Abstract
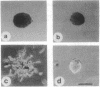
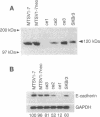
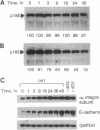
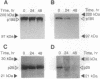
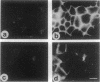
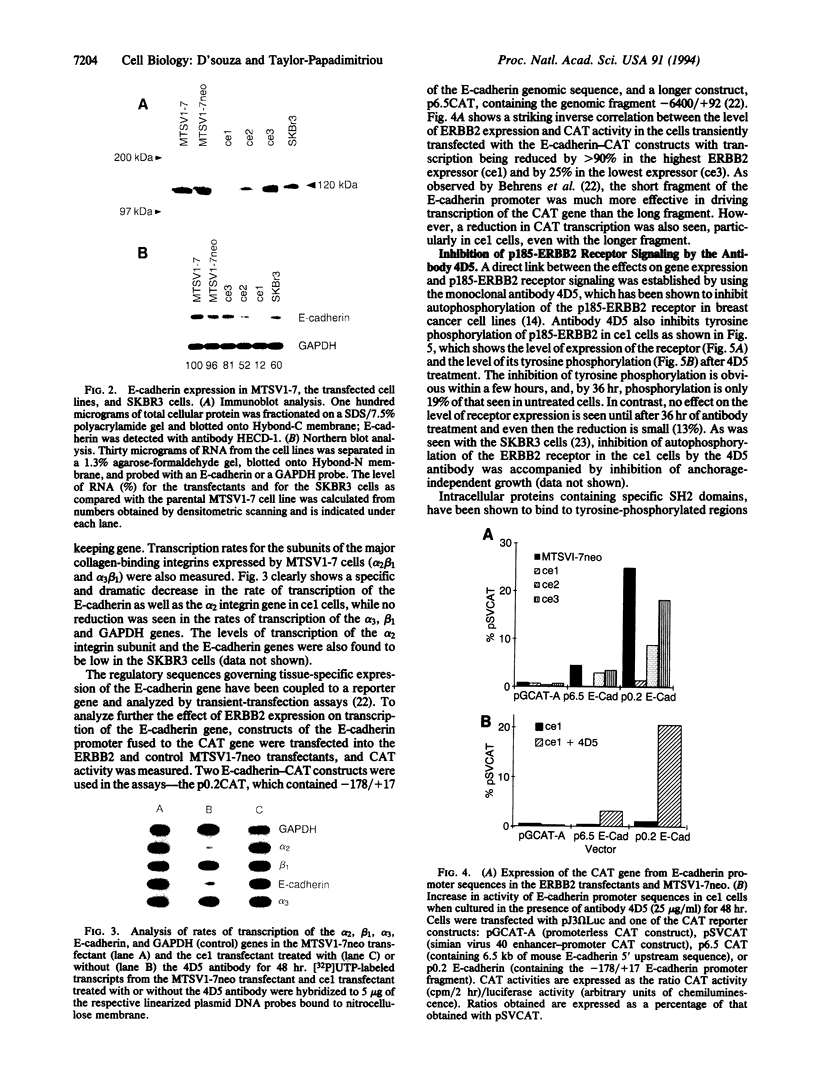
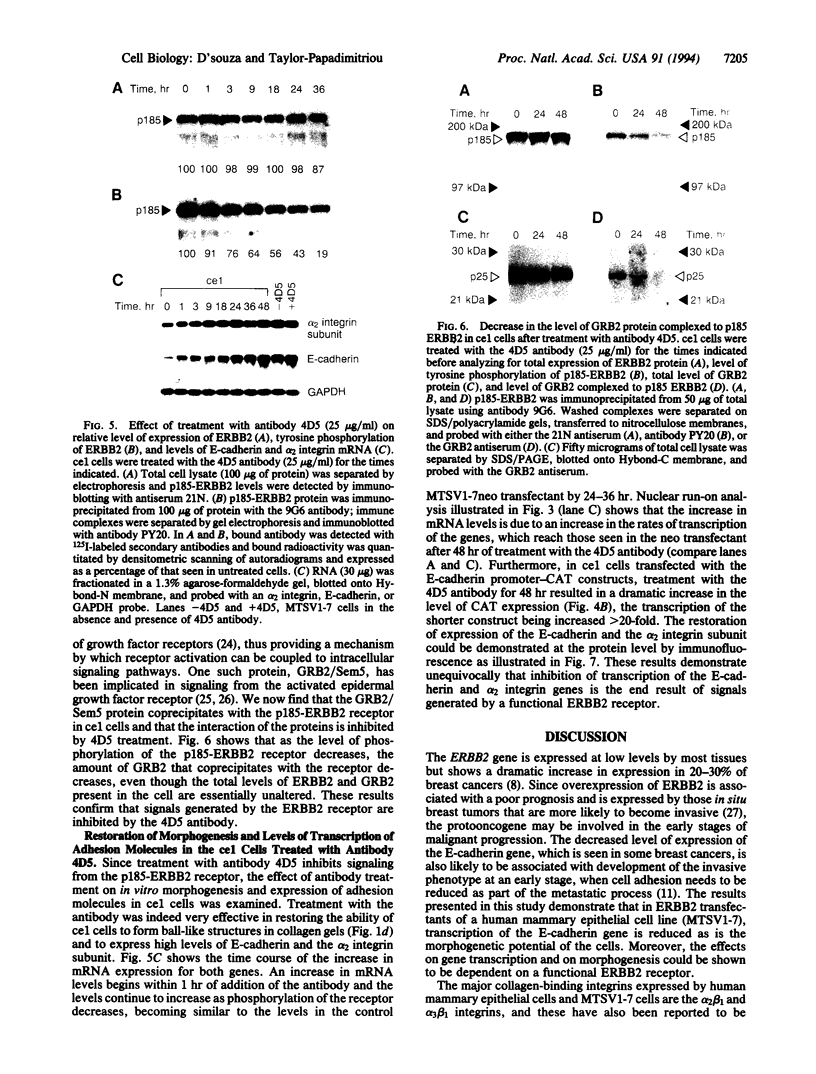
Overexpression of the ERBB2 receptor in transfectants of a human mammary epithelial cell line (MTSV1-7) is associated with a reduced ability to undergo morphogenesis in vitro and with a decreased level of expression of the E-cadherin and alpha 2 integrin genes. The inhibition of expression of the adhesion molecules has been shown to be at the level of transcription by using nuclear run-on assays and by following transcription of a reporter gene fused to 5' sequences of the E-cadherin gene. To relate the effects on gene transcription to a functional ERBB2 protein, signaling from the receptor was inhibited by the antibody 4D5, which blocks phosphorylation of ERBB2 on tyrosine residues and association of the protein with the GRB2/Sem5 protein. After treatment with the antibody 4D5, the ERBB2 transfectants regain the ability to form three-dimensional structures in collagen gels and the rates of transcription of the genes encoding the E-cadherin and the alpha 2 integrin subunit are restored to the levels seen in MTSV1-7neo cells. These results demonstrate that the inhibition of morphogenesis and transcription of specific adhesion molecules in human mammary epithelial cells can be affected by signals generated by the ERBB2 receptor and suggest a role for ERBB2 overexpression in tumor progression and metastasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartek J., Bartkova J., Kyprianou N., Lalani E. N., Staskova Z., Shearer M., Chang S., Taylor-Papadimitriou J. Efficient immortalization of luminal epithelial cells from human mammary gland by introduction of simian virus 40 large tumor antigen with a recombinant retrovirus. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3520–3524. doi: 10.1073/pnas.88.9.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J., Löwrick O., Klein-Hitpass L., Birchmeier W. The E-cadherin promoter: functional analysis of a G.C-rich region and an epithelial cell-specific palindromic regulatory element. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11495–11499. doi: 10.1073/pnas.88.24.11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky F., Gilbert C., Shearer M., Taylor-Papadimitriou J. Collagen-induced rapid morphogenesis of human mammary epithelial cells: the role of the alpha 2 beta 1 integrin. J Cell Sci. 1992 Jul;102(Pt 3):437–446. doi: 10.1242/jcs.102.3.437. [DOI] [PubMed] [Google Scholar]
- Buday L., Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993 May 7;73(3):611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Frebourg T., Brison O. Plasmid vectors with multiple cloning sites and cat-reporter gene for promoter cloning and analysis in animal cells. Gene. 1988 May 30;65(2):315–318. doi: 10.1016/0378-1119(88)90468-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Sliwkowski M. X., Akita R. W., Henzel W. J., Lee J., Park J. W., Yansura D., Abadi N., Raab H., Lewis G. D. Identification of heregulin, a specific activator of p185erbB2. Science. 1992 May 22;256(5060):1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- Hotchin N. A., Watt F. M. Transcriptional and post-translational regulation of beta 1 integrin expression during keratinocyte terminal differentiation. J Biol Chem. 1992 Jul 25;267(21):14852–14858. [PubMed] [Google Scholar]
- Hudziak R. M., Lewis G. D., Winget M., Fendly B. M., Shepard H. M., Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. 1989 Mar;9(3):1165–1172. doi: 10.1128/mcb.9.3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglehart J. D., Kraus M. H., Langton B. C., Huper G., Kerns B. J., Marks J. R. Increased erbB-2 gene copies and expression in multiple stages of breast cancer. Cancer Res. 1990 Oct 15;50(20):6701–6707. [PubMed] [Google Scholar]
- King C. R., Borrello I., Porter L., Comoglio P., Schlessinger J. Ligand-independent tyrosine phosphorylation of EGF receptor and the erbB-2/neu proto-oncogene product is induced by hyperosmotic shock. Oncogene. 1989 Jan;4(1):13–18. [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Kovarik A., Peat N., Wilson D., Gendler S. J., Taylor-Papadimitriou J. Analysis of the tissue-specific promoter of the MUC1 gene. J Biol Chem. 1993 May 5;268(13):9917–9926. [PubMed] [Google Scholar]
- Kumar R., Shepard H. M., Mendelsohn J. Regulation of phosphorylation of the c-erbB-2/HER2 gene product by a monoclonal antibody and serum growth factor(s) in human mammary carcinoma cells. Mol Cell Biol. 1991 Feb;11(2):979–986. doi: 10.1128/mcb.11.2.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lonardo F., Di Marco E., King C. R., Pierce J. H., Segatto O., Aaronson S. A., Di Fiore P. P. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol. 1990 Nov;2(11):992–1003. [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Lupu R., Colomer R., Zugmaier G., Sarup J., Shepard M., Slamon D., Lippman M. E. Direct interaction of a ligand for the erbB2 oncogene product with the EGF receptor and p185erbB2. Science. 1990 Sep 28;249(4976):1552–1555. doi: 10.1126/science.2218496. [DOI] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Oka H., Shiozaki H., Kobayashi K., Inoue M., Tahara H., Kobayashi T., Takatsuka Y., Matsuyoshi N., Hirano S., Takeichi M. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993 Apr 1;53(7):1696–1701. [PubMed] [Google Scholar]
- Peles E., Bacus S. S., Koski R. A., Lu H. S., Wen D., Ogden S. G., Levy R. B., Yarden Y. Isolation of the neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumor cells. Cell. 1992 Apr 3;69(1):205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- Pignatelli M., Hanby A. M., Stamp G. W. Low expression of beta 1, alpha 2 and alpha 3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991 Sep;165(1):25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Puissant C., Houdebine L. M. An improvement of the single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Biotechniques. 1990 Feb;8(2):148–149. [PubMed] [Google Scholar]
- Segatto O., Lonardo F., Pierce J. H., Bottaro D. P., Di Fiore P. P. The role of autophosphorylation in modulation of erbB-2 transforming function. New Biol. 1990 Feb;2(2):187–195. [PubMed] [Google Scholar]
- Semba K., Kamata N., Toyoshima K., Yamamoto T. A v-erbB-related protooncogene, c-erbB-2, is distinct from the c-erbB-1/epidermal growth factor-receptor gene and is amplified in a human salivary gland adenocarcinoma. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6497–6501. doi: 10.1073/pnas.82.19.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon D. J., Clark G. M., Wong S. G., Levin W. J., Ullrich A., McGuire W. L. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Zutter M. M., Mazoujian G., Santoro S. A. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990 Oct;137(4):863–870. [PMC free article] [PubMed] [Google Scholar]