokinetics of DA will be necessary to extrapolate health risks from animal studies to humans.
Future studies of DA should take a broad-based approach to the behavioral methods that are selected as functional metrics. Our review of the literature has shown that the adverse consequences of DA exposure are not limited to a single behavioral effect and treatment-related changes have been documented in cognitive, motor and emotionality domains. Although most experimental studies of DA have focused on spatial leaning as the primary functional outcome, a more comprehensive assessment of learning and memory should be considered. Other aspects of behavior such as reactivity, temperament, social behavior and measures of emotionality would be meaningful and well-derived. In a research report on the neuropathology of DA in primates, Schmued and colleagues [47] noted that the hippocampal and limbic lesions produced by DA exposure would lead one to expect permanent memory loss and emotional changes or aberrations. In addition, lesions in the piriform cortex would suggest functional loss in olfactory perception. To date, there have been no sensory studies of DA exposure on vision, hearing, olfaction or somatosensory functioning in adults or exposed offspring. There are also experimental data that suggest the endocrine system may be adversely impacted by exposure and possible hormonal effects of DA should be investigated in pregnant females and offspring [27].
The possibility of delayed or latent neurotoxicity associated with DA exposure should also be explored. Research with monkeys and rodents demonstrates that functional losses can be persistent and injuries to the CNS can be progressive over time. Data from humans and sea lions suggest the possibility of latent or silent neurotoxicity, as in the appearance of DA-related seizures after a significant period of good health [8,41]. The concept of delayed expression of injury or disease, common in oncology, is now widely accepted as a basic tenet in the field of neurotoxicology. Latent toxicity has been demonstrated for other neurotoxicants such as methylmercury [42] and triethyltin [5]. Rather than producing a transient pharmacological effect, exposure to a neurotoxicant has the potential to cause CNS injury that may be expressed only after a period of biological “silence” [43]. Effects on health and behavior can be persistent and the neurotoxic impact may not be fully expressed at the time of exposure, laying the groundwork for latent or silent effects. A substantial period of time may pass between exposure and the expression of these effects. To accurately record the health effects of DA, lifespan studies will be necessary to track changes in health and behavior over time. Age-related vulnerabilities, such as that observed with DA neurotoxicity, have important public health implications and require special consideration when environmental regulatory policies are considered.
In conclusion, scientific programs aimed at the elucidation of the neurobehavioral effects of DA should focus on chronic, low-dose exposure paradigms and longitudinal assessment with neurobehavioral and sensory test methods. Greater emphasis should be placed on exploring the fetal effects of this complex biotoxin as extant data suggests persistent and progressive changes in exposed offspring. To model human and wildlife exposure more closely, experimental designs should include oral dosing methods more frequently. This route of exposure has been poorly explored in the literature and is the most relevant to real world scenarios. Additional studies focused on the dose-response effects of chronic, low-level exposures during pregnancy and/or early postnatal development are needed to adequately characterize the effects of DA on developmental processes. Future studies will also need to include systematic assessments of pregnant dams for overt toxicity when evaluating the fetal effects of this compound. The level of DA determined to be unsafe for human consumption by the Federal Food and Drug Administration (FDA) is 20 ppm in shellfish meat tissue. This guideline is based on data from adults and does not provide for the possibility of enhanced vulnerability in the fetus or child. Given that DA is a lethal and dangerous neurotoxicant in neonatal rats after parenteral treatment (70), developmental studies of oral DA exposure will be required to adequately understand fetal risk. Research programs that embrace these experimental parameters will be necessary to properly define the biological and neuropsychological risks associated with DA exposure in adult humans and sensitive subgroups such as infants, children and the aged.
Acknowledgements
The authors would like to thank Caroline Kennedy and Noelle Liberato for their skillful assistance with the preparation of this manuscript. We would also like to acknowledge the support of the Pacific Northwest Center for Human Health and Ocean Studies, the Center on Human Development and Disabilities, the Center for Child Environmental Health Risks and the Washington National Primate Resarch Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams AL, Doucette TA, Ryan CL. Altered pre-pulse inhibition in adult rats treated neonatally with domoic acid. Amino Acids. 2008;35:157–60. doi: 10.1007/s00726-007-0603-3. [DOI] [PubMed] [Google Scholar]
- 2.Adams AL, Doucette TA, James R, Ryan CL. Persistent changes in learning and memory in rats following neonatal treatment with domoic acid. Physiol Behav. 2009;96:505–12. doi: 10.1016/j.physbeh.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Appel NM, Rapoport SI, O'Callaghan JP. Sequelae of parenteral domoic acid administration in rats: comparison of effects on different anatomical markers in brain. Synapse. 1997;25:350–8. doi: 10.1002/(SICI)1098-2396(199704)25:4<350::AID-SYN6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Auer RN. Excitotoxic mechanisms, and age-related susceptibility to brain damage in ischemia, hypoglycemia and toxic mussel poisoning. Neurotoxicol. 1991;12:541–6. [PubMed] [Google Scholar]
- 5.Barone S, Jr, Stanton ME, Mundy WR. Neurotoxic effects of neonatal triethyltin (TET) exposure are exacerbated with aging. Neurobiol Aging. 1995;16:723–35. doi: 10.1016/0197-4580(95)00089-w. [DOI] [PubMed] [Google Scholar]
- 6.Brett MM. Food poisoning associated with biotoxins in fish and shellfish. Curr Opin Infect Dis. 2003;16:461–5. doi: 10.1097/00001432-200310000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Burt MA, Ryan CL, Doucette TA. Altered responses to novelty and drug reinforcement in adult rats treated neonatally with domoic acid. Physiol Behav. 2008;28:327–36. doi: 10.1016/j.physbeh.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Cendes F, Andermann F, Carpenter S, Zatorre RJ, Cashman NR. Temporal lobe epilepsy caused by domoic acid intoxication: evidence for glutamate receptor-mediated excitotoxicity in humans. Ann Neurol. 1995;37:123–6. doi: 10.1002/ana.410370125. [DOI] [PubMed] [Google Scholar]
- 9.Clayton EC, Peng YG, Means LW, Ramsdell JS. Working memory deficits induced by single but not repeated exposures to domoic acid. Toxicon. 1999;37:1025–39. doi: 10.1016/s0041-0101(98)00230-x. [DOI] [PubMed] [Google Scholar]
- 10.Dakshinamurti K, Sharma SK, Sundaram M, Watanabe T. Hippocampal changes in developing postnatal mice following intrauterine exposure to domoic acid. J Neurosci. 1993;13:4486–95. doi: 10.1523/JNEUROSCI.13-10-04486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucette TA, Bernard PB, Yuill PC, Tasker RA, Ryan CL. Low doses of non-NMDA glutamate receptor agonists alter neurobehavioral development in the rat. Neurotoxicol Teratol. 2003;25:473–479. doi: 10.1016/s0892-0362(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 12.Doucette TA, Bernard PB, Husum H, Perry MA, Ryan CL, Tasker RA. Low doses of domoic acid during postnatal development produce permanent changes in rat behavior and hippocampal morphology. Neurotox Res. 2004;6:555–63. doi: 10.1007/BF03033451. [DOI] [PubMed] [Google Scholar]
- 13.Doucette TA, Ryan CL, Tasker RA. Gender-based changes in cognition and emotionality in a new rat model of epilepsy. Amino Acids. 2007;32:317–22. doi: 10.1007/s00726-006-0418-7. [DOI] [PubMed] [Google Scholar]
- 14.Erdner DL, Dyble J, Parsons ML, Stevens RC, Hubbard KA, Wrabel ML, Moore SK, Lefebvre KA, Anderson DM, Bienfang P, Bidigare RR, Parker MS, Moelle P, Brand LE, Trainer VL. Centers for Oceans and Human Health: a unified approach to the challenge of harmful algal blooms. Environ Health. 2008;7(Suppl 2):S2. doi: 10.1186/1476-069X-7-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming LE, Easom J, Baden D, Rowan A, Levin B. Emerging harmful algal blooms and human health: Pfiesteria and related organisms. Toxicol Pathol. 1999;27:573–81. doi: 10.1177/019262339902700511. [DOI] [PubMed] [Google Scholar]
- 16.Foster AC, Mena EE, Monaghan DT, Cotman CW. Synaptic localization of kainic acid binding sites. Nature. 1981;289:73–5. doi: 10.1038/289073a0. [DOI] [PubMed] [Google Scholar]
- 17.Friedman MA, Levin BE. Neurobehavioral effects of harmful algal bloom (HAB) toxins: a critical review. J Int Neuropsychol Soc. 2005;11:331–8. doi: 10.1017/S1355617705050381. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein T, Mazet JA, Zabka TS, Langlois G, Colegrove KM, Silver M, Bargu S, Van Dolah F, Leighfield T, Conrad PA, Barakos J, Williams DC, Dennison S, Haulena M, Gulland FM. Novel symptomatology and changing epidemiology of domoic acid toxicosis in California sea lions (Zalophus californianus): an increasing risk to marine mammal health. Proc Biol Sci. 2008;275:267–76. doi: 10.1098/rspb.2007.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulland FM, Haulena M, Fauquier D, Langlois G, Lander ME, Zabka T, Duerr R. Domoic acid toxicity in Californian sea lions (Zalophus californianus): clinical signs, treatment and survival. Vet Rec. 2002;150:475–80. doi: 10.1136/vr.150.15.475. [DOI] [PubMed] [Google Scholar]
- 20.Hampson DR, Manalo JL. The activation of glutamate receptors by kainic acid and domoic acid. Nat. Toxins. 1998;6:153–158. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<153::aid-nt16>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 21.Iverson F, Truelove J, Nera E, Tryphonas L, Campbell J, Lok E E. Domoic acid poisoning and mussel-associated intoxication: preliminary investigations into the response of mice and rats to toxic mussel extract. Food Chem Toxicol. 1989;27:377–84. doi: 10.1016/0278-6915(89)90143-9. [DOI] [PubMed] [Google Scholar]
- 22.James KJ, Gillman M, Amandi MF, López-Rivera A, Puente PF, Lehane M, Mitrovic S, Furey A. Amnesic shellfish poisoning toxins in bivalve molluscs in Ireland. Toxicon. 2005;46:852–8. doi: 10.1016/j.toxicon.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Jeffery B, Barlow T, Moizer K, Paul S, Boyle C. Amnesic shellfish poison. Food Chem Toxicol. 2004;42:545–57. doi: 10.1016/j.fct.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Knap A, Dewailly E, Furgal C, Galvin J, Baden D, Bowen RE, Depledge M, Duguay L, Fleming LE, Ford T, Moser F, Owen R, Suk WA, Unluata U. Indicators of ocean health and human health: developing a research and monitoring framework. Environ Health Perspect. 2002;110:839–45. doi: 10.1289/ehp.02110839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuhlmann AC, Guilarte TR. The peripheral benzodiazepine receptor is a sensitive indicator of domoic acid neurotoxicity. Brain Res. 1997;751:281–8. doi: 10.1016/s0006-8993(96)01409-6. [DOI] [PubMed] [Google Scholar]
- 26.Lefebvre KA, Bargu S, Kieckhefer T, Silver MW. From sanddabs to blue whales: the pervasiveness of domoic acid. Toxicon. 2002;40:971–7. doi: 10.1016/s0041-0101(02)00093-4. [DOI] [PubMed] [Google Scholar]
- 27.Levin ED, Pizarro K, Pang WG, Harrison J, Ramsdell JS. Persisting behavioral consequences of prenatal domoic acid exposure in rats. Neurotoxicol Teratol. 2005;27:719–25. doi: 10.1016/j.ntt.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 28.Levin ED, Pang WG, Harrison J, Williams P, Petro A, Ramsdell JS. Persistent neurobehavioral effects of early postnatal domoic acid exposure in rats. Neurotoxicol Teratol. 2006;28:673–80. doi: 10.1016/j.ntt.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Luckas B, Dahlmann J, Erler K, Gerdts G, Wasmund N, Hummert C, Hansen PD. Overview of key phytoplankton toxins and their recent occurrence in the North and Baltic Seas. Environ Toxicol. 2005;20:1–17. doi: 10.1002/tox.20072. [DOI] [PubMed] [Google Scholar]
- 30.Maucher JM, Ramsdell JS. Domoic acid transfer to milk: evaluation of a potential route of neonatal exposure. Environ Health Perspect. 2005;113:461–4. doi: 10.1289/ehp.7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maucher JM, Ramsdell JS. Ultrasensitive detection of domoic acid in mouse blood by competitive ELISA using blood collection cards. Toxicon. 2005;45:607–13. doi: 10.1016/j.toxicon.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Maucher JM, Ramsdell JS. Maternal-fetal transfer of domoic acid in rats at two gestational time points. Environ Health Perspect. 2007;115:1743–6. doi: 10.1289/ehp.10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris R. Sea lions hit by high levels of acid poison California. New York Times (Science and Environment) 2007 Jun;6 [Google Scholar]
- 34.Nakajima S, Potvin JL. Neural and behavioral effects of domoic acid, an amnesic shellfish toxin, in the rat. Can J Psychol. 1992;46:569–81. doi: 10.1037/h0084334. [DOI] [PubMed] [Google Scholar]
- 35.Peng YG, Ramsdell JS. Brain Fos induction is a sensitive biomarker for the lowest observed neuroexcitatory effects of domoic acid. Fundam Appl Toxicol. 1996;31:162–8. doi: 10.1006/faat.1996.0087. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Mendes P, Cinini SM, Medeiros MA, Tufik S, Mello LE. Behavioral and histopathological analysis of domoic acid administration in marmosets. Epilepsia. 2005;46:148–51. doi: 10.1111/j.1528-1167.2005.01023.x. [DOI] [PubMed] [Google Scholar]
- 37.Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990a;322:1775–80. doi: 10.1056/NEJM199006213222504. [DOI] [PubMed] [Google Scholar]
- 38.Perl TM, Bédard L, Kosatsky T, Hockin JC, Todd EC, McNutt LA, Remis RS. Amnesic shellfish poisoning: a new clinical syndrome due to domoic acid. Can Dis Wkly Rep. 1990b;16(Suppl 1E):7–8. [PubMed] [Google Scholar]
- 39.Petrie BF, Pinsky C, Standish NM, Bose R, Glavin GB. Parenteral domoic acid impairs spatial learning in mice. Pharmacol Biochem Behav. 1992;41:211–4. doi: 10.1016/0091-3057(92)90084-s. [DOI] [PubMed] [Google Scholar]
- 40.Ramsdell JS. The molecular and integrative basis to domoic acid toxicity. In: Botana L, editor. Phycotoxins: Chemisty and Biochemistry. Blackwell Publishing; Ames, IA, USA: 2007. pp. 223–50. [Google Scholar]
- 41.Ramsdell JS, Zabka TS. In utero domoic acid toxicity: a fetal basis to adult disease in the California sea lion (Zalophus californianus. Mar Drugs. 2008;6:262–90. doi: 10.3390/md20080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicol. 1996;17:583–96. [PubMed] [Google Scholar]
- 43.Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. 2000;108:511–33. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rhodes L, Scholin C, Garthwaite I. Pseudo-nitzschia in New Zealand and the role of DNA probes and immunoassays in refining marine biotoxin monitoring programmes. Nat Toxins. 1998;6:105–11. [PubMed] [Google Scholar]
- 45.Scallet AC, Binienda Z, Caputo FA, Hall S, Paule MG, Rountree RL, Schmued L, Sobotka T, Slikker W., Jr Domoic acid-treated cynomolgus monkeys (M. fascicularis): effects of dose on hippocampal neuronal and terminal degeneration. Brain Res. 1993;627:307–13. doi: 10.1016/0006-8993(93)90335-k. [DOI] [PubMed] [Google Scholar]
- 46.Scholin CA, Gulland F, Doucette GJ, Benson S, Busman M, Chavez FP, Cordaro J, DeLong R, De Vogelaere A, Harvey J, Haulena M, Lefebvre K, Lipscomb T, Loscutoff S, Lowenstine LJ, Marin R, 3rd, Miller PE, McLellan WA, Moeller PD, Powell CL, Rowles TPPP, Silvagni M, Silver T, Spraker V, Trainer V, Van Dolah FM. Mortality of sea lions along the central California coast linked to a toxic diatom bloom. Nature. 2000;403:80–4. doi: 10.1038/47481. [DOI] [PubMed] [Google Scholar]
- 47.Schmued LC, Scallet AC, Slikker W., Jr Domoic acid-induced neuronal degeneration in the primate forebrain revealed by degeneration specific histochemistry. Brain Res. 1995;695:64–70. doi: 10.1016/0006-8993(95)00799-v. [DOI] [PubMed] [Google Scholar]
- 48.Sierra-Beltrán A, Palafox-Uribe M, Grajales-Montiel J, Cruz-Villacorta A, Ochoa JL. Sea bird mortality at Cabo San Lucas, Mexico: evidence that toxic diatom blooms are spreading. Toxicon. 1997;35:447–53. doi: 10.1016/s0041-0101(96)00140-7. [DOI] [PubMed] [Google Scholar]
- 49.Slikker W W, Jr, Scallet AC, Gaylor DW. Biologically-based dose-response model for neurotoxicity risk assessment. Toxicol Lett. 1998;102-103:429–33. doi: 10.1016/s0378-4274(98)00335-x. [DOI] [PubMed] [Google Scholar]
- 50.Sobotka TJ, Brown R, Quander DY, Jackson R, Smith M, Long SA, Barton CN, Rountree RL, Hall S, Eilers P, Johannessen JN, Scallet AC. Domoic acid: neurobehavioral and neurohistological effects of low-dose exposure in adult rats. Neurotoxicol Teratol. 1996;18:659–70. doi: 10.1016/s0892-0362(96)00120-1. [DOI] [PubMed] [Google Scholar]
- 51.Stommel EW, Watters MR. Marine neurotoxins: Ingestible toxins. Curr Treat Options Neurol. 2004;6:105–114. doi: 10.1007/s11940-004-0020-9. [DOI] [PubMed] [Google Scholar]
- 52.Strain SM, Tasker RA. Hippocampal damage produced by systemic injections of domoic acid in mice. Neurosci. 1991;44:343–52. doi: 10.1016/0306-4522(91)90059-w. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki CA, Hierlihy SL. Renal clearance of domoic acid in the rat. Food Chem Toxicol. 1993;10:701–6. doi: 10.1016/0278-6915(93)90140-t. [DOI] [PubMed] [Google Scholar]
- 54.Takemoto T, Daigo K. Constituents of Chondria. Chem Pharm Bulletin. 1958;6:578–580. [Google Scholar]
- 55.Takemoto T, Daigo K, Kondo Y, Kondo Y, K. Chem Abstr. 1967;66:28604. [Google Scholar]
- 56.Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990a;322:1781–7. doi: 10.1056/NEJM199006213222505. [DOI] [PubMed] [Google Scholar]
- 57.Teitelbaum J, Zatorre RJ, Carpenter S, Gendron D, Cashman NR. Neurological sequelae of domoic acid intoxication. Can Dis Wkly Rep. 1990b;16(Suppl 1E):9–12. [PubMed] [Google Scholar]
- 58.Trainer VL, Adams NG, Bill BD, Anulacion BF, Wekell JC. Concentration and dispersal of a Pseudo-nitzschia bloom in Penn Cove, Washington, USA. Nat Toxins. 1998;6:113–26. doi: 10.1002/(sici)1522-7189(199805/08)6:3/4<113::aid-nt14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 59.Todd EC. Emerging diseases associated with seafood toxins and other water-borne agents. Ann N Y Acad Sci. 1994;740:77–94. doi: 10.1111/j.1749-6632.1994.tb19855.x. [DOI] [PubMed] [Google Scholar]
- 60.Truelove J, Iverson F. Serum domoic acid clearance and clinical observations in the cynomolgus monkey and Sprague-Dawley rat following a single i.v. dose. Bull Environ Contam Toxicol. 1994;52:479–86. doi: 10.1007/BF00194132. [DOI] [PubMed] [Google Scholar]
- 61.Truelove J, Mueller R, Pulido O, Iverson F. Subchronic toxicity study of domoic acid in the rat. Food Chem Toxicol. 1996;34:525–9. doi: 10.1016/0278-6915(96)81814-x. [DOI] [PubMed] [Google Scholar]
- 62.Truelove J, Muelle R, Pulido O, Martin L, Fernie S, Iverson F. 30-day oral toxicity study of domoic acid in cynomolgus monkeys: lack of overt toxicity at doses approaching the acute toxic dose. Nat Toxins. 1997;5:111–4. doi: 10.1002/1522-7189(1997)5:3<111::AID-NT5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 63.Tyrphonas L, Truelove J, Iverson F, Nera L. Acute neurotoxocity of domic acid in the rat. Toxicol Pathol. 1990;18:1–9. doi: 10.1177/019262339001800101. [DOI] [PubMed] [Google Scholar]
- 64.Tryphonas L, Truelove J, Iverson F, Nera EA, Iverson F. Acute parenteral neurotoxicity of domoic acid in cynomolgus monkeys (M. fascicularis) Toxicol Pathol. 1990;18:297–303. doi: 10.1177/019262339001800208. [DOI] [PubMed] [Google Scholar]
- 65.Tryphonas L, Truelove J, Iverson F. Experimental oral toxicity of domoic acid in cynomolgus monkeys (Macaca fascicularis) and rats. Preliminary investigations. Food Chem Toxicol. 1990;28:707–15. doi: 10.1016/0278-6915(90)90147-f. [DOI] [PubMed] [Google Scholar]
- 66.Van Dolah FM. Marine algal toxins: origins, health effects, and their increased occurrence. Environ Health Perspect. 2000;108(Suppl 1):133–41. doi: 10.1289/ehp.00108s1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang GJ, Schmued LC, Andrews AM, Scallet AC, Slikker W W, Jr, Binienda Z. Systemic administration of domoic acid-induced spinal cord lesions in neonatal rats. J Spinal Cord Med. 2000;23:31–9. doi: 10.1080/10790268.2000.11753506. [DOI] [PubMed] [Google Scholar]
- 68.Walz PM, Garrison DL, Graham WM, Cattey MA, Tjeerdema RS, Silver MW. Domoic acid-producing diatom blooms in Monterey Bay, California: 1991-1993. Nat Toxins. 1994;2:271–9. doi: 10.1002/nt.2620020505. [DOI] [PubMed] [Google Scholar]
- 69.Wekell JC, Gauglitz EJ, Jr, Barnett HJ, Hatfield CL, Simons D, Ayres D. Occurrence of domoic acid in Washington state razor clams (Siliqua patula) during 1991-1993. Nat Toxins. 1994;2:197–205. doi: 10.1002/nt.2620020408. [DOI] [PubMed] [Google Scholar]
- 70.Xi D, Peng YG, Ramsdell JS. Domoic acid is a potent neurotoxin to neonatal rats. Nat Toxins. 1997;5:74–9. doi: 10.1002/(SICI)(1997)5:2<74::AID-NT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]