Abstract
Although modern dental repair materials show excellent mechanical and adhesion properties, they still face two major problems: First, any microbes that remain alive below the composite fillings actively decompose dentin and thus, subsequently cause secondary caries. Second, even if those microbes are killed, the extracellular proteases such as MMP, remain active and can still degrade collagenousdental tissue. In order to address both problems, a poly(2-methyloxazoline) with a biocidal quaternary ammonium and a polymerizable methacrylate terminal was explored as additive for a commercial dental adhesive. It could be demonstrated that the adhesive rendered the adhesive contact-active antimicrobial against S. mutans at a concentration of only 2.5 wt% and even constant washing with water for 101 days did not diminish this effect. Increasing the amount of the additive to 5 wt% allowed killing S. mutans cells in the tubuli of bovinedentin upon application of the adhesive. Further, the additive fully inhibited bacterial collagenase at a concentration of 0.5 wt% and reduced human recombinant collagenase MMP-9 to 13% of its original activity at that concentration. Human MMPs naturally bound to dentin were inhibited by more than 96% in a medium containing 5 wt% of the additive. Moreover, no adverse effect on the enamel/dentine shear bond strength was detected in combination with a dental composite.
Keywords: polyoxazoline, antimicrobial, dental adhesive, self-etch, collagenase inhibitor, surface binding, composite
Introduction
Treatment of clinically manifest dental decay requires mechanincal excavation of decayed dental hard tissue. The cavity left by excavation may contain residual S. mutans or other carious organisms. Most authorities suggest treating that dentin with an antimicrobiol dental adhesive to secure subsequently applied filling resin composite to underlying dentin. The resulting dentin/adhesive/composite interface is composed of dentin, a hybrid layer consisting of a collagen network extended into the adhesive polymer, the adhesive and the composite seal, respectively. Although, very advanced dental materials have been developed so far, there are still cases of long-term failure of such restorations. Solutions to that problem can certainly improve life quality of patients after restoration and save costs of replacement dentistry.
Degradation of the hybrid layer between adhesive and dentin is a multifactorial problem, that has been linked with an incomplete monomer infiltration into the dentin substrate,1 inhomogeneous monomer distribution through the interdiffusion zone, incomplete polymerization, alteration of the organic matrix due to surface preparation, enzymatic polymer degradation,2 nanophase separation,3 and hydrolysis of the dentin matrix by matrix metalloproteases (MMPs).
Water uptake by both the adhesive layer and the composite seems to be the main reason for long-term in-vivo tooth/composite-bond degradation.,4–6SEM/TEM-studies investigating the deterioration of interfaces between adhesive/composite and tooth surfacefound unprotected and partly disrupted collagen fibrils.7, 8.
Deterioration of the dentin substrate and collagen fibrils of the hybrid layer can be induced by predominantly anaerobic, cariogenic bacterial strains (Lactobacillaceae and Streptococcaceae) colonizing irregularities at the margins of composite filling. Those microbes are often located in the straight parallel tubuli that define the characteristic structure of dentin9and may therefore be hard to remove completely by mechanical excavationcarious tissue. Their acidic by-products may invoke a recurrent caries process by dissolution of the hydroxyapatite interphase in dentin.10 Such acid-induced demineralization removes apatite crystallites, exposing collagen fibrils and activating the previously inactive MMPs causing protease-induced decomposition of the collagenous organic matrix.
In order to fight residual bacterial cells trapped in dentin below the fillings, several groups have introduced biocidal additives for dental repair materials.11, 12 Mostly these approaches consist of release systems based on chlorhexidine,13–16 furanons,17 zinc ions,18 fluoride19, and quaternary ammonium compounds20. More recently, numerous biocidal monomers for incorporation into dental materials have been reported.21–24 Also incorporated polyquats, such asalkylated polyethyleneimine were shown to equip dental resins with long-lasting antimicrobial properties.25
The degradation process of the collagen matrix is catalyzed by extracellular protease, namely C-terminal telopeptidase collagen hydrolyzing matrix metalloproteinases (MMPs) such as MMP-2 and MMP-9.26–28 Additional to microbial MMPs, the dentin also contains the human collagenase MMP-8 (Sukula et al., 2007). Most of the latter dentinal MMPs are produced and released from odontoblasts in the form of inactive proenzymes and require activation through the so-called cysteine switch.29In the caries process, pH-changes caused by lactic acid-release activate the host-derived (dentinal) pro-MMPs.26 Recently, it has been shown30, 31 that dentinal pro-MMPs can be activated by acidic monomers that are common components of self-etching, etch- and rinse- dental adhesive systems.32, 33 In detail, etch- and rinse adhesives,34, 35 as well as self-etching adhesives30 have been confirmed to activate gelatinases A and B (MMP-2/MMP-9) and collagenase (MMP-8) in human dentin.36 Lehmann et al. found that MMP-2 and pro-MMP-9 are expressed from odontoblastic cells immediately after stimulating them with self-etching adhesives.37 Consequently, in order to increase the durability of the dentin/adhesive/composite joint, it is not sufficient to kill cariogenic bacterial cells in the dentin cavity, but one must also inactivate dentin in MMPs.
Several MMP-inhibitors have been added to dentin adhesive systems. Recently, low-molecular weight QUART-methacrylates have been described as powerful inhibitors of soluble and matrix-bound MMPs.38 Gendron et al.39 observed complete MMP-inhibition with even marginal concentrations of chlorhexidine. For this reason, many investigations (in vitro/in vivo) have been conducted showing chlorhexidine’s improving effect on the dentin/adhesive/composite interface by protecting the hybrid layer.16, 40–42 In this context, more specific synthetic MMP-inhibitors like galardin have also been tested.43
In this study, we explore a macromeric biocide based on poly(2-methyl oxazoline) for its ability to simultaneously render a commercial dental adhesive antimicrobially anti-microbial to enable it to kill S. mutans cells on contact in the tubuli of tooth, and to inhibit bacterial and human collagenases and gelatinases.
Experimental Part
Materials
All reactions, purifications and polymerizations were carried out under argon atmosphere. Chloroform (AppliChem GmbH, Darmstadt, Germany) was shaken with concentrated H2SO4and dried by passage through a column of activated alumina, resulting in residual moisture of only < 0.5 ppm (determined by Karl-Fischer titration). The dried solvent was stored over Linde-type 4Å molecular sieves. The antimicrobial initiator 4-(bromomethyl)-N-dodecyl-N,N-dimethylbenzene ammonium bromide (DDA-X) was synthesized analogously to previous works on antimicrobial telechelic poly(oxazoline)s.44 The monomers 2-methyl-1,3-oxazoline (MOX, Sigma-Aldrich Chemie GmbH, Munich, Germany) and 2-ethyl-1,3-oxazoline (EOX, Sigma-Aldrich) were freshly distilled from CaH2 prior to use. Ethylenediamine (EDA, Sigma Aldrich) was purified twice in a freeze-and thaw procedure. N,N-dimethyldodecylamine (DDA, Sigma-Aldrich) and N-[3-(dimethyl-amino)-propyl]-methacrylamide (AMA, Sigma-Aldrich) were distilled under reduced pressure from butylated hydroxytoluene (BHT, Sigma-Aldrich). Pyridine was shaken with sodium hydroxide, distilled in vacuum and stored over Linde type 4Å molecular sieves (Sigma-Aldrich). All other compounds were of analytical grade or purer and used without further purification. Whole bovine teeth (provided by Ivoclar Vivadent AG, Schaan, Liechtenstein) were sliced, autoclaved, sterilized and stored in aqueous ethanol (5/95, V/V) under dark and cool (4°C) conditions. Commercially available AdheSE One F (Ivoclar Vivadent AG) was used as self-etch dental adhesive matrix; Tetric EvoCeram A3 (Ivoclar Vivadent AG) was used as a commonly shaded composite. Streptococcus mutans (type strain ATCC 25175) was provided by the German Resource Centre for Biological Material (DSMZ, Braunschweig, Germany).
Measurements
1H and 13C NMR spectra were recorded in CDCl3, DMSO-d6, and methanol-d4, respectively, using a DRX-400 spectrometer (Bruker Corp., Ettlingen,Germany) with a 5 mm sample head operating at 400.13 MHz for 1H and 100.63 MHz for 13C. Size exclusion chromatography (SEC) was performed on a GPCMax (Malvern Instruments, Herrenberg, Germany) equipped with an refractive index (RI) detector (adjusted to 55°C) using a TSKgel GMHHR-M (Tosoh, 5.0 µm pores, 2× + 1× precolumn) column set. Saline N,N-dimethylformamide (DMF+LiBr, 20 mmol) was used as eluent at 60°C with a flow rate of 0.70 mL·min−1. Calibration was performed with poly(styrene) standards (Malvern Instruments). UV/vis analyses were performed on a Specord 210 (Analytik Jena AG, Jena, Germany) UV/vis spectrometer at 25°C.
Standard Procedure of the Polymerization
The required concentration of methyl p-toluenesulfonate(MeOTs, Sigma-Aldrich) or the antimicrobial initiator 4-(bromomethyl)-N-dodecyl-N,N-dimethylbenzene ammonium bromide, respectively was calculated on base of the degree of polymerization DPset and the starting concentration of monomer [M0] according to [MeOTs/DDA-X] = [M0]·DPset−1. In order to synthesize a DDA-X-initiated poly(methyl-oxazoline) with DPsetof 30 monomeric units, DDA-X (0.56 g, 1.17 mmol) was dissolved in chloroform (12 mL) at roomtemperature and methyloxazoline (MOX) was added (3.00 g, 3.00 mL, 35.25 mmol). The microwave-assisted polymerizations were carried out in Discover Synthesis microwaves (CEM GmbH, Linfort, Germany). The reaction temperature was constantly monitored with a vertically focused IR temperature sensor.The closed vessels were heated to 100°C under magnetic stirring.
The corresponding reaction times varied with DPset in the range of 2–4 h in the case of PMOX- and in the rangeof 4–6 h in the case of PEOX-systems.
Termination and Purification
A 20-fold molar excess (with respect to the initiator amount) of the terminating agent (EDA, DDA, PYR) was added to the living polymers and heated at 40–50°C for 24 hrs. The hydroxy-terminals upon the poly(oxazolines) were introduced using a saturated aqueous K2CO3 solution added to the living polymeric reaction mixtures at 25°C. In the case of the sensitive N-[3-(dimethylamino)-propyl]-methacrylamide (AMA),butylated hydroxytoluene (BHT, 0.05 wt%, related to the respective amount) was used for double-bond protection. The raw polymeric products were purified by precipitation in diethyl ether and dialyzed against methanol using benzoylated cellulose membranes (ZelluTrans, Carl Roth, Karlsruhe, Germany, 1,000 g·mol−1 molecular weight cut-off). After solvent removal, yields of 88 to 93 wt% were obtained. The products were characterized by 1H/13C-NMR spectroscopy and size exclusion chromatography (SEC), the basic NMR signals of the polymeric backbones are listed below. The molecular weights and the MIC values are given in Table 1.
Table 1.
Analytical data of all telechelic poly(2-R-oxazoline) I-(PROX)n-T systems with R=M (methyl-) and E (ethyl-) including the evaluation of their biocidal- and MMP-inhibiting efficacies.
| reference/polymer | MNMRa [g·mol−1] |
DPNMRa | Mn, SEC [g·mol−1] |
Mw, SEC [g·mol−1] |
DPSEC | PDI | MICS. m. [µg/mL]b |
|---|---|---|---|---|---|---|---|
| DTACc | 263.89 | n.d. | n.d. | n.d. | n.d. | n.d. | 4.9 |
| Me-PMOX37-OHd | n.d. | n.d. | 3,200 | 3,750 | 37 | 1.16 | > 2,500 |
| Me-PEOX24-OHd | n.d. | n.d. | 2,400 | 2,700 | 24 | 1.15 | > 2,500 |
| Me-PEOX53-PYRe | 5,400 | 53 | 5,200 | 6,000 | 51 | 1.16 | - |
| Me-PMOX22-DDAe | 2,100 | 22 | 2,300 | 2,700 | 24 | 1.15 | 39.1 |
| DDA-X-PMOX17-EDA | 2,000 | 17 | 2,300 | 2,600 | 21 | 1.13 | 4.9 |
| DDA-X-PMOX28-AMA | 3,000 | 28 | 2,700 | 3,200 | 24 | 1.19 | 9.8 |
| DDA-X-PMOX37-AMA | 3,800 | 37 | 3,300 | 3,900 | 31 | 1.20 | 19.5 |
Degree of polymerization, determined as ratio of the characteristic initiator signal (DDA-X) at 0.84 ppm (t, 3H, −CH3) vs the average integral of the PROX backbone signal around 2.0 (PMOX, b, n3H, NCOCH3) and around 1.0 (PEOX, b, n3H, NCOCH2CH3), respectively,
Determined (in triplicate) against the Gram-positive germ S. mutans; the maximum standard deviation was the next higher value,
Dodecyltrimethylammonium chloride,
Neither the initiator- (Me-), nor the hydroxyl-terminal (−OH) were sufficiently visible/integrable in NMR; DP calculation was performed on SEC-basis,
This polymer is the same as described in reference.45
Poly(2-methyloxazoline)s PMOx
1H NMR (CDCl3) = 3.65-3.00 (b, n·4H, N(CH2)2), 2.15-1.85 (b, n·3H, NCOCH3).
13C NMR (CDCl3) = 170.7-169.6 (NCOCH3), 47.0-42.3 (N(CH2)2), 21.5-20.7 (NCOCH3).
Poly(2-ethyloxazoline)s PEtOx
1H NMR (CDCl3) = 3.85-3.10 (b, n·4H, N(CH2)2), 2.65-2.00 (b, n·2H, NCOCH2CH3), 1.35-0.80 (b,n·3H, NCOCH2CH3).
13C NMR (CDCl3) = 174.8-173.8 (NCOCH2CH3), 47.0-44.8 (N(CH2)2), 26.6-25.7 (NCOCH2CH3), 9.8-9.1 (NCOCH2CH3).
All signals generated by the initiator MeOTs, the antimicrobial initiator DDA-X and by the terminating agents PYR, DDA and AMA are described below. For the hydroxyl terminal, not quantifiable signal could be found.
PMOx and PEtOx Started with MeOTs
1H NMR (CDCl3) = 3.00 (m, 3H, CH3). The products also contain residues of OTs− at the following positions: 7.61 (d [AA’], 2H, CH3Car(CH2)2(CH2)2CarSO2O−), 7.13 (d [BB’], 2H, CH3Car(CH2)2(CH2)2CarSO2O−), 2.31 (s, 3H, CH3Car(CH2)2(CH2)2CarSO2O−).
13C NMR (CDCl3) = 31.2 (CH3). The products also contain residues of OTs- at the following positions (with low signal-to-noise ratio): 136.2 (CH3Car(CH2)2(CH2)2CarSO2O−), 134.8 (CH3Car(CH2)2(CH2)2CarSO2O−), 127.3 (CH3Car(CH2)2(CH2)2CarSO2O−), 126.8 (CH3Car(CH2)2(CH2)2CarSO2O−).
PMOx, Started with DDA-X
1H NMR (CDCl3) = 7.51 (m, 2H, C(CHar)2(CHar)2CCH2PMOx), 7.34 (m, 2H, C(CHar)2(CHar)2CCH2PMOx), 4.56 (b, 2H, Ar-CH2PMOx), 1.77 (m, 2H, N+(CH3)2CH2CH2CH2C8H16CH3), 1.24 (b, 18H, N+(CH3)2CH2CH2CH2C8H16CH3), 0.84 (t, 3H, N+(CH3)2CH2CH2CH2C8H16CH3).
13C NMR (CDCl3)= 141.8 (CarCH2PMOx), 137.8 (N+(CH3)2CH2Car), 128.1 (Car(CH)2(CH)2CarCH2PMOx), 125.5 (N+(CH3)2CH2Car(CH)2(CH)2Car), 61.9 (N+(CH3)2CH2Ar), 54.9 (ArCH2N+(CH3)2CH2), 50.3 (ArCH2PMOx), 45.9 (N+(CH3)2), 35.6 (N(CH3)2CH2C10H20CH3), 22.0-20.3 (N+(CH3)2CH2C10H20CH3), 16.2 (N+(CH3)2CH2C10H20CH3).
Signals of the PYR-Termination on PMOx
1H NMR (CDCl3) = 9.55 (m, 2H, N+(CH)2(CH)2CH), 8.35 (m, 1H, N+(CH)2(CH)2CH), 7.95 (m, 2H, N+(CH)2(CH)2CH), 5.00 (m, 2H, CH2CH2N+).
13C NMR (CDCl3)= 144.0 (N+(CH)2(CH)2CH), 135.2 (N+(CH)2(CH)2CH), 129.2 (N+(CH)2(CH)2CH), 57.2 (PROXCH2CH2N+).
Signals of the DDA-Termination on PMOx and PEtOx
1H NMR (CDCl3) = 3.28 (m, 6H, CH2N+(CH3)2), 3.21 (m, 2H, CH2N+(CH3)2), 3.15 (m, 2H, N+(CH3)2CH2CH2C8H18CH3), 1.78 (m, 2H, N+(CH3)2CH2CH2C8H18CH3), 1.26 (m, 18H, N+(CH3)2CH2CH2C8H18CH3), 0.85 (t, 3H, N+(CH3)2CH2CH2C8H18CH3).
13C NMR (CDCl3) = 68.0 (CH2N+(CH3)2), 63.1 (CH2N+(CH3)2CH2CH2C8H18CH3), 48.7 (N+(CH3)2), 26.9 (CH2N+(CH3)2CH2CH2C8H18CH3), ~29 (CH2N+(CH3)2CH2CH2C8H18CH3), 14.1 (CH2N+(CH3)2CH2CH2C8H18CH3).
Signals of the AMA-Termination on PMOX)
1H NMR (methanol-d4) = 7.59 (m, 1H, NHCO, 5.73 (m, 1H, HA(cis)HB(trans)CCCH3), 5.38 (m, 1H, HA(cis)HB(trans)CCCH3), 3.81 (m, 4H, CH2CH2N+(CH3)2CH2), 3.18 (m, 6H, N+(CH3)2), 1.92 (m, 3H, NHCOCCH3).
13C NMR (methanol-d4) = 169.0 (NHCO), 139.0 (COCCH3CHAB), 120.4 (COCCH3CHAB), 60.2 (CH2CH2N+(CH3)2), 53.1 (N+(CH3)2CH2), 52.4 (N+(CH3)2).
Gel-Electrophoresis
The reducing sodium dodeclsulfate polyacrylamide gel electrophoreses (SDS-PAGE) were performed according to Laemmli.46 The quaternary antimicrobial compound dodecyltrimethylammonium chloride (DTAC, 50 mmol·L−1)44 and ethylenediamine-tetraacetate disodium dihydrate (EDTA-Na2, 50 mmol·L−1) were used as low-molecular weight reference inhibitors. OH-terminated, methyltosylate (MeOTs) initiated PMOx (Me-PMOX37-OH, Mn=3200 g·mol−1, Mw=3750 g·mol−1, PDI=1.16) was used as the high-molecular reference. For the inhibition experiments, the inhibitor’s solution (100 µL, in distilled water) was mixed with a solution of Type I collagenase from C. histolyticum (150 µL, 2.0 mg·mL−1, 0.016 µmol·L−1, calculated with 125 kDa) in distilled water.
Every reaction mixture (1st) was pre-incubated in a tempered thermomixing unit (30 min, 37 °C, 1400 rpm; Thermomixer Comfort, Eppendorf). Then 1.0 mL of the gelatin solution (2.0·mg·mL−1, in 0.1 mol·L−1 citrate buffer, pH=6.3) was added to the collagenase/inhibitor mixture. The resulting mixture (2nd) was incubated once more (5 min, 37°C, 1400 rpm), a sample was taken thereof (17.0 µL), mixed with loading buffer (17.0 µL), heated (96°C, 10 min) and finally treated with CLELAND’s reagent47 dithiothreitol (DTT, 1.0 mol·L−1, 3.4 µL). The finished mixtures (3rd) were loaded on a 10 % tris(hydroxymethyl)aminomethane (TRIS)-Glycine gel. Roti-Mark 10–150 (Carl Roth, Karlsruhe, Germany) was used as protein-ladder. The gel run was performed in a P8PS mini gel system (Owl Separation Systems, Rochester, NY, USA) connected to an EV231 power supply (Consort bvba, Turnhout, Belgium). The run in the stacking gel was carried out with constant voltage (60 V). After the bromophenol blue-band reached the resolving gel the current was increased to 160 V. For Coomassie® staining, the obtained gel was shaken overnight in an acetic solution of Coomassie Brilliant Blue R 250 (Carl Roth, Karlsruhe, Germany; 290 mg·L−1, in 40/10 v/v, ethanol/glacial acetic acid, distilled water, ad 100). De-colorizing was performed in a less acidic milieu (25/8 v/v, ethanol/glacial acetic acid, distilled water, ad 100) until the washing solution was colorless.48
MMP-9 Inhibition Assay
The inhibition of human rhMMP-9 by DDA-X-PMOX28-AMA in different concentrations was explored by using a generic matrix metalloproteinases MMP assay kit (Sensolyte, AnaSpec Inc., Freemont, CA, USA). Human rhMMP-9 splits a thiopeptide substrate that releases a sulfhydryl group. The sulfhydryl group reacts with 5,5’-dithiobis-(2-nitrobenzoic acid) to produce a colored reaction product (2-nitro-5-thiobenzoic acid). The absorbance of that product was measured with UV/Vis (412 nm) in a 96-well plate reader. The rhMMP-9 (AnaSpec Inc.) was activated with trypsin (10 µg·mL−1) at 37°C for 2 h immediately before the assay. After activation (2 h), the trypsin was inactivated by soybean trypsin inhibitor.
Each well contained active rhMMP-9 (10 µL, 19.6 ng/well) and the putative MMP inhibitor DDA-X-PMOX28-AMA solution (40 µL). This mixture was pre-incubated for 20 min. After pre-incubation, the thiopeptide solution (50 µL, 0.2 mmol·L−1, in proprietary buffer) was added to reach a total volume of 100 µL for each well. Reagents were mixed in a plate mixer for 30 s and absorbance was measured every 10 min for 60 min. All rhMMP-9 assays were performed in quinta-replicates. Since the normality and homoscedasticity assumptions appeared to be valid, the percent inhibition of DDA-X-PMOX28-AMA was analyzed by a one-way analysis of variance (ANOVA) followed by TUKEY multiple comparison tests at α = 0.05.
Bovine Dental Collagen Degradation Test
Whole bovine teeth were sliced into standardized discs using a water-cooled wire saw. The specimens were completely demineralized by treatment with diluted nitric acid (5 vol. %, 4–6 h) and washed/neutralized several times with sterile citrate buffer (0.1 mol·L−1 citric acid, 10 mmol·L−1 calcium acetate, pH=6.3, pH adjusted with aqueous NaOH). In order to avoid bacterial contamination, the samples were stored at low temperature (4°C) in sterile citrate buffer and used within one day. All the following processing steps were carried out under sterile conditions. Prior to use, every sample was washed with distilled water and dried with oil-free compressed air. The standardized, completely demineralized bovine dentine disc was incubated in the citrate buffer solution containing 1.0 wt.% of DDA-X-PMOx28-AMA for 1 h, taken out, and blown with a gentle stream of oil-free compressed air for at least 10 s. The negative test series was treated the same way with the citrate buffer solution. All samples were incubated simultaneously in 10 mL of a Clostridium histolyticum type I bacterial collagenase solution (1.0 mg·mL−1, 37°C) in the citrate buffer. The samples were taken out after 48h, washed with water, air-dried and weighted.
Human Dental Collagen Peptides Release Assay
Dentin beams were prepared from extracted human wisdom teeth. The enamel and superficial dentin was removed from the crown of the teeth using a diamond impregnated copper saw (Isomet, Buehler Ltd., Lake Bluff, IL, USA), cooled with water. One millimeter thick dentin disks are prepared from deep dentin and then, cut into 6 × 2 × 1 mm standardized beams of dentin (2–3 beams per single disk). The dentin beams were completely demineralized using 10 wt% aqueous phosphoric acid (pH=1.0) at 25 °C with tumbling for 18 hrs. The density of the beams falls from 2.1 mg·µL−1 to 1.02 mg·µL−1 when they are completely demineralized. Complete demineralization was confirmed by energy dispersive X-ray spectroscopy (EDX) and compared to an aluminum step-wedge.
Control beams were incubated in a simulated body fluid (1 mL, SBF) containing sodium chloride (NaCl, 150 mmol·L−1), calcium chloride (CaCl2, 2.5 mmol·L−1), 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt (HEPES-Na, 10 mmol·L−1), zinc chloride (ZnCl2, 0.05 mmol·L−1) and sodium azide (NaN3, 3.0 mmol·L−1) to prevent microbial growth. Prior to incubation, the completely demineralized dentin beams were rinsed in distilled water to remove salts and placed in a sealed container of anhydrous calcium sulfate (CaSO4,Drierite, W.A. Hammond Co., Xenia, OH, USA) to remove all traces of water until a constant dry mass could be obtained using a microbalance weighing unit (Mettler Toledo GmbH, Giessen, Germany). This was achieved within 24 h. The beams were then rehydrated in the SBF for 1 hr. Experimental beams were pre-incubated in DDA-X-PMOX37-AMA (5.0 or 15.0 wt%) in the same SBF medium (1.0 mL) for 30 min. After 30 min, the beams were removed from the pretreatment solution and blotted against filter paper pre-moistened with the pretreatment solution to remove excess of the inhibitor containing solution.
These fully conditioned beams were dropped into inhibitor-free SBF (1.0 mL), capped and placed in the shaking water bath (1 Hz) at 37°C for 30 days. At the end of that time, the tubes were opened, the beams removed from the media, rinsed in distilled water to remove salts and dried in anhydrous CaSO4 to obtain a post-incubation dry mass. Aliquots of the medium were mixed with equal volumes of aqueous hydrogen chloride (HCl, 12.0 mol·L−1), sealed in glass ampules and hydrolyzed to amino acids (120°C, 18 hrs.). After hydrolysis, the ampules were opened and placed in glass desiccators containing both anhydrous CaSO4 to absorb water vapor, and sodium hydroxide (NaOH) pellets to trap HCl vapor released from the hydrolysate. The hydroxyproline content of each hydrolysate was analyzed spectrophotometrically at 558 nm.49
Adhesive Surface Coatings
Degreased and cleansed (acetone, water content <100 ppm) microscope slides (Marienfeld-Superior, Germany) were briefly (5 s) dip coated into an alcoholic suspension (60/35 v/v, isopropanol/ethanol, distilled water ad 100) of 3-(trimethoxysilyl)propyl methacrylate (Sigma-Aldrich), activated with potassium hydroxide (KOH, 5 mmol·L−1). DDA-X-PMOX37-AMAwas mixed with the commercially available self-etch dental adhesive AdheSE One F (Ivoclar Vivadent AG) under protective gas atmosphere (argon) and a blue light filter.
The transparent compositions were applied on the double-bond functionalized microscope slides within a defined, rhomboid test area (0.8 in2, 5.16 cm2). The adhesive surface coatings were blue-light cured (10–20 s) according to the instructions of the adhesive/lamp manufacturer (Ivoclar Vivadent) on a lamp intensity of 650mW·cm−1 (λ>380 nm). As reference, unmodified AdheSE One F was used. In all cases, fully coherent and visually clear adhesive coatings were obtained.
Bacterial Surface Susceptibility
The DDA-X-PMOX28-AMA-containing adhesive coatings on the microscope slides were individually placed in distilled water (10 mL) and shaken at 37°C. The water of every sample was changed once a day. After 1/2/4/6/10/20/40/50 and 101 days, samples were taken out and dried with compressed air under sterile conditions. A stock solution of S. mutans (Gram-positive, strain ATCC 25175) was prepared from a stock pellet (from DSMZ, see Material section) in a standard nutrient broth (Merck, 50 mL, pH=7.38). After incubation (37°C, 12 h), the bacterial concentration of the suspension was determined via UV/vis to approx. 1010 cells·L−1. One portion of the suspension (40 mL) was precipitated carefully by centrifugation (10 min, 1500 rpm), re-suspended in sterile phosphate-buffered saline (PBS, sodium dihydrogen phosphate dihydrate, 0.1 mol·L−1, pH=7.38) and double diluted to approx. 108 cells·L−1. A small amount (1 mL) of this suspension was filtered through glass wool and re-re-suspended in isotonic, sterile saline (9 mL, 154 mmol·L−1) to give 107 cells·L−1. The adhesive-coated microscope slides were sprayed with this S. mutans suspension using sterile compressed air and immediately, covered with pre-cooled standard nutrient agar (Merck, 9 wt%, max. 39°C, 40 mL). The solidified agar plates were incubated for 6–8 h at 37°C, stained with an aqueous 2,3,5-triphenyl-tetrazolium chloride solution (TTC, 3.0 wt%, 2 h) and photographed.
Enamel/Dentin Bond Strength Measurements
DDA-X-PMOX28-AMA (2.50 wt%) was mixed with commercial AdheSE One F to give homogenous and clear solutions. As a low molecular weight reference, N,N-dimethyldodecylamine (DDA, 2.50 wt%) in AdheSE One F was used. Bovine dentin or enamel was exposed by water-irrigated grinding with P120 & P1000 SiC-grit sandpaper (Buehler-Met, Lake Bluff, USA) and the adhesives applied to the surfaces by agitation for the prescribed duration of 20s using a dental microbrush (Microbrush, Grafton, USA). After air-thinning with oil-free compressed air, the adhesive layers were blue-light cured for 10 s using a halogen dental curing light with 650 mW·cm−1 output (Astralis 7, Ivoclar Vivadent AG, Liechtenstein). A plug of dental composite, Tetric EvoCeram A3 (Ivoclar Vivadent) was applied to the adhesive layer in two increments using a standard Ultradent jig according to ISO 29022 and light-polymerized for 40 s. After aging for 24h in water at 37°C the samples were tested for bond strength using a Zwick Z010 universal testing machine (Zwick GmbH, Ulm, Germany) at 0.8 mm/sec cross-head speed.
Results and Discussion
This study aimed towards a dental repair system that contains an additive, which inhibits collagenases and renders the surface contact-active antimicrobial. Our research on antimicrobial polymers is based on poly(2-alkyloxazoline)s, which carry an antimicrobial end group and another functional group at the distal end of the polymer, the so-called satellite group.50, 51 The latter group can be used to control the biocidal activity of the whole polymer44, 45, even switching it on and off.52 Further, the satellite group can be used to attach the polymer to surfaces.53 As shown previously, poly(2-methyloxazoline) with the biocidal group N-dodecyl-N,N-dimethylxylyl ammonium bromide (DDA-X) attached to one and a methacrylamide group (AMA) attached to the other terminal can be copolymerized into a poly(2-hydroxyethylacrylate) based network. During the process, the polymer concentrates at the surface and is covalently attached there, rendering the material contact-active antimicrobial against the nosocomial bacterial strain Staphylococcus aureus.54 In order to explore if this concept works with relevant self-etch dental adhesives,we added DDA-X-PMOX28-AMAin different concentrations (0.5, 1.0, 2.5, and 5.0 wt%)to the commercial self-etch adhesive AdheSE® One F and applied the homogenous mixtures on double-bond functionalized microscope slides. The coatings were cured with blue-light. In order to find out if the additive is leeching out and to explore the long-term activity, the coating was washed with water at 37°C including a daily change of water. AdheSE® One F coatings without additive were treated equally as negative control. All coatings were washed with water at 37°C for 2 d prior to testing the antimicrobial activity. For the latter, we used the established colony test55 by spraying a bacterial cell suspension of cariogenic bacterium Streptococcus mutans on the surface and let the colonies form under growth agar. Figure 1 depicts a microscope slide partially coated with the adhesive containing 2.5 wt% additive after the microbial susceptibility test procedure.
Figure 1.
Glass slide partially coated with AdheSE® One F containing 2.5 wt% DDA-X-PMOx28-AMA, photo-cured, washed with water for 2 d, dried, sprayed with S. mutans cells in 0.1 M PBS buffer (pH 7.0, 105 cells/mL), incubated under growth ager (1.5 wt% agar) at 37°C overnight, and stained with TTC.
It is clearly visible that no colonies formed on the adhesive-coated area, while 1000–2000 colonies are visible in the respective surrounding non-coated area. With respect to this number, the antimicrobially equipped coating prevents at least 99.9 % of the S. mutans cells from growing. Further, no inhibition zone is visible, which indicates, although not proves, contact-activity of the surface. While the same was found for the coating with 5 wt% additive, the adhesives with 0.5 and 1.0 wt%, respectively, and the one without additive were fully overgrown with S. mutans colonies.
Antimicrobial contact-activity is difficult to prove experimentally, because biocidal surfaces with a low release rate, e.g. silver nanoparticle56 or quarternary ammonium57 loaded materials, in some cases do not show an inhibition zone or do not kill microbes in the surrounding bacterial suspension.Both of the latter are the common observations that indicate contact-activity. To effectively exclude a release activity of the adhesive modified with 2.5 wt% DDA-X-PMOx28-AMA, microscopy slides were subjected to extensive washing for up to101 d with daily change of water. Even after this time, the coating isfully active against S. mutans cells. Taking the minimal release rate of quarternary ammonium compounds into account, a release coatingis calculated to lose its activity after 42 d given zero order release kinetics. Since the modified adhesive coating clearly exceeds this time, the only explanation for its biocidal behavior is contact-activity.
A common criticism on contact-active antimicrobial surfaces is that such a surface will eventually be deactivated by adhering dead bacterial cells. In order to explore this scenario,we added a confluent suspension of S. mutans onto the biocidal adhesive coating (2.5 wt% additive) and incubated it for 48 h. Then, the formed biofilm was rinsed off with water. This procedure was repeated on the same sample. After the second biofilm-removal, the sample was sprayed with a S. mutans and incubated under growth agar overnight. Surprisingly, the biocidal adhesive did not lose its activity against S. mutans after this procedure.
Next, we explored if the biocidal adhesive is also capable of killing S. mutans cells that remain in the dentin tubuli after mechanical excavation. For this, a sterilized slice of bovine tooth 2 mm in thickness was treated with 5 µl of a confluent S. mutans suspension in growth medium for 5 min to create a simulated caries infection. The lightly air-dried bacteria-treated area was then scrubbed with the adhesive for 20s as prescribed in manufacturer's instructions and light-cured. Then the tooth slice was immersed in bacterial growth medium and incubated at 37°C for 1 d and viable bacterial cells detected by the ensuing turbidity of the growth medium. As seen in Figure 2, the adhesive without additive could not prevent the bacterial cells from proliferating in the tubuli and eventually enter the growth medium. The same is true for the adhesive with 2.5 wt% additive. However, 5.0 wt% of the additive in the adhesive effectively killed the S. mutans cells in the tubuli as well.
Figure 2.
Photographs of eppendorf tubes containing cow tooth slices incubated at 37°C for 24 h. The slices were treated with 5 µL of confluent S. mutans growth medium, air-dried, coated with AdheSE® One F containing different concentrations of DDA-X-PMOx28-AMA, the adhesive layer photo-cured for 10 s using a dental blue-light polymerization unit and placed in the growth medium.
Having established that DDA-X-PMOx28-AMA is an appropriate additive to render a commercial dental adhesive antimicrobial contact-active against the caries causing bacterium S. mutans and showing that it is able to kill remaining bacteria in the tubuli of teeth, we explored the potential of the polymer to additionally inhibit collagenases, which have been shown to cause degradation of adhesive infiltrated collagen fibrils.
Since the DDA-X-PMOx28-AMA contains three different structural units, the polymeric chain and two different end groups, a series of polymers were synthesized to systematically explore the influence of these elements on the collagenase inhibiting potential.
The quaternary low molecular weight compound, N,N-dimethyldodecylammonium chloride (DTAC) and the well-known MMP-inhibitor ethylenediaminetetraacetate disodium salt dihydrate (EDTA- Na2) were used as positive controls.
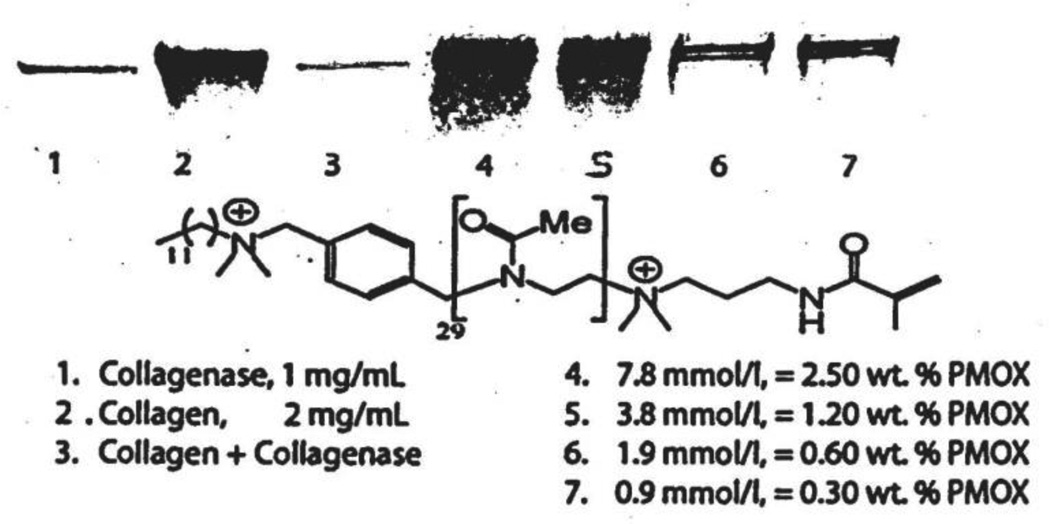
Collagenase inhibition was measured by a degradation procedure of human placental collagen (30 µg), monitored by SDS-PAGE.58 The additive concentration was investigated in a range of 0.3 – 2.5 wt%. As seen in Figure 3, lane 1-C, histolyticum collagenase (30 µg) produced a line light band due to the protease in the enzyme. Lane 2 shows that this gelatin produces a broad band of Coomassie-positve staining most intense in the region of 150 kDa and above. The Type I collagenase from C. histolyticum in Figure 3, lane B shows four discrete bands with approx. 125, 103, 40 and 29 kDa, of which the 125 kDa band is the most intense one. Predictably, no trace of gelatin could be observed with the incubated gelatin/collagenase mixture in Figure 3, lane C.
Figure 3.
SDS-PAGE gels showing only the origin of lanes 1–9. Lane 1 – 30 µg of C. histolyticum collagenase gave a faint band of protein staining due to the presence of the enzyme itself. Lane 2 – 30 µg of human placental collagen that gave a dark staining broad band of collagen staining. Lane 3 –the same concentrations of collagenase and collagen were incubated for 2 hr at 37°C and then placed on the origin. The lack of protein staining indicates that the collagenase split the collagen into multiple peptide fragments that migrated off the origin into the gel. Lane 4 – containing the same concentration of collagenase and collagen, plus 2.5 wt% poly(2-methyloxazoline) Me-PMOx37-OH. After incubation at 37°C for 2 h, the origin showed the same amount of collagen as was seen at the origin of lane 2, indicating that 2.5% PMOx had inhibited the collagenase in the mixture. Lane 5 – collagen plus collagenase plus 1.2 wt% PMOx left about half of the collagen on the origin indicating that about half of the collagenase was inhibited. In lane 6, the collagen plus collagenase mixture contained 0.6 wt% PMOx; this resulted in less intense collagen staining. In lane 7, 0.3 wt% PMOx was used, resulting in less intense collagen staining.
The low molecular weight positive control DTAC (1.6 mmol·L−1)inhibited the C. histolyticum collagenase as seen in lane D. The same result was found for EDTA at the same concentration (not shown), which indicates MMP activity of the bacterial collagenase. In contrast to that, no decrease in the collagenase activity is detectable on the gel in Figure 3, lane E when using the MeOTs-initiated, OH-terminated species Me-PMOX37-OH in an equimolar concentration (2.5 wt%), indicating that this compound is not a collagenase inhibitor, i.e., the PMOX itself does not influence the enzyme activity.
As seen in Lane F, the polymer DDA-X-PMOx28-AMA used in the same molar concentration as DTAC is clearly inhibiting the collagenase. The concentration of this polymer is below the concentration required to render the dental adhesive antimicrobially active.
The results of the collagenese inhibition tests with all polymers in the concentration range of 0.5–2.5 wt% are summarized in Table 2. Inhibition in the qualitative test is presumed, if the collagen band appears to have the same band with the same intensity in the SDS-PAGE as that seen in lane A, Figure 3 of gelatin alone. That is, because the PMOx inhibited the collagenase, the gelatin concentration remained high.. The results in Table 2 clearly show that only polymers with hydrophobic quarternary ammonium groups inhibited the collagenase. The inhibition is obviously caused by this group as expected from the inhibition potential of DTAC, which chemically resembles the DDA end group.
Table 2.
Collagenase inhibition activity of telechelic poly(2-R-oxazoline)s with defined functional end groups at the initiator side I, and the termination side T. PYR = pyridinium, DDA = dimethyldodecylammonium, EDA = ethylene diamine.
| reference/polymer | Ia | R | Tb | MMP-inhibitionc |
|---|---|---|---|---|
| DTAC | - | - | - | 0.03 wt% |
| Me-PMOX37-OH | CH3 | CH3 | OH | - |
| Me-PEOX24-OH | CH3 | C2H5 | OH | - |
| Me-PEOX53-PYR | CH3 | C2H5 | PYR | - |
| Me-PMOX22-DDA | CH3 | CH3 | DDA | 0.5 wt% |
| DDA-X-PMOX17-EDA | DDA-X | CH3 | EDA | 0.5 wt% |
| DDA-X-PMOX28-AMA | DDA-X | CH3 | AMA | 0.5 wt% |
| DDA-X-PMOX37-AMA | DDA-X | CH3 | AMA | 0.5 wt% |
Functional end group, introduced by initiation at the beginning of the polymerization,
Functional end group, introduced by termination,
Estimation of the MMP-inhibiting efficacy against buffered bacterial Collagenase from C. histolyticum Type I; determined using a SDS-PAGE.
For instance, a quaternary pyridinium bromide at the terminal of Me-PEOX53-PYR does not give the polymer a collagenase-inhibiting effect at concentration of up to 2.5 wt%. Obviously a cationic group is not sufficient for collagenase inhibition, but it has to be accompanied by a hydrophobic group nearby.
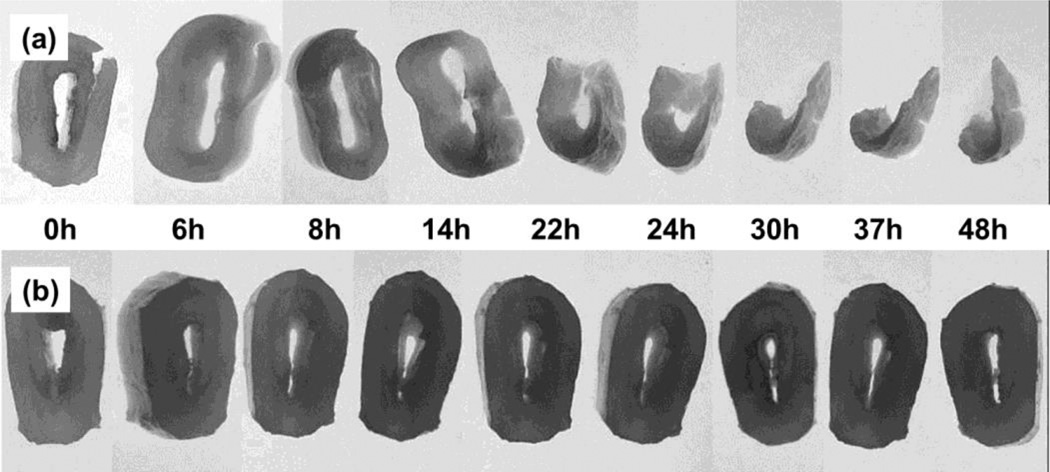
Having established DDA-X-PMOx-AMA as an MMP inhibiting polymer, we decided to explore this activity on the degradation of dental bovine collagen. To this end, demineralized bovine dentin discs were impregnated with a solution of DDA-X-PMOX28-AMAin citrate buffer for 1 h. The still moist disk and a non-treated one were then incubated at 37°C in a collagenase/citrate (1 mg collagenase·mL−1) solution. The disks were photographed at several time periods (see Fig. 4) and finally dried and weighted.
Figure 4.
Photographs of decalcified cow dentine disks impregnated in citrate buffer with 1 mg collagenase. a) Non-treated disks. b) disks incubated with a 1.0 wt% DDA-X-PMOx28-AMA/citrate solution for 1 hat room temperature and air dried.
As seen in the upper row in Figure 4, the non-treated sample is visibly degraded after 14 h and lost more than 70% of its weight after 48h. In contrast, the DDA-X-PMOx28-AMA treated samples show no sign of degradation in this time.
Additionally, the inhibition of recombinant human matrix metalloproteinase rhMMP-9 was explored using a quantitative test setup. The results of DDA-X-PMOX37-AMA inhibition of soluble rhMMP-9 activity are shown in Figure 5.
Figure 5.
Graphical representation of the rhMMP-9 inhibition values (in %) with increasing amounts (0.5–20 wt%) of the telechelic collagenase inhibitor DDA-X-PMOx37-AMA. The error bars indicate the maximum uncertainity of the used method.
Interestingly, DDA-X-PMOX37-AMAshows a fairly low inhibition of human matrix metalloproteinase rhMMP-9. In comparison to the seemingly full inhibition of bacterial collagenase at 0.5 wt%, the rhMMP-9 was only by 14.30 % at this concentration. The maximum inhibition of 66.9 % was found at 15 wt%.
The most critical collagenase for dentin degradation is that of the MMPs native to dentinal collagen. In order to explore if the antimicrobial additive inhibits those enzymes, human dentin beams were demineralized using diluted nitric acid and the demimeralization process monitored with X-ray that allows thedentin native MMPs to be uncovered and activated.40 These collagen beams were incubated in a simulated body fluid (SBF) with 0,5 and 15 wt% DDA-X-PMOX37-AMA, respectively. The mass loss and the peptide release were monitored over a period of 30 d. After incubation, the beams’ remaining mass after drying and the hydroxyproline (Hyp) content were determined. Since the normality and homoscedasticity assumption of the data were violated, both the loss of dry mass and the release of Hyp-containing peptides were evaluated using the KRUSKAL-WALLIS one-way ANOVA and DUNN’s multiple comparison tests at α = 0.05. Table 3 summarizes the loss of dry mass and the release of collagen peptides into the incubation medium (SBF) over a 30 day period.
Table 3.
Loss of dry mass, release of human dentin collagen peptides and hydroxyproline (Hyp)-release into a simulated body fluid (SBF) over a 30 day period. Detected on an untreated control and on two impregnation concentrations of DDA-X-PMOx37-AMA 7 (5.0 and 15.0 wt%). N = 10 individual beams in each group.
| treatments | loss of dry mass [wt%] |
inhibition [%] |
hydroxyproline release | |
|---|---|---|---|---|
| [µg·mg−1], dry mass | [%], inhibition | |||
| control (no inhibitor) | −16.16 ± 2.54a | 0 | 10.43 ± 1.89 A | 0 |
| 5.0 wt% | −6.00 ± 2.63 b | 62.9 | 0.33 ± 0.12 B | 96.8 |
| 15.0 wt% | −4.77 ± 1.70 b | 70.5 | 0.22 ± 0.12 B | 97.9 |
The results indicate that control beams of demineralized dentin lose 16 wt% of their dry mass over a 30 day period, while beams incubated with DDA-X-PMOX37-AMAshow 62.9 % at 5.0 wt% and 70.5 % at 15.0 wt% lower mass loss. Presumably, the loss of dry mass of the matrix was due to solubilization of collagen. In the control group, the medium yielded 10.43 µg of hydroxyproline (Hyp)·mg−1 of dry mass. In beams pretreated with 5.0 and 15.0 wt%, respectively of the PMOX-based inhibitor, only yielded 0.33 and 0.22 µg Hyp·mg−1, respectively, dry mass of matrix. When expressed as a percent of the controls, the degradation of the DDA-X-PMOx37-AMA -treated beams was almost completely inhibited by 96.8 and 97.9 %, respectively.
The stronger inhibition of matrix-bound endogenous MMPs compared to soluble rhMMP-9 might be due to electrostatic binding of the PMOx-based high molecular weight inhibitor to the collagen fibrils of the dentin matrix as well as to the matrix-bound MMPs. The collagen-bound PMOx might have slowly desorbed from the matrix and kept the MMPs saturated with the inhibitor. Such speculation should be tested in future studies of the binding characteristics of DDA-X-PMOx-AMA systems to insoluble type I collagen.
In order to explore if the dental adhesive is still effective with the additive, its influence on the enamel and dentine bond strength of AdheSE® One F was measured. The macromer DDA-X-PMOx37-AMA was added to AdheSE® One F (2.5 wt.%) and the respective shear bond strengths (SBS, notched-edge method, N = 8 probes each) were determined for both formulations. Figure 6 compares the obtained SBS results on bovine enamel and dentine after 24 hr.
Figure 6.
Graphical comparison of the results of the shear bond strength (SBS, notched-edge method) measurements conducted on the AdheSE® One F dental adhesive reference without any additives (left doublet of bars) and on a modified AdheSE® One F matrix containing 2.5 wt.% of the macromeric MMP inhibitor DDA-X-PMOx37-AMA (right doublet of bars). As dental template (enamel / dentine), bovine teeth were used. The error bars indicate the maximum uncertainity of the used method calculated from N = 8 probes per sample and template.
In comparison to the reference, no significant decrease in both bond strengths was detected; the measured values were in a common range for corresponding dental adhesive compositions.
Conclusion
The polymeric additive with a polymerizable end group, a hydrophilic inert poly(2-methyloxazoline) chain, and an antimicrobial quaternary ammonium end group was explored regarding its usefulness as an additive to a commercial dental adhesive with antimicrobial and collagenase inhibiting properties. When using 2.5 wt% the adhesive, the material became contact-active antimicrobial against S. mutans cells and did not lose this property after 101 d of washing with water at 37°C. Increasing the concentration to 5 wt% additionally allowed killing S. mutans remaining in the tubuli below the cured adhesive. Additionally, this concentration is sufficient to fully inhibit human collagenase MMPs bound to dentin. From these experiments, we conclude that using DDA-X-PMOx-AMA as additive in dental adhesives may help prolonging longevity of dental composite restoration by reducing incidence of secondary caries as well as MMP-caused degradation of dentinal collagen.
Acknowledgements
The authors thank Dr. Heintze and M. Forjanic from Ivoclar Vivadent, for the enamel/dentine bond strength verifications. This work was supported, in part, by R01 DE015306 from the National Institute of Dental and Craniofacial Research (P.I. DHP).
References
- 1.Yoshida E, Uno S, Nodasaka Y, Kaga M, Hirano S. Relationship between water status in dentin and interfacial morphology in all-in-one adhesives. Dental Materials. 2007;23(5):556–560. doi: 10.1016/j.dental.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Park JG, Ye Q, Topp EM, Lee CH, Kostoryz EL, Misra A, Spencer P. Dynamic mechanical analysis and esterase degradation of dentin adhesives containing a branched methacrylate. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2009;91B(1):61–70. doi: 10.1002/jbm.b.31374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Q, Park JG, Topp E, Wang Y, Misra A, Spencer P. In vitro performance of nano-heterogeneous dentin adhesive. J. Dent. Res. 2008;87(9):829–833. doi: 10.1177/154405910808700911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, Qian F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Operative Dentistry. 2004;29(6):705–712. [PubMed] [Google Scholar]
- 5.Foxton RM, Melo L, Stone DG, Pilecki P, Sherriff M, Watson TF. Long-term durability of one-step adhesive-composite systems to enamel and dentin. Operative Dentistry. 2008;33(6):651–657. doi: 10.2341/07-166. [DOI] [PubMed] [Google Scholar]
- 6.Sadek FT, Castellan CS, Braga RR, Mai S, Tjaderhane L, Pashley DH, Tay FR. One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dental Materials. 2010;26(4):380–386. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 7.De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, Van Meerbeek B. A critical review of the durability of adhesion to tooth tissue: Methods and results. J. Dent. Res. 2005;84(2):118–132. doi: 10.1177/154405910508400204. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto M, Fujita S, Kaga M, Yawaka Y. In vitro durability of one-bottle resin adhesives bonded to dentin. Dental Materials Journal. 2007;26(5):677–686. doi: 10.4012/dmj.26.677. [DOI] [PubMed] [Google Scholar]
- 9.Fik CP, Meuris M, Salz U, Bock T, Tiller JC. Ultrahigh-aspect ratio microfiber-furs as plant-surface mimics derived fromteeth. Adv. Mater. (Weinheim, Ger.) 2011;23(31):3565-+. doi: 10.1002/adma.201101102. [DOI] [PubMed] [Google Scholar]
- 10.Van Houte J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994;73:672–681. doi: 10.1177/00220345940730031301. [DOI] [PubMed] [Google Scholar]
- 11.Beyth N, Farah S, Domb AJ, Weiss EI. Antibacterial dental resin composites. Reactive & Functional Polymers. 2014;75:81–88. [Google Scholar]
- 12.Chen L, Shen H, Suh Byoung I. Antibacterial dental restorative materials: a state-of-the-art review. American Journal of Dentistry. 2012;25(6):337–346. [PubMed] [Google Scholar]
- 13.Mehdawi IM, Pratten J, Spratt DA, Knowles JC, Young AM. High strength re-mineralizing, antibacterial dental composites with reactive calcium phosphates. Dental Materials. 2013;29(4):473–484. doi: 10.1016/j.dental.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Hook Edward R, Owen Olivia J, Bellis Candice A, Holder James A, O'Sullivan Dominic J, Barbour Michele E. Development of a novel antimicrobial-releasing glass ionomer cement functionalized with chlorhexidine hexametaphosphate nanoparticles. Journal of Nanobiotechnology. 2014;12:3. doi: 10.1186/1477-3155-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung D, Spratt DA, Pratten J, Gulabivala K, Mordan NJ, Young AM. Chlorhexidine-releasing methacrylate dental composite materials. Biomaterials. 2005;26(34):7145–7153. doi: 10.1016/j.biomaterials.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Breschi L, Mazzoni A, Nato F, Carrilho M, Visintini E, Tjaderhane L, Ruggeri A, Tay FR, Dorigo ED, Pashley DH. Chlorhexidine stabilizes the adhesive interface: A 2-year in vitro study. Dental Materials. 2010;26(4):320–325. doi: 10.1016/j.dental.2009.11.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng Y, Howard L, Chong VJ, Sun J, Gregory RL, Xie D. A novel furanone-modified antibacterial dental glass ionomer cement. Acta Biomaterialia. 2012;8(8):3153–3160. doi: 10.1016/j.actbio.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Aydin Sevinc B, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2010;94B(1):22–31. doi: 10.1002/jbm.b.31620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research, Part B: Applied Biomaterials. 2012;100B(4):1151–1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botelho Michael G. Compressive strength of glass ionomer cements with dental antibacterial agents. SADJ : Journal of the South African Dental Association = tydskrif van die Suid-Afrikaanse Tandheelkundige Vereniging. 2004;59(2):51–53. [PubMed] [Google Scholar]
- 21.He J, Soderling E, Lassila LVJ, Vallittu PK. Incorporation of an antibacterial and radiopaque monomer into dental resin system. Dental Materials. 2012;28(8):e110–e117. doi: 10.1016/j.dental.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 22.Kurata S, Hamada N, Kanazawa A, Endo T. Study on antibacterial dental resin using tri-n-butyl(4-vinylbenzyl) phosphonium chloride. Dental Materials Journal. 2011;30(6):960–966. doi: 10.4012/dmj.2011-157. [DOI] [PubMed] [Google Scholar]
- 23.Pupo YM, Farago PV, Nadal JM, Esmerino LA, Maluf DF, Zawadzki SF, Michel MD, dos Santos FA, Gomes OMM, Gomes JC. An innovative quaternary ammonium methacrylate polymer can provide improved antimicrobial properties for a dental adhesive system. Journal of Biomaterials Science, Polymer Edition. 2013;24(12):1443–1458. doi: 10.1080/09205063.2013.766784. [DOI] [PubMed] [Google Scholar]
- 24.Thome T, Mayer MPA, Imazato S, Geraldo-Martins VR, Marques MM. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. Journal of Dentistry (Oxford, United Kingdom) 2009;37(9):705–711. doi: 10.1016/j.jdent.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Beyth N, Yudovin-Farber I, Bahir R, Domb Abraham J, Weiss Ervin I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27(21):3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Chaussain-Miller C, Fioretti F, Goldberg M, Menashi S. The role of matrix metalloproteinases (MMPs) in human caries. J. Dent. Res. 2006;85(1):22–32. doi: 10.1177/154405910608500104. [DOI] [PubMed] [Google Scholar]
- 27.Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, Ito S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004;83(3):216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- 28.Tezvergil-Mutluay A, Agee KA, Hoshika T, Carrilho M, Breschi L, Tjaderhane L, Nishitani Y, Carvalho RM, Looney S, Tay FR, Pashley DH. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dental Materials. 2010;26(11):1059–1067. doi: 10.1016/j.dental.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanwart HE, Birkedalhansen H. The cysteine switch - a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. U. S. A. 1990;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishitani Y, Yoshiyama M, Wadgaonkar B, Breschi L, Mannello F, Mazzoni A, Carvalho RM, Tjaderhane L, Tay FR, Pashley DH. Activation of gelatinolytic/collagenolytic activity in dentin by self-etching adhesives. European Journal of Oral Sciences. 2006;114(2):160–166. doi: 10.1111/j.1600-0722.2006.00342.x. [DOI] [PubMed] [Google Scholar]; Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Archives of Oral Biology. 2007;52:121–127. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Tay FR, Pashley DH, Loushine RJ, Weller RN, Monticelli F, Osorio R. Self-etching adhesives increase collagenolytic activity in radicular dentin. Journal of Endodontics. 2006;32(9):862–868. doi: 10.1016/j.joen.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Moszner N, Salz U, Zimmermann J. Chemical aspects of self-etching enamel-dentin adhesives: A systematic review. Dental Materials. 2005;21(10):895–910. doi: 10.1016/j.dental.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Van Landuyt KL, Snauwaert J, De Munck J, Peurnans M, Yoshida Y, Poitevin A, Coutinho E, Suzuki K, Lambrechtsa P, Van Meerbeek B. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28(26):3757–3785. doi: 10.1016/j.biomaterials.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 34.De Munck J, Van den Steen PE, Mine A, Van Landuyt KL, Poitevin A, Opdenakker G, Van Meerbeek B. Inhibition of Enzymatic Degradation of Adhesive-Dentin Interfaces. J. Dent. Res. 2009;88(12):1101–1106. doi: 10.1177/0022034509346952. [DOI] [PubMed] [Google Scholar]
- 35.Mazzoni A, Pashley DH, Nishitani Y, Breschi L, Marinello F, Tjaderhane L, Toledano M, Pashley EL, Tay FR. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials. 2006;27(25):4470–4476. doi: 10.1016/j.biomaterials.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Mazzoni A, Mannello F, Tay FR, Tonti GAM, Papa S, Mazzotti G, Di Lenarda R, Pashley DH, Breschi L. Zymographic analysis and characterization of MMP-2 and-9 forms in human sound dentin. J. Dent. Res. 2007;86(5):436–440. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- 37.Lehmann N, Debret R, Romeas A, Magloire H, Degrange M, Bleicher F, Sommer P, Seux D. Self-etching increases matrix metalloproteinase expression in the dentin-pulp complex. J. Dent. Res. 2009;88(1):77–82. doi: 10.1177/0022034508327925. [DOI] [PubMed] [Google Scholar]
- 38.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. J. Dent. Res. 2011;90(4):535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gendron R, Grenier D, Sorsa T, Mayrand D. Inhibition of the activities of matrix metalloproteinases 2, 8, and 9 by chlorhexidine. Clinical and Diagnostic Laboratory Immunology. 1999;6(3):437–439. doi: 10.1128/cdli.6.3.437-439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrilho MRO, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjaderhane L, Reis AF, Hebling J, Mazzoni A, Breschi L, Pashley D. In vivo preservation of the hybrid layer by chlorhexidine. J. Dent. Res. 2007;86(6):529–533. doi: 10.1177/154405910708600608. [DOI] [PubMed] [Google Scholar]
- 41.Hebling J, Pashley DH, Tjaderhane L, Tay FR. Chlorhexidine arrests subclinical degradation of dentin hybrid layers in vivo. J. Dent. Res. 2005;84(8):741–746. doi: 10.1177/154405910508400811. [DOI] [PubMed] [Google Scholar]
- 42.Zhou JF, Tan JG, Yang X, Xu XM, Li DL, Chen L. MMP-Inhibitory Effect of Chlorhexidine Applied in a Self-etching Adhesive. Journal of Adhesive Dentistry. 2011;13(2):111–115. doi: 10.3290/j.jad.a18783. [DOI] [PubMed] [Google Scholar]
- 43.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjaderhane L, Visintini E, Cadenaro M, Tay FR, Dorigo EDS, Pashley DH. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dental Materials. 2010;26(6):571–578. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fik CP, Krumm C, Muennig C, Baur TI, Salz U, Bock T, Tiller JC. Impact of functional satellite groups on the antimicrobial activity and hemocompatibility of telechelic poly(2-methyloxazoline)s. Biomacromolecules. 2011 doi: 10.1021/bm201403e. [DOI] [PubMed] [Google Scholar]
- 45.Waschinski CJ, Tiller JC. Poly(oxazoline)s with telechelic antimicrobial functions. Biomacromolecules. 2005;6(1):235–243. doi: 10.1021/bm049553i. [DOI] [PubMed] [Google Scholar]
- 46.Laemmli UK. Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature. 1970;227(5259):680-&. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 47.Cleland WW. Dithiothreitol new protective reagent for Sh groups. Biochemistry. 1964;3(4):480-&. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- 48.Fazekas SDS, Webster RG, Datyner A. 2 New Staining Procedures for Quantitative Estimation of Proteins on Electrophoretic Strips. Biochim. Biophys. Acta. 1963;71(2):377-&. doi: 10.1016/0006-3002(63)91092-8. [DOI] [PubMed] [Google Scholar]
- 49.Jamall IS, Finelli VN, Hee SSQ. A Simple Method to Determine Nanogram Levels of 4-Hydroxyproline in Biological Tissues. Anal. Biochem. 1981;112(1):70–75. doi: 10.1016/0003-2697(81)90261-x. [DOI] [PubMed] [Google Scholar]
- 50.Waschinski CJ, Barnert S, Theobald A, Schubert R, Kleinschmidt F, Hoffmann A, Saalwachter K, Tiller JC. Insights in the antibacterial action of poly(methyloxazoline)s with a blocidal end group and varying satellite groups. Biomacromolecules. 2008;9(7):1764–1771. doi: 10.1021/bm7013944. [DOI] [PubMed] [Google Scholar]
- 51.Waschinski CJ, Herdes V, Schueler F, Tiller JC. Influence of satellite groups on telechelic antimicrobial functions of polyoxazolines. Macromol. Biosci. 2005;5(2):149–156. doi: 10.1002/mabi.200400169. [DOI] [PubMed] [Google Scholar]
- 52.Krumm C, Harmuth S, Hijazi M, Neugebauer B, Kampmann A-L, Geltenpoth H, Sickmann A, Tiller JC. Antimicrobial Poly(2-methyloxazoline)s with bioswitchable activity via satellite group modification. Angewandte Chemie International Edition. 2014 doi: 10.1002/anie.201311150. [DOI] [PubMed] [Google Scholar]
- 53.Bieser AM, Thomann Y, Tiller JC. Contact-Active Antimicrobial and Potentially Self-Polishing Coatings Based on Cellulose. Macromol. Biosci. 2011;11(1):111–121. doi: 10.1002/mabi.201000306. [DOI] [PubMed] [Google Scholar]
- 54.Waschinski CJ, Zimmermann J, Salz U, Hutzler R, Sadowski G, Tiller JC. Design of contact-active antimicrobial acrylate-based materials using biocidal macromers. Adv. Mater. (Weinheim, Ger.) 2008;20(1):104-+. [Google Scholar]
- 55.Siedenbiedel F, Fuchs A, Moll T, Weide M, Breves R, Tiller JC. Star-shaped poly(styrene)-block-poly(4-vinyl-N-methylpyridiniumiodide) for semipermanent antimicrobial coatings. Macromol. Biosci. 2013;13(10):1447–1455. doi: 10.1002/mabi.201300219. [DOI] [PubMed] [Google Scholar]
- 56.Ho CH, Odermatt EK, Berndt I, Tiller JC. Long-term active antimicrobial coatings for surgical sutures based on silver nanoparticles and hyperbranched polylysine. J. Biomater. Sci.-Polym. Ed. 2013;24(13):1589–1600. doi: 10.1080/09205063.2013.782803. [DOI] [PubMed] [Google Scholar]
- 57.Tiller JC, Sprich C, Hartmann L. Amphiphilic conetworks as regenerative controlled releasing antimicrobial coatings. J. Control. Release. 2005;103(2):355–367. doi: 10.1016/j.jconrel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Thompson JM, Agee K, Sidow SJ, McNally K, Lindsey K, Borke J, Elsalanty M, Tay FR, Pashley DH. Inhibition of endogenous dentin matrix metalloproteinases by ethylenediaminetetraacetic acid. Journal of Endodontics. 2012;38(1):62–65. doi: 10.1016/j.joen.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]