Abstract
Tuberculosis (TB) remains the second most common cause of death due to a single infectious agent. The cell envelope of Mycobacterium tuberculosis (Mtb), the causative agent of the disease in humans, is a source of unique glycoconjugates and the most distinctive feature of the biology of this organism. It is the basis of much of Mtb pathogenesis and one of the major causes of its intrinsic resistance to chemotherapeutic agents. At the same time, the unique structures of Mtb cell envelope glycoconjugates, their antigenicity and essentiality for mycobacterial growth provide opportunities for drug, vaccine, diagnostic and biomarker development, as clearly illustrated by recent advances in all of these translational aspects. This review focuses on our current understanding of the structure and biogenesis of Mtb glycoconjugates with particular emphasis on one of most intriguing and least understood aspect of the physiology of mycobacteria: the translocation of these complex macromolecules across the different layers of the cell envelope. It further reviews the rather impressive progress made in the last ten years in the discovery and development of novel inhibitors targeting their biogenesis.
Keywords: Glycosyltransferase, phosphatidylinositol mannosides, lipoarabinomannan, arabinogalactan, acyltrehaloses, peptidoglycan, (lipo)polysaccharides, flippase
Introduction
Mycobacteria are known to produce a variety of cytosolic and cell envelope-associated (glyco)lipids and (lipo)polysaccharides of exceptional structures that play various essential roles both in their physiology and interactions with the host in the course of infection. Cytosolic glycoconjugates (e.g., glycogen, glucosylglycerate, polymethylated polysaccharides, mycothiols), for instance, are thought to be important in maintaining a reducing environment in the cytosol, protecting the cells from osmotic and nitrogen stress, regulating fatty acid synthesis and as a carbohydrate reserve (Newton et al., 2008; Jackson and Brennan, 2009; Kaur et al., 2009; Behrends et al., 2012). The bulk of the glycoconjugates produced by mycobacteria, however, is found in their cell envelope providing shape and rigidity to the cells and contributing to their impermeability to biocides and nutrients. They also confer unique staining properties to the cells that aid in the microscopy-based diagnosis of mycobacterial diseases and ultimately direct much of the interactions of mycobacteria with the host. While the interest in mycobacterial glycoconjugates originally stemmed from their structural diversity and antigenicity, continued research in this field has been driven by their important contribution to pathogenesis as well as from the standpoint of developing drugs, vaccines, diagnostics and biomarkers. In this regard, developments in the genomics and genetics of mycobacteria in the 1990s have provided a major impetus to the study of Mycobacterium tuberculosis (Mtb) cell envelope glycoconjugates culminating in significant progress made in the last two decades in elucidating the biosynthetic pathways leading to their elongation, assembly and export. Concomitantly, the ability to generate isogenic knock-outs, knock-ins and knock-downs of Mtb proficient or, on the contrary, deficient in the synthesis or export of specific glycoconjugates has allowed for the definition of novel therapeutic targets and a better understanding of their roles in pathogenesis. This review focuses on the cell envelope glycoconjugates of Mtb with particular emphasis on recent findings concerning their structures, biogenesis and biological activities. It further discusses the common themes that are beginning to emerge with regard to the coupling of their biosynthesis and export, and finally reviews ongoing drug discovery efforts aimed at targeting their biogenesis.
The major cell envelope glycoconjugates of Mtb
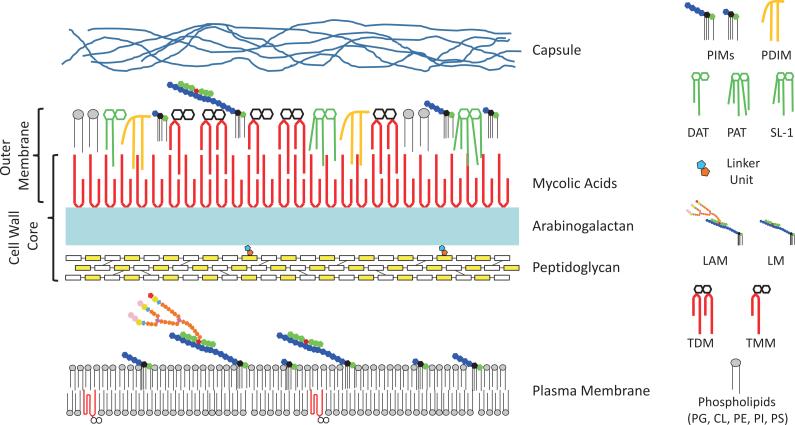
The mycobacterial cell envelope is made up of three major entities [Fig. 1]. The innermost layer is the plasma membrane that seems typical of bacterial membranes except for the presence of Mycobacterium-specific (glyco)lipids, lipoglycans and (lipo)proteins. Outside the plasma membrane is the cell wall core comprised of peptidoglycan (PG) in covalent attachment via phosphoryl-N-acetylglucosaminosyl-rhamnosyl linkage units with the heteropolysaccharide arabinogalactan (AG), which is in turn esterified at its non-reducing ends to α-alkyl, β-hydroxy long-chain (C60-C90) mycolic acids. The cell wall core, also known as the mycolyl arabinogalactan-peptidoglycan (mAGP) complex, is essential for viability and the site of resistance and susceptibility to many anti-TB drugs (Barry et al., 2007; Jackson et al., 2013). The AG-bound mycolic acids form the bulk of the inner leaflet of the outer membrane, with the outer layer consisting of a variety of non-covalently attached (glyco)lipids, lipoglycans (lipomannan and lipoarabinomannan) and (lipo)proteins some of which are glycosylated. The organization and composition of this asymmetrical outer bilayer known as the ‘mycomembrane’ or ‘outer membrane’ (OM) (Hoffmann et al., 2008; Zuber et al., 2008) confer to mycobacteria a high intrinsic resistance to many therapeutic agents and host defense mechanisms (Minnikin et al., 1982; Jarlier and Nikaido, 1994). Finally, a loosely attached capsular-like structure outside the OM of Mtb was shown to mainly consist of polysaccharides and proteins with only minor amounts of lipids (Lemassu and Daffé, 1994; Ortalo-Magné et al., 1995; Sani et al., 2010). The three major polysaccharides found in the capsular material of Mtb consist of a high molecular weight α-D-glucan with a structure similar to that of glycogen, a D-arabino-D-mannan (AM), and a D-mannan (Lemassu and Daffé, 1994; Ortalo-Magné et al., 1995; Dinadayala et al., 2004). Importantly, the nature and amounts of outer membrane and capsular materials vary with the Mtb isolates and this diversity in terms of surface composition is likely to significantly impact the way that Mtb interacts with the host (Cywes et al., 1997; Ehlers and Daffé, 1998; Daffé and Etienne, 1999; Torrelles and Schlesinger, 2010).
Figure 1. Schematic representation of the Mtb cell envelope.
Many of the classes of lipids and glycolipids discussed in the review are represented schematically and are shown in probable locations in the cell envelope. The overall schematic and individual structures are not drawn to scale. Proteins and peptides are not shown for the sake of clarity. The color code used in the representation of LM and LAM is the same as in Fig. 6. PE, phosphatidylethanolamine, PI, phosphatidyl-myo-inositol; CL, cardiolipin; PS, phosphatidylserine; PG, phosphatidylglycerol.
The glycoconjugates of the cell wall core
(1) Peptidoglycan
PG structure
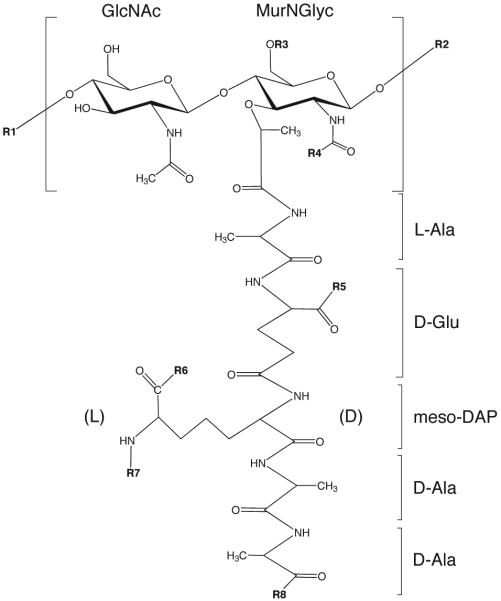
PG is a complex glycopolymer forming a rigid layer outside the plasma membrane allowing the bacterium to maintain its shape and to resist the effects of changes in osmotic pressure. As in other bacteria, the synthesis and turnover of PG in Mtb are intimately coordinated with cell division. In mycobacteria, PG also serves as a scaffold for the rest of the cell envelope [Fig. 1]. The detailed structure and biosynthesis of the PG of Mtb have been reviewed recently (Pavelka et al., 2014). The PG of mycobacteria belongs to the A1γ chemotype as does that of Escherichia coli and a number of other bacteria. It consists of a glycan backbone of alternating units of N-acetylglucosamine (GlcNAc) and modified muramic acid (Mur) in a β-(1,4) linkage, with tetrapeptide side chains attached to the lactyl moiety of Mur typically consisting of L-alanyl-D-glutamine-meso-diaminopimelyl-D-alanyl-D-alanine that may be cross-linked [Fig. 2]. Most of the cross-links in Mtb (60-80%) consist of ‘3,3’ linkages between the meso-diaminopimelate (meso-DAP) residues of adjacent peptides (Kumar et al., 2012); the second type of linkages found are known as ‘4,3’ linkages and occur between the D-Ala at position 4 of one peptide and the meso-DAP at position 3 of an adjacent peptide. Contrary to the earlier impression that the proportion of 3,3 linkages increased as Mtb bacilli reached stationary phase (Lavollay et al., 2008), the percentage of 3,3 to 4,3 linkages is in fact relatively constant throughout the growth of Mtb (Kumar et al., 2012). The overall high degree of cross-linking typically found in Mycobacterium spp. (70-80% compared to 30-50% in E. coli) (Matsuhashi, 1966) provides added structural integrity to the cells. A particularity of the muramic acid residues found in the PG of mycobacteria and closely related actinobacteria is that they can be either N-acetylated (MurNAc) or N-glycolylated (MurNGlyc). N-glycolylation contributes to the resistance of mycobacteria to lysozyme (Raymond et al., 2005) and potentiates the innate immune recognition of their PG by Nod2 (Coulombe et al., 2009) but does not affect the pathogenicity of Mtb (Hansen et al., 2013). Other variations in the peptide chain include the amidation of free carboxyl group of D-Glu and that of the free carboxyl group of meso-DAP. It is thought that some of the structural particularities of mycobacterial PG are related to its role in stabilizing the mAGP complex (Mahapatra et al., 2005). AG is attached to PG through a phosphodiester link to position 6 of about 10-12% of the Mur residues. The specific linker unit ensuring this covalent attachment is made of a rhamnosyl residue attached to a GlcNAc-1-phosphate residue (McNeil et al., 1990) [Fig. 3].
Figure 2. Structures of a representative monomer of mycobacterial PG prior to peptide trimming.
R1, N-glycolylmuramic acid residue of another monomer; R2, N-acetylglucosamine residue of another monomer; R3, H or the linker unit of AG; R4, H, COCH3 (N-acetyl) or COCH2OH (N-glycolyl); R5, R6, R8, OH, NH2 or OCH3; R7, H, or cross-linked to penultimate D-Ala or to the D-center of another meso-DAP residue.
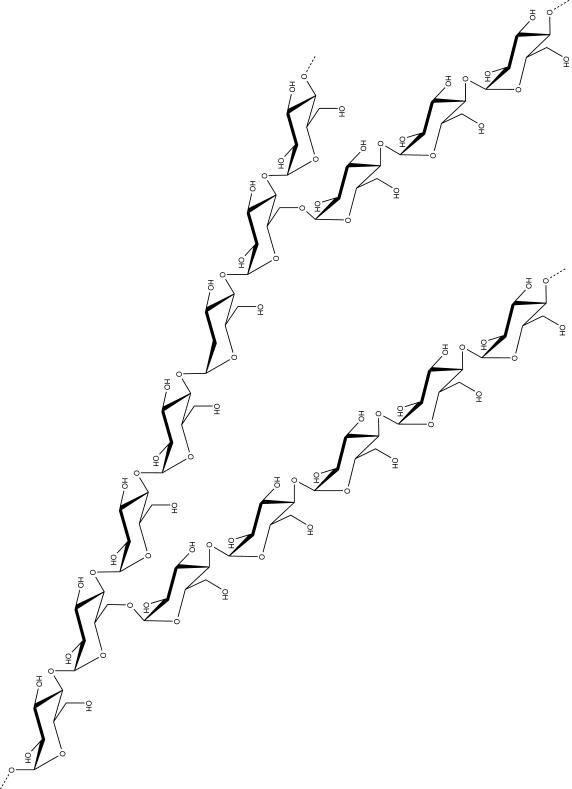
Figure 3. Structure of arabinogalactan.
See text for details.
PG synthesis
PG synthesis requires a series of essential steps: (i) the cytoplasmic synthesis of precursor molecules; (ii) the translocation of the PG precursor known as lipid II to the periplasmic face of the plasma membrane and (iii) its incorporation into the existing PG.
The cytoplasmic synthesis of PG precursors in Mtb is for the most part similar to that of other bacteria (for a recent review, Pavelka et al., 2014). Consistently, most of the genes involved have been found primarily by homology. The formation of UDP-MurNAc from UDP-GlcNAc in a two-step reaction catalyzed by MurA (Rv1315) and MurB (Rv0482) is the first committed step in the biosynthesis of Park’s nucleotide (UDP-N-acetylmuramyl-L-alanyl-D-glutamyl-meso-diaminopimelyl-D-alanyl-D-alanine). UDP-GlcNAc is the product of the phosphoglucosamine mutase GlmM (Rv3441c) and the UDP-N-acetylglucosamine pyrophosphorylase GlmU (Rv1018c) (Zhang et al., 2008; Li et al., 2012). The next steps in the biosynthesis of Park’s nucleotide consist of the stepwise additions of each amino acid of the peptide chain to the D-lactoyl group of Mur residues in reactions catalyzed by the Mur family of ligases. MurC (Rv2152c) catalyzes the addition of the first D-Ala residue; MurD (Rv2155c), the addition of a D-Glu residue; MurE (Rv2158c), the addition of meso-DAP; and MurF (Rv2157c), the addition of the last two D-Ala residues as a dipeptide. The latter dipeptide is the product of the D-Ala-D-Ala ligase (DdlA; Rv2981c). D-Ala and D-Glu are produced from L-Ala and L-Glu by the alanine racemase Alr (Rv3423c) and the glutamate racemase MurI (Rv1338), respectively. meso-DAP is produced from L-aspartate in a series of eight reactions involving the enzymes Ask (Rv3709c), Asd (Rv3708c), DapA (Rv2753c), DapB (Rv2773c), DapC (Rv0858c), DapD (Rv1201c), DapE (Rv1202) and DapF (Rv2726c).
As in other bacteria, the fully assembled sugar-peptide moiety of the Park’s nucleotide is then transferred to a lipid carrier by the phospho-N-acetylmuramyl pentapeptide translocase MraY (Rv2156c) forming lipid I. Unlike other bacteria, however, the lipid carrier used by mycobacteria in the biosynthesis of PG and other major cell envelope glycoconjugates (e.g., polar forms of phosphatidyl-myo-inositol mannosides, AG, lipomannan, lipoarabinomannan, glycoproteins) is decaprenyl-phosphate (Dec-P) instead of the usual undecaprenyl phosphate. The biosynthesis of Dec-P in Mtb was reviewed recently (Daffé et al., 2014). The glycosyltransferase MurG (Rv2153c) next transfers GlcNAc from UDP-GlcNAc to lipid I yielding lipid II. It is at the level of lipid II that the peptides may undergo modifications (amidation, methylation and glycolylation) and that the N-acetyl groups of the MurNAc residues may be oxidized to N-glycolyl by the UDP-MurNAc hydroxylase NamH (Rv3818) (Raymond et al., 2005). Although candidate genes for the modification of the peptide have been identified, their involvement in the process has not yet been validated experimentally. The physiological significance of the differentially modified lipid II molecules that arise from these modifications is currently not known, nor is it clear that all of these modifications occur in the mature PG.
The identity of the ‘flippase(s)’ required to translocate lipid II to the periplasmic face of the plasma membrane and that of the transporter required to import back the decaprenyl diphosphate (or Dec-P) released upon the incorporation of the lipid II precursors into the mature AG have not yet been established. Genetic and bioinformatic evidence based on studies conducted in other bacteria suggest that FtsW- and MviN- (MurJ) like proteins may be involved (Ruiz, 2008; Mohammadi et al., 2011). FtsW- and MviN-like proteins seem to be present in all PG-producing eubacteria. MviN proteins are members of the multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) flippase superfamily that bears sequence similarity with the Wzx/WzxE flippases involved in the synthesis of the majority of LPS O-antigens and in that of the enterobacterial common antigen (ECA) (Hvorup et al., 2003; Ruiz, 2008). FtsW-like proteins belong to the shape/elongation/division and sporulation (SEDS) protein family (Henriques et al., 1998) which in Gram positive and Gram negative organisms have been shown to control PG synthesis during cell elongation, division and sporulation (Henriques et al., 1998; Pastoret et al., 2004; Real et al., 2008). Recent biochemical studies conducted on the FtsW protein of E. coli are consistent with this protein displaying lipid II flippase activity (Mohammadi et al., 2011). The Mtb MOP superfamily protein Rv3910 which harbors an N-terminal MviN-like domain was shown to be essential for growth. The knock-down of this gene in M. smegmatis causes altered cell morphology in addition to growth inhibition, and leads to the accumulation of PG precursors in the cells (Gee et al., 2012). Whether Rv3910 acts a lipid II flippase, however, remains to be established. Likewise, the FtsW-like protein encoded in the genome of Mtb H37Rv by Rv2154c maps in a cluster of genes dedicated to PG synthesis including mraY (Rv2156c) and murG (Rv2153c) responsible, respectively, for the formation of lipids I and II. Rv2154c was shown to physically interact with the penicillin-binding protein PbpB (Rv2163c) (see further) and FtsZ (Rv2150c) thereby likely facilitating septal PG synthesis during cell division (Datta et al., 2002; Datta et al., 2006), but its precise function in PG synthesis remains elusive. Finally, a third FtsW-like lipid II flippase candidate found in Mtb is encoded by rodA (Rv0017c). RodA is an essential protein in Bacillus subtilis required for the elongation of the lateral wall of the cells and the maintenance of their rod shape (Henriques et al., 1998). The recent finding that RodA of Corynebacterium glutamicum exclusively localizes to the cell poles and is required for optimal growth and cell length is consistent with this protein serving an analogous function in Actinobacteria even though the dispensability of this protein for viability in this species indicates that compensatory activities exist (Sieger et al., 2013). In other rod-shaped model organisms such as E. coli and B. subtilis, it is assumed that the requirement of the divisome and cell elongation machineries for distinct lipid II flippases accounts for the existence of more than one of these transporters in the cells (Sieger et al., 2013). It is thus possible that Mtb expresses more than one lipid II flippase and that MviN (Rv3910), FtsW (Rv2154c) and RodA (Rv0017c) all contribute to this function in the context of cell division and/or cell elongation. Clearly, the identification of Mtb lipid II flippase candidates is an exciting breakthrough for its potential to lead to a better understanding of cell elongation and division.
A number of transglycosylases, transpeptidases and carboxypeptidases including eight penicillin-binding proteins (PBPs), mediate the polymerization of the sugar backbone and cross-linking of the peptides of PG in Mtb (Pavelka et al., 2014). The role of some of these enzymes in PG biosynthesis, however, is not fully understood. The Mtb PBPs PbpA (Rv0016c) and PbpB (FtsI; Rv2163c) also play critical roles in cell division (Pavelka et al., 2014). A specific family of enzymes known as L,D-transpeptidases accounts for the formation of the 3,3 crosslinks in PG. The Mtb genome encodes five of these enzymes, four of which (LdtA [Rv0116c]; LdtB [Rv2518c]; Mt4 [Rv0192]; and Mt5 [Rv0483]) exhibit L,D-transpeptidase activity in vitro (Lavollay et al., 2008; Gupta et al., 2010; Cordillot et al., 2013). Unlike the classical D,D-transpeptidases which are penicillin-binding proteins and can be inhibited by various classes of β-lactam antibiotics (Goffin et al., 2002), L,D-transpeptidases are typically resistant to most β-lactams with the exception of the carbapenem class (Lavollay et al., 2008; Gupta et al., 2010; Kumar et al., 2012; Cordillot et al., 2013; Pavelka et al., 2014). Interestingly, carbapenems were reported to form covalent adducts with LdtA, LdtB and Mt4 but not Mt5 (Cordillot et al., 2013).
PG turnover
Not much is known about PG breakdown and recycling in mycobacteria. CwlM (Rv3915) and Rv3717 are amidases of Mtb that cleave PG between the N-acetylmuramyl acid residues and the first L-Ala of the peptide chain (Deng et al., 2005; Prigozhin et al., 2013). In addition, the genome of Mtb potentially encodes seven NPL/P60 family endopeptidases, the two best-studied members of which are RipA (Rv1477) and RipB (Rv1478) that cleave PG fragments between D-Glu and meso-DAP (Both et al., 2011; Pavelka et al., 2014). Finally, Mtb has five resuscitation-promoting factor (rpf)-like genes whose protein products share common structural features with the so-called ‘lysozyme-like’ fold, suggesting that they may cleave the glycan chain of PG (Kaprelyants et al., 2012). Rpf proteins have partially overlapping activity in vitro and in vivo, are all highly induced during resuscitation, and are required to restore the culturability of non-replicating persistent bacilli (Kaprelyants et al., 2012). The mechanism through which Rpf proteins stimulate cell reactivation and growth is still unclear. While the cleavage of PG by Rfp proteins may directly account for the initiation of replication after a period of latency, it was also proposed that the muropeptides released as a result of the action of Rpf proteins may act as signaling molecules in the host or stimulate the Ser/Thr protein kinase PknB to indirectly regulate cell envelope biosynthesis and cell division (Molle and Kremer, 2010; Mir et al., 2011; Kaprelyants et al., 2012).
PG biosynthesis in the context of drug discovery
With multidrug resistance on the rise, recent years have seen a marked intensification of TB drug discovery efforts with the result that many new lead compounds are now at various stages of the drug discovery and preclinical development pipeline. These efforts not only keep pointing at cell envelope biogenesis as one of the Achille’s heel of Mtb (for a recent review, Jackson et al., 2013) but are also leading the TB field to revisit earlier impressions that drug targeting the biogenesis of the cell envelope may not be synergistic with other drugs, useful against MDR-Mtb isolates, or active against persistent bacilli. Interestingly, PG synthesis is one of the cell envelope pathways that has undergone the greatest resurgence of interest as a promising target for new chemotherapeutic approaches. Of course, D-cycloserine has long been a useful second-line drug in the treatment of TB and nowadays in the treatment of MDR-TB despite its well known effects on the central nervous system. It competitively inhibits two enzymes in the synthesis of the peptide chain of PG, alanine racemase (Alr) which forms D-alanine from L-alanine, and D-alanine:D-alanine synthase (DdlA) in all eubacteria including mycobacteria. Towards the development of more effective inhibitors of DdlA, the crystal structures of this enzyme under its apo form and in complex with D-cycloserine were recently solved (Bruning et al., 2011). In addition, thiadiazolidinone inhibitors of Alr (IC50 of 0.03 to 28 μM) were found to inhibit the growth of Mtb at concentrations ranging from 1.6 to 100 μg/ml (Lee et al., 2013). Other recent efforts have focused on a series of synthetic N-methyl-2-alkenyl-4-quinolones showing IC50 values in the range of 100 μM against the Mtb MurE ligase in vitro and 5 to 25 μg/ml MICs against Mtb in culture (Guzman et al., 2011). An assay suitable for the high throughput screening of inhibitors of GlmU (involved in the formation of GlcNAc) was developed, and an NIH-sponsored screening was performed (http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=1376) which was later analyzed for optimization of hits (Singla et al., 2011). Other inhibitors of GlmU were recently described (Tran et al., 2013). Even though their mode of action in whole Mtb cells has yet to be confirmed, the synthesis of capuramycin analogs as inhibitors of bacterial phospho-N-acetylmuramyl pentapeptide translocases (MraY) has led to the identification of several analogs with potent activity against drug susceptible and MDR Mtb isolates (MIC of 2 to 4 μg/ml) as well as several other mycobacterial pathogens both in vitro and in vivo (Koga et al., 2004; Reddy et al., 2008; Nikonenko et al., 2009). One of these analogs, SQ641, is now at the stage of preclinical development (http://www.newtbdrugs.org). The importance of Rfps in restoring the culturability of non-replicating persistent bacilli and the structural similarity between the conserved catalytic domain of these proteins and that of cell wall lytic enzymes has prompted a search for Rpf inhibitors based on known inhibitors of the latter proteins. Nitrophenylthiocyanate derivatives were tested and found to inhibit the mycobacterial purified Rpfs. While devoid of activity against acute TB in vivo, some of them impair the resuscitation of dormant Mtb cells both in vitro and in mice and may thus represent a promising new scaffold for drugs targeting persistent Mtb bacilli (Kaprelyants et al., 2012). The recent success of carbapenems and clavulanate combinations in the treatment of active and latent TB (Hugonnet et al., 2009; England et al., 2012) further emphasizes the potential of PG as a target to kill persistent and MDR bacilli and has resulted in a re-visitation of the prospects of introducing β-lactam-β-lactamase inhibitors into standard TB chemotherapy. As noted earlier, the L,D-transpeptidases involved in the (3,3)-crosslinking of PG are resistant to most β-lactams with the exception of the carbapenem class. The fact that the disruption of ldtB negatively impacts virulence and increases the susceptibility of Mtb to amoxicillin-clavulanate both in vitro and during the chronic phase of infection (Gupta et al., 2010) suggests, however, that a combination of L,D-transpeptidase inhibitor, clavulanate and classical β-lactams could effectively target replicating and persistent bacilli. The structure of LdtB was recently determined and drugs targeting this enzyme are being sought (Erdemli et al., 2012). The discovery that synthesis of the major lipid carrier, Dec-P, in Mtb proceeds through the methylerythritol phosphate pathway which has no homolog in humans has provided stimulus for the identification and characterization of inhibitors of the relevant enzymes. Fosmidomycin, which is currently in clinical trials for the treatment of malaria in humans, is a competitive inhibitor of the second enzyme of the pathway, 1-deoxy-D-xylulose-5-phosphate reductoisomerase (DXR, IspC). Promising results on Mtb were obtained with lipophilic prodrug derivatives of this compound (Uh et al., 2011) and the availability of several crystal structures of the Mtb DXR enzyme and DXR-fosmidomycin complexes has opened the way to the structure-based design of more potent analogs. Finally, another approach to targeting PG metabolism has focused on inhibiting the Ser/Thr kinases involved in the regulation of this pathway (Molle and Kremer, 2010; Mir et al., 2011; Gee et al., 2012). Accordingly, libraries of compounds were screened against PknA or PknB in vitro and several promising inhibitors were found (Magnet et al., 2010; Danilenko et al., 2011; Lougheed et al., 2011; Chapman et al., 2012; http://www.newtbdrugs.org). Preliminary evidence suggests, however, that the treatment of Mtb with some of these inhibitors leads to the inhibition of multiple targets (Magnet et al., 2010; Lougheed et al., 2011).
(2) Arabinogalactan
Structure of AG
The most recent model of Mtb AG, based on the analysis of this heteropolysaccharide from in vitro-grown Mtb H37Rv, indicates that it contains on average 79 glycosyl residues distributed between a galactan domain made of 23 Galf residues, two arabinan domains each containing about 26 Araf residues, and a specific linker unit made of a rhamnosyl residue attached to a N-acetylglucosaminosyl-1-phosphate residue which serves in the covalent attachment of AG to PG (Bhamidi et al., 2011) [Fig. 3]. It was estimated that 1.3 AG molecules were present per 10 repeating units of PG in Mtb (Bhamidi et al., 2011). The characteristic non-reducing termini of the arabinan domain of AG consist of an Ara6 motif, Arafβ–(1,2)-Arafα–(1,5)(Arafβ–(1,2)Arafα–(1,3))-Arafα–(1,5)-Arafα–(1, where both the terminal β-Araf and the penultimate 2-α-Araf serve as the anchoring points for the mycolic acids. Approximately two-thirds of these attachment sites are occupied with mycolate residues in in vitro-grown Mtb (McNeil et al., 1991; Bhamidi et al., 2011). The inner core of the arabinan domain is essentially made of stretches of α–(1,5)-linked Araf residues with a critically positioned α–(3,5)-branch site. In addition, galactosamine (α-D-GalpNH2 thereafter referred to GalN) and succinyl substituents were found specifically attached at O-2 of a portion of the internal (3,5)-branched D-Araf residues in the AG of Mtb (Draper et al., 1997; Lee et al., 2006; Bhamidi et al., 2008; Peng et al., 2012). α-D-GalN was estimated to occur at the level of about one residue per entire AG molecule and succinyl groups at the level of one to three residues per AG molecule (Bhamidi et al., 2008). Interestingly, a similar GalN residue was found to substitute the AG of M. avium, M. kansasii, M. bovis BCG (Draper et al., 1997) and M. leprae but not that of M. smegmatis, M. neoaurum and M. phlei (Draper et al., 1997; Lee et al., 2006; Bhamidi et al., 2008; Bhamidi et al., 2011), suggesting that fast-growing Mycobacterium spp. are devoid of GalN substituent.
AG biosynthesis
The synthesis of AG begins with the cytoplasmic formation of the linker unit on a decaprenyl monophosphate (Dec-P) carrier lipid followed by the addition of Galf residues still on the cytosolic face of the plasma membrane and that of Araf residues on the periplasmic side of the membrane (Mikušová et al., 1996; Mikušová et al., 2000; Yagi et al., 2003) [Fig. 4]. Many of the enzymes involved in this process have been identified (Kaur et al., 2009). A WecA-like transferase encoded by Rv1302 in the genome of Mb H37Rv transfers GlcNAc-1-phosphate from UDP-GlcNAc to Dec-P to form Dec-P-P-GlcNAc (also known as GL-1) (Mikušová et al., 1996; Jin et al., 2010). The attachment of a rhamnosyl residue from the sugar nucleotide dTDP-Rha to the 3-position of GlcNAc is catalyzed by WbbL1 yielding GL-2 (Dec-P-P-GlcNAc-Rha), “the linker unit” (Mills et al., 2004). dTDP-Rha is synthesized from glucose-1-phosphate through a four-step reaction catalyzed by RmlA (Rv0334), RmlB (Rv3464), RmlC (Rv3465) and RmlD (Rv3266c) (Ma et al., 1997; Stern et al., 1999; Hoang et al., 1999; Ma et al., 2001). GL-2 then serves as an acceptor for the sequential cytoplasmic addition of Galf residues from UDP-Galf catalyzed by two bifunctional galactosyltransferases, GlfT1 (Rv3782) and GlfT2 (Rv3808c). UDP-Galf is generated from UDP-Galp by the UDP-Galp mutase Glf (Rv3809c) (Weston et al., 1997; Mikušová et al., 2000). GlfT1 is endowed with β-(1,4) and β-(1,5) galactosyltransferase activities and transfers the first two Galf residues to GL-2 (Belanova et al., 2008). The remaining alternating 5- and 6-linked Galf residues are added by the bifunctional galactosyltransferase GlfT2 (Kremer et al., 2001; Rose et al., 2006; Belanova et al., 2008; Wheatley et al., 2012). The identity of the transporter responsible for the translocation of the fully elaborated or nascent lipid-linked galactan chain to the periplasmic side of the plasma membrane has not yet been firmly established although an ABC-transporter has been proposed for this function (Dianiskova et al., 2011). The arabinosylation of AG next takes place on the periplasmic side of the plasma membrane catalyzed by membrane-associated decaprenyl-phosphate arabinose (Dec-P-Ara)-dependent arabinosyltransferases (AraTs). Dec-P-Ara is the only known arabinose donor in the building of the arabinan domains of the two essential cell envelope glycoconjugates, AG and lipoarabinomannan (LAM) (Wolucka et al., 1994). It is synthesized from 5-phosphoribose-1-pyrophosphate (Scherman et al., 1995; Scherman et al., 1996) - the product of the phosphoribosyl-pyrophosphate synthetase PrsA (Alderwick et al., 2011a) - through four reaction steps involving the Dec-P 5-phosphoribosyltransferase, UbiA (Rv3806c) (Huang et al., 2005; Huang et al., 2008; Alderwick et al., 2005), the phosphoribosyl-monophosphodecaprenol phosphatase Rv3807c (Jiang et al., 2011), and DprE1 (Rv3790) and DprE2 (Rv3791) responsible for the epimerization of decaprenyl-phosphate ribose to Dec-P-Ara (Mikušová et al., 2005). The AraTs involved in the formation of the arabinan domain of AG identified to date include AftA (Rv3792), responsible for the transfer of the very first Araf residues to the galactan domain of AG (Alderwick et al., 2006a), the terminal β–(1,2)-capping AraT AftB (Rv3805c) (Seidel et al., 2007), AftC (Rv2673) involved in the internal α–(1,3)-branching of AG (Birch et al., 2008) and the EmbA (Rv3794) and EmbB (Rv3795) proteins involved in the formation of the Ara6 motif of AG (Escuyer et al., 2001; Khasnobis et al., 2006). We successfully overexpressed and purified a soluble form of AftC from M. smegmatis and showed that it retains α–(1,3)-branching AraT activity in vitro upon reconstitution into proteoliposomes containing mycobacterial lipids (Zhang et al., 2011). By analogy with the Emb protein of C. glutamicum (NCgl0184) (Alderwick et al., 2005) and EmbC (Rv3793) which is required for the elongation of the arabinan domain of LAM (Berg et al., 2005; Shi et al., 2006) (see PIM, LM, LAM section), it was proposed that EmbA and/or EmbB (or an EmbA/EmbB dimer) acted as the α-(1,5) AraTs responsible for the elongation of the arabinan domain of AG (Bhamidi et al., 2008). However, direct evidence for this assumption is still lacking. Moreover, elongating α–(1,5) AraT activities - some of which are apparently unrelated to the Emb proteins - have been detected in cell-free assays using mycobacterial cell wall preparations and synthetic arabinan acceptors (Lee et al., 1997; Lee et al., 1998; Zhang et al., 2007). Finally, another functional Dec-P-Ara-dependent AraT with α–(1,3) branching activity on linear α–(1,5)-linked neoglycolipid acceptors was identified as AftD (Rv0236c) (Škovierová et al., 2009). aftD is an essential gene in M. smegmatis. Alterations in its level of expression caused defects in cell division, reduced growth, altered colony morphology and accumulation of trehalose dimycolates in the cell envelope. Overexpression of aftD in M. smegmatis, in contrast, induced the accumulation of arabinosylated compounds with carbohydrate backbones reminiscent of that of LAM. Collectively, these results suggest that AftD is involved in the synthesis of the arabinan domains of AG and LAM, although its precise function in these pathways remains to be defined.
Figure 4. Schematic diagram of arabinogalactan biosynthesis.
The synthesis of AG begins with the cytoplasmic formation of the linker unit on a decaprenyl monophosphate carrier lipid followed by the addition of Galf residues still on the cytosolic face of the plasma membrane and that of Araf residues and other decorating motifs (e.g., GalN motif) on the periplasmic side of the membrane. See text for details.
Importantly, the enzymes involved in the formation of Dec-P (Eoh et al., 2007), dTDP-Rha (Ma et al., 2002; Li et al., 2006), UDP-Galf (Pan et al., 2001), GL-I (Jin et al., 2010; Ishizaki et al., 2013), GL-II (Mills et al., 2004), the galactan domain (Pan et al., 2001), Dec-P-Ara (Crellin et al., 2011; Kolly et al., 2014) and the arabinan domain of AG (Alderwick et al., 2005; Amin et al., 2008; Shi et al., 2008; Škovierová et al., 2009) are all essential for mycobacterial growth providing opportunities for new chemotherapeutic strategies against Mtb (see further).
By analogy to the biosynthetic pathway responsible for the modification of lipid A with a D-GalN unit in Francisella (Kanistanon et al., 2008; Wang et al., 2009; Song et al., 2009), we identified and functionally characterized two glycosyltransferases, Rv3631 and Rv3779, responsible for the synthesis and transfer of the GalN motif of AG (Škovierová et al., 2010) [Fig. 4]. Rv3631 displays polyprenol-phospho-GalNAc (Dec-P-GalNAc) synthase activity, generating on the cytoplasmic face of the plasma membrane Dec-P-GalNAc from Dec-P and UDP-GalNAc. Dec-P-GalNAc or its deacylated counterpart, Dec-P-Gal, then serve as the sugar donors used by the GT-C glycosyltransferase Rv3779 in the periplasmic transfer of GalN (or GalNAc) onto the arabinan domain of AG (Škovierová et al., 2010). The deacetylase required to generate Dec-P-GalN from Dec-P-GalNAc and the “flippase” required to translocate Dec-P-GalNAc (or Dec-P-GalN) from the cytosolic to the periplasmic side of the plasma membrane have not yet been identified. The enzyme responsible for the transfer of succinyl residues to the arabinan domain of AG is also presently not known.
Topology of the AG biosynthetic pathway and evidence for the existence of multiprotein complexes
In spite of the significant advances made in the last 15 years in understanding the biosynthesis of AG and underlying genetics, the fundamentals of how the different domains of AG are assembled, if on a lipid carrier, growing stepwise from the reducing towards the non-reducing end through the sequential addition of glycosyl residues or assemble through the polymerization of building blocks, are at present not fully understood. Based on available evidence, the sequential addition of arabinosyl and galactosyl residues is favored over the polymerization of building blocks such as described in the biosynthesis of some bacterial O-antigens, glycoproteins and capsular polysaccharides (Raetz and Whitfield, 2002; Rick et al., 2003; Whitfield, 2006; Alaimo et al., 2006; Raetz et al., 2007; Ruiz et al., 2008; Mohammadi et al., 2011). Experimental evidence further points to the concurrent galactosylation and arabinosylation of lipid-linked AG precursors, at least in cell-free assays (Mikušová et al., 2000), and possibly in intact cells (Larrouy-Maumus et al., 2012). Given that galactosylation and arabinosylation events are topologically split across the plasma membrane, this finding could suggest a ‘synthase-dependent’ type of pathway for AG biosynthesis wherein the nascent lipid-linked galactan chain is progressively extruded across the plasma membrane as it is elongated (Raetz and Whitfield, 2002). The transporter involved has not yet been identified although an ABC-transporter has been proposed to participate in this function (Dianiskova et al., 2011). The periplasmic arabinosylation of AG further raises the question of the flipping of Dec-P-Ara from the cytosolic face of the membrane where this sugar donor is synthetized (Mikušová et al., 2005) to the outer leaflet of the membrane where it can then be used by GT-C superfamily arabinosyltransferases. ErmE-like Small Multidrug Resistance (SMR) transporters, typically 105-121 amino acids in size and containing four transmembrane domains (Bay et al., 2008), participate in the translocation of lipid-linked phosphate sugars across the plasma membrane in a variety of microorganisms. Examples include the E. coli transporter encoded by arnE/arnF that flips undecaprenyl phosphate 4-amino-4-deoxy-L-arabinose across the plasma membrane (Yan et al., 2007), and the GtrA protein produced by the bacteriophage SfX of Shigella flexneri which was proposed to mediate the translocation of undecaprenyl phosphate glucose used in the glucosylation of O-antigen (Guan et al., 1999; Korres et al., 2005). The presence of an SMR transporter-like gene, Rv3789, located immediately upstream dprE1 and dprE2 (responsible for the formation of Dec-P-Ara) in the genome of Mtb was suggestive of the involvement of Rv3789 in the re-orientation of Dec-P-Ara to the periplasm [Fig. 4]. Consistently, our results have shown that the disruption of the ortholog of Rv3789 in M. smegmatis resulted in a truncation of the arabinan domains of both AG and LAM that accompanied the accumulation of Dec-P-Ara in the mutant cells (Larrouy-Maumus et al., 2012). Further supporting the characterization of Rv3789 as a translocase, AG and LAM synthesis was restored in the mutant not only upon expression of the Rv3789 gene from Mtb but also upon that of the undecaprenyl phosphate aminoarabinose flippase arnE/F genes from E. coli. Interestingly, our studies on Rv3789 also served to establish for the first time that, similar to capsular polysaccharide, lipopolysaccharide, glycoprotein and PG synthesis in other prokaryotic organisms (Whitfield, 2006; Marolda et al., 2006; Sperandeo et al., 2009; Clarke et al., 2009), the biogenesis of AG in mycobacteria most likely relied on multiprotein complexes made of transporters and biosynthetic enzymes for efficient elongation and export. Indeed, Rv3789 was found to physically interact with the galactosyltransferase GlfT1, likely accounting for the membrane association of this enzyme and the dramatically reduced polymerization rate of the galactan domain of AG in the Rv3789 knock-out mutant (Larrouy-Maumus et al., 2012). Alternatively, assuming that lipid-linked AG precursors need to undergo concurrent galactosylation and arabinosylation in the process of elongation and extrusion across the plasma membrane, one may hypothesize that the decreased arabinosylation activity of the mutant consecutive to the disruption of Rv3789 negatively impacts the galactosylation rate of the precursors on the cytoplasmic face of the plasma membrane. In spite of its pivotal role in the biogenesis of the arabinan domains of AG and LAM, Rv3789 is not essential for the growth of M. smegmatis and Mtb indicating that multiple transporters with overlapping Dec-P-Ara flipping activities exist in mycobacteria.
Attachment of AG to PG
The last step in the biosynthesis of AG and PG is the covalent attachment of these two macromolecules via phosphoryl-N-acetylglucosaminosyl-rhamnosyl linkage units [Fig. 3]. The ligase(s) responsible for the formation of a 1-O-phosphoryl linkage between the GlcNAc residue of the linker unit of the mature AG and the 6-position of a MurNAc residue of PG has(have) not yet been identified. AG-PG ligation has been demonstrated in cell-free preparations of M. smegmatis (Yagi et al., 2003) and shown to require newly synthesized PG undergoing concomitant cross-linking (Hancock et al., 2002). Thus, ligation likely occurs either at the lipid II level, as seen for other PG modifications, or while the nascent PG is being formed. The observation that a Dec-P-Ara-deficient (ubiA knock-out) mutant of C. glutamicum which is unable to synthesize the arabinan domain of AG was viable and still capable of producing a simplified cell wall consisting of the galactan chain of AG attached to PG suggests that neither the arabinosylation of AG or its mycolylation are prerequisites for its attachment to PG (Alderwick et al., 2005; Alderwick et al., 2006b).
Biological significance of the minor covalent modifications of AG
The biological significance of the galactosamine and succinyl residues esterifying some of the interior branched (3,5)-Araf residues of AG is at present not known. It has been proposed that the protonated GalN (GalNH3+) interacts with the negatively charged succinyl residues leading to a more rigid and tightened AG structure (Bhamidi et al., 2008). The apparent lack of succinylation on the mycolylated arabinan chains (Bhamidi et al., 2008) could further suggest that succinylation negatively controls mycolylation. This possibility, however, needs to be considered with care given that the succinyl group is rather far from the site of mycolylation and succinylation might follow mycolylation rather than precede it. The possibility has also been raised that the protonated GalN (GalNH3+) interacts with anionic substances such as phosphates of glycero(glyco)lipids and the phosphatidyl-myo-inositol anchor of LM and LAM (Draper et al., 1997) thereby potentially affecting the organization of these compounds in the OM and the way they interact with the host in the course of infection. Our recent work involving wild-type Mtb versus isogenic GalN-deficient mutants (Škovierová et al., 2010) with human peripheral blood monocyte-derived dendritic cells (PBM-DCs) provides support for this hypothesis. Indeed, these studies have shown that the presence of the GalN substituent on AG abrogates a complete maturation/activation DC phenotype (as determined by decreased CD80/86, CD40 and HLA-DR expression) and stimulates increased IL-10 secretion while showing no difference in initial interaction and phagocytosis of the bacilli (W. Wheat, R. Dhouib, S. Angala, M. Jackson, unpublished results). Since purified AG from either wild-type or GalN-deficient mutants do not alter human DC maturation, it is therefore postulated that GalN may impose a topological modulation of the Mtb cell surface that provides better access to DC-SIGN or perhaps other receptors such as mannose receptor (MR) on macrophages and DCs preventing maturation signaling and resulting in the down-regulation of the initial immune response. More studies aimed at testing these hypotheses are in progress.
AG biosynthesis in the context of drug discovery
Ethambutol (EMB) has been known as an effective anti-TB drug since the early days of chemotherapy and is now a component of the ‘short-course chemotherapy’ involving isoniazid, rifampicin, pyrazinamide and EMB. EMB inhibits the synthesis of the arabinan domains of LAM and AG through the inhibition of the Emb arabinosyltransferases EmbA, EmbB and EmbC (Belanger et al., 1996; Goude et al., 2009). This observation and the pivotal roles played by other glycosyltransferases of the GT-C superfamily in the biosynthesis of AG has stimulated the design of innovative assays for inhibitor screening against these enzymes (Zhang et al., 2010; Zhang et al., 2011). In the last five years, whole cell-based screening of compounds against Mtb has produced several inhibitors of the essential epimerase DprE1 required for the formation of Dec-P-Ara (Makarov et al., 2009; Christophe et al., 2009; Magnet et al., 2010; Stanley et al., 2012; Wang et al., 2013). The molecular mechanism of action of some of these compounds has been thoroughly investigated (Trefzer et al., 2010; Trefzer et al., 2012; Neres et al., 2012; Batt et al., 2012) and several Dec-P-Ara inhibitors are now reported to be in the hit-to-lead or pre-clinical development phases (Jackson et al., 2013; http://www.newtbdrugs.org). Other ongoing approaches to AG inhibition consist of targeting the synthesis of Dec-P (the common lipid carrier in the biosynthesis of AG, PG and other major cell envelope glycoconjugates) as described in the previous (PG) section. Finally, recent studies have shown that the caprazamycin derivative CPZEN-45 which displays potent activity against Mtb in vitro (MIC of 0.2 to 1.5 μg/ml) is an inhibitor of the decaprenyl-phosphate-GlcNAc-1-phosphate transferase WecA, which catalyzes the first committed step in the biosynthesis of AG (Ishizaki et al., 2013).
Phosphatidylinositol mannosides and lipoglycans
Structures of PIM, LM and LAM
Mannosyl-phosphatidyl-myo-inositol-based glycolipids (PIM) and related lipoglycans comprising lipomannan (LM) and lipoarabinomannan (LAM) are found in abundant quantities in the cell envelope of mycobacteria and closely related Actinomycetes. PIMs, LM and LAM are non-covalently-linked components of the cell envelope. They are anchored in the inner and outer membranes via their phosphatidyl-D-myo-inositol unit (Ortalo-Magné et al., 1996; Pitarque et al., 2008). The existence of mannosylated phosphoglycolipids now known as the phosphatidylinositol mannosides (PIM) in mycobacteria has been known since the 1930s (Anderson and Roberts, 1930). Structural studies by a number of investigators have since established the complete structures of mono-, di-, tri-, tetra-, penta- and hexamannoside variants of these lipids in Mtb, M. bovis BCG, M. smegmatis and M. phlei (for reviews, Gilleron et al., 2008; Guerin et al., 2010). The basic core of PIMs consists of an acylated sn-glycerol-3-phospho-(1-D-myo-inositol) moiety (phosphatidyl-myo-inositol; PI) further glycosylated at the C-2 and C-6 positions of myo-inositol with one to six mannopyranose (α-D-Manp) residues [Fig. 5]. Their structures are extremely diverse with respect to the number and position of acylations they carry (C16:0, C18:0, C18:1, and C19 tuberculostearic acid are the major fatty acid forms found in PIM). The two most common forms of PIMs found in all mycobacterial species, are the tri- and tetraacylated PIM2 and PIM6 [Fig. 5]. Triacylated-PIMs (Ac1PIM2/Ac1PIM6) harbor two fatty acyl chains on the glycerol moiety (usually C16:0 and C19) and an additional acyl chain linked either to the C-6 position of the Manp residue linked to C-2 of myo-inositol or to C-3 position of myo-inositol. Tetraacylated-PIMs (Ac2PIM2/Ac2PIM6) are acylated on both sugar residues (Gilleron et al., 2001; Gilleron et al., 2003). The complete structural analysis of acylated PIMs from M. bovis BCG has been determined using advanced mass spectrometric approaches (Gilleron et al., 2006a, Hsu et al., 2007a, Hsu et al., 2007b).
Figure 5. Structures of the two major tetracylated forms of PIM2 and PIM6.
The forms of PIM2 and PIM6 represented here both harbor three palmitic and one tuberculostearic acyl chains.
Suggestive of a metabolic relationship with PIMs, the reducing end of LM and LAM consists of PI wherein the myo-inositol residue is mannosylated at positions C-2 and C-6 and the glycerol moiety, myo-inositol and Manp residue linked to C-2 of myo-inositol are esterified with similar fatty acyl chains as in PIMs (Chatterjee et al., 1992a; Hunter and Brennan, 1990; Khoo et al., 1995a; Gilleron et al., 2006b; Nigou et al., 1997) [Fig. 6]. LM and LAM share a common linear α-(1,6)-linked mannan backbone made up of 20-25 Manp residues elaborated by α-(1,2)-monomannose side chains. Our most recent structural data indicate that a stretch of uninterrupted linear α-6-linked mannosyl units attached to the inositol unit precedes the occurrence of contiguously occurring α-(1,2)-monomannose branches on the main chain (D. Kaur et al., manuscript in preparation). The major LAM glycoforms contain about 110 glycosyl residues (approximately 60 Araf and 50 Manp units) and consist of what appears to be a single D-arabinan chain attached to the α-(1,6) D-mannan backbone through an α-(1,2) linkage in Mtb (Chatterjee et al., 1993; D. Kaur et al., manuscript in preparation; S. K. Angala, manuscript in preparation). Our recent structural analyses confirmed this α-(1,2) linkage in LAM purified from M. smegmatis (S. K. Angala et al., unpublished results). The D-arabinan portion of LAM is very similar to that of AG in that the same linkages of Araf units are found and both structures share an Ara18 motif extending from the α–(3,5)-Araf interior residues (Shi et al., 2006; Bhamidi et al., 2008) [Fig. 6]. However, the D-arabinan structure of LAM has been found to be more variable than that of AG in terms of the length of this particular motif (Ara18- Ara22) (Shi et al., 2006). Further, in contrast to the presence of two arabinan chains per molecule of AG (Bhamidi et al., 2011), LAM seems to carry a single arabinan domain (Kaur D. et al., manuscript in preparation). Other distinctive features of the D-arabinan of LAM are found in its non-reducing termini which, in addition to the branched Ara6 motif found in AG, may consist of linear Ara4 [Fig. 6]. LAM further displays considerable structural micro-heterogeneity at its non-reducing arabinan termini. While in slow-growing mycobacterial species such as Mtb, M. leprae, M. avium, M. bovis, M. kansasii, M. xenopi, M. marinum and M. bovis BCG, these termini are capped with one to three α-(1,2)-Manp-linked residues giving rise to mannosylated LAM (known as ManLAM) (Gilleron et al., 2008), the LAM of fast-growing species may either be capped with phospho-inositol (yielding PILAM) as in M. smegmatis and M. fortuitum (Khoo et al., 1995b) or not carry any capping motifs (AraLAM) as in M. chelonae (Guérardel et al., 2002) [Fig. 6]. More recently, some of the Manp caps of Mtb ManLAM were found to be decorated with an α-(1,4)-linked methyl-thio-D-xylose (MTX) residue (Treumann et al., 2002, Ludwiczak et al., 2002; Turnbull et al., 2004, Joe et al., 2006; Turnbull and Stalford, 2012). Interestingly, the same MTX motif was found in M. kansasii ManLAM but attached to the mannan backbone rather than to the Manp caps (Guérardel et al., 2003). Finally, the arabinan chains of ManLAM from Mtb and M. bovis BCG may be substituted with lactate or succinate residues at the C-2 position of the α–(3,5)-Araf interior residues (Hunter et al., 1986, Delmas et al., 1997).
Figure 6. Structures of LM and LAM.
See text for details. MPI, mannosylated phosphatidyl-myo-inositol anchor.
Biosynthesis of PIM, LM and LAM
(a) PIM biosynthesis
Since the publication of the first genome sequence of Mtb in 1998 (Cole et al., 1998), major efforts have been committed to defining the molecular bases of the biosynthesis of apolar PIMs. Using a combination of biochemical assays, recombinant genetic approaches and structural biology, the gene products of pimA (Rv2610c in Mtb H37Rv), pimB’ (Rv2188c) and Rv2611c were defined as the enzymes involved in the cytoplasmic synthesis of apolar forms of PIMs (PIM1, PIM2, Ac1PIM1, Ac1PIM2, Ac2PIM1, Ac2PIM2) (for a review, Guerin et al., 2010) [Fig. 7]. Work from our laboratory defined the first mannosylation step involved in the biosynthesis of PIMs; we showed that the mannosyltransferase (ManT) PimA transfers a Manp residue from GDP-Manp to the C-2 position of the myo-inositol ring of PI to form PIM1 on the cytosolic face of the plasma membrane (Korduláková et al., 2002; Guerin et al., 2009a). PimA is an essential enzyme in M. smegmatis and Mtb. Several structures of PimA under its apo form and in complex with GDP and GDP-Manp have been reported and its peripheral interactions with the plasma membrane and conformational changes undergone during catalysis deciphered (Guerin et al., 2007; Guerin et al., 2009b; Giganti et al., 2013; Abesa-Jove et al., 2014). The second ManT of the pathway is a GDP-Manp-dependent ManT named PimB’ (Rv2188c in Mtb H37Rv) that transfers a single Manp residue to the C-6 position of the myo-inositol ring of PIM1 to form PIM2 (Lea-Smith et al., 2008; Guerin et al., 2009a). Unlike M. smegmatis pimB’ knock-out mutants, pimB’ null mutants of C. glutamicum were found to be viable despite their loss of ability to produce PIM2, LM and LAM (Lea-Smith et al., 2008, Mishra et al., 2008a; Guerin et al., 2009a). Crystal structures of PimB’ from C. glutamicum in complex with GDP and GDP-Man were reported (Batt et al., 2010). The attachment of an acyl chain to the C-6 position of the Manp residue linked to C-2 of myo-inositol in PIM1 and PIM2 is catalyzed by an acyltransferase encoded by Rv2611c in Mtb H37Rv (Korduláková et al., 2003). Rv2611c is an essential enzyme in Mtb (Barilone et al., unpublished results). Its disruption is achievable in M. smegmatis but leads to severe growth defects (Korduláková et al., 2003). Assays using purified PimA and PimB’ proteins indicated that Rv2611c favors PIM2 over PIM1 as a substrate (Guerin et al., 2009a). PimC (RvD2-ORF1 in Mtb CDC1551), a non-essential GDP-Manp-dependent ManT present in only some Mtb isolates, catalyzes the formation of Ac1PIM3 from Ac1PIM2 (Kremer et al., 2002). The identity of the analogous PimC enzyme in Mtb isolates lacking an ortholog of RvD2-ORF1 is at present not known and nor is that of the acyltransferase catalyzing the transfer of an acyl group to C-3 of the myo-inositol ring. Assuming that the third conserved and possibly essential ManT of the PIM pathway is also a GDP-Manp-utilizing ManT, it is likely that the synthesis of Ac1PIM3 (and perhaps Ac2PIM3) is completed on the cytoplasmic face of the plasma membrane. Once synthesized, these PIM products are thought to be flipped to the periplasmic face of the plasma membrane by an as yet unknown transporter in order to serve as substrates for further integral membrane ManTs of the GT-C superfamily reliant on polyprenyl (C35/C50)-monophospho-mannose rather than GDP-Manp as the Manp donor (Berg et al., 2007). Heptaprenyl- and decaprenyl-monophospho-mannose (Dec-P-Man) are synthesized from GDP-Manp and polyprenyl phosphates by the polyprenol monophospho-mannose synthase encoded by ppm1 (Rv2051c) in Mtb (Gurcha et al., 2002). Ppm1 is an essential enzyme in both Mtb and M. smegmatis (Zhang et al., 2012; Rana et al., 2012). The ManT (PimD) responsible for the formation of tetra-mannosylated forms of PIMs from Ac1PIM3/Ac2PIM3 is not known. The addition of two α-(1,2)-linked Manp residues to Ac1PIM4/Ac2PIM4 leads to the synthesis of higher order forms of PIMs commonly referred to as “polar PIMs” [Fig. 5]. PimE was identified as the Dec-P-Man-dependent α-(1,2) ManT responsible for the formation of Ac1PIM5/Ac2PIM5 from Ac1PIM4/Ac2PIM4 (Morita et al., 2006) [Fig. 7]. Whether this enzyme can also transfer a second α-(1,2)-linked Manp to Ac1PIM5/Ac2PIM5 to form Ac1PIM6/Ac2PIM6, the end products of the PIM pathway, or whether another enzyme participates in this process is currently not known but our preliminary enzyme assays with purified PimE favor the second hypothesis (Larrouy-Maumus et al., unpublished results).
Figure 7. Schematic diagram of PIM, LM and LAM biosynthesis.
The biosynthesis of PIM, LM and LAM is initiated on the cytoplasmic side of the plasma membrane by GDP-Manp-utilizing ManTs that catalyze attachment of mannosyl residues to the myo-inositol ring of PI. Di- or tri-mannosylated forms of PIMs are then flipped to the periplamic face of the membrane where they undergo further elongation catalyzed by integral membrane polyprenyl-monophospho-mannose-dependent ManTs and β-D-arabinofuranosyl-1-monophosphoryl-decaprenol (DPA)-dependent AraTs to generate polar forms of PIMs, LM, and ManLAM. See text for details.
(b) Biosynthesis of LM and LAM
Ac1PIM4/Ac2PIM4 appear to be the last common intermediates in the biosynthesis of PIM, LM and LAM. Extension of this subpopulation of PIMs with chains of α-(1,6)-linked Manp and further modification with α-(1,2)-monomannose side chains lead to the formation of LM [Fig. 7]. LpqW (Rv1166) is a putative lipoprotein involved in regulating access of Ac1PIM4/Ac2PIM4 to either PimE (to form polar PIMs) or the α-(1,6) ManTs responsible for the elongation of LM. An M. smegmatis lpqW knock-out mutant produced wild-type forms of PIMs but had a reduced capacity to synthesize LM and LAM (Kovacevic et al., 2006). This mutant was found to be unstable and to accumulate secondary mutations in pimE that resulted in a block in the synthesis of polar PIMs and restored synthesis of LM and LAM (Crellin et al., 2008). Determination of the three dimensional structure of LpqW from M. smegmatis revealed the existence of a putative Ac1PIM4 binding site (Marland et al., 2006) suggesting that this protein may function as a glycolipid chaperone, regulating the access of ManTs to this substrate. Recent genetic and enzymatic studies conducted on an lpqW (NCgl1054) knock-out mutant of C. glutamicum now suggest that LpqW may in fact regulate the activity of the α-(1,6) ManT, Cg-MptB, involved in the initial steps of the elongation of LM from tetramannosylated PIMs (Rainczuk et al., 2012).
The extension of Ac1PIM4/Ac2PIM4 by Dec-P-Man-dependent α-(1,6) ManTs on the periplasmic face of the plasma membrane leads to the biosynthesis of LM. The first committed enzyme in this process was identified in C. glutamicum as Cg-MptB (NClg1505) (Mishra et al., 2008b) but the corresponding enzyme in Mtb has not yet been identified. Indeed, the closest Mtb H37Rv ortholog, Rv1459c (which shares about 35% identity with Cg-MptB), failed to complement a C. glutamicum mptB knock-out mutant and disruption of the orthologous gene in M. smegmatis (MSMEG_3120) had no effect on LM and LAM biosynthesis. The GT-C glycosyltransferase Rv1459c thus appears to have a distinct, albeit as yet unknown, function in Mtb. MptA (Rv2174 in Mtb H37Rv) was characterized as a GT-C superfamily Dec-P-Man-dependent α-(1,6) ManT responsible for the elongation of the mannan backbone of LM in mycobacteria and corynebacteria (Kaur et al., 2007; Mishra et al., 2007). Disruption of mptA (MSMG_4241) in M. smegmatis leads to a phenotype marked by a virtual absence of LM and LAM and a build-up of truncated forms of LM in the mutant strain with only 5 to 20 Manp residues as compared to wild-type LM consisting of 21-34 Manp residues, but with only few changes in the branching pattern (Kaur et al., 2007). The Dec-P-Man-dependent α-(1,2) ManT involved in the branching of LM was identified as Rv2181 (MSMEG_4247 in M. smegmatis) (Kaur et al., 2006; Kaur et al., 2008). Interestingly, while the disruption of Rv2181 in Mtb abrogates the monomannose branching of the mannan backbone of LM and LAM (Kaur et al., 2008), knocking-out the orthologous gene in M. smegmatis leads to a mutant unable to synthesize LM and producing a shorter LAM devoid of α-(1,2) branches (Kaur et al., 2006). By modulating the level of expression of MSMEG_4241 (mptA) and MSMEG_4247 in M. smegmatis, it was shown that the elongation and branching of the mannan backbone of LM and LAM are tightly coordinated, with the overexpression of MSMEG_4247 leading to the synthesis of dwarfed LM and LAM presenting a shorter mannan backbone as well as a significantly smaller arabinan domain in the case of LAM (Sena et al., 2010). Our recent structural analyses of the dwarfed LAM produced by a M. smegmatis MSMEG_4247 knock-out strain (Kaur et al., 2006) revealed a single arabinosylation site on the mannan backbone (D. Kaur et al., manuscript in preparation). The priming arabinosyltransferase (AraT) responsible for the transfer of the first Araf residue of LAM has not yet been identified although we were able to detect a matching enzymatic activity in cell-free extracts prepared from M. smegmatis (S. K. Angala et al., manuscript in preparation). Dec-P-Ara being the only known Araf donor in mycobacteria (Wolucka et al., 1994), it is expected that all of the arabinosylation of LAM, like that of AG, takes place on the periplasmic side of the plasma membrane catalyzed by integral membrane AraTs of the GT-C superfamily of glycosyltransferases (Berg et al., 2007) [Fig. 7]. One of these enzymes, known as EmbC, was found to be critical in this process (Zhang et al., 2003; Berg et al., 2005; Shi et al., 2006). EmbC is an essential enzyme in Mtb (Goude et al., 2008) where it serves as one of the targets of the TB drug EMB (Goude et al., 2009). The knock-out of embC, however, is achievable in M. smegmatis resulting in a mutant deficient in LAM synthesis (Zhang et al., 2003). EmbC is predicted to carry 13 transmembrane spanning helices followed by an hydrophilic extracytoplasmic carbohydrate-binding C-terminal domain. Like EmbA and EmbB involved in AG biosynthesis, EmbC harbors a proline-rich motif homologous to that of bacterial polysaccharide co-polymerases (Berg et al., 2007). Consistently, biochemical analyses of M. smegmatis recombinant strains expressing truncated and point-mutated variants of EmbC indicated that this protein is most likely multi-functional possessing polymerization and chain length regulating functions in addition to AraT activity (Berg et al., 2005; Shi et al., 2006). The C-terminal domain of EmbC was recently co-crystallized with a synthetic di-arabinoside acceptor substrate (Alderwick et al., 2011b). Another critical AraT in the biosynthesis of LAM is the α-(1,3)-branching AraT AftC. Disruption of aftC in M. smegmatis results in a mutant producing a truncated form of LAM whose arabinan domain is devoid of (3,5)-Araf-branching residues (Birch et al., 2010). Thus, AftC participates in the branching of the arabinan domains of both AG and LAM (Birch et al., 2008). Evidence based on the analysis of the cell envelope content of M. smegmatis recombinant strains expressing different levels of aftD (Rv0236c) suggests that this essential GT-C enzyme, which displays α-(1,3)-branching AraT activity on synthetic α-(1,5) arabinosyl acceptors in vitro, may also participate in the synthesis of the arabinan domains of both AG and LAM although its precise function remains to be determined (Škovierová et al., 2009). The presence of two to three Araf residues attached to the mannan backbone of LM in the embC knock-out mutant of M. smegmatis supports the existence of a “priming” AraT activity independent of EmbC (Zhang et al., 2003); the corresponding enzyme has not yet been identified.
The ManT responsible for transferring the first Manp residue of the mannoside caps of ManLAM to the non-reducing termini of the arabinan domain was identified as Rv1635c (also known as CapA) (Dinadayala et al., 2006). The further elongation of the mannoside cap with at least one Manp residue requires the promiscuous α-(1,2) ManT Rv2181 which is also responsible for the monomannoside branching of LM (Kaur et al., 2008) [Fig. 7]. The enzymes required for the biosynthesis and transfer of a MTX motif to the t-Manp residue of the mannoside caps of ManLAM have not yet been identified although biosynthetic models have recently been proposed (Turnbull and Stalford, 2012). Likewise, the biosynthetic origin of the succinyl residues linked to the arabinan domain of LAM remains to be determined.
Regulatory mechanisms
As illustrated above, the biosynthetic steps leading to the formation of PIMs, LM and LAM are highly complex and tightly coordinated to ensure the production of appropriate levels of fully elaborated molecules. Although our current understanding of PIM/LM/LAM biosynthesis suggests the existence of multiple points of controls of these pathways, our knowledge of the regulatory mechanisms involved is still limited. EmbR, a protein homologous to the OmpR class of transcriptional regulators, was implicated in the positive regulation of the embCAB operon and LM/LAM biosynthesis (Belanger et al., 1996; Sharma et al., 2006a; Alderwick et al., 2006c). EmbR is phosphorylated by the Ser/Thr kinase PknH, enhancing its binding to the promoter region of embCAB (Molle et al., 2003). EmbR is also phosphorylated by the Ser/Thr kinases PknA, PknB and PknJ and is dephosphorylated by the Ser/Thr phosphatase PstP (Sharma et al., 2006b; Molle & Kremer, 2010; Jang et al., 2010). Beyond these transcriptional and post-translational aspects, the compartmentalization of the PIM, LM and LAM pathways is expected to play an important role in regulating the access of enzymes to their substrates (Morita et al., 2011). Finally, elegant work on LpqW has highlighted the critical role of this protein in regulating the activity of the ManTs acting at the juncture of the polar PIM and LM elongation pathways (Kovacevic et al., 2006; Crellin et al., 2008; Rainczuk et al., 2012).
Topology of the PIM/LM/LAM biosynthetic pathway
As in the case of AG, the fundamentals of how the different domains of LAM are assembled and exported are at present not fully understood. As outlined in the preceding sections, the early steps of PIM biosynthesis take place on the cytosolic face of the plasma membrane until di- or tri-mannosylated forms of PIMs are translocated across the plasma membrane to serve as substrates for further mannosylation reactions catalyzed by PimE and other GT-C polyprenyl-phosphate mannose-dependent glycosyltransferases [Fig. 7]. Since the unassisted transbilayer movement of polar (glyco)lipids across the plasma membrane is energetically unfavorable (Daleke et al., 2007; Sanyal et al., 2008) such a compartmentalization implies that an as yet unknown translocase (or “flippase”) translocates PIM intermediates from the cytoplasmic to the periplasmic side of the plasma membrane. Evidence to date then points to LM and LAM elongation proceeding through the sequential addition of mannosyl and arabinosyl residues to a PIM4 substrate from the reducing towards the non-reducing end on the periplasmic face of the plasma membrane. Our recent studies have established the SMR-like transporter Rv3789 as a likely Dec-P-Ara translocase required for the optimal arabinosylation of AG and LAM (Larrouy-Maumus et al., 2012) (see Arabinogalactan section). Finally, the export of PIM, LM and LAM from the inner membrane to the outer membrane and cell surface most likely requires dedicated translocation machineries but none of the components of this(ese) machineries have yet been formally identified. Evidence based on physical interactions and co-crystallography suggests that the lipoprotein LprG (Rv1411c) which shares structural resemblance to LppX, a lipoprotein thought to carry phthiocerol dimycocerosates (PDIM) across the periplasm (Sulzenbacher et al., 2006), may participate in the transport of PIM, LM and LAM to the cell surface (Drage et al., 2010) [Fig. 7]. Further biochemical studies are required, however, to confirm the involvement of LprG in this process and precisely delineate its substrate specificity.
Physiological functions and biological activities of PIM, LM and LAM
PI and PIMs make up as much as 56% of all phospholipids in the cell wall and 37% of those in the plasma membrane of M. bovis BCG (Goren, 1984) and are thus important structural components of the mycobacterial cell envelope. Emerging data indicate that PIM not only play important roles in the permeability of the cell envelope but also in inner membrane integrity and regulation of cell septation and division (Parish et al., 1997; Korduláková et al., 2002; Patterson et al., 2003; Morita et al., 2005; Morita et al., 2006). The dramatic changes in β-lactam susceptibility and acid-fast staining properties of mycobacterial cells that accompany structural defects in LM and ManLAM indicate that these lipoglycans play equally important roles in cell envelope integrity, also impacting the pathogenicity of Mtb (Fukuda et al., 2013). The amount of higher order PIMs (PIM5-PIM6) recovered from M. smegmatis cells increases with the age of the culture, apparently at the expense of the apolar forms (PIM1 –PIM4) and LAM, the synthesis of which was shown to decrease in M. smegmatis as the bacilli approached stationary phase (Penumarti and Khuller, 1983; Morita et al., 2005; Dhiman et al., 2011). Important changes affecting the amounts and structures of PIMs and LAM were also reported to occur in Mtb during in vitro growth (Yang et al., 2013). The physiological significance of these changes is not known.
In addition to their physiological and structural roles, a substantial number of biological activities have been associated with PIMs, LM and LAM. These have been the object of several reviews (Briken et al., 2004; Gilleron et al., 2008; Torrelles and Schlesinger, 2010; Mishra et al., 2011; Neyrolles and Guilhot, 2011) and we will only summarize here some of the most significant findings that have occurred in the field in the last couple of years. The contribution of PIMs, LM and LAM to the infection process accompanies virtually every step of the lifecycle of Mtb inside the host. The fact that even subtle variations in the structures of these molecules (including their degree of acylation and mannan branching, the lengths of the mannan and arabinan chains, and the nature of the substituents capping the non-reducing end of the arabinan domain) dramatically impact their biological activities (Gilleron et al., 2001; Gilleron et al., 2006; Gilleron et al., 2008; Nigou et al., 2008; Torrelles and Schlesinger, 2010; Mishra et al., 2011; Stoop et al., 2013) suggests that they are probably important modulators of host-pathogen interactions in the course of infection. The mannoside caps of ManLAM bind to the mannose receptor (MR) thereby contributing to the phagocytosis of Mtb by human macrophages. The mannoside caps of ManLAM also bind to the C-type lectin DC-SIGN present on dendritic cells (DCs) resulting in anti-inflammatory effects that have been proposed to contribute to the immune evasion of Mtb. LM, in contrast, associates with DC-SIGN but not MR. Contrary to earlier impressions that ManLAM dominated the interactions of Mtb with antigen-presenting cells, however, Mtb and M. bovis BCG mutants deficient in the mannose-capping of LAM showed no impairment in DC-SIGN binding, interactions with macrophages in vitro, virulence in mice and immunogenicity (Appelmelk et al., 2008; Afonso-Barroso et al., 2012). The presence of multiple other MR and DC-SIGN ligands at the cell surface of Mtb, including glycoproteins (see Glycoproteins section), polar forms of PIMs (PIM5-PIM6) and capsular polysaccharides (arabinomannan, mannan) (see Capsular Polysaccharides section) sharing with ManLAM terminal α–(1,2)-linked oligomannoside appendages, is thought to account for this absence of phenotype. Polar forms of PIMs in particular have been shown to participate in DC-SIGN binding (Torrelles et al., 2006; Driessen et al., 2009) and phagocytosis events through the MR limiting phagosome-lysosome fusion. In addition to C-type lectins, Mtb interacts with Toll-like receptors (TLR); LM and, to a lesser extent, ManLAM have been shown to be potent TLR-2 ligands (Nigou et al., 2008). Once inside phagocytic cells, Mtb resides in a phagosome that fails to fuse with lysosomes. The ability of ManLAM and/or derived products to intercalate within host cell membranes was proposed as a possible mechanism through which these molecules may impair phagosome maturation (Welin et al., 2008). Inside the cells, Mtb releases significant amounts of cell envelope components among which PIMs, LM and ManLAM that traffic within the cells and may be released through exocytosis (Russell, 2011). These molecules may not only be taken up by bystander antigen-presenting cells, they also act as modulators of the functions of the host cell and surrounding tissue required for granuloma formation and protection. ManLAM for instance is known to negatively modulate the production of nitric oxide, oxygen radicals and inflammatory cytokines by macrophages and DCs, inhibit Mtb-induced apoptosis, and interfere with signaling pathways of T-lymphocytes affecting cytokine production (Shabaana et al., 2005) and cell migration (Richmond et al., 2012). Thus, whereas PI-LAM is generally considered to induce pro-inflammatory responses, ManLAM has anti-inflammatory effects. LM, on the other hand, induces apoptosis and a pro-inflammatory response through TLR-2. Preliminary studies employing purified MTX and MTX-Man disaccharide have begun to investigate the possible biological roles of the α-D-methylthioxylofuranosyl (MTX) substituent of ManLAM. It was found that MTX-Man has immunomodulatory properties inhibiting the production of TNF-α and IL-12p70 by activated human THP-1 monocytes (Joe et al., 2006; Turnbull and Stalford, 2012). MTX and its sulfinyl analog (α-D-methyl-sulfinyl-xylofuranosyl; MSX), on the other hand, have the ability to sequester hydroxyl radicals thereby potentially promoting the intracellular survival of Mtb (Turnbull and Stalford, 2012). The extent to which these properties associated to the MTX and MSX substituents of LAM impact the pathogenesis of Mtb remains to be determined; such experiments will have to await the construction of isogenic mutants deficient in the biosynthesis of these motifs. As in the case of AG, the biological significance of the succinyl residues esterifying some of the interior branched (3,5)-Araf residues of LAM (Delmas et al., 1997) is not known. Finally, in addition to their involvement in innate immune mechanisms, PIMs, LM and ManLAM are also recognized as antigens by the adaptative immune system upon presentation to T-lymphocytes by MHC-I-like molecules of the CD1 family. Biochemical and structural studies have begun to elucidate the molecular basis of their processing and presentation (Porcelli and Modlin, 1999; Fischer et al., 2004; de la Salle et al., 2005; Torrelles et al., 2004; Torrelles et al., 2011; Garcia-Alles et al., 2011; Torrelles et al., 2012; Cala-De Paepe et al., 2012).
In spite of their astonishing biological activities in vitro, the precise contribution of PIMs, LM and LAM to TB pathogenesis when carried by whole Mtb bacilli is far from being clear. The essentiality of much of the PIM/LM/LAM pathway for Mtb growth limits the number of informative isogenic mutants that can be generated for cellular and in vivo studies. Moreover, with the exception of one mutant dramatically affected in its cell envelope integrity (Fukuda et al., 2013), the few Mtb PIM/LM/LAM recombinant strains that have constructed thus far failed to significant differ from their wild-type parent strain in terms of interactions with host cells, virulence or immunogenicity (Driessen et al., 2009; Afonso-Barroso et al., 2012). The presence of multiple glycoconjugates with partially overlapping activities at the surface of Mtb, the genetic diversity of Mtb isolates and the existence of regulatory mechanisms affecting the production of these compounds render the precise delineation of the roles of PIMs, LM and LAM in host-pathogen interactions extremely complex (Pitarque et al., 2005; Appelmelk et al., 2008; Driessen et al., 2009; Torrelles and Schelsinger, 2010; Afonso-Barroso et al., 2012).
PIM, LM and LAM biosynthesis in the context of drug discovery and biomarker development
The essential character of PIMs, LM and LAM, their restricted distribution to mycobacteria and closely-related Actinomycetes, and demonstrated impact on the structure and permeability of the cell envelope of Mtb make the biosynthetic enzymes of these molecules attractive candidates for the development of specific Mtb inhibitors with the potential to synergize with or potentiate the activity of other drugs used in combination. Accordingly, target-to-drug approaches are pursuing various essential enzymes acting at early (e.g., PimA, PimB’) or late (e.g., Emb proteins and other lipid-linked sugar-utilizing glycosyltransferases) stages of the pathway (Zhang et al., 2010; Zhang et al., 2011). The most advanced LAM inhibitors to date (apart from EMB) are those targeting Dec-P-Ara synthesis as described earlier (see AG section). Beyond these therapeutic applications, LAM is also actively being pursued as a potential biomarker to monitor TB infection as well as the efficacy of treatments and vaccination (Wallis, 2013). Finally, the potent bioactivity of the α–(1,2)-linked oligomannoside appendages found in ManLAM, mannoproteins, polar forms of PIMs and capsular polysaccharides has stimulated innovative approaches toward the development of synthetic immunomodulators for the treatment of lung inflammatory diseases (Blattes et al., 2013).
Acyltrehaloses
Trehalose and acyltrehaloses in Mtb
Trehalose is a non-reducing disaccharide of glucose (1-O-α-D-glucopyranosyl-α-D-glucopyranoside) found in bacteria, yeast, fungi, plants and invertebrates, but not in mammalian cells. In mycobacteria, it serves as biosynthetic precursor for a range of glycolipids that populate both the inner and outer membranes of the cell envelope. The acyltrehaloses found in the cell envelope of Mtb include trehalose monomycolates (TMM), trehalose dimycolates (TDM), sulfolipids (SL), diacyltrehaloses (DAT), triacyltrehaloses (TAT) and polyacyltrehaloses (PAT) [Fig. 8]. In addition, M. canettii, a representative of smooth tubercle bacilli that seems to have originated from the same pool of ancestors as Mtb but rarely causes human disease (Supply et al., 2013) produces trehalose-based lipooligosaccharides (LOS) (Daffé et al., 1991). A characteristic feature of DAT, TAT, PAT and LOS is the presence of long-chain multi-methyl branched fatty acids esterifying the trehalose moiety [Fig. 8]. These long chain methyl-branched fatty acids are produced by multifunctional polyketide synthases similar to the type I multienzyme fatty acid synthase (FAS-I) of eukaryotes. However, unlike FAS-I, these polyketide synthases preferentially use methyl-malonyl-CoA instead of malonyl-CoA for fatty acid elongation, thereby introducing methyl branches into fatty acyl chains.
Figure 8. Structures of the acyltrehaloses of Mtb.
In TMM and TDM, trehalose is here shown esterified with alpha-mycolic acid chains. In SL-I (2,3,6,6’-tetraacyl α–α’-trehalose-2’-sulfate), trehalose is sulfated at the 2’ position and esterified with palmitic acid and the multimethyl-branched phthioceranic and hydroxyphthioceranic acids. In DAT (2,3-di-O-acyltrehalose), trehalose is esterified with palmitic acid and the multimethyl-branched mycosanoic acid. In PAT (penta-acyltrehalose), trehalose is esterified with stearic acid and the multimethyl-branched mycolipenic acids. The oligosaccharide of the LOS of Mtb Canettii strains consists of 2-O-methyl-α-L-Fucp-(1,3)-β–D-Glcp-(1,3)-2-O-methyl-α-L-Rhap-(1,3)-2-O-methyl-L-Rhap-(1,3)-β-D-Glcp-(1,3)-4-O-methyl-α-L-Rhap-(1,3)-6-O-methyl-α-D-Glc-(1,1)-α-D-Glc. R are 2,4-dimethylhexadecanoic acid and 2,4,6,8-tetramethyloctadecanoic acid residues.
Trehalose biosynthesis
Three different pathways have been described for the biosynthesis of trehalose. Most prokaryotes rely on the OtsA-OtsB pathway wherein OtsA is a trehalose-6-phosphate synthase catalyzing the condensation of glucose-6-phosphate and UDP-glucose to form trehalose-6-phosphate, and OtsB is a dephosphorylase releasing free trehalose from trehalose-6-phosphate (Kaasen et al., 1992). An alternative pathway that generates trehalose from glycogen has been identified in Arthrobacter, Rhizobium and Sulfolobus acidocaldarius. This pathway involves the TreY-TreZ enzymes in which the terminal α–(1,4)-linked residue of the glucose polymer is converted to an α–(1,1) linkage by the maltooligosyltrehalose synthase TreY. The terminal disaccharide is then cleaved by the hydrolase enzyme TreZ releasing trehalose (Maruta et al., 1996a, Maruta et al., 1996b, Maruta et al., 1996c). Finally, a third pathway was found in Pimelobacter and Arthrobacter in which the trehalose synthase TreS catalyzes the reversible isomerization of the α-(1,4) linkage of maltose to the α-(1,1) linkage of trehalose (Nishimoto et al., 1996, Nakada et al., 1995). The Mycobacterium genus is unique in possessing all three pathways for the synthesis of trehalose (De Smet et al., 2000). While the three pathways are functionally redundant in M. smegmatis (Woodruff et al., 2004), the OtsAB pathway was found to be predominant in Mtb (Murphy et al., 2005). Disruption of otsA (Rv3490) in Mtb resulted in growth defects both in vitro and in vivo and otsB2 (Rv3372) was demonstrated to be an essential gene of Mtb (Murphy et al., 2005). Furthermore, recent genetic and biochemical evidence (Kalscheuer et al., 2010; Miah et al., 2013) supported by structural data (Caner et al., 2013) indicates that TreS predominantly functions in the reverse orientation in M. smegmatis and Mtb, catalyzing the formation of maltose from trehalose which is then used in the biosynthesis of α-(1,4)-glucans (Kalscheuer et al., 2010) (see Capsular Polysaccharides section).
Trehalose monomycolates (TMM) and trehalose dimycolates (TDM)
These two glycolipids are produced by all mycobacterial species examined to date. In TMM, trehalose is esterified at the 6-position by a mycolic acid chain while TDM, also known as “cord factor”, is esterified at the 6- and 6’-positions by two mycolic acid chains [Fig. 8]. As indicated above, mycolic acids are long-chain (C60-C90) α-alkyl-β-hydroxy- fatty acids and are essential components of the mycobacterial outer membrane [Fig. 1]. The structure and biosynthesis of mycolic acids has been reviewed elsewhere (Barry et al., 1998; Takayama et al., 2005; Marrakchi et al., 2008). Upon elongation, modification and assembling in the cytosol, the completed mycolic acid chains are transferred to the 6- and 6’-positions of trehalose through an as yet unknown mechanism, generating TDM. TMM was recently shown to be the form under which mycolic acids are exported to the cell envelope in a process involving the integral membrane Resistance-Nodulation and Division (RND) superfamily transporter, MmpL3 (Grzegorzewicz et al., 2012). The genetic or chemical inactivation of MmpL3 causes the arrest of TMM translocation to the cell surface and cell death. Intriguingly, MmpL3 was identified as the target of a number of small molecules inhibitors with activity against Mtb bacilli in culture, including the TB drug candidate SQ109, pointing to the chemical vulnerability of this critical step of the formation of their OM (Grzegorzewicz et al., 2012; Stanley et al., 2012; La Rosa et al., 2012; Remuinan et al., 2013; Ioerger et al., 2013; Poce et al., 2013; Konddredi et al., 2013; Onajole et al., 2013; Rao et al., 2013). The precise role of MmpL3 in TMM export, whether required to translocate TMM across the plasma membrane (“flippase” activity) or to carry TMM from the outer leaflet of the plasma membrane to the periplasmic space or OM (intermembrane transport) remains to be determined. Based on what is known of the transport mechanism of RND transporters in Gram-negative bacteria (Paulsen et al., 1996; Tseng et al., 1999; Murakami, 2008), the hypothesis of an intermembrane translocation is favored. Either way, the complexity of translocating TMM across the different layers of the cell envelope suggests that MmpL3 probably functions with other membrane proteins, periplasmic adapters, lipoproteins and/or OM proteins to deliver TMM in the vicinity of the OM where the mycolic acyl chain carried by this glycolipid can then be transferred to another molecule of TMM to form TDM, or to AG. Identifying these other components of the TMM translocation machinery and understanding the substrate specificity and mechanism of transport of MmpL3 are just some of the gaps in our knowledge of the building of the mycobacterial OM that need to be addressed. The transfer of mycolic acids from TMM onto the non-reducing termini of the arabinan chains of AG or onto TDM is catalyzed by the mycolyltransferases of the Ag85 family (Ag85A, Ag85B and Ag85C). These enzymes appear to have partially redundant functions and, consistently, their individual genetic inactivation has no impact on the viability of Mtb (Jackson et al., 1999; Armitige et al., 2000; Puech et al., 2002). The reactions catalyzed by the Ag85 family of enzymes result in the periplasmic release of free trehalose as a by-product. The ABC transporter LpqY-SugABC recycles trehalose back to the cytoplasm in Mtb (Kalscheuer et al., 2011).
TDM has long been associated with the virulence of Mtb. It was first noticed in the 1950s that Mtb isolates subjected to surface lipid extraction, while retaining viability, became avirulent and unable to form cords (Bloch, 1950). This fraction was named “cord factor” and later shown to be composed primarily of TDM (Noll et al., 1956). A number of biological activities have been attributed to TDM over the years, as this glycolipid seems to be a major contributor of the inflammation seen in the course of mycobacterial infections. Purified TDM can by itself induce lesions characterized by chronic granulomatous in?ammation in mice and rabbits (Hamasaki et al., 2000; Sakaguchi et al., 2000). TDM also contributes to the protection of Mtb from killing by macrophages, is a potent modulator of the activation of macrophages and increases the resistance of mycobacteria to antibiotics (Silva et al., 1985; Katti et al., 2008; Axelrod et al., 2008; Indrigo et al., 2002, Indrigo et al., 2003). The study of defined Mtb knock-out mutants deficient in the modification of mycolic acids with cyclopropane rings and oxygenated functions has provided evidence of the impact of the fine structures of the mycolyl substituents of TDM on the biological activities of this glycolipid (Rao et al., 2005; Linares et al., 2012). Only recently have TDM receptors been identified at the surface of macrophages. The inducible C-type lectin Mincle (also called Clec4e) recognizes TDM but not other mycobacterial glycolipids such as PIM, LM or LAM (Ishikawa et al., 2009); moreover, the binding of TDM to Mincle is required for activation of macrophages and granuloma formation in mice (Ishikawa et al., 2009; Lang, 2013). Mincle was shown to be essential for inflammation in vivo when purified TDM or heat-killed Mtb was administered to mice (Ishikawa et al., 2009; Schoenen et al., 2010). Nevertheless, Mtb-infected Mincle-deficient mice did not differ from control mice in their inflammatory response or ability to control the infection (Heitmann et al., 2013) suggestive of the existence of (an)other TDM receptor(s) at the surface of antigen-presenting cells. The C-type lectin MCL (also called Clec4d) which is thought to have arisen from gene duplication of Mincle (Miyake et al., 2013) was recently identified as another TDM receptor. In contrast to Mincle, MCL is constitutively expressed in myeloid cells and is required for the development of TDM-induced acquired immune responses in mouse. TDM-induced granuloma formation is also severely impaired in MCL-deficient mice. Importantly, MCL appears to play a critical role in the induction of Mincle following TDM stimulation (Miyake et al., 2013).
Sulfolipids (SL)
Sulfolipids are a family of polyacylated trehalose-2-sulfate glycolipids esterified with two to four acyl chains (for reviews, Goren and Brennan, 1979; Goren, 1990; Bertozzi and Schelle, 2008). The major SL form found in Mtb is sulfolipid-1 (SL-1), a tetra-acylated glycolipid with a middle-chain saturated fatty acyl chain (palmitic or stearic acid) on the 2’-position of trehalose and different combinations of the hepta- and octa-methyl-branched phthioceranic or hydroxyphthioceranic acids (C31 to C46) on 3’-, 6- and 6’-positions [Fig. 8]. Monomethyl-branched unsaturated C16 to C20 fatty acids have also been found as minor constituents of SL (Dubey et al., 2003). This family of lipids is specific to Mtb.
The first committed step in SL biosynthesis is the transfer of a sulfate group to the 2-position of trehalose in a reaction catalyzed by the sulfotransferase Stf0 (Mougous et al., 2004). The acyltransferase PapA2 then transfers a palmitoyl or stearoyl chain from palmitoyl- or stearoyl-CoA on the 2’-position of trehalose yielding a monoacylated SL (Kumar et al., 2007; Bhatt et al., 2007). The polyketide synthase Pks2 is responsible for the synthesis of phthioceranates and hydroxyphthioceranates using an activated fatty acid starter unit provided by the fatty acyl-AMP-ligase FadD23 (Sirakova et al., 2001; Lynett and Stokes, 2007). Upon elongation by Pks2, a (hydroxy)phthioceranyl chain is then transferred to the 3’-position of trehalose by the acyltransferase PapA1, yielding a diacylated SL also known as SL1278 (Kumar et al., 2007; Bhatt et al., 2007). It is thought that PapA1 directly transfers (hydroxy)phthioceranoyl groups from Pks2 and that the acylated Pks2 acyl carrier protein domain is its substrate (Kumar et al., 2007). The last two acylations on the 6- and 6’-positions of trehalose are catalyzed by Chp1, a cutinase-like protein anchored in the plasma membrane with its catalytic domain facing the cytoplasm (Seeliger et al., 2012). Unlike PapA1 and PapA2, Chp1 does not use an activated thioester acyl donor, but rather catalyzes regioselective transacylations between two SL1278 molecules to generate the mature tetra-acylated SL-1. This mechanism is reminiscent of the one utilized by the Ag85 mycoloyltransferases for the synthesis of TDM from two molecules of TMM. Finally, gene knock-out studies indicated that the polyketide synthase encoded by pks8+pks17 is responsible for the production of the monomethyl-branched unsaturated C16 to C20 fatty acids found in some forms of SL (Dubey et al., 2003).
The fadD23, papA1, papA2 and chp1 genes cluster on the Mtb chromosome [Fig. 9]. Interspersed between these genes are two more ORFs encoding integral membrane proteins, mmpL8 and sap. MmpL8, like MmpL3 involved in the translocation of TMM (see above), is an RND superfamily transporter. Sap (Sulfolipid-1-Addressing Protein) appears to facilitate the translocation of SL-1 to the cell surface. Its disruption in Mtb causes the intracellular build-up of SL1278 although the mutant is still capable of synthesizing small amounts of SL-1 (Seeliger et al., 2012). Similar to the sap mutant, an mmpL8 knock-out strain was shown to accumulate SL1278 intracellularly and to fail to export SL-I or SL1278 the cell surface (Seeliger et al., 2012). The accumulation of SL1278 precursor in the mmpL8 mutant suggests that the presence of MmpL8 in the membrane is required for Chp1 to complete the acylation of SL-I. It was proposed that MmpL8 may serve as a scaffold for the coupled synthesis and transport of SL (Seeliger et al., 2012) [Fig. 10]. The recent isolation of a protein complex made of Pks2, MmpL8, PapA1 and FadD23 from membrane preparations of M. bovis BCG provides strong support for this model (Zheng et al., 2011). While it is expected that the translocation of SL-1 to the cell surface, similar to the situation with TMM, requires additional inner membrane, periplasmic and/or OM transporters, the identity of these protein is at presently not known.
Figure 9.

A schematic representation of the SL, DAT/PAT and LOS biosynthetic gene clusters of Mtb H37Rv (SL; DAT/PAT) and Mtb canettii (LOS).
Figure 10. Biogenesis of SL, DAT/PAT and PDIM/PGL in Mtb.
The enzymes and transporters that have been involved in the elongation, assembly and export of SL, DAT/PAT and PDIM/PGL and their localization in the bacterium are represented.
FadD enzymes are fatty acyl-AMP ligases; PapA and Chp enzymes are acyltransferases; Pks, Mas and PpsA-E are the polyketide synthases responsible for the elongation of the polymethyl-branched fatty acids found in DAT/PAT, SL and PDIM/PGL; TesA is a thioesterase; Stf0 is a sulfotransferase; Antigens 85 (Ag85) are mycolyltransferases; DrrABC is an ABC-transporter; LppX is a periplasmic lipoprotein required for the translocation of PDIM to the outer membrane; MmpL proteins are integral membrane RND superfamily transporters required for the translocation of acyltrehaloses and PDIM to the periplasmic space and outer membrane. The precise extent of (glyco)lipid translocation mediated by MmpL proteins, LppX and DrrABC has has not yet been defined. See text for further details.
The restriction of SL to Mtb has led to think that these lipids might play a role in pathogenesis. Studies by Goren more than 40 years ago, established a correlation between the presence of SL in Mtb isolates and their virulence in guinea pigs (Goren, 1974a). Several studies were carried out since then to establish the roles of SL during infection. In vitro studies using purified SL-1 have implicated this glycolipid in the prevention of phagosome-lysosome fusion, the activation of human neutrophils and the modulation of cytokine production by leukocytes (Goren et al., 1976; Pabst et al., 1988; Zhang et al., 1988; Zhang et al., 1991; Brozna et al., 1991; Goren, 1990). Despite these observations, in vivo and ex vivo studies with Mtb mutants defective in various aspects of SL biosynthesis failed to reveal any significant virulence or pathogenicity defects associated with these mutations in mice, guinea pigs and cultured macrophages (Rousseau et al., 2003a; Kumar et al., 2007). On the other hand, four studies have shown that mmpL8 knock-out mutants of Mtb which accumulates the diacylated precursor sulfolipid SL1278 are attenuated for virulence in mouse models of infection (Converse et al., 2003; Domenech et al., 2004; Domenech et al., 2005; Lamichhane et al., 2005). SL1278 and other diacylated forms of SL were recognized as CD1b-restricted T-cell antigens and studies using a panel of synthetic analogs have begun to explore their structure-function relationship (Gilleron et al., 2004; Guiard et al., 2009). Recently, Gilmore et al. (2012) provided evidence that a sft0 null mutant of Mtb, which fails to synthesize any forms of SL, survived better than its wild-type parent in human but not murine macrophages, possibly as a result of the increased resistance of this strain to human cationic antimicrobial peptides (defensins). These results suggest that SL may only have a clear impact on infection in the human host. Recent studies have highlighted the role of methyl-branched fatty acid-containing lipids such as SL, PDIM, DAT and PAT in alleviating the propionate-mediated stress undergone by Mtb when the bacterium switches to host cholesterol as a major carbon source during infection (Singh et al., 2009; Lee et al., 2013). The propionyl-CoA generated upon β-oxidation of cholesterol is converted to methylmalonyl-CoA by the propionyl-CoA carboxylase which is subsequently used by dedicated polyketide synthases such as Pks2, Mas and Pks3/4 in the elongation of the methyl-branched fatty acids found in SL, PDIM and DAT/PAT, respectively (see further sections). The regulator facilitating this metabolic switching to fatty acids was identified as WhiB3 (Singh et al., 2009).
The synthesis of SL appears to be tightly regulated during the course of infection (Graham et al., 1999; Rodriguez et al., 2013). The two-component system regulator PhoP-PhoR is required for the production of SL, DAT and PAT and a point mutation in the phoP gene of Mtb H37Ra accounts for the absence of these glycolipids from this strain (Gonzalo Asensio et al., 2006; Walters et al., 2006; Chesne-Seck et al., 2008). Consistent with these results, PhoP was found to activate the transcription of several Mtb genes including those involved in the production and transport of SL such as mmpL8, papA1 and pks2 (Walters et al., 2006; Goyal et al., 2011; Cimino et al., 2012). The regulatory protein WhiB3 also regulates the synthesis of SL, DAT, PAT and PDIM as indicated above. A whiB3 mutant of Mtb produces 3 to 10-fold less SL, DAT and PAT compared to the wild-type parental strain (Singh et al., 2009). Collectively, these studies highlight the complexity of the regulation of acyltrehalose biosynthesis in Mtb and the pleiotropic roles that these glycolipids are likely to play at various stages of the infection.
Diacyltrehaloses (DAT) and Polyacyltrehaloses (PAT)
The 2,3-di-O-acyltrehaloses (DAT) consist of trehalose esterified at the 2-position by a middle-chain saturated fatty acid (palmitic, stearic or tuberculostearic acid) and at the 3-position by a long-chain methyl-branched fatty acid [Fig. 8]. The methyl-branched fatty acids found in DAT consist of di-methyl-branched mycosanoic acids (C21-C25) and, less commonly, of tri-methyl-branched C25-C27 mycolipenic (phthienoic) and mono-hydroxylated tri-methyl-branched C24-C28 mycolipanolic acids (Minnikin et al., 1985; Lemassu et al., 1991; Besra et al., 1992). Polyacyltrehaloses (PAT) comprise a family of penta-acylated trehaloses where the trehalose moiety is esterified at the 2-position with a middle-chain saturated fatty acyl chain (palmitic, stearic or tuberculostearic acid) and at the 3-, 6-, 4’ and 6’-positions with four methyl-branched mycolipenic acids, although minor amounts of mycolipanolic acids can also be found (Minnikin et al., 1985; Daffé et al., 1988). In addition, the presence of 2,3,6-triacyltrehaloses (TAT) has been reported in Mtb. In TAT, trehalose is esterified by two middle-chain saturated fatty acids and one mycolipenic or mycolipanolic acid (Muñoz et al., 1997). DAT, TAT and PAT with this type of fatty acid composition are exclusively found in species of the Mtb complex. Monomethyl-branched unsaturated C16 to C20 fatty acids have also been found as minor constituents esterifying PAT and DAT (Dubey et al., 2003).
Little is known about the biosynthesis of DAT and PAT although a dedicated biosynthetic gene cluster has been identified and found to resemble that of the better studied SL-1 (Hatzios et al., 2009). Of the genes present in this cluster, only pks3/4 and papA3 have been confirmed to participate in the biosynthesis of DAT and PAT thus far. pks3/4 encodes the polyketide synthase responsible for the elongation of mycosanoic and mycolipenic acids. Accordingly, an Mtb pks3/4 knock-out mutant was reported to be deficient in PAT and DAT production (Dubey et al., 2002; Rousseau et al., 2003b). In vitro studies with the purified PapA3 protein have shown that this acyltransferase is capable of sequentially transferring two palmitoyl groups onto positions 2 and 3 of trehalose yielding a diacylated trehalose molecule structurally similar to Mtb DAT. Consistently, the genetic disruption of papA3 abolished the synthesis of PAT in Mtb (Hatzios et al., 2009). Remarkably, the PAT biosynthetic gene cluster also encompasses genes potentially encoding for a lipid transporter of the RND superfamily (mmpL10), a fatty-acyl AMP ligase (fadD21) and an acyltransferase (Rv1184c; chp2) [Fig. 9]. In order to investigate the function of the products of these genes, Mtb knock-out mutants were generated in our laboratory [unpublished results]. Preliminary studies indicate that the fadD21 null mutant fails to synthesize both DAT and PAT, suggestive of a role for the acyl-AMP ligase FadD21 in the loading of activated fatty acid starter units onto Pks3/4 for the synthesis of mycosanoic and mycolipenic acids. The mmpL10 mutant, in contrast, is unable to synthesize PAT and accumulates DAT intracellularly, pointing to the involvement of this membrane transporter in the translocation of DAT to the cell surface. The chp2 mutant is unable to synthesize PAT and builds up large amounts of DAT, part of which are found at the cell surface. Interestingly, topological studies on Chp2 indicated that it is a membrane-anchored enzyme with a catalytic domain facing the periplasm. Enzyme assays further indicate that Chp2 is capable of synthesizing PAT from DAT. Thus, similar to Chp1 in the biosynthesis of SL-1, Chp2 appears to catalyze three sequential transacylations between DAT molecules yielding the fully elaborated PAT [unpublished results]. Since PapA3 is a cytosolic enzyme, it follows that DAT is synthesized in the cytoplasm prior to being flipped across the plasma membrane to serve as a substrate for Chp2 [Fig. 10]. The fact that PAT are synthesized on the periplasmic side of the membrane and then transported to the OM further suggests that DAT flipping and DAT/PAT translocation between membranes are two separate events. Further investigations are required to determine whether MmpL10 is involved in the first and/or second event(s) and identify the missing components of the translocation machinery. Finally, as reported above, the polyketide synthase Pks8-17 is responsible for the production of the monomethyl-branched unsaturated C16 to C20 fatty acids found in some forms of SL, DAT and PAT (Dubey et al., 2003). The synthesis of DAT and PAT, like that of SL, is under control of the two-component system PhoP-PhoR and the regulatory protein WhiB3 (Gonzalo Asensio et al., 2006; Walters et al., 2006; Chesne-Seck et al., 2008, Singh et al., 2009; Goyal et al., 2011; Cimino et al., 2012).
Several studies have investigated the physiological roles and biological relevance of DAT and PAT in Mtb infection. Phenotypic observations made on a pks3/4 DAT/PAT-deficient mutant indicated a role for these lipids in the retention of the capsular material at the cell surface of Mtb (Dubey et al., 2002; Rousseau et al., 2003b), possibly accounting for the changes observed in the binding and uptake of the mutant by phagocytic and non-phagocytic cells (Rousseau et al., 2003b). In vitro, DAT are strong immunomodulatory molecules, inhibiting the proliferation of murine and human CD4+ and CD8+ T-cells, and the expression of Th-1 cytokines in murine cells through the disruption of the MAPK signaling pathway (Saavedra et al., 2001; Saavedra et al., 2006; Palma-Nicolás et al., 2010). In another study, purified DAT (but not SL or PDIM) inhibited in a dose-dependent manner LPS- and Mtb-induced IL-12p40 and TNF-α production in human monocytes (Lee et al., 2007). Mycolipenic acids, the major acyl substituents found in PAT, TAT and some forms of DAT, have been shown to be potent inhibitors of leukocyte migration in vitro (Husseini and Eldberg, 1952). Notwithstanding, a pks3/4 knock-out mutant of Mtb deficient in DAT and PAT synthesis replicated similarly to its wild-type parent in mice (Rousseau et al., 2003b). Conversely, two independent high-density transposon mutagenesis-based studies aimed at identifying genes required for the optimal replication and survival of Mtb in mice (Sassetti and Rubin, 2003; Lamichhane et al., 2005) identified mmpL10 as a virulence gene; one of these studies also identified fadD21 and chp2 mutants as attenuated in vivo. Clearly, further infection studies using individual knock-out mutants and different animal models are required to provide a definite answer as to the role of DAT and PAT in Mtb pathogenesis. As in the case of SL, possible explanations for the discrepancy between the potent biological activities of DAT and PAT in vitro and their apparent lack of impact on Mtb infection in vivo may be found in the failure of animal models of infection to accurately mimic human TB infection and the potential functional redundancy of OM polymethyl-branched fatty acids-containing (glyco)lipids. In support of the latter assumption, a recent study comparing the effects of the individual and combined inactivation of the SL, PDIM and DAT/PAT pathways on Mtb infection revealed a functional overlap between these molecules with PDIM having a dominant effect over DAT/PAT and SL (Passemar et al., 2013). As noted in the SL section, one of the biological functions shared by these lipids is apparently to alleviate the propionate-mediated stress undergone by the bacilli during growth on host cholesterol as a major carbon source (Singh et al., 2009; Lee et al., 2013). In addition, the contribution of these lipids to blocking the phagosome acidification of infected macrophages suggests that their presence at the cell surface may promote the intracellular survival of Mtb (Brodin et al., 2010; Passemar et al., 2013).
Lipooligosaccharides (LOS)
LOS are produced by various fast- and slow-growing Mycobacterium species. They are found in M. canettii and related M. tuberculosis complex strains (Daffé et al., 1991) but are otherwise apparently absent from Mtb sensu stricto. LOS share a poly-O-acylated trehalose core glycosylated by a mono- or, more frequently, a oligosaccharidyl unit which is species-specific [Fig. 8] (Daffé and Lemassu, 2000). The trehalose moiety of LOS is acylated by polymethyl-branched fatty acids that can either be saturated (e. g. in M. canettii) or unsaturated (e. g. in M. smegmatis).
Although LOS were discovered more than 30 years ago (Hunter et al., 1983; Saadat & Ballou, 1983), nothing was known of their biosynthesis until recently. While most studies on LOS biosynthesis have focused on M. smegmatis and M. marinum, the conservation of the core genes involved in M. canettii suggests that the biosynthesis of LOS in this species follows a similar pattern. The identification of a LOS biosynthetic gene cluster in M. marinum has shed light into the set of steps leading to the synthesis of these glycolipids (Burguière et al., 2005; Ren et al., 2007). The composition of this gene cluster resembles those described previously for SL and DAT/PAT biosynthesis with genes encoding two polyketide synthases (Pks5 and Pks5.1), a fatty-acyl AMP ligase (FadD25), a lipid transport membrane protein of the RND superfamily (MmpL12) and a polyketide-associated acyltransferase (PapA4) [Fig. 9]. Additionally, the cluster encompasses several genes encoding glycosyltransferases, methyltransferases and other glycosyl modifying enzymes likely to be involved in the synthesis and modification of the species-specific oligosaccharidyl unit (Ren et al., 2007; Alibaud et al., 2014). A M. marinum mutant carrying a transposon insertion in papA4 fails to synthesize LOS, confirming the involvement of this acyltransferase in the pathway (Rombouts et al., 2011). Interestingly, while a homologous gene cluster is present in the genome of Mtb H37Rv, pks5.1 is missing from this cluster and the H37Rv ortholog of papA4 is predicted to encode a truncated protein of only 165 amino acids instead of the 465 residue protein encoded for instance by papA4 from M. marinum. The papA4 gene of the LOS-producing strain M. canettii, in contrast, encodes a full-size protein and this strain is also endowed with an ortholog of pks5.1 (Rombouts et al., 2011). Therefore, the lack of functional Pks5.1 and PapA4 most likely accounts for the inability of Mtb H37Rv to produce LOS. Pks5 was shown to synthesize the methyl-branched 2,4-dimethyl-2-eicosenoic acid found in LOS and the disruption of pks5 in M. smegmatis abolished LOS synthesis in this species (Etienne et al., 2009).
LOS are highly antigenic molecules (Daffé et al., 1991). Recent observations suggest that they play an important role in retaining proteins at the cell surface of some Mycobacterium species such as M. marinum (van der Woude et al., 2012). Their precise role in colony morphology is still a matter of debate and may depend on the species (Belisle & Brennan, 1989; Lemassu et al., 1992; Burguière et al., 2005). In M. marinum for instance, LOS have clearly been associated with colony morphology, sliding motility, biofilm formation and the ability of this Mycobacterium to enter macrophages (Ren et al., 2007). The M. marinum LOS are also endowed with immunomodulatory activities (Rombouts et al., 2009) and modulate virulence in the zebrafish embryo model of infection (van der Woude et al., 2012).
Acyltrehaloses in serodiagnosis, drug discovery and vaccine development
The essentiality of TMM and TDM for mycobacterial growth makes the enzymes and transporters involved in their biogenesis targets of choice for drug development. Many inhibitors of this pathway in fact already exist if one considers all the compounds either used clinically (e.g., isoniazid, ethionamide) or under development that target the biosynthesis of mycolic acids. A comprehensive review of past and ongoing efforts to target this pathway was recently published (North et al., 2013). Among the targets sought, the mycoloyltransferases of the antigen 85 complex have received some attention lately. High-throughput screening assays were reported for these enzymes (Elamin et al., 2009; Boucau et al., 2009; Sanki et al., 2009; Favrot et al., 2013) and used to identify inhibitors. The molecular mechanism of inhibition of antigens 85 by one of them, known as ebselen (MIC of 20 μg/ml against Mtb in culture), was elucidated and found to be particularly interesting in the sense that it is unlikely to promote the emergence of drug-resistant isolates (Favrot et al., 2013). Small molecule binders of antigen 85C identified by magnetic resonance spectroscopy also showed activity against Mtb in vitro and inside macrophages in the 100 μM range (Warrier et al., 2012). Importantly, the return of interest in whole cell-based screening that the TB field has witnessed in recent years also has led to the identification of a variety of chemotypes active against the TMM transporter, MmpL3 (Grzegorzewicz et al., 2012; Stanley et al., 2012; La Rosa et al., 2012; Remuinan et al., 2013; Ioerger et al., 2013; Poce et al., 2013; Konddredi et al., 2013; Onajole et al., 2013; Rao et al., 2013) including SQ109, a drug candidate currently undergoing phase II clinical trials (Sacksteder et al., 2012; Tahlan et al., 2012). The reason why so many different chemical scaffolds apparently inhibit the MmpL3-mediated translocation of TMM is at present unclear. Another approach to targeting acyltrehalose biosynthesis would to screen for inhibitors of OtsB2 since this enzyme was shown to be essential for Mtb growth (Murphy et al., 2005). To the best of our knowledge, no OtsB2 inhibitor has yet been reported. Finally, a regulatory system of interest in the context of drug development is the two-component transcriptional regulator PhoP-PhoR (Rv0757-Rv0758) which regulates the biosynthesis of multiple virulence factors including SL, DAT and PAT (Gonzalo-Asencio et al., 2006; Walters et al., 2006; Frigui et al., 2008; Gonzalo-Asencio et al., 2008; Ryndak et al., 2008; Chesne-Seck et al., 2008; Lee et al., 2008). No inhibitors of PhoP-PhoR have yet been described.
Beyond drug discovery, acyltrehaloses, either as purified antigens or in the context of attenuated live strains, are also being studied from the perspective of their vaccine potential. Ongoing studies include the testing of CD1-restricted sulfolipid antigens (G. Puzo, pers. comm.) and that of attenuated phoP knock-out mutants of Mtb (Nambiar et al., 2012).
The acyltrehaloses of Mtb (TDM, SL, DAT, TAT) are potent inducers of the humoral immune response (Lemassu et al., 1991; Muñoz et al., 1997). Accordingly, their potential as serodiagnostic tools for the detection of TB has been explored (Simonney et al., 1995; Simonney et al., 1996; Julian et al., 2002; Julian et al., 2004). While TB patients seem to exhibit heterogeneous IgG and IgA antibody responses against these glycolipid antigens, the results of these studies were still encouraging in that ELISA tests based on these antigens tended to be more sensitive than protein-based ELISAs in the detection of smear-negative TB patients and in that of patients co-infected with HIV (Simonney et al., 1995; Simonney et al., 1996; Julian et al., 2002; Julian et al., 2004).
para-hydroxybenzoic acids and phenolic glycolipids
Structures and distribution of para-hydroxybenzoic acid derivatives and phenolic glycolipids in Mtb
The structures of the phenolic glycolipids (PGLs) and para-hydroxybenzoic acid derivatives (pHBADs) of Mtb are shown on Fig. 11. pHBADs and PGLs share the same glycosylated aromatic nucleus. PGLs are found in the capsule and OM of Mtb (Ortalo-Magné et al., 1996); pHBADs, in contrast, are released in culture filtrates and tend not to remain associated with the cell envelope (Constant et al., 2002). While all Mtb isolates analyzed to date have retained the ability to produce and secrete pHBADs, most Mtb strains do not produce PGL due to a frameshift mutation in the polyketide synthase gene pks15/1 which is required for the assembly of the lipid moiety of the molecule (Constant et al., 2002). In fact, PGL production appears to be restricted to M. canettii and some Mtb isolates of the East Asian/Beijing lineage (Daffé et al., 1987; Constant et al., 2002; Reed et al., 2004; Huet et al., 2009). The PGLs of Mtb are glycosylated phenolic derivatives of phthiocerol dimycocerosates (PDIM), themselves abundant components of the OM of Mtb contributing to its impermeability (Camacho et al., 2001) [Fig. 11].
Figure 11. Structures of the phthiocerol dimycocerosates (PDIM), phenolic glycolipids (PGL) and p-hydroxybenzoic acid derivatives (p-HBADs) of Mtb.
The lipid core of PGL from Mtb is composed of phenolphthiocerol esterified by mycocerosic acids (p, p’=3-5; n, n’=16-18; m2=15-17 ; m1= 20-22; R= CH2-CH3 or CH3). The trisaccharide substituent of PGL and p-HBAD-II (i.e., the fully elaborated form of p-HBAD produced by Mtb) consists of 2,3,4-tri-O-methyl-α-L-Fucp-(1,3)-α–L-Rhap-(1,3)-2-O-methyl-α-L-Rhap.
Roles in the physiology and virulence of Mtb
The roles of pHBADs, PDIM and PGL in the permeability barrier, intracellular survival, modulation of the host immune response and pathogenicity of Mtb have been the object of several recent reviews (Jackson et al., 2007; Guilhot et al., 2008; Daffé et al., 2014) and will therefore not be detailed here. Interestingly, in an attempt to correlate the lipid content with the virulence of Mtb isolates, Goren et al. characterized in 1974 a methoxylated phenolphthiocerol (a non-glycosylated variant of PGL), the so-called “attenuation indicator lipid” (Goren et al., 1974b). Recent studies have identified this lipid and its unmethylated form in East Asian/Beijing isolates and found that they accumulate in all Indo-Oceanic Mtb strains examined (Krishnan et al., 2011). Similarly, Beijing strains were reported to accumulate variants of PDIM and eventually PGL, known as phthiotriol and glycosylated phenolphthiotriol dimycocerosates (Huet et al., 2009). The correlation between the occurrence of these lipids and variations in virulence remains, however, unclear (Huet et al., 2009; Krishnan et al., 2011). It was proposed that the different lineages of Mtb (Gagneux and Small, 2007) may have evolved regionally to tailor their OM lipid composition to the genetic background of their human host (Neyrolles and Guilhot, 2011).
Biogenesis of PGL and pHBADs
Coupled genetic and biochemical strategies have allowed much of the biosynthetic pathways of PDIM, PGL and pHBADs to be elucidated (for recent reviews, Guilhot et al., 2008; Malaga et al., 2008; Daffé et al., 2014). The nature of the enzymes involved suggests that most if not all of the elongation and assembly of these compounds takes place on the cytoplasmic side of the plasma membrane. The biosynthesis of the mycocerosic acid and (phenol)phthiocerol moieties of PDIM and PGL follows a similar pattern as the polymethyl-branched fatty acids found in SL, DAT and PAT (see previous section) and involves dedicated type I polyketide synthases, associated FadD-type fatty acyl-AMP ligases for the activation of long-chain (C16-C24) starter fatty acids as acyl-adenylates, and a PapA enzyme (PapA5) to transfer the newly synthesized mycocerosates to their phthiocerol or phenolphthiocerol acceptors. Consistent with their conserved structures [Fig. 11], the biosynthesis of the glycosyl moiety of PGL and pHBADs involves the same set of enzymes including three glycosyltransferases (Rv2962c, Rv2957, Rv2958c) and four O-methyltransferases (Rv2954c, Rv2955c, Rv2956 and Rv2959c). Upon synthesis, PDIM, PGL and pHBADs are exported by a dedicated translocation machinery. All of the work on this topic thus far has focused on PDIM but it is reasonable to assume that the same transporters are involved in the export of PGLs. Both the RND transporter MmpL7 and the ABC-transporter DrrABC are required for the translocation of PDIM to the OM. In addition, the lipoprotein LppX has been found to be required for PDIM to reach the cell surface (Sulzenbacher et al., 2006). LppX shares a similar fold with the periplasmic chaperone LolA and the outer membrane lipoprotein LolB which, in Gram negative bacteria, are involved in the localization of lipoproteins to the OM. It is thought that LppX acts downstream from MmpL7 and DrrABC, carrying PDIM across the periplasm. Using a yeast two-hybrid system, Jain and Cox (2005) showed that MmpL7 interacts with the polyketide synthase PpsE involved in the synthesis of the (phenol)phthiocerol moieties of PDIM and PGL. Based on this finding, a model was proposed wherein the synthesis and transport of PDIM are coupled (Jain and Cox, 2005) [Fig. 10]. In many ways, the biogenesis of PDIM and PGL thus resembles that of polymethyl-branched fatty acid-containing acyltrehaloses, involving similar sets of enzymes and transporters interacting with one another. Most of the genes involved in the biosynthetic pathways of PDIM, PGL and pHBADs are clustered on a 73-kb fragment of the Mtb chromosome (for a recent review, Daffé et al., 2014).
PGL biosynthesis in the context of diagnosis and drug discovery
Although not essential for growth, PDIM and biosynthetically-related PGL contribute to a significant extent to the ability of Mtb to replicate and survive in vivo. These lipids also play important roles in the permeability barrier of the cell envelope (Camacho et al., 2001) suggesting that compounds inhibiting their synthesis could synergize with or potentiate existing anti-TB drugs. A compound inhibiting the production of PGL in whole Mtb cells has been reported (Ferreras et al., 2008). As noted earlier in this review, promising compounds targeting the Ser/Thr kinases of Mtb that regulate the synthesis of PDIM and PGL (among other physiological processes) (Molle and Kremer, 2010) are also under development. In addition, several studies have explored the potential of PGL as serodiagnostic tools for TB detection (Simonney et al., 1995; Simonney et al., 1996; Constant et al., 2002). As most clinical isolates of Mtb do not produce PGL (Daffé et al., 1987; Daffé et al., 1988; Constant et al., 2002), it is likely that the antibodies detected in patients were in fact directed against pHBADs (Constant et al., 2002).
Mannosyl-β-1-phosphomycoketides
Mannosyl-β-1-phosphomycoketides (MPM) are glycoconjugates found in minute quantities (in the range of 1 nM concentration) inside the cells and released in the culture medium of pathogenic slow-growing Mycobacterium spp. including Mtb, M. bovis BCG, M. africanum, M. canetti, M. avium, M. avium paratuberculosis, M. marinum and M. ulcerans (Matsunaga and Sugita, 2012). They are apparently not found in rapidly growing mycobacteria (Matsunaga et al., 2004). MPM consist of a mannosyl-β-1-phosphate moiety reminiscent of polyprenol phosphomannose (PPM) and an alkyl chain of varying length (C30-C34) made of a fully saturated 4, 8, 12, 16, 20-pentamethylpentacosyl unit (the mycoketide) [Fig. 12]. The alkyl chain of MPM is elongated by the polyketide synthase Pks12 (Rv2048c) (Matsunaga et al., 2004; Chopra et al., 2008), the largest predicted protein of Mtb (430 KDa). Pks12 consists of two complete sets of fatty acid synthase (FAS)-like catalytic domains capable together of using alternating C2 (malonyl-CoA) and C3 (methylmalonyl-CoA) units to elongate the alkyl backbone of mycoketides. After 5 cycles of C3 and C2 chain elongation, the alkyl chain is thought to be released from Pks12 upon hydrolysis yielding mycoketidic acid which is further reduced to the corresponding long-chain alcohol, mycoketide, and finally phosphorylated and mannosylated to generate MPM (Matsunaga and Sugita, 2012). The identity of the enzymes catalyzing the hydrolysis, reduction, phosphorylation and mannosylation steps is not known.
Figure 12.

Structure of the predominant mannosyl-β-1-phosphomycoketide from Mtb H37Rv.
In line with the restricted distribution of MPM to pathogenic slow-growing Mycobacterium spp., studies comparing the virulence of MPM-deficient mutants of Mtb, M. avium and M. marinum to that of their wild-type parent in animal models of infection have provided support for their involvement in pathogenicity (Matsunaga and Sugita, 2012). MPM have the ability to activate human CD1c-restricted T-cells (Moody et al., 2000; Ly et al., 2013). In addition, they have been proposed to contribute to the suppression of phagosomal acidification and to act as signaling molecules regulating cell division and virulence (Matsunaga and Sugita, 2012).
Glycoproteins
Several proteins of Mtb complex species have been identified as glycoproteins on the basis of lectin binding (Espitia et al., 1989; Garbe et al., 1993; Gonzalez-Zamorano et al., 2009; Sartain and Belisle, 2009) or by using liquid chromatography-mass spectrometry approaches and bioinformatic analyses (Smith et al., 2013), but the detailed structure of the glycosyl appendages of only two of them have been characterized so far. The 45-47 kDa (Apa) antigen of Mtb was shown to be modified at threonine residues with one to three linear α–(1,2)-linked oligomannosides, whereas the MBP83 antigen of M. bovis is modified at threonine residues with one to three linear α–(1,3)-linked oligomannosides (Dobos et al., 1996; Michell et al., 2003). Mtb proteins may also be O-glycosylated on serine residues as shown in the case of the superoxide dismutase SodC (Sartain and Belisle, 2009). The glycosylation pattern of Apa and MBP83 is reminiscent of eukaryotic short-chain mannoproteins (Lengeler et al., 2007). Consistently, the yeast-like protein-O-mannosyltransferase Rv1002c was identified in Mtb as the enzyme responsible for the first mannosylation step of Apa (VanderVen et al., 2005). Analogous to eukaryotic systems, Sec-translocation is required for the mannosylation of extracytoplasmic proteins to occur in Mtb (VanderVen et al., 2005). Rv1002c belongs to the GT-C superfamily of glycosyltransferases as would be expected for a protein transferring a mannosyl residue from the mannose donor, Dec-P-Man, on the periplasmic side of the plasma membrane. The glycosyltransferases responsible for the further elongation of the α-(1,2) or α-(1,3)-linked oligomannoside motifs have not yet been identified in Mtb. The presence of identical linear α–(1,2)-linked oligomannosides in polar forms of PIMs (PIM6) or capping the non-reducing termini of the arabinosyl side chains of ManLAM led us to investigate the putative involvement of the α-(1,2) mannosyltransferases Rv2181 (Kaur et al., 2006; Kaur et al., 2008) and PimE (Rv1159) (Morita et al., 2006) in this process. Interestingly, while the disruption of pimE or Rv2181 in Mtb failed to reveal any effect on the glycosylation pattern of Apa in the mutant strains (G. Larrouy-Maumus, M. Jackson, D. Kaur, K. Dobos et al., unpublished results), disruption of the pimE gene in M. smegmatis yielded a mutant devoid of triglycosylated forms of FasC (a major mannosylated secreted protein in this species) and producing almost exclusively the monoglycosylated forms of the protein with only trace amounts of the diglycosylated forms (Liu et al., 2013). Importantly, wild-type mannosylation was restored in the mutant complemented with the pimE gene of Mtb. Disruption of MSMEG_4247 (the ortholog of Rv2181 in M. smegmatis), in contrast, had no effect on the glycosylation pattern of FasC (Liu et al., 2013). Collectively, these data point to the involvement of PimE in the deposition of the second and perhaps third Manp residue of the glycosyl appendages of mannoproteins, even though compensatory enzymatic activities may exist in Mtb accounting for the absence of protein mannosylation phenotype in the Mtb knock-out mutant.
In contrast to eukaryotic protein-O-mannosyltransferase, Rv1002c is not an essential protein of Mtb and M. smegmatis indicating that protein O-mannosylation is not essential for viability in mycobacteria (Liu et al., 2013). Disruption of Rv1002c in Mtb, however, dramatically alters the growth properties of the bacterium in certain liquid and solid media and negatively impacts its virulence in cellular and animal models of infection (Liu et al., 2013). Protein O-mannosylation thus plays critical roles in the physiology and pathogenesis of the tubercle bacillus. One of its proposed functions is to regulate the proteolytic processing and subcellular localization of exported proteins (Herrmann et al., 1996; Sartain and Belisle, 2009; Wilkinson et al., 2009). As in other bacterial pathogens, the glycosylation of mycobacterial proteins also influences their interactions with the host (Torrelles and Schlesinger, 2010). The mannosyl appendages of Apa for instance have been implicated in the ability of this protein to induce a delayed-type hypersensitivity response in guinea pigs, stimulate primed T-cells in vitro, and bind C-type lectins such as the surfactant protein A and, potentially, DC-SIGN (Romain et al., 1999; Horn et al., 1999; Pitarque et al., 2005; Ragas et al., 2007).
Capsular polysaccharides
The capsular material of Mtb
The outermost compartment of the cell envelope of Mtb consists of a loosely bound structure referred to as ‘capsule’ (Daffé and Draper, 1998) primarily made of proteins and polysaccharides (~ 97% of the total material) with only small amounts of lipids (Lemassu and Daffé, 1994; Ortalo-Magné et al., 1995). While the nature of this material has essentially been studied in axenically grown bacilli, the major capsular polysaccharides of Mtb have also been found coating the bacilli during infection (Schwebach et al., 2001; Schwebach et al., 2002). The amount of capsular polysaccharides produced by Mtb varies between isolates and this diversity is thought to impact the way that Mtb interacts with host cells (Cywes et al., 1997; Ehlers and Daffé, 1998; Daffé and Etienne, 1999; Torrelles and Schlesinger, 2010). The three major capsular polysaccharides of Mtb are an α-D-glucan, a D-mannan and a D-arabino-D-mannan representing, respectively, approximately 70%, 15% and 13% of the total polysaccharide content of the capsule (Lemassu and Daffé, 1994; Ortalo-Magné et al., 1995). In addition, traces (2-5%) of xylose were also detected (Ortalo-Magné et al., 1995). All are devoid of acyl substituents and are not covalently linked to the rest of the cell envelope.
α-D-glucan
α-D-glucan is a linear polymer of α–(1,4)-Glcp substituted at some 6-positions by oligoglucoside chains comprised of 1 to 9 Glc residues (Dinadayala et al., 2008) [Fig. 13]. It is structurally very similar to the intracellular glycogen of Mtb and M. bovis BCG although its 3D-structure appears to be more compact and its molecular mass, as determined by analytical ultracentrifugation, slightly higher (13 × 106 versus 7.5 × 106 Da) (Dinadayala et al., 2004; Dinadayala et al., 2008; Sambou et al., 2008). Consistently, the biosynthetic pathways of both glucopolymers share common enzymes that resemble the glycogen biosynthetic enzymes of E. coli. Enzymatic studies and phenotypic analyses of knock-out mutants of Mtb identified the α-(1,4)-glucosyltransferases Rv3032 and GlgA (Rv1212c), the ADP-glucose pyrophosphorylase GlgC (Rv1213) and the branching enzyme GlgB (Rv1326c) as components of their biosynthetic machinery (Sambou et al., 2008; Garg et al., 2007) [Fig. 14]. In addition, UDP-glucose which also serves as a Glc donor in the biosynthesis of α-(1,4)-linked glucans (Stadthagen et al., 2007) may be formed from glucose-1-phosphate and UTP by the UDP-glucose pyrophosphorylase GalU (Rv0993) (Lai et al., 2008). Disruption of glgA reduced the capsular α-D-glucan content of Mtb by half while that of glgC reduced by half both the α-D-glucan and glycogen contents of the cells (Sambou et al., 2008). Attempts to create a double glgA-Rv3032 mutant were unsuccessful indicating that a functional copy of at least one of the two α-(1,4)-glucosyltransferases is required for Mtb growth. The targeted inactivation of the only Rv3032 gene yielded a mutant with dramatically reduced glycogen and methylglucose lipopolysaccharide (MGLP) contents (Stadthagen et al., 2007). MGLPs are intracellular 6-O-methylglucose lipopolysaccharides of intermediate size which share with α-D-glucan and glycogen an α-(1,4)-linked glucan backbone. The precise physiological function of these cytosolic lipopolysaccharides is currently not known although a regulatory role in fatty acid biosynthesis has been proposed (Jackson and Brennan, 2009). The disruption of glgB was not achievable (Sambou et al., 2008) most likely due to the toxic accumulation of maltose-1-phosphate that follows the inactivation of this gene (Kalscheuer et al., 2010). Nevertheless, altering the level of expression of this gene in Mtb or replacing the Mtb glgB gene by that of E. coli resulted in profound quantitative and qualitative effects on the α-D-glucan and glycogen of the cells, thereby implicating GlgB in the branching of both polysaccharides in Mtb (Sambou et al., 2008). In contrast to the reactions leading to polymerization and branching, nothing is known of the initiation reactions of α-D-glucan and glycogen. In particular, it is not known whether they involve an endogenous acceptor such polyprenyl-phosphate, diacylglycerophosphate or D-3-phosphoglycerate (Whitfield, 2006; Kaur et al., 2009), or if they directly occur on the α-(1,4)-glucosyltransferases as reported for the glycogen synthase of Agrobacterium tumefaciens (Ugalde et al., 2003). In addition to the classical Glg pathway, a second pathway for the biogenesis of α-(1,4)-glucans exists in Mtb. This four-step pathway generates α-(1,4)-glucans from trehalose and involves the trehalose synthase TreS, the maltokinase Pep2, the maltose-1-phosphate maltosyltransferase GlgE, and GlgB [Fig. 14] (Pan et al., 2004; Pan et al., 2008; Kalscheuer et al., 2010, Elbein et al., 2010; Mendes et al., 2010; Miah et al., 2013). Disruption of glgE, like that of glgB, results in the accumulation of maltose-1-phosphate which is toxic to the cells. The crystal structures of TreS from both Mtb and M. smegmatis were recently reported (Roy et al., 2013; Caner et al., 2013) and the mechanism of association of the C-ter domain of this enzyme with oligosaccharides and possibly glycogen investigated (Caner et al., 2013). Importantly, it was found that TreS forms a hetero-octameric complex with Pep2. The formation of this complex boosts the maltokinase activity of Pep2 suggesting that it may serve to regulate α-(1,4)-glucan synthesis (Roy et al., 2013). Another point of control of this pathway appears to be at the level of GlgE which was recently reported to be negatively phosphoregulated by the Ser/Thr kinase PknB (Leiba et al., 2013).
Figure 13.
Structure of the capsular α-D-glucan of Mtb.
Figure 14. Biosynthesis of α-D-glucans in Mtb.
See text for details.
Consistent with the intracellular localization of glycogen and MGLPs, GlgA and Rv3032 are nucleotide sugar-utilizing glycosyltransferases predicted to catalyze the elongation of polysaccharides on the cytosolic face of the plasma membrane, and GlgB, GlgC, GlgE, Pep2 and TreS are also predicted to be cytosolic proteins. Consistently, α-D-glucan is likely to be elongated and branched on the cytosolic side of the plasma membrane before or in the process of being translocated across the plasma membrane. Given the major challenge represented by the translocation of such a high-molecular-weight hydrophilic molecule across the different layers of the mycobacterial cell envelope, the existence of molecular scaffolds providing continuity between the cytoplasm and the surface of Mtb functionally similar to the transport systems of Gram-negative group 2 and 3 capsules (Whitfield, 2006), seems likely. To this date, however, none of the components of the dedicated translocation machinery have been identified.
Recent studies using cellular models of infection have highlighted the roles of capsular polysaccharides in host-pathogen interactions. α-D-glucan and D-mannan mediate the nonopsonic binding of Mtb to CR3 (Cywes et al., 1997). CR3 is one of the main phagocytic receptors on monocytes and neutrophils and bacilli uptake through this receptor is thought to promote intracellular survival by suppressing the production of IL-12 and limiting respiratory burst (Ehlers et al., 1998; Fenton et al., 2005). Further studies have highlighted the antiphagocytic properties of the Mtb capsule and suggested that this structure may serve to limit and control the interactions of the bacilli with macrophages (Stokes et al., 2004). More recently, Sani et al. (2010) showed that the presence of capsular material at the surface of M. bovis BCG enhanced the binding of the bacterium to human monocyte-derived macrophages and modulated the pro-inflammatory cytokine response of these cells. Along the same lines, Gagliardi et al. (2007) showed that Mtb capsular α-D-glucan blocked CD1 expression and suppressed IL-12 production in monocyte-derived DCs. The ability of α-D-glucan to bind the C-type lectin DC-SIGN may account at least in part for its biological activities (Geurtsen et al., 2009). Other studies have shown that antibodies to Mtb capsular polysaccharides can modify the course of infection to the benefit of the host (Glatman-Freedman and Casadevall, 1998; Teitelbaum et al., 1998). Owing to its glycogen-like structure, α-D-glucan was also proposed to be involved in Mtb’s evasion of the immune system by molecular mimicry (Lemassu and Daffé, 1994). Finally, studies aimed at elucidating the basis of the immunotherapeutic properties of M. bovis BCG against bladder cancer have highlighted the anti-tumor activity of α-D-glucan (Wang et al., 1995; Zlotta et al., 2000). Since most of these studies have focused on the interactions of non-isogenic strains of Mtb or purified α-D-glucan with cellular models, it is important to keep in mind that they may not accurately reflect the relevance and individual contribution of capsular polysaccharides in mycobacterial infections. Further studies are warranted to determine the contribution of α-D-glucan to the pathogenicity of Mtb when carried by whole bacilli. Such studies will require isogenic mutants of Mtb specifically deficient in the production of capsular α-D-glucan (and producing wild-type levels of glycogen and MGLPs) that are presently not available.
D-mannan and D-arabino-D-mannan
The structure of D-mannan is identical to that of the mannan domain of LM, and the structure of AM is identical to that of the arabinomannan domain of LAM [Fig. 6]. It is likely that D-mannan and AM are released from LM and LAM upon hydrolysis of their phosphatidyl-myo-inositol anchor. As pointed out earlier in this review, D-mannan and AM are expected to share with LM and LAM common properties in their interactions with the host.
Capsular polysaccharides in the context of drug discovery
Two enzymes involved in the formation of the α-(1,4)-glucans of Mtb, namely the branching enzyme GlgB and the α-(1,4)-glucan:maltose 1-phosphate maltosyl transferase GlgE, may represent good targets for drug development since their inactivation is expected to result in the lethal accumulation of maltose-1-phosphate (Sambou et al., 2008; Kalscheuer et al., 2010). To the best of our knowledge, no inhibitors of these enzymes have yet been reported.
Conclusions and Future Prospects
The progress made in elucidating the biosynthetic pathways of the major cell envelope glycoconjugates of Mtb since the beginning of the mycobacterial genomic/genetic era in the late 1990s has been substantial and so has our understanding of the roles of these molecules in the physiology and pathogenesis of this paramount bacterial pathogen. Yet, important challenges lay ahead.
Several key biosynthetic enzymes have yet to be identified. In the PIM pathway, these include the α-(1,6)-ManT(s) initiating the elongation of the mannan backbone of LM from PIM3; the α-(1,2)-ManT responsible for the formation of PIM6 from PIM5; and the acyltransferase catalyzing the acylation of position 3 of myo-Ins. In the LM and LAM pathways, an activity consistent with the priming AraT that transfers the first Araf residue to the mannan backbone of LM was recently detected in cell-free assays but the identity of the corresponding enzyme is not known. The number and identities of the α-(1,5)-AraTs involved in the elongation of the arabinan domains of AG and LAM and the precise contribution of the Emb proteins in this process also remain to be defined. In light of the renewed interest in PG synthesis and recycling as a target for new and repurposed drugs (Jackson et al., 2013), more work is required to fully define the enzymes involved. With regards to glycoproteins, further work will need to determine whether Mtb proteins may be modified with other sugars than mannose and the identity the underlying glycosyltransferases, whether shared with other biosynthetic pathways as seems to be the case with the α-(1,2)-ManT PimE in M. smegmatis (Liu et al., 2013), or otherwise. Another biosynthetic step of considerable interest for its potential to uncover new drug targets is the attachment of AG to PG. The missing enzymes involved in all of these key aspects of the physiology of Mtb may be found in the numerous as yet unannotated ORFs of the Mtb genome, including some bearing conserved motifs suggestive of sugar-modifying enzymes (Pavelka et al., 2014; Berg et al., 2007; Slayden et al., 2013). Concomitantly, our continuously evolving view of the fine structures of Mtb glycoconjugates may reveal the existence of previously unsuspected ‘decorating’ enzymes, such as the ones involved in the biosynthesis of the MTX motif of ManLAM or the succinylation and galactosaminylation of the arabinan chains of AG and ManLAM. Based on the restricted distribution of some of these modifications to pathogenic Mycobacterium species and what is known of the biological activities of minor covalent modifications of lipopolysaccharides in other bacterial pathogens (Raetz et al., 2007; Kanistanon et al., 2008; Hamad et al., 2012), one may expect these discrete substituents to play a role in pathogenesis. Validation of this assumption, however, awaits in most cases the availability of Mtb mutants specifically deficient in the production of these motifs.
Beyond the identification and characterization of individual enzymes, more substantial challenges to be faced in terms of biogenesis reside in defining the sequential order of the reactions leading to the elongation, assembly and export of Mtb glycoconjugates, the processes involved in chain termination, and the nature and spatial organization of the translocation machineries. As illustrated by this review, recent years have seen a number of breakthroughs made on these fronts in the context of PG, PIM, LM/LAM, AG, glycoprotein and acyltrehalose biosynthesis allowing for more accurate models of these pathways [Fig. 4,5,6,10]. A pattern that has begun to emerge from these studies is that, analogous to other prokaryotic and eukaryotic systems, much of the biosynthesis of glycoconjugates in mycobacteria seems to rely on multiprotein complexes (e. g., acyltrehaloses, AG, PG), and involves tightly coordinated polymerization, modification and translocation events on both sides of the plasma membrane (e. g., AG, LM/LAM, glycoproteins). In some cases, biosynthesis and export may be coupled (e. g., acyltrehaloses, glycoproteins). Unlike other systems, however, the polymerization of building blocks in the assembly of complex glycoconjugates has not yet been reported in Mtb, and evidence to date instead points to the sequential addition of mannosyl, arabinosyl and galactosyl residues in the biosynthesis of AG, LM and LAM. Moreover, mycobacteria seem to have evolved somewhat unusual translocation mechanisms to export their (lipo)polysaccharides and glycolipids possibly reflecting the unique structure and composition of their cell envelope (e. g, MmpL proteins and periplasmic LppX-like lipoproteins in the transport of acyltrehaloses, lipids, siderophores and PIMs). Certainly, the missing components of the translocation machineries of AG, PIM, LM, LAM, MPM, PGL, acyltrehaloses and capsular polysaccharides are to be found among the numerous putative transporters of unknown function encoded by the Mtb genome (Slayden et al., 2013). Yet, the poor sequence similarity typically shared by prokaryotic transporters and general lack of identifiable motifs in their primary sequence complicates their identification. Moreover, our preliminary evidence indicates that the relaxed substrate specificity and thus redundancy of many of these transporters (e. g., the Dec-P-Ara flippase Rv3789) represents a major obstacle to their functional characterization as it limits the usefulness of genetic strategies based on the phenotypic analysis of knock-out or knock-down mutants.
Clearly, much remains to be done on the topic of mycobacterial glycoconjugates, particularly in establishing the composition of the multiprotein complexes involved in their biogenesis and elucidating the pivotal processes responsible for the translocation of biosynthetic intermediates and end products of these pathways across the different layers of the Mtb cell envelope. Pursuing this fascinating avenue of research is required for a complete understanding of the physiology and pathogenesis of Mtb as much as for the development of new drugs, vaccines, diagnostics and biomarkers.
PIM is used to describe the global family of PIM that carries one to four fatty acids and one to six Manp residues. In AcXPIMY, x refers to the number of acyl groups esterified to available hydroxyls on the Manp or myo-inositol residues, y refers to the number of Manp residues; e.g. Ac1PIM6 corresponds to the phosphatidylinositol hexamannoside PIM6 carrying two acyl groups attached to the glycerol (the diacylglycerol substituent) and one acyl group esterified to the Manp residue.
The Mtb gene nomenclature used is that of the Mtb strain H37Rv. The M. smegmatis gene nomenclature used in this review is that currently in use for the M. smegmatis strain mc2155.
Acknowledgments
The authors wish to thank Drs. M. McNeil (Colorado State University), Devinder Kaur, and Germain Puzo (IPBS-CNRS, Toulouse, France) for sharing unpublished data.
Research on Mtb glycoconjugates conducted in the authors’ laboratory is supported through the National Institutes of Health / National Institute of Allergy and Infectious Diseases grants AI064798, AI063054 and AI085992. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AG
arabinogalactan
- AM
D-arabino-D-mannan
- Araf
arabino-furanose
- AraT
arabinosyltransferase
- Dec-P
decaprenyl-monophosphate
- Dec-PP
decaprenyl diphosphate
- Dec-P-Man
decaprenyl-monophospho-mannose
- DAT
diacyltrehaloses
- Dec-P-Ara
decaprenyl-monophospho-arabinose
- EMB
ethambutol
- Galp
galactopyranose
- Galf
galacto-furanose
- LM
lipomannan
- LAM
lipoarabinomannan
- LPS
lipopolysaccharide
- Manp
mannopyranose
- ManT
mannosyltransferase
- myo-Ins
myo-Inositol
- PAT
polyacyltrehaloses
- PDIM
phthiocerol dimycocerosates
- PG
peptidoglycan
- PI
phosphatidyl-myo-inositol
- PIMs
phosphatidyl-myo-inositol mannosides
- SL
sulfolipids
Footnotes
Declaration of interest
The authors report no declarations of interest.
References
- AFONSO-BARROSO A, CLARK SO, WILLIAMS A, ROSA GT, NOBREGA C, SILVA-GOMES S, VALE-COSTA S, UMMELS R, STOKER N, MOVAHEDZADEH F, VAN DER LEY P, SLOOTS A, COT M, APPELMELK BJ, PUZO G, NIGOU J, GEURTSEN J, APPELBERG R. Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses. Cell Microbiol. 2012;15:660–674. doi: 10.1111/cmi.12065. [DOI] [PubMed] [Google Scholar]
- ALAIMO C, CATREIN I, MORF L, MAROLDA CL, CALLEWAERT N, VALVANO MA, FELDMAN MF, AEBI M. Two distinct but interchangeable mechanisms for flipping of lipid-linked oligosaccharides. EMBO J. 2006;25:967–976. doi: 10.1038/sj.emboj.7601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALBESA-JOVE D, GIGANTI D, JACKSON M, ALZARI PM, GUERIN ME. Structure-function relationships of membrane-associated GT-B glycosyltransferases. Glycobiology. 2014;24:108–24. doi: 10.1093/glycob/cwt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDERWICK LJ, DOVER LG, SEIDEL M, GANDE R, SAHM H, EGGELING L, BESRA GS. Arabinan-deficient mutants of Corynebacterium glutamicum and the consequent flux in decaprenylmonophosphoryl-D-arabinose metabolism. Glycobiology. 2006b;16:1073–81. doi: 10.1093/glycob/cwl030. [DOI] [PubMed] [Google Scholar]
- ALDERWICK LJ, LLOYD GS, GHADBANE H, MAY JW, BHATT A, EGGELING L, FUTTERER K, BESRA GS. The C-terminal domain of the Arabinosyltransferase Mycobacterium tuberculosis EmbC is a lectin-like carbohydrate binding module. PLoS Pathog. 2011b;7:e1001299. doi: 10.1371/journal.ppat.1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDERWICK LJ, LLOYD GS, LLOYD AJ, LOVERING AL, EGGELING L, BESRA GS. Biochemical characterization of the Mycobacterium tuberculosis phosphoribosyl-1-pyrophosphate synthetase. Glycobiology. 2011a;21:410–25. doi: 10.1093/glycob/cwq173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDERWICK LJ, MOLLE V, KREMER L, COZZONE AJ, DAFFORN TR, BESRA GS, FUTTERER K. Molecular structure of EmbR, a response element of Ser/Thr kinase signaling in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2006c;103:2558–63. doi: 10.1073/pnas.0507766103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDERWICK LJ, RADMACHER E, SEIDEL M, GANDE R, HITCHEN P, MORRIS HR, DELL A, SAHM H, EGGELING L, BESRA GS. Deletion of Cg-emb in Corynebacterianeae leads to a novel truncated cell wall arabinogalactan, whereas inactivation of Cg-ubiA results in an arabinan-deficient mutant with a cell wall galactan core. J. Biol. Chem. 2005;280:32362–32371. doi: 10.1074/jbc.M506339200. [DOI] [PubMed] [Google Scholar]
- ALDERWICK LJ, SEIDEL M, SAHM H, BESRA GS, EGGELING L. Identification of a novel arabinosyl transferase (AftA) involved in cell wall arabinan biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2006a;281:15653–15661. doi: 10.1074/jbc.M600045200. [DOI] [PubMed] [Google Scholar]
- ALIBAUD L, PAWELCZYK J, GANNOUN-ZAKI L, SINGH VK, ROMBOUTS Y, DRANCOURT M, DZIADEK J, GUERARDEL Y, KREMER L. Increased phagocytosis of Mycobacterium marinum mutants defective in lipooligosaccharide production: a structure-activity relationship study. J Biol Chem. 2014;289:215–28. doi: 10.1074/jbc.M113.525550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMIN AG, GOUDE R, SHI L, ZHANG J, CHATTERJEE C, PARISH T. EmbA is an essential arabinosyltransferase in Mycobacterium tuberculosis. Microbiology. 2008;154:240–248. doi: 10.1099/mic.0.2007/012153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDERSON RJ, ROBERTS EG. The chemistry of the lipoids of tubercle bacilli. XIV. The occurence of inosite in the phosphatide from human tubercle bacilli. J. Am. Chem. Soc. 1930;52:5023–5029. [Google Scholar]
- APPELMELK BJ, DEN DUNNEN J, DRIESSEN NN, UMMELS R, PAK M, NIGOU J, LARROUY-MAUMUS G, GURCHA SS, MOVAHEDZADEH F, GEURTSEN J, BROWN EJ, EYSINK SMEETS MM, BESRA GS, WILLEMSEN PTJ, LOWARY TL, VAN KOOYK Y, MAASKANT JJ, STOCKER NG, VAN DER LEY P, PUZO G, VANDENBROUCKE-GRAULS CMJE, WIELAND CW, VAN DER POLL T, GEIJTENBEEK TBH, VAN DER SAR AM, BITTER W. The mannose cap of mycobacterial lipoarabinomannan does not dominate the Mycobacterium-host interaction. Cell Microbiol. 2008;10:930–944. doi: 10.1111/j.1462-5822.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- ARMITIGE LY, JAGANNATH C, WANGER AR, NORRIS SJ. Disruption of the genes encoding antigen 85A and antigen 85B of Mycobacterium tuberculosis H37Rv: effect on growth in culture and in macrophages. Infect. Immun. 2000;68:767–678. doi: 10.1128/iai.68.2.767-778.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AXELROD S, OSCHKINAT H, ENDERS J, SCHLEGEL B, BRINKMANN V, KAUFMANN SH, HAAS A, SCHAIBLE UE. Delay of phagosome maturation by a mycobacterial lipid is reversed by nitric oxide. Cell Microbiol. 2008;10:1530–45. doi: 10.1111/j.1462-5822.2008.01147.x. [DOI] [PubMed] [Google Scholar]
- BARRY CE, CRICK DC, MCNEIL MR. Targeting the formation of the cell wall core of Mycobacterium tuberculosis. Infectious Disorders Drug Targets. 2007;7:182–202. doi: 10.2174/187152607781001808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRY CE, III, LEE RE, MDLULI K, SAMPSON AE, SCHROEDER BG, SLAYDEN RA, YUAN Y. Mycolic acids: structure, biosynthesis and physiological functions. Prog. Lipid Res. 1998;37:143–179. doi: 10.1016/s0163-7827(98)00008-3. [DOI] [PubMed] [Google Scholar]
- BATT SM, JABEEN T, BHOWRUTH V, QUILL L, LUND PA, EGGELING L, ALDERWICK LJ, FUTTERER K, BESRA GS. Structural basis of inhibition of Mycobacterium tuberculosis DprE1 by benzothiazinone inhibitors. Proc Natl Acad Sci U S A. 2012;109:11354–9. doi: 10.1073/pnas.1205735109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BATT SM, JABEEN T, MISHRA AK, VEERAPEN N, KRUMBACH K, EGGELING L, BESRA GS, FUTTERER K. Acceptor substrate discrimination in phosphatidyl-myo-inositol mannoside synthesis: structural and mutational analysis of mannosyltransferase Corynebacterium glutamicum PimB'. J Biol Chem. 2010;285:37741–52. doi: 10.1074/jbc.M110.165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAY DC, ROMMENS KL, TURNER RJ. Small multidrug resistance proteins: a multidrug transporter family that continues to grow. Biochim Biophys Acta. 2008;1778:1814–38. doi: 10.1016/j.bbamem.2007.08.015. [DOI] [PubMed] [Google Scholar]
- BEHRENDS V, WILLIAMS KJ, JENKINS VA, ROBERTSON BD, BUNDY JG. Free glucosylglycerate is a novel marker of nitrogen stress in Mycobacterium smegmatis. J Proteome Res. 2012;11:3888–96. doi: 10.1021/pr300371b. [DOI] [PubMed] [Google Scholar]
- BELANGER AE, BESRA GS, FORD ME, MIKUSOVA K, BELISLE JT, BRENNAN PJ, INAMINE JM. The embAB genes of Mycobacterium avium encode an arabinosyl transferase involved in cell wall arabinan biosynthesis that is the target for the antimycobacterial drug ethambutol. Proc. Natl. Acad. Sci. USA. 1996;93:11919–11924. doi: 10.1073/pnas.93.21.11919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELANOVA M, DIANISKOVA P, BRENNAN PJ, COMPLETO GC, ROSE NL, LOWARY TL, MIKUSOVA K. Galactosyl transferases in mycobacterial cell wall synthesis. J. Bacteriol. 2008;190:1141–1145. doi: 10.1128/JB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELISLE JT, BRENNAN PJ. Chemical basis of rough and smooth variation in mycobacteria. J Bacteriol. 1989;171:3465–70. doi: 10.1128/jb.171.6.3465-3470.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERG S, KAUR D, JACKSON M, BRENNAN PJ. The glycosyltransferases of Mycobacterium tuberculosis- roles in the synthesis of arabinogalactan, lipoarabinomannan, and other glycoconjugates. Glycobiology. 2007;17:35R–56R. doi: 10.1093/glycob/cwm010. [DOI] [PubMed] [Google Scholar]
- BERG S, STARBUCK J, TORRELLES JB, VISSA VD, CRICK DC, CHATTERJEE C, BRENNAN PJ. Roles of the conserved proline and glycosyltransferase motifs of EmbC in biosynthesis of lipoarabinomannan. J. Biol. Chem. 2005;280:5651–5663. doi: 10.1074/jbc.M411418200. [DOI] [PubMed] [Google Scholar]
- BERTOZZI CR, SCHELLE MW. Sulfated metabolites from Mycobacterium tuberculosis: Sulfolipid-1 and beyond. In: DAFFÉ M, REYRAT J-M, editors. The mycobacterial cell envelope. ASM Press; Washington DC: 2008. [Google Scholar]
- BESRA GS, BOLTON R, MCNEIL MR, RIDELL M, SIMPSON KE, GLUSHKA J, VAN HALBEEK H, BRENNAN PJ, MINNIKIN DE. Structure elucidation and antigenicity of a novel family of glycolipid antigens from Mycobacterium tuberculosis H37Rv. Biochemistry. 1992;31:9832–9837. doi: 10.1021/bi00155a040. [DOI] [PubMed] [Google Scholar]
- BHAMIDI S, SCHERMAN MS, JONES V, CRICK DC, BELISLE JT, BRENNAN PJ, MCNEIL MR. Detailed structural and quantitative analysis reveals the spatial organization of the cell walls of in vivo grown Mycobacterium leprae and in vitro grown Mycobacterium tuberculosis. J Biol Chem. 2011;286:23168–77. doi: 10.1074/jbc.M110.210534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHAMIDI S, SCHERMAN MS, RITHNER CD, PRENNI JE, CHATTERJEE D, KHOO K-H, MCNEIL MR. The identification and location of succinyl residues and the characterization of the interior arabinan region allows for a model of the complete primary structure of Mycobacterium tuberculosis mycolyl arabinogalactan. J. Biol. Chem. 2008;283:12992–13000. doi: 10.1074/jbc.M800222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHATT K, GURCHA SS, BHATT A, BESRA GS, JACOBS WR., JR. Two polyketide-synthase-associated acyltransferases are required for sulfolipid biosynthesis in Mycobacterium tuberculosis. Microbiology. 2007;153:513–520. doi: 10.1099/mic.0.2006/003103-0. [DOI] [PubMed] [Google Scholar]
- BIRCH HL, ALDERWICK LJ, APPELMELK BJ, MAASKANT J, BHATT A, SINGH A, NIGOU J, EGGELING L, GEURTSEN J, BESRA GS. A truncated lipoglycan from mycobacteria with altered immunological properties. Proc Natl Acad Sci U S A. 2010;107:2634–9. doi: 10.1073/pnas.0915082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRCH HL, ALDERWICK LJ, BHATT A, RITTMANN D, KRUMBACH K, SINGH A, BAI Y, LOWARY TL, EGGELING L, BESRA GS. Biosynthesis of mycobacterial arabinogalactan: identification of a novel a(1->3) arabinofuranosyltransferase. Mol Microbiol. 2008;69:1191–1206. doi: 10.1111/j.1365-2958.2008.06354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLATTES E, VERCELLONE A, EUTAMENE H, TURRIN CO, THEODOROU V, MAJORAL JP, CAMINADE AM, PRANDI J, NIGOU J, PUZO G. Mannodendrimers prevent acute lung inflammation by inhibiting neutrophil recruitment. Proc Natl Acad Sci U S A. 2013;110:8795–800. doi: 10.1073/pnas.1221708110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCH H. Studies on the virulence of tubercle bacilli; isolation and biological properties of a constituent of virulent organisms. J Exp Med. 1950;91:197–218. doi: 10.1084/jem.91.2.197. pl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTH D, SCHNEIDER G, SCHNELL R. Peptidoglycan remodeling in Mycobacterium tuberculosis: comparison of structures and catalytic activities of RipA and RipB. J Mol Biol. 2011;413:247–60. doi: 10.1016/j.jmb.2011.08.014. [DOI] [PubMed] [Google Scholar]
- BOUCAU J, SANKI AK, VOSS BJ, SUCHECK SJ, RONNING DR. A coupled enzymatic assay measuring Mycobacterium tuberculosis antigen 85C enzymatic activity. Anal. Biochem. 2009;385:120–127. doi: 10.1016/j.ab.2008.10.018. [DOI] [PubMed] [Google Scholar]
- BRODIN P, POQUET Y, LEVILLAIN F, PEGUILLET I, LARROUY-MAUMUS G, GILLERON M, EWANN F, CHRISTOPHE T, FENISTEIN D, JANG J, JANG MS, PARK SJ, RAUZIER J, CARRALOT JP, SHRIMPTON R, GENOVESIO A, GONZALO-ASENSIO JA, PUZO G, MARTIN C, BROSCH R, STEWART GR, GICQUEL B, NEYROLLES O. High content phenotypic cell-based visual screen identifies Mycobacterium tuberculosis acyltrehalose-containing glycolipids involved in phagosome remodeling. PLoS Pathog. 2010;6:e1001100. doi: 10.1371/journal.ppat.1001100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROZNA JP, HORAN M, RADEMACHER JM, PABST KM, PABST MJ. Monocyte responses to sulfolipid from Mycobacterium tuberculosis: inhibition of priming for enhanced release of superoxide, associated with increased secretion of interleukin-1 and tumor necrosis factor alpha and altered protein phosphorylation. Infect. Immun. 1991;59:2542–2548. doi: 10.1128/iai.59.8.2542-2548.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNING JB, MURILLO AC, CHACON O, BARLETTA RG, SACCHETTINI JC. Structure of the Mycobacterium tuberculosis D-alanine:D-alanine ligase, a target of the antituberculosis drug D-cycloserine. Antimicrob Agents Chemother. 2011;55:291–301. doi: 10.1128/AAC.00558-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGUIERE A, HITCHEN P, DOVER LG, KREMER L, RIDELL M, ALEXANDER DC, LIU J, MORRIS HR, MINNIKIN DE, DELL A, BESRA GS. LosA, a key glycosyltransferase involved in the biosynthesis of a novel family of glycosylated acyltrehalose lipooligosaccharides from Mycobacterium marinum. J. Biol. Chem. 2005;280:42124–42133. doi: 10.1074/jbc.M507500200. [DOI] [PubMed] [Google Scholar]
- CALA-DE PAEPE D, LAYRE E, GIACOMETTI G, GARCIA-ALLES LF, MORI L, HANAU D, DE LIBERO G, DE LA SALLE H, PUZO G, GILLERON M. Deciphering the role of CD1e protein in mycobacterial phosphatidyl-myo-inositol mannosides (PIM) processing for presentation by CD1b to T lymphocytes. J Biol Chem. 2012;287:31494–502. doi: 10.1074/jbc.M112.386300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMACHO LR, CONSTANT P, RAYNAUD C, LANEELLE MA, TRICCAS JA, GICQUEL B, DAFFE M, GUILHOT C. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis. Evidence that this lipid is involved in the cell wall permeability barrier. J Biol Chem. 2001;276:19845–54. doi: 10.1074/jbc.M100662200. [DOI] [PubMed] [Google Scholar]
- CANER S, NGUYEN N, AGUDA A, ZHANG R, PAN YT, WITHERS SG, BRAYER GD. The structure of the Mycobacterium smegmatis trehalose synthase reveals an unusual active site configuration and acarbose-binding mode. Glycobiology. 2013;23:1075–83. doi: 10.1093/glycob/cwt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN TM, BOULOC N, BUXTON RS, CHUGH J, LOUGHEED KE, OSBORNE SA, SAXTY B, SMERDON SJ, TAYLOR DL, WHALLEY D. Substituted aminopyrimidine protein kinase B (PknB) inhibitors show activity against Mycobacterium tuberculosis. Bioorg Med Chem Lett. 2012;22:3349–53. doi: 10.1016/j.bmcl.2012.02.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE D, HUNTER SW, MCNEIL M, BRENNAN PJ. Lipoarabinomannan. Multiglycosylated form of the mycobacterial mannosylphosphatidylinositols. J. Biol. Chem. 1992;267:6228–6233. [PubMed] [Google Scholar]
- CHATTERJEE D, KHOO KH, MCNEIL MR, DELL A, MORRIS HR, BRENNAN PJ. Structural definition of the non-reducing termini of mannose-capped LAM from Mycobacterium tuberculosis through selective enzymatic degradation and fast atom bombardment-mass spectrometry. Glycobiology. 1993;3:497–506. doi: 10.1093/glycob/3.5.497. [DOI] [PubMed] [Google Scholar]
- CHESNE-SECK ML, BARILONE N, BOUDOU F, GONZALO ASENSIO J, KOLATTUKUDY PE, MARTIN C, COLE ST, GICQUEL B, GOPAUL DN, JACKSON M. A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J Bacteriol. 2008;190:1329–34. doi: 10.1128/JB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPRA T, BANERJEE S, GUPTA S, YADAV G, ANAND S, SUROLIA A, ROY RP, MOHANTY D, GOKHALE RS. Novel intermolecular iterative mechanism for biosynthesis of mycoketide catalyzed by a bimodular polyketide synthase. PLoS Biology. 2008;6:1584–1598. doi: 10.1371/journal.pbio.0060163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTOPHE T, JACKSON M, JEON HK, FENISTEIN D, CONTRERAS-DOMINGUEZ M, KIM J, GENOVESIO A, CARRALOT JP, EWANN F, KIM EH, LEE SY, KANG S, SEO MJ, PARK EJ, SKOVIEROVA H, PHAM H, RICCARDI G, NAM JY, MARSOLLIER L, KEMPF M, JOLY-GUILLOU ML, OH T, SHIN WK, NO Z, NEHRBASS U, BROSCH R, COLE ST, BRODIN P. High content screening identifies decaprenyl-phosphoribose 2' epimerase as a target for intracellular antimycobacterial inhibitors. PLoS Pathog. 2009;5:e1000645. doi: 10.1371/journal.ppat.1000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIMINO M, THOMAS C, NAMOUCHI A, DUBRAC S, GICQUEL B, GOPAUL DN. Identification of DNA binding motifs of the Mycobacterium tuberculosis PhoP/PhoR two-component signal transduction system. PLoS One. 2012;7:e42876. doi: 10.1371/journal.pone.0042876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE BR, GREENFIELD LK, BOUWMAN C, WHITFIELD C. Coordination of polymerization, chain termination, and export in assembly of the Escherichia coli lipopolysaccharide O9a antigen in an ABC-transporter-dependent pathway. J. Biol. Chem. 2009;284:30662–30672. doi: 10.1074/jbc.M109.052878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE ST, BROSCH R, PARKHILL J, GARNIER T, CHURCHER C, HARRIS D, GORDON SV, EIGLMEIER K, GAS S, BARRY CE, III, TEKAIA F, BADCOCK K, BASHAM D, BROWN D, CHILLINGWORTH T, CONNOR R, DAVIES R, DEVLIN K, FELTWELL T, GENTLES S, HAMLIN N, HOLROYD S, HORNSBY T, JAGELS K, KROGH A, MCLEAN J, MOULE S, MURPHY L, OLIVER K, OSBORNE J, QUAIL MA, RAJANDREAM M-A, ROGERS J, RUTTER S, SEEGER K, SKELTON J, SQUARES R, SQUARES S, SULSTON JE, TAYLOR K, WHITEHEAD S, BARRELL BG. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- CONSTANT P, PEREZ E, MALAGA W, LANEELLE MA, SAUREL O, DAFFE M, GUILHOT C. Role of the pks15/1 gene in the biosynthesis of phenolglycolipids in the Mycobacterium tuberculosis complex. Evidence that all strains synthesize glycosylated p-hydroxybenzoic methyl esters and that strains devoid of phenolglycolipids harbor a frameshift mutation in the pks15/1 gene. J Biol Chem. 2002;277:38148–58. doi: 10.1074/jbc.M206538200. [DOI] [PubMed] [Google Scholar]
- CONVERSE SE, MOUGOUS JD, LEAVELL MD, LEARY JA, BERTOZZI CR, COX JS. MmpL8 is required for sulfolipid-1 biosynthesis and Mycobacterium tuberculosis virulence. Proc. Natl. Acad. Sci. USA. 2003;100:6121–6126. doi: 10.1073/pnas.1030024100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORDILLOT M, DUBEE V, TRIBOULET S, DUBOST L, MARIE A, HUGONNET JE, ARTHUR M, MAINARDI JL. In vitro cross-linking of Mycobacterium tuberculosis peptidoglycan by L,D-transpeptidases and inactivation of these enzymes by carbapenems. Antimicrob Agents Chemother. 2013;57:5940–5. doi: 10.1128/AAC.01663-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COULOMBE F, DIVANGAHI M, VEYRIER F, DE LESELEUC L, GLEASON JL, YANG Y, KELLIHER MA, PANDEY AK, SASSETTI CM, REED MB, BEHR MA. Increased NOD2-mediated recognition of N-glycolyl muramyl dipeptide. J Exp Med. 2009;206:1709–16. doi: 10.1084/jem.20081779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRELLIN PK, BRAMMANANTH R, COPPEL RL. Decaprenylphosphoryl-beta-D-ribose 2'-epimerase, the target of benzothiazinones and dinitrobenzamides, is an essential enzyme in Mycobacterium smegmatis. PLoS One. 2011;6:e16869. doi: 10.1371/journal.pone.0016869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRELLIN PK, KOVACEVIC S, MARTIN KL, BRAMMANANTH R, MORITA YS, BILLMAN-JACOBE H, MCCONVILLE MJ, COPPEL RL. Mutations in pimE restore lipoarabinomannan synthesis and growth in a Mycobacterium smegmatis lpqW mutant. J. Bacteriol. 2008;190:3690–3699. doi: 10.1128/JB.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYWES C, HOPPE HC, DAFFÉ M, EHLERS MRW. Nonopsonic binding of Mycobacterium tuberculosis to human complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect. Immun. 1997;65:4258–4266. doi: 10.1128/iai.65.10.4258-4266.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAFFÉ M, CRICK DC, JACKSON M. Genetics of capsular polysaccharides and cell envelope (glyco)lipids. Microbiol Spectrum. 2014;2(4) doi: 10.1128/microbiolspec.MGM2-0021-2013. MGM2-0021-2013. doi:10.1128/microbiolspec.MGM2-0021-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAFFÉ M, DRAPER P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- DAFFÉ M, ETIENNE G. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber Lung Dis. 1999;79:153–169. doi: 10.1054/tuld.1998.0200. [DOI] [PubMed] [Google Scholar]
- DAFFÉ M, LACAVE C, LANÉELLE M-A, GILLOIS M, LANÉELLE G. Polyphthienoyl trehalose, glycolipids specific for virulent strains of the tubercle bacillus. Eur. J. Biochem. 1988;172:579–584. doi: 10.1111/j.1432-1033.1988.tb13928.x. [DOI] [PubMed] [Google Scholar]
- DAFFÉ M, LACAVE C, LANEELLE MA, LANEELLE G. Structure of the major triglycosyl phenol-phthiocerol of Mycobacterium tuberculosis (strain Canetti) Eur J Biochem. 1987;167:155–60. doi: 10.1111/j.1432-1033.1987.tb13317.x. [DOI] [PubMed] [Google Scholar]
- DAFFÉ M, LANÉELLE M-A. Distribution of phthiocerol diester, phenolic mycosides and related compounds in mycobacteria. J. Gen. Microbiol. 1988;134:2049–2055. doi: 10.1099/00221287-134-7-2049. [DOI] [PubMed] [Google Scholar]
- DAFFÉ M, LEMASSU A. Glycobiology of the mycobacterial surface. Structures and biological activities of the cell envelope glycoconjugates. In: DOYLE RJ, editor. Glycomicrobiology. Kluwer Academic / Plenum Publishers; New York, NY, USA: 2000. [Google Scholar]
- DAFFÉ M, MCNEIL MR, BRENNAN PJ. Novel type-specific lipooligosaccharides from Mycobacterium tuberculosis. Biochemistry. 1991;30:378–388. doi: 10.1021/bi00216a011. [DOI] [PubMed] [Google Scholar]
- DALEKE DD. Phospholipid flippases. J. Biol. Chem. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- DANILENKO VN, OSOLODKIN DI, LAKATOSH SA, PREOBRAZHENSKAYA MN, SHTIL AA. Bacterial eukaryotic type serine-threonine protein kinases: from structural biology to targeted anti-infective drug design. Curr Top Med Chem. 2011;11:1352–69. doi: 10.2174/156802611795589566. [DOI] [PubMed] [Google Scholar]
- DATTA P, DASGUPTA A, BHAKTA S, BASU J. Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J Biol Chem. 2002;277:24983–7. doi: 10.1074/jbc.M203847200. [DOI] [PubMed] [Google Scholar]
- DATTA P, DASGUPTA A, SINGH AK, MUKHERJEE P, KUNDU M, BASU J. Interaction between FtsW and penicillin-binding protein 3 (PBP3) directs PBP3 to mid-cell, controls cell septation and mediates the formation of a trimeric complex involving FtsZ, FtsW and PBP3 in mycobacteria. Mol Microbiol. 2006;62:1655–73. doi: 10.1111/j.1365-2958.2006.05491.x. [DOI] [PubMed] [Google Scholar]
- DE LA SALLE H, MARIOTTI S, ANGENIEUX C, GILLERON M, GARCIA-ALLES L-F, MALM D, BERG T, PAOLETTI S, MAITRE B, MOUREY L, SALAMERO J, CAZENAVE J-P, HANAU D, MORI L, PUZO G, DE LIBERO G. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- DE SMET KAL, WESTON A, BROWN IN, YOUNG DB, ROBERTSON BD. Three pathways for trehalose biosynthesis in mycobacteria. Microbiology. 2000;146:199–208. doi: 10.1099/00221287-146-1-199. [DOI] [PubMed] [Google Scholar]
- DELMAS C, GILLERON M, BRANDO T, VERCELLONE A, GHEORGHIU M, RIVIÈRE M, PUZO G. Comparative structural study of the mannosylated-lipoarabinomannans from Mycobacterium bovis BCG vaccine strains: characterization and localization of succinates. Glycobiology. 1997;7:811–817. doi: 10.1093/glycob/7.6.811. [DOI] [PubMed] [Google Scholar]
- DENG LL, HUMPHRIES DE, ARBEIT RD, CARLTON LE, SMOLE SC, CARROLL JD. Identification of a novel peptidoglycan hydrolase CwlM in Mycobacterium tuberculosis. Biochim Biophys Acta. 2005;1747:57–66. doi: 10.1016/j.bbapap.2004.09.021. [DOI] [PubMed] [Google Scholar]
- DHIMAN RK, DINADAYALA P, RYAN GJ, LENAERTS AJ, SCHENKEL AR, CRICK DC. Lipoarabinomannan localization and abundance during growth of Mycobacterium smegmatis. J. Bacteriol. 2011;193:5802–5809. doi: 10.1128/JB.05299-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIANIŠKOVÁ P, KORDULÁKOVÁ J, ŠKOVIEROVÁ H, KAUR D, JACKSON M, BRENNAN PJ, MIKUŠOVÁ K. Investigation of ABC transporter from mycobacterial arabinogalactan biosynthetic cluster. Gen. Physiol. Biophys. 2011;30:239–250. doi: 10.4149/gpb_2011_03_239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINADAYALA P, KAUR D, BERG S, AMIN AG, VISSA VD, CHATTERJEE D, BRENNAN PJ, CRICK DC. Genetic basis for the synthesis of the immunomodulatory mannose caps of lipoarabinomannan in Mycobacterium tuberculosis. J. Biol. Chem. 2006;281:20027–20035. doi: 10.1074/jbc.M603395200. [DOI] [PubMed] [Google Scholar]
- DINADAYALA P, LEMASSU A, GRANOVSKI P, CÉRANTOLA S, WINTER N, DAFFÉ M. Revisiting the structure of the anti-neoplastic glucans of Mycobacterium bovis bacille Calmette-Guérin. J. Biol. Chem. 2004;279:12369–12378. doi: 10.1074/jbc.M308908200. [DOI] [PubMed] [Google Scholar]
- DINADAYALA P, SAMBOU T, DAFFÉ M, LEMASSU A. Comparative structural analyses of the alpha-glucan and glycogen from Mycobacterium bovis. Glycobiology. 2008;18:502–508. doi: 10.1093/glycob/cwn031. [DOI] [PubMed] [Google Scholar]
- DOBOS KM, KHOO KH, SWIDEREK KM, BRENNAN PJ, BELISLE JT. Definition of the full extent of glycosylation of the 45-Kilodalton glycoprotein of Mycobacterium tuberculosis. J. Bacteriol. 1996;178:2498–2506. doi: 10.1128/jb.178.9.2498-2506.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMENECH P, REED MB, BARRY CE., III Contribution of the Mycobacterium tuberculosis MmpL protein family to virulence and drug resistance. Infect. Immun. 2005;73:3492–3501. doi: 10.1128/IAI.73.6.3492-3501.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOMENECH P, REED MB, DOWD CS, MANCA C, KAPLAN G, BARRY CE., III The role of MmpL8 in sulfatide biogenesis and virulence of Mycobacterium tuberculosis. J. Biol. Chem. 2004;279:21257–21265. doi: 10.1074/jbc.M400324200. [DOI] [PubMed] [Google Scholar]
- DRAPER P, KHOO K-H, CHATTERJEE D, DELL A, MORRIS HR. Galactosamine in walls of slow-growing mycobacteria. Biochem. J. 1997;327:519–525. doi: 10.1042/bj3270519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRIESSEN NN, UMMELS R, MAASKANT JJ, GURCHA SS, BESRA GS, AINGE GD, LARSEN DS, PAINTER GF, VANDENBROUCKE-GRAULS CMJE, GEURTSEN J, APPELMELK BJ. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect. Immun. 2009;77:4538–4547. doi: 10.1128/IAI.01256-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBEY VS, SIRAKOVA TD, CYNAMON MH, KOLATTUKUDY PE. Biochemical function of msl5 (pks8 plus pks17) in Mycobacterium tuberculosis H37Rv: Biosynthesis of monomethyl branched unsaturated fatty acids. J. Bacteriol. 2003;185:4620–4625. doi: 10.1128/JB.185.15.4620-4625.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBEY VS, SIRAKOVA TD, KOLATTUKUDY PE. Disruption of msl3 abolishes the synthesis of mycolipanoic and mycolipenic acids required for polyacyltrehalose synthesis in Mycobacterium tuberculosis H37Rv and causes cell aggregation. Mol. Microbiol. 2002;45:1451–1459. doi: 10.1046/j.1365-2958.2002.03119.x. [DOI] [PubMed] [Google Scholar]
- EHLERS MRW, DAFFÉ M. Interactions between Mycobacterium tuberculosis and host cells: are mycobacterial sugars the key? Trends in Microbiology. 1998;6:328–335. doi: 10.1016/s0966-842x(98)01301-8. [DOI] [PubMed] [Google Scholar]
- ELAMIN AA, STEHR M, OEHLMANN W, SINGH M. The mycolyltransferase 85A, a putative drug target of Mycobacterium tuberculosis: development of a novel assay and quantification of glycolipid-status of the mycobacterial cell wall. J Microbiol Methods. 2009;79:358–63. doi: 10.1016/j.mimet.2009.10.010. [DOI] [PubMed] [Google Scholar]
- ELBEIN AD, PASTUSZAK I, TACKETT AJ, WILSON T, PAN YT. The last step in the conversion of trehalose to glycogen: A mycobacterial enzyme that transfers maltose from maltose-1-phosphate to glycogen. J. Biol. Chem. 2010;285:9803–9812. doi: 10.1074/jbc.M109.033944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLAND K, BOSHOFF HI, ARORA K, WEINER D, DAYAO E, SCHIMEL D, VIA LE, BARRY CE., 3RD Meropenem-clavulanic acid shows activity against Mycobacterium tuberculosis in vivo. Antimicrob Agents Chemother. 2012;56:3384–7. doi: 10.1128/AAC.05690-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EOH H, BROWN AC, BUETOW L, HUNTER WN, PARISH T, KAUR D, BRENNAN PJ, CRICK DC. Characterization of the Mycobacterium tuberculosis 4-diphosphocytidyl-2-C-methyl-D-erythitol synthase: potential for drug development. J. Bacteriol. 2007;189:8922–8927. doi: 10.1128/JB.00925-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERDEMLI SB, GUPTA R, BISHAI WR, LAMICHHANE G, AMZEL LM, BIANCHET MA. Targeting the Cell Wall of Mycobacterium tuberculosis: Structure and Mechanism of L,D-Transpeptidase 2. Structure. 2012;20:2103–15. doi: 10.1016/j.str.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESCUYER VE, LETY M-A, TORRELLES JB, KHOO K-H, TANG J-B, RITHNER CD, FREHEL C, MCNEIL MR, BRENNAN PJ, CHATTERJEE C. The role of the embA and embB gene products in the biosynthesis of the terminal hexaarabinofuranosyl motif of Mycobacterium smegmatis arabinogalactan. J. Biol. Chem. 2001;276:48854–48862. doi: 10.1074/jbc.M102272200. [DOI] [PubMed] [Google Scholar]
- ESPITIA C, MANCILLA R. Identification, isolation and partial characterization of Mycobacterium tuberculosis glycoprotein antigens. Clin. Exp. Immunol. 1989;77:378–383. [PMC free article] [PubMed] [Google Scholar]
- ETIENNE G, MALAGA W, LAVAL F, LEMASSU A, GUILHOT C, DAFFE M. Identification of the polyketide synthase involved in the biosynthesis of the surface-exposed lipooligosaccharides in mycobacteria. J Bacteriol. 2009;191:2613–21. doi: 10.1128/JB.01235-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVROT L, GRZEGORZEWICZ AE, LAJINESS DH, MARVIN RK, BOUCAU J, ISAILOVIC D, JACKSON M, RONNING DR. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nat Commun. 2013;4:2748. doi: 10.1038/ncomms3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENTON MJ, RILEY LW, SCHLESINGER LS. Receptor-mediated recognition of Mycobacterium tuberculosis by host cells. In: COLE ST, DAVIS EISENACH K, MCMURRAY DN, JACOBS WR JR., editors. Tuberculosis and the tubercle bacillus. ASM Press; Washington, DC: 2005. [Google Scholar]
- FERRERAS JA, STIRRETT KL, LU X, RYU JS, SOLL CE, TAN DS, QUADRI LE. Mycobacterial phenolic glycolipid virulence factor biosynthesis: mechanism and small-molecule inhibition of polyketide chain initiation. Chem Biol. 2008;15:51–61. doi: 10.1016/j.chembiol.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER K, SCOTET E, NIEMEYER M, KOEBERNICK H, ZERRAHN J, MAILLET S, HURWITZ R, KURSAR M, BONNEVILLE M, KAUFMANN SHE, SCHAIBLE UE. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA. 2004;101:10685–10690. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIGUI W, BOTTAI D, MAJLESSI L, MONOT M, JOSSELIN E, BRODIN P, GARNIER T, GICQUEL B, MARTIN C, LECLERC C, COLE ST, BROSCH R. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008;4:e33. doi: 10.1371/journal.ppat.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUDA T, MATSUMURA T, ATO M, HAMASAKI M, NISHIUCHI Y, MURAKAMI Y, MAEDA Y, YOSHIMORI T, MATSUMOTO S, KOBAYASHI K, KINOSHITA T, MORITA YS. Critical roles for lipomannan and lipoarabinomannan in cell wall integrity of mycobacteria and pathogenesis of tuberculosis. MBio. 2013;4:e00472–12. doi: 10.1128/mBio.00472-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAGLIARDI MC, LEMASSU A, TELONI R, MARIOTTI S, SARGENTINI V, PARDINI M, DAFFE M, NISINI R. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the fucntion of dendritic cells derived from infected monocyte. Cell Microbiol. 2007;9:2081–2092. doi: 10.1111/j.1462-5822.2007.00940.x. [DOI] [PubMed] [Google Scholar]
- GAGNEUX S, SMALL PM. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis. 2007;7:328–37. doi: 10.1016/S1473-3099(07)70108-1. [DOI] [PubMed] [Google Scholar]
- GARBE T, HARRIS D, VORDERMEIER M, LATHIGRA R, IVANYI J, YOUNG D. Expression of the Mycobacterium tuberculosis 19-Kilodalton antigen in M. smegmatis: immunological analysis and evidence of glycosylation. Infect. Immun. 1993;61:260–267. doi: 10.1128/iai.61.1.260-267.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA-ALLES LF, COLLMANN A, VERSLUIS C, LINDNER B, GUIARD J, MAVEYRAUD L, HUC E, IM JS, SANSANO S, BRANDO T, JULIEN S, PRANDI J, GILLERON M, PORCELLI SA, DE LA SALLE H, HECK AJ, MORI L, PUZO G, MOUREY L, DE LIBERO G. Structural reorganization of the antigen-binding groove of human CD1b for presentation of mycobacterial sulfoglycolipids. Proc Natl Acad Sci U S A. 2011;108:17755–60. doi: 10.1073/pnas.1110118108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARG SK, ALAM MS, KISHAN KVR, AGRAWAL P. Expression and characterization of α-(1,4)-glucan branching enzyme Rv1326c of Mycobacterium tuberculosis H37Rv. Protein Expr. Purif. 2007;51:198–208. doi: 10.1016/j.pep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- GEE CL, PAPAVINASASUNDARAM KG, BLAIR SR, BAER CE, FALICK AM, KING DS, GRIFFIN JE, VENGHATAKRISHNAN H, ZUKAUSKAS A, WEI JR, DHIMAN RK, CRICK DC, RUBIN EJ, SASSETTI CM, ALBER T. A phosphorylated pseudokinase complex controls cell wall synthesis in mycobacteria. Sci Signal. 2012;5:ra7. doi: 10.1126/scisignal.2002525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEURTSEN J, CHEDAMMI S, MESTERS J, COT M, DRIESSEN NN, SAMBOU T, KAKUTANI R, UMMELS R, MAASKANT J, TAKATA H, BABA O, TERASHIMA T, BOVIN N, VANDENBROUCKE-GRAULS CMJE, NIGOU J, PUZO G, LEMASSU A, DAFFE M, APPELMELK BJ. Identification of mycobacterial α-glucan as a novel ligand for DC-SIGN: Involvement of mycobacterial capsular polysaccharides in host immune modulation. J. Immunol. 2009;183:5221–5231. doi: 10.4049/jimmunol.0900768. [DOI] [PubMed] [Google Scholar]
- GIGANTI D, ALEGRE-CEBOLLADA J, URRESTI S, ALBESA-JOVE D, RODRIGO-UNZUETA A, COMINO N, KACHALA M, LOPEZ-FERNANDEZ S, SVERGUN DI, FERNANDEZ JM, GUERIN ME. Conformational plasticity of the essential membrane-associated mannosyltransferase PimA from mycobacteria. J Biol Chem. 2013;288:29797–808. doi: 10.1074/jbc.M113.462705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLERON M, JACKSON M, NIGOU J, PUZO G. Structure, activities and biosynthesis of the Phosphatidyl-myo-Inositol-based lipoglycans. In: DAFFÉ M, REYRAT J-M, editors. The Mycobacterial Cell Envelope. ASM Press; Washington, DC: 2008. [Google Scholar]
- GILLERON M, LINDNER B, PUZO G. MS/MS approach for characterization of the fatty acid distribution on mycobacterial phosphatidyl-myo-inositol mannosides. Anal. Chem. 2006a;78:8543–8548. doi: 10.1021/ac061574a. [DOI] [PubMed] [Google Scholar]
- GILLERON M, NIGOU J, NICOLLE D, QUESNIAUX V, PUZO G. The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem. Biol. 2006b;13:39–47. doi: 10.1016/j.chembiol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- GILLERON M, QUESNIAUX VFJ, PUZO G. Acylation state of the phosphatidylinositol hexamannosides from Mycobacterium bovis Bacillus Calmette Guérin and Mycobacterium tuberculosis H37Rv and its implication in Toll-like receptor response. J. Biol. Chem. 2003;278:29880–29889. doi: 10.1074/jbc.M303446200. [DOI] [PubMed] [Google Scholar]
- GILLERON M, RONET C, MEMPEL M, MONSARRAT B, GACHELIN G, PUZO G. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis Bacillus Calmette Guérin and ability to induce granuloma and recruit natural killer T cells. J. Biol. Chem. 2001;276:34896–34904. doi: 10.1074/jbc.M103908200. [DOI] [PubMed] [Google Scholar]
- GILLERON M, STENGER S, MAZORRA Z, WITTKE F, MARIOTTI S, BÖHMER G, PRANDI J, MORI L, PUZO G, DE LIBERO G. Diacylated sulfoglycolipids are novel mycobacterial antigens stimulating CD1-restricted T cells during infection with Mycobacterium tuberculosis. J. Exp. Med. 2004;199:649–659. doi: 10.1084/jem.20031097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILMORE SA, SCHELLE MW, HOLSCLAW CM, LEIGH CD, JAIN M, COX JS, LEARY JA, BERTOZZI CR. Sulfolipid-1 biosynthesis restricts Mycobacterium tuberculosis growth in human macrophages. ACS Chem Biol. 2012;7:863–70. doi: 10.1021/cb200311s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLATMAN-FREEDMAN A, CASADEVALL A. Serum therapy for tuberculosis revisited: reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin. Microbiol. Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOFFIN C, GHUYSEN JM. Biochemistry and comparative genomics of SxxK superfamily acyltransferases offer a clue to the mycobacterial paradox: presence of penicillin-susceptible target proteins versus lack of efficiency of penicillin as therapeutic agent. Microbiol Mol Biol Rev. 2002;66:702–38. doi: 10.1128/MMBR.66.4.702-738.2002. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALEZ-ZAMORANO M, MENDOZA-HERNANDEZ G, XOLALPA W, PARADA C, VALLECILLO AJ, BIGI F, ESPITIA C. Mycobacterium tuberculosis glycoproteomics based on ConA-lectin affinity capture of mannosylated proteins. J Proteome Res. 2009;8:721–33. doi: 10.1021/pr800756a. [DOI] [PubMed] [Google Scholar]
- GONZALO ASENSIO J, MAIA C, FERRER NL, BARILONE N, LAVAL F, SOTO CY, WINTER N, DAFFE M, GICQUEL B, MARTIN C, JACKSON M. The virulence-associated two-component PhoP-PhoR system controls the biosynthesis of polyketide-derived lipids in Mycobacterium tuberculosis. J Biol Chem. 2006;281:1313–6. doi: 10.1074/jbc.C500388200. [DOI] [PubMed] [Google Scholar]
- GONZALO-ASENSIO J, MOSTOWY S, HARDERS-WESTERVEEN J, HUYGEN K, HERNANDEZ-PANDO R, THOLE J, BEHR M, GICQUEL B, MARTIN C. PhoP: a missing piece in the intricate puzzle of Mycobacterium tuberculosis virulence. PLoS One. 2008;3:e3496. doi: 10.1371/journal.pone.0003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOREN MB. Biosynthesis and structures of phospholipids and sulfatides. In: KUBICA GP, WAYNE LG, editors. The mycobacteria. A sourcebook. Marcel Dekker, Inc; New York and Basel: 1984. [Google Scholar]
- GOREN MB. Mycobacterial fatty acid esters of sugars and sulfosugars. In: KATES M, editor. Handbook of Lipid Research. Glycolipids, phosphoglycolipids and sulfoglycolipids. Plenum Press; New York, London: 1990. [Google Scholar]
- GOREN MB, BRENNAN PJ. Mycobacterial lipids: chemistry and biologic activities. In: YOUMANS GP, editor. Tuberculosis. W. B. Saunders Company; Philadelphia, London, Toronto: 1979. [Google Scholar]
- GOREN MB, BROKL O, ROLLER P, FALES HM, DAS BC. Sulfatides of Mycobacterium tuberculosis: the structure of the principal sulfatide (SL-I) Biochem. 1976;15:2728–2734. doi: 10.1021/bi00658a003. [DOI] [PubMed] [Google Scholar]
- GOREN MB, BROKL O, SCHAEFER WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: correlation of virulence with elaboration of sulfatides and strongly acidic lipids. Infect. Immun. 1974a;9:142–149. doi: 10.1128/iai.9.1.142-149.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOREN MB, BROKL O, SCHAEFER WB. Lipids of putative relevance to virulence in Mycobacterium tuberculosis: Phthiocerol dimycocerosate and the attenuation indicator lipid. Infect. Immun. 1974b;9:150–158. doi: 10.1128/iai.9.1.150-158.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUDE R, AMIN AG, CHATTERJEE D, PARISH T. The critical role of embC in Mycobacterium tuberculosis. J. Bacteriol. 2008;190:4335–4341. doi: 10.1128/JB.01825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOUDE R, AMIN AG, CHATTERJEE D, PARISH T. The arabinosyltransferase EmbC is inhibited by ethambutol in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2009;53:4138–46. doi: 10.1128/AAC.00162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOYAL R, DAS AK, SINGH R, SINGH PK, KORPOLE S, SARKAR D. Phosphorylation of PhoP protein plays direct regulatory role in lipid biosynthesis of Mycobacterium tuberculosis. J Biol Chem. 2011;286:45197–208. doi: 10.1074/jbc.M111.307447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAHAM JE, CLARK-CURTISS JE. Identification of Mycobacterium tuberculosis RNAs synthesized in response to phagocytosis by human macrophages by selective capture of transcribed sequences (SCOTS) Proc. Natl. Acad. Sci. USA. 1999;96:11554–11559. doi: 10.1073/pnas.96.20.11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRZEGORZEWICZ AE, PHAM H, GUNDI VAKB, SCHERMAN MS, NORTH EJ, HESS T, JONES V, GRUPPO V, BORN SEM, KORDULÁKOVÁ J, CHAVADI SS, MORISSEAU C, LENAERTS AJ, LEE RE, MCNEIL MR, JACKSON M. Inhibition of mycolic acid transport across the Mycobacterium tuberculosis plasma membrane. Nat. Chem. Biol. 2012;8:334–341. doi: 10.1038/nchembio.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUAN S, BASTIN DA, VERMA NK. Functional analysis of the O antigen glucosylation gene cluster of Shigella flexneri bacteriophage SfX. Microbiology. 1999;145:1263–1273. doi: 10.1099/13500872-145-5-1263. [DOI] [PubMed] [Google Scholar]
- GUÉRARDEL Y, MAES E, BRIKEN V, CHIRAT F, LEROY Y, LOCHT C, STRECKER G, KREMER L. Lipomannan and lipoarabinomannan from a clinical isolate of Mycobacterium kansasii: Novel structural features and apoptosis-inducing properties. J. Biol. Chem. 2003;278:36637–36651. doi: 10.1074/jbc.M305427200. [DOI] [PubMed] [Google Scholar]
- GUÉRARDEL Y, MAES E, ELASS E, LEROY Y, TIMMERMAN P, BESRA GS, LOCHT C, STRECKER G, KREMER L. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. J. Biol. Chem. 2002;277:30635–30648. doi: 10.1074/jbc.M204398200. [DOI] [PubMed] [Google Scholar]
- GUERIN ME, KAUR D, SOMASHEKAR BS, GIBBS S, GEST P, CHATTERJEE D, BRENNAN PJ, JACKSON M. New insights into the early steps of phosphatidylinositol mannosides biosynthesis in mycobacteria. PimB' is an essential enzyme of Mycobacterium smegmatis. J. Biol. Chem. 2009a;284:25687–25696. doi: 10.1074/jbc.M109.030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERIN ME, SCHAEFFER F, CHAFFOTTE A, GEST P, GIGANTI D, KORDULAKOVA J, VAN DER WOERD M, JACKSON M, ALZARI PM. Substrate-induced conformational changes in the essential peripheral membrane-associated mannosyltransferase PimA from mycobacteria: implications for catalysis. J Biol Chem. 2009b;284:21613–25. doi: 10.1074/jbc.M109.003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERIN ME, KORDULAKOVA J, ALZARI PM, BRENNAN PJ, JACKSON M. Molecular basis of phosphatidyl-myo-inositol mannoside biosynthesis and regulation in mycobacteria. J Biol Chem. 2010;285:33577–83. doi: 10.1074/jbc.R110.168328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUERIN ME, KORDULAKOVA J, SCHAEFFER F, SVETLIKOVA Z, BUSCHIAZZO A, GIGANTI D, GICQUEL B, MIKUSOVA K, JACKSON M, ALZARI PM. Molecular recognition and interfacial catalysis by the essential phosphatidylinositol mannosyltransferase PimA from mycobacteria. J. Biol. Chem. 2007;282:20705–20714. doi: 10.1074/jbc.M702087200. [DOI] [PubMed] [Google Scholar]
- GUIARD J, COLLMANN A, GARCIA-ALLES LF, MOUREY L, BRANDO T, MORI L, GILLERON M, PRANDI J, DE LIBERO G, PUZO G. Fatty acyl structures of Mycobacterium tuberculosis sulfoglycolipid govern T cell response. J Immunol. 2009;182:7030–7. doi: 10.4049/jimmunol.0804044. [DOI] [PubMed] [Google Scholar]
- GUILHOT C, CHALUT C, DAFFÉ M. Biosynthesis and roles of phenolic glycolipids and related molecules in Mycobacterium tuberculosis. In: DAFFÉ M, REYRAT J-M, editors. The mycobacterial cell envelope. ASM Press; Washington DC: 2008. [Google Scholar]
- GUPTA R, LAVOLLAY M, MAINARDI JL, ARTHUR M, BISHAI WR, LAMICHHANE G. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat Med. 2010;16:466–9. doi: 10.1038/nm.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GURCHA SS, BAULARD AR, KREMER L, LOCHT C, MOODY DB, MUHLECKER W, COSTELLOS CE, CRICK DC, BRENNAN PJ, BESRA GS. Ppm1, a novel polyprenol monophosphomannose synthase from Mycobacterium tuberculosis. Biochem J. 2002;365:441–450. doi: 10.1042/BJ20020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUZMAN JD, WUBE A, EVANGELOPOULOS D, GUPTA A, HUFNER A, BASAVANNACHARYA C, RAHMAN MM, THOMASCHITZ C, BAUER R, MCHUGH TD, NOBELI I, PRIETO JM, GIBBONS S, BUCAR F, BHAKTA S. Interaction of N-methyl-2-alkenyl-4-quinolones with ATP-dependent MurE ligase of Mycobacterium tuberculosis: antibacterial activity, molecular docking and inhibition kinetics. J Antimicrob Chemother. 2011;66:1766–72. doi: 10.1093/jac/dkr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMAD MA, DI LORENZO F, MOLINARO A, VALVANO MA. Aminoarabinose is essential for lipopolysaccharide export and intrinsic antimicrobial peptide resistance in Burkholderia cenocepacia(dagger) Mol Microbiol. 2012;85:962–74. doi: 10.1111/j.1365-2958.2012.08154.x. [DOI] [PubMed] [Google Scholar]
- HAMASAKI N, ISOWA K, KAMADA K, TERANO Y, MATSUMOTO T, ARAKAWA T, KOBAYASHI K, YANO I. In vivo administration of mycobacterial cord factor (Trehalose 6, 6'-dimycolate) can induce lung and liver granulomas and thymic atrophy in rabbits. Infect Immun. 2000;68:3704–9. doi: 10.1128/iai.68.6.3704-3709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANCOCK IC, CARMAN S, BESRA GS, BRENNAN PJ, WAITE E. Ligation of arabinogalactan to peptidoglycan in the cell wall of Mycobacterium smegmatis requires concomitant synthesis of the two wall polymers. Microbiology. 2002;148:3059–67. doi: 10.1099/00221287-148-10-3059. [DOI] [PubMed] [Google Scholar]
- HANSEN JM, GOLCHIN SA, VEYRIER FJ, DOMENECH P, BONECA IG, AZAD AK, RAJARAM MV, SCHLESINGER LS, DIVANGAHI M, REED MB, BEHR MA. N-Glycolylated Peptidoglycan Contributes to the Immunogenicity but Not Pathogenicity of Mycobacterium tuberculosis. J Infect Dis. 2013 doi: 10.1093/infdis/jit622. [DOI] [PubMed] [Google Scholar]
- HATZIOS SK, SCHELLE MW, HOLSCLAW CM, BEHRENS CR, BOTYANSZKI Z, LIN FL, CARLSON BL, KUMAR P, LEARY JA, BERTOZZI CR. PapA3 is an acyltransferase required for polyacyltrehalose biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2009;284:12745–51. doi: 10.1074/jbc.M809088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEITMANN L, SCHOENEN H, EHLERS S, LANG R, HOLSCHER C. Mincle is not essential for controlling Mycobacterium tuberculosis infection. Immunobiology. 2013;218:506–16. doi: 10.1016/j.imbio.2012.06.005. [DOI] [PubMed] [Google Scholar]
- HENRIQUES AO, GLASER P, PIGGOT PJ, MORAN CP., JR. Control of cell shape and elongation by the rodA gene in Bacillus subtilis. Mol Microbiol. 1998;28:235–47. doi: 10.1046/j.1365-2958.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- HERRMANN J-L, O'GAORA P, GALLAGHER A, THOLE JER, YOUNG DB. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO. J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- HOANG TT, MA Y, STERN RJ, MCNEIL MR, SCHWEIZER H. Construction and use of low-copy number T7 expression vectors for purification of problem proteins: purification of Mycobacterium tuberculosis RmlD and Pseudomonas aeruginosa LasI and Rh1I proteins, and functional analysis of purified Rh1I. Gene. 1999;237:361–371. doi: 10.1016/s0378-1119(99)00331-5. [DOI] [PubMed] [Google Scholar]
- HOFFMANN C, LEIS A, NIEDERWEIS M, PLITZKO JM, ENGELHARDT H. Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure. Proc. Natl. Acad. Sci. USA. 2008;105:3963–3967. doi: 10.1073/pnas.0709530105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORN C, NAMANE A, PESCHER P, RIVIERE M, ROMAIN F, PUZO G, BARZU O, MARCHAL G. Decreased capacity of recombinant 45/47-kDa molecules (Apa) of Mycobacterium tuberculosis to stimulate T lymphocyte responses related to changes in their mannosylation pattern. J. Biol. Chem. 1999;274:32023–32030. doi: 10.1074/jbc.274.45.32023. [DOI] [PubMed] [Google Scholar]
- HSU FF, TURK J, OWENS RM, RHOADES ER, RUSSELL DG. Structural characterization of phosphatidyl-myo-inositol mannosides from Mycobacterium bovis Bacillus Calmette Guerin by multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. I. PIMs and lyso-PIMs. J Am Soc Mass Spectrom. 2007a;18:466–78. doi: 10.1016/j.jasms.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU FF, TURK J, OWENS RM, RHOADES ER, RUSSELL DG. Structural characterization of phosphatidyl-myo-inositol mannosides from Mycobacterium bovis Bacillus Calmette Guerin by multiple-stage quadrupole ion-trap mass spectrometry with electrospray ionization. II. Monoacyl- and diacyl-PIMs. J Am Soc Mass Spectrom. 2007b;18:479–92. doi: 10.1016/j.jasms.2006.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG H, BERG S, SPENCER JS, VEREECKE D, D'HAEZE W, HOLSTERS M, MCNEIL MR. Identification of amino acids and domains required for catalytic activity of DPPR synthase, a cell wall biosynthetic enzyme of Mycobacterium tuberculosis. Microbiology. 2008;154:736–743. doi: 10.1099/mic.0.2007/013532-0. [DOI] [PubMed] [Google Scholar]
- HUANG H, SCHERMAN MS, D'HAEZE W, VEREECKE D, HOLSTERS M, CRICK DC, MCNEIL MR. Identification and active expression of the Mycobacterium tuberculosis gene encoding 5-phospho-a-D-ribose-1-diphosphate: decaprenyl-phosphate 5-phosphoribosyltransferase, the first enzyme committed to decaprenylphosphoryl-D-arabinose synthesis. J. Biol. Chem. 2005;280:24539–24543. doi: 10.1074/jbc.M504068200. [DOI] [PubMed] [Google Scholar]
- HUET G, CONSTANT P, MALAGA W, LANEELLE MA, KREMER K, VAN SOOLINGEN D, DAFFE M, GUILHOT C. A lipid profile typifies the Beijing strains of Mycobacterium tuberculosis: identification of a mutation responsible for a modification of the structures of phthiocerol dimycocerosates and phenolic glycolipids. J Biol Chem. 2009;284:27101–13. doi: 10.1074/jbc.M109.041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGONNET JE, TREMBLAY LW, BOSHOFF HI, BARRY CE, 3RD, BLANCHARD JS. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science. 2009;323:1215–8. doi: 10.1126/science.1167498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER SW, BRENNAN PJ. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J. Biol. Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- HUNTER SW, GAYLORD H, BRENNAN PJ. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J. Biol. Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- HUNTER SW, MURPHY RC, CLAY K, GOREN MB, BRENNAN PJ. Trehalose-containing lipooligosaccharides. A new class of species-specific antigens from Mycobacterium. J Biol Chem. 1983;258:10481–7. [PubMed] [Google Scholar]
- HUSSEINI H, ELBERG S. Cellular reactions to phthienoic acid and related branched-chain acids. Am. Rev. Tuberc. 1952;65:655–672. doi: 10.1164/art.1952.65.6.655. [DOI] [PubMed] [Google Scholar]
- HVORUP RN, WINNEN B, CHANG AB, JIANG Y, ZHOU XF, SAIER MH., JR. The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) exporter superfamily. Eur J Biochem. 2003;270:799–813. doi: 10.1046/j.1432-1033.2003.03418.x. [DOI] [PubMed] [Google Scholar]
- INDRIGO J, HUNTER RL, JR., ACTOR JK. Influence of trehalose 6,6'-dimycolate (TDM) during mycobacterial infection of bone marrow macrophages. Microbiology. 2002;148:1991–8. doi: 10.1099/00221287-148-7-1991. [DOI] [PubMed] [Google Scholar]
- INDRIGO J, HUNTER RL, JR., ACTOR JK. Cord factor trehalose 6,6'-dimycolate (TDM) mediates trafficking events during mycobacterial infection of murine macrophages. Microbiology. 2003;149:2049–59. doi: 10.1099/mic.0.26226-0. [DOI] [PubMed] [Google Scholar]
- IOERGER TR, O'MALLEY T, LIAO R, GUINN KM, HICKEY MJ, MOHAIDEEN N, MURPHY KC, BOSHOFF HI, MIZRAHI V, RUBIN EJ, SASSETTI CM, BARRY CE, 3RD, SHERMAN DR, PARISH T, SACCHETTINI JC. Identification of New Drug Targets and Resistance Mechanisms in Mycobacterium tuberculosis. PLoS One. 2013;8:e75245. doi: 10.1371/journal.pone.0075245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA E, ISHIKAWA T, MORITA YS, TOYONAGA K, YAMADA H, TAKEUCHI O, KINOSHITA T, AKIRA S, YOSHIKAI Y, YAMASAKI S. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med. 2009;206:2879–88. doi: 10.1084/jem.20091750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZAKI Y, HAYASHI C, INOUE K, IGARASHI M, TAKAHASHI Y, PUJARI V, CRICK DC, BRENNAN PJ, NOMOTO A. Inhibition of the First Step in Synthesis of the Mycobacterial Cell Wall Core, Catalyzed by the GlcNAc-1-phosphate Transferase WecA, by the Novel Caprazamycin Derivative CPZEN-45. J Biol Chem. 2013;288:30309–19. doi: 10.1074/jbc.M113.492173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON M, BRENNAN PJ. Polymethylated polysaccharides from Mycobacterium species revisited. J. Biol. Chem. 2009;284:1949–1953. doi: 10.1074/jbc.R800047200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON M, MCNEIL MR, BRENNAN PJ. Progress in targeting cell envelope biogenesis in Mycobacterium tuberculosis. Future Microbiol. 2013;8:855–75. doi: 10.2217/fmb.13.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACKSON M, RAYNAUD C, LANÉELLE MA, GUILHOT C, LAURENT-WINTER C, ENSERGUEIX D, GICQUEL B, DAFFÉ M. Inactivation of the antigen 85C gene profoundly affects the mycolate content and alters the permeability of the Mycobacterium tuberculosis cell envelope. Mol Microbiol. 1999;31:1573–1587. doi: 10.1046/j.1365-2958.1999.01310.x. [DOI] [PubMed] [Google Scholar]
- JACKSON M, STADTHAGEN G, GICQUEL B. Long-chain multiple methyl-branched fatty acid-containing lipids of Mycobacterium tuberculosis: biosynthesis, transport, regulation and biological activities. Tuberculosis. 2007;87:78–86. doi: 10.1016/j.tube.2006.05.003. [DOI] [PubMed] [Google Scholar]
- JAIN M, COX JS. Interaction between polyketide synthase and transporter suggests coupled synthesis and export of virulence lipid in M. tuberculosis. PLoS Pathog. 2005;1:e2. doi: 10.1371/journal.ppat.0010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANG J, STELLA A, BOUDOU F, LEVILLAIN F, DARTHUY E, VAUBOURGEIX J, WANG C, BARDOU F, PUZO G, GILLERON M, BURLET-SCHILTZ O, MONSARRAT B, BRODIN P, GICQUEL B, NEYROLLES O. Functional characterization of the Mycobacterium tuberculosis serine/threonine kinase PknJ. Microbiology. 2010;156:1619–31. doi: 10.1099/mic.0.038133-0. [DOI] [PubMed] [Google Scholar]
- JARLIER V, NIKAIDO H. Mycobacterial cell wall: Structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 1994;123:11–18. doi: 10.1111/j.1574-6968.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- JIANG T, HE L, ZHAN Y, ZANG S, MA Y, ZHAO X, ZHANG C, XIN Y. The effect of MSMEG_6402 gene disruption on the cell wall structure of Mycobacterium smegmatis. Microb Pathog. 2011;51:156–60. doi: 10.1016/j.micpath.2011.04.005. [DOI] [PubMed] [Google Scholar]
- JIN Y, XIN Y, ZHANG W, MA Y. Mycobacterium tuberculosis Rv1302 and Mycobacterium smegmatis MSMEG_4947 have WecA function and MSMEG_4947 is required for the growth of M. smegmatis. FEMS Microbiol Lett. 2010;310:54–61. doi: 10.1111/j.1574-6968.2010.02045.x. [DOI] [PubMed] [Google Scholar]
- JOE M, SUN D, TAHA H, COMPLETO GC, CROUDACE JE, LAMMAS DA, BESRA GS, LOWARY TL. The 5-deoxy-5-methylthio-xylofuranose residue in mycobacterial lipoarabinomannan. Absolute stereochemistry, linkage position, conformation, and immunomodulatory activity. J Am Chem Soc. 2006;128:5059–5072. doi: 10.1021/ja057373q. [DOI] [PubMed] [Google Scholar]
- JULIAN E, MATAS L, ALCAIDE J, LUQUIN M. Comparison of antibody responses to a potential combination of specific glycolipids and proteins for test sensitivity improvement in tuberculosis serodiagnosis. Clin Diagn Lab Immunol. 2004;11:70–6. doi: 10.1128/CDLI.11.1.70-76.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN E, MATAS L, PEREZ A, ALCAIDE J, LANEELLE MA, LUQUIN M. Serodiagnosis of tuberculosis: comparison of immunoglobulin A (IgA) response to sulfolipid I with IgG and IgM responses to 2,3-diacyltrehalose, 2,3,6-triacyltrehalose, and cord factor antigens. J Clin Microbiol. 2002;40:3782–8. doi: 10.1128/JCM.40.10.3782-3788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAASEN I, FALKENBERG P, STYRVOLD OB, STROM AR. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by katF (AppR) J Bacteriol. 1992;174:889–98. doi: 10.1128/jb.174.3.889-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALSCHEUER R, SYSON K, VEERARAGHAVAN U, WEINRICK B, BIERMANN KE, LIU Z, SACCHETTINI JC, BESRA GS, BORNEMANN S, JACOBS WR., JR. Self-poisoning of Mycobacterium tuberculosis by targeting GlgE in an alpha-glucan pathway. Nature Chemical Biology. 2010;6:376–384. doi: 10.1038/nchembio.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALSCHEUER R, WEINRICK B, VEERARAGHAVAN U, BESRA GS, JACOBS WR., JR. Trehalose-recycling ABC transporter LpqY-SugA-SugB-SugC is essential for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2011;107:21761–6. doi: 10.1073/pnas.1014642108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANISTANON D, HAJJAR AM, PELLETIER MR, GALLAGHER LA, KALHORN T, SHAFFER SA, GOODLETT DR, ROHMER L, BRITTNACHER MJ, SKERRETT SJ, ERNST RK. A Francisella mutant in lipid A carbohydrate modification elicits protective immunity. PLoS Pathog. 2008;4:e24. doi: 10.1371/journal.ppat.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPRELYANTS AS, MUKAMOLOVA GV, RUGGIERO A, MAKAROV VA, DEMINA GR, SHLEEVA MO, POTAPOV VD, SHRAMKO PA. Resuscitation-promoting factors (Rpf): in search of inhibitors. Protein Pept Lett. 2012;19:1026–34. doi: 10.2174/092986612802762723. [DOI] [PubMed] [Google Scholar]
- KATTI MK, DAI G, ARMITIGE LY, RIVERA MARRERO C, DANIEL S, SINGH CR, LINDSEY DR, DHANDAYUTHAPANI S, HUNTER RL, JAGANNATH C. The Delta fbpA mutant derived from Mycobacterium tuberculosis H37Rv has an enhanced susceptibility to intracellular antimicrobial oxidative mechanisms, undergoes limited phagosome maturation and activates macrophages and dendritic cells. Cell Microbiol. 2008;10:1286–303. doi: 10.1111/j.1462-5822.2008.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR D, BERG S, DINADAYALA P, GICQUEL B, CHATTERJEE D, MCNEIL MR, VISSA V, CRICK DC, JACKSON M, BRENNAN PJ. Biosynthesis of mycobacterial lipoarabinomannan: Role of a branching mannosyltransferase. Proc. Natl. Acad. Sci. USA. 2006;103:13664–13669. doi: 10.1073/pnas.0603049103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR D, GUERIN ME, SKOVIEROVA H, BRENNAN PJ, JACKSON M. Chapter 2. Biogenesis of the cell wall and other glycoconjugates of Mycobacterium tuberculosis. Adv Appl Microbiol. 2009;69:23–78. doi: 10.1016/S0065-2164(09)69002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUR D, MCNEIL MR, KHOO K-H, CHATTERJEE D, CRICK DC, JACKSON M, BRENNAN PJ. New insights into the biosynthesis of mycobacterial lipomannan arising from deletion of a conserved gene. J. Biol. Chem. 2007;282:27133–27140. doi: 10.1074/jbc.M703389200. [DOI] [PubMed] [Google Scholar]
- KAUR D, OBREGÓN-HENAO A, PHAM H, CHATTERJEE D, BRENNAN PJ, JACKSON M. Lipoarabinomannan of Mycobacterium; mannose capping by a multifunctional terminal mannosyltransferase. Proc. Natl. Acad. Sci. USA. 2008;105:17973–17977. doi: 10.1073/pnas.0807761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHASNOBIS S, ZHANG J, ANGALA SK, G AA, MCNEIL MR, CRICK DC, CHATTERJEE D. Characterization of a specific arabinosyltransferase activity involved in mycobacterial arabinan synthesis. Chem. Biol. 2006;13:787–795. doi: 10.1016/j.chembiol.2006.05.016. [DOI] [PubMed] [Google Scholar]
- KHOO K-H, DELL A, MORRIS HR, BRENNAN PJ, CHATTERJEE D. Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium tuberculosis: definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology. 1995a;5:117–127. doi: 10.1093/glycob/5.1.117. [DOI] [PubMed] [Google Scholar]
- KHOO KH, DELL A, MORRIS HR, BRENNAN PJ, CHATTERJEE D. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem. 1995b;270:12380–9. doi: 10.1074/jbc.270.21.12380. [DOI] [PubMed] [Google Scholar]
- KOGA T, FUKUOKA T, DOI N, HARASAKI T, INOUE H, HOTODA H, KAKUTA M, MURAMATSU Y, YAMAMURA N, HOSHI M, HIROTA T. Activity of capuramycin analogues against Mycobacterium tuberculosis, Mycobacterium avium and Mycobacterium intracellulare in vitro and in vivo. J Antimicrob Chemother. 2004;54:755–60. doi: 10.1093/jac/dkh417. [DOI] [PubMed] [Google Scholar]
- KOLLY GS, BOLDRIN F, SALA C, DHAR N, HARTKOORN RC, VENTURA M, SERAFINI A, MCKINNEY JD, MANGANELLI R, COLE ST. Assessing the essentiality of the decaprenyl-phospho-d-arabinofuranose pathway in Mycobacterium tuberculosis using conditional mutants. Mol Microbiol. 2014 doi: 10.1111/mmi.12546. [DOI] [PubMed] [Google Scholar]
- KONDREDDI RR, JIRICEK J, RAO SP, LAKSHMINARAYANA SB, CAMACHO LR, RAO R, HERVE M, BIFANI P, MA NL, KUHEN K, GOH A, CHATTERJEE AK, DICK T, DIAGANA TT, MANJUNATHA UH, SMITH PW. Design, Synthesis, and Biological Evaluation of Indole-2-carboxamides: A Promising Class of Antituberculosis Agents. J Med Chem. 2013;56:8849–59. doi: 10.1021/jm4012774. [DOI] [PubMed] [Google Scholar]
- KORDULÁKOVÁ J, GILLERON M, MIKUSOVA K, PUZO G, BRENNAN PJ, GICQUEL B, JACKSON M. Definition of the first mannosylation step in phosphatidylinositol synthesis: PimA is essential for growth of mycobacteria. J. Biol. Chem. 2002;277:31335–31344. doi: 10.1074/jbc.M204060200. [DOI] [PubMed] [Google Scholar]
- KORDULÁKOVÁ J, GILLERON M, PUZO G, BRENNAN PJ, GICQUEL B, MIKUSOVA K, JACKSON M. Identification of the required acyltransferase step in the biosynthesis of the phosphatidylinositol mannosides of Mycobacterium species. J. Biol. Chem. 2003;278:36285–36295. doi: 10.1074/jbc.M303639200. [DOI] [PubMed] [Google Scholar]
- KORRES H, MAVRIS M, MORONA R, MANNING PA, VERMA NK. Topological analysis of GtrA and GtrB proteins encoded by the serotype -converting cassette of Shigella flexneri. Biochem Biophys Res Commun. 2005;328:1252–1260. doi: 10.1016/j.bbrc.2005.01.087. [DOI] [PubMed] [Google Scholar]
- KOVACEVIC S, ANDERSON D, MORITA YS, PATTERSON J, HAITES R, MCMILLAN BN, COPPEL RL, MCCONVILLE MJ, BILLMAN-JACOBE H. Identification of a novel protein with a role in lipoarabinomannan biosynthesis in mycobacteria. J Biol Chem. 2006;281:9011–9017. doi: 10.1074/jbc.M511709200. [DOI] [PubMed] [Google Scholar]
- KREMER L, DOVER LG, MOREHOUSE C, HITCHIN P, EVERETT M, MORRIS HR, DELL A, BRENNAN PJ, MCNEIL MR, FLAHERTY C, DUNCAN K, BESRA GS. Galactan biosynthesis in Mycobacterium tuberculosis. Identification of a bifunctional UDP-galactofuranosyltransferase. J. Biol. Chem. 2001;276:26430–26440. doi: 10.1074/jbc.M102022200. [DOI] [PubMed] [Google Scholar]
- KREMER L, GURCHA SS, BIFANI P, HITCHEN PG, BAULARD A, MORRIS HR, DELL A, BRENNAN PJ, BESRA GS. Characterization of a putative α-mannosyltransferase involved in phosphatidylinositol trimannoside biosynthesis in Mycobacterium tuberculosis. Biochem. J. 2002;363:437–447. doi: 10.1042/0264-6021:3630437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNAN N, MALAGA W, CONSTANT P, CAWS M, TRAN TH, SALMONS J, NGUYEN TN, NGUYEN DB, DAFFE M, YOUNG DB, ROBERTSON BD, GUILHOT C, THWAITES GE. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One. 2011;6:e23870. doi: 10.1371/journal.pone.0023870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR P, ARORA K, LLOYD JR, LEE IY, NAIR V, FISCHER E, BOSHOFF HI, BARRY CE., 3RD Meropenem inhibits D,D-carboxypeptidase activity in Mycobacterium tuberculosis. Mol Microbiol. 2012;86:367–81. doi: 10.1111/j.1365-2958.2012.08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUMAR P, SCHELLE MW, JAIN M, LIN FL, PETZOLD CJ, LEAVELL MD, LEARY JA, COX JS, BERTOZZI CR. PapA1 and PapA2 are acyltransferases essential for the biosynthesis of the Mycobacterium tuberculosis virulence factor sulfolipid-1. Proc. Natl. Acad. Sci. USA. 2007;104:11221–11226. doi: 10.1073/pnas.0611649104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LA ROSA V, POCE G, CANSECO JO, BURONI S, PASCA MR, BIAVA M, RAJU RM, PORRETTA GC, ALFONSO S, BATTILOCCHIO C, JAVID B, SORRENTINO F, IOERGER TR, SACCHETTINI JC, MANETTI F, BOTTA M, DE LOGU A, RUBIN EJ, DE ROSSI E. MmpL3 Is the Cellular Target of the Antitubercular Pyrrole Derivative BM212. Antimicrob Agents Chemother. 2012;56:324–331. doi: 10.1128/AAC.05270-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAI X, WU J, CHEN S, ZHANG X, WANG H. Expression, purification, and characterization of a functionally active Mycobacterium tuberculosis UDP-glucose pyrophosphorylase. Protein Expr Purif. 2008;61:50–6. doi: 10.1016/j.pep.2008.05.015. [DOI] [PubMed] [Google Scholar]
- LAMICHHANE G, TYAGI S, BISHAI WR. Designer arrays for defined mutant analysis to detect genes essential for survival of Mycobacterium tuberculosis in mouse lungs. Infect Immun. 2005;73:2533–2540. doi: 10.1128/IAI.73.4.2533-2540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANG R. Recognition of the mycobacterial cord factor by Mincle: relevance for granuloma formation and resistance to tuberculosis. Front Immunol. 2013;4:5. doi: 10.3389/fimmu.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARROUY-MAUMUS G, ŠKOVIEROVÁ H, DHOUIB R, ANGALA SK, ZUBEROGOITIA S, PHAM H, DRUMOND VILLELA A, MIKUŠOVÁ K, NOGUERA A, GILLERON M, VALENTINOVA L, KORDULÁKOVÁ J, BRENNAN PJ, PUZO G, NIGOU J, JACKSON M. A small multidrug resistance-like transporter involved in the arabinosylation of arabinogalactan and lipoarabinomannan in mycobacteria. J Biol Chem. 2012;287:39933–39941. doi: 10.1074/jbc.M112.400986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVOLLAY M, ARTHUR M, FOURGEAUD M, DUBOST L, MARIE A, VEZIRIS N, BLANOT D, GUTMANN L, MAINARDI JL. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by L,D-transpeptidation. J Bacteriol. 2008;190:4360–6. doi: 10.1128/JB.00239-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEA-SMITH DJ, MARTIN KL, PYKE JS, TULL D, MCCONVILLE MJ, COPPEL RL, CRELLIN PK. Analysis of a new mannosyltransferase required for the synthesis of phosphatidylinositol mannosides and lipoarabinomannan reveals two lipomannan pools in Corynebacterineae. J. Biol. Chem. 2008;283:6773–6782. doi: 10.1074/jbc.M707139200. [DOI] [PubMed] [Google Scholar]
- LEE A, WU S-W, SCHERMAN MS, TORRELLES JB, CHATTERJEE C, MCNEIL MR, KHOO K-H. Sequencing of the oligoarabinosyl units released from mycobacterial arabinogalactan by endogenous arabinanase: Identification of distinctive and novel structural motifs. Biochemistry. 2006;45:15817–15828. doi: 10.1021/bi060688d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE JS, KRAUSE R, SCHREIBER J, MOLLENKOPF HJ, KOWALL J, STEIN R, JEON BY, KWAK JY, SONG MK, PATRON JP, JORG S, ROH K, CHO SN, KAUFMANN SH. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe. 2008;3:97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- LEE KS, DUBEY VS, KOLATTUKUDY PE, SONG CH, SHIN AR, JUNG SB, YANG CS, KIM SY, JO EK, PARK JK, KIM HJ. Diacyltrehalose of Mycobacterium tuberculosis inhibits lipopolysaccharide- and mycobacteria-induced proinflammatory cytokine production in human monocytic cells. FEMS Microbiol Lett. 2007;267:121–8. doi: 10.1111/j.1574-6968.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- LEE RE, BRENNAN PJ, BESRA GS. Mycobacterial arabinan biosynthesis: the use of synthetic arabinoside acceptors in the development of an arabinosyl transfer assay. Glycobiology. 1997;7:1121–1128. doi: 10.1093/glycob/7.8.1121. [DOI] [PubMed] [Google Scholar]
- LEE RE, BRENNAN PJ, BESRA GS. Synthesis of β-D-arabinofuranosyl-1-monophosphoryl polyprenols: examination of their function as mycobacterial arabinosyl transferase donors. Biorog. Med. Chem. Lett. 1998;8:951–954. doi: 10.1016/s0960-894x(98)00147-4. [DOI] [PubMed] [Google Scholar]
- LEE W, VANDERVEN BC, FAHEY RJ, RUSSELL DG. Intracellular Mycobacterium tuberculosis Exploits Host-derived Fatty Acids to Limit Metabolic Stress. J Biol Chem. 2013;288:6788–800. doi: 10.1074/jbc.M112.445056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE Y, MOOTIEN S, SHOEN C, DESTEFANO M, CIRILLO P, ASOJO OA, YEUNG KR, LEDIZET M, CYNAMON MH, ARISTOFF PA, KOSKI RA, KAPLAN PA, ANTHONY KG. Inhibition of mycobacterial alanine racemase activity and growth by thiadiazolidinones. Biochem Pharmacol. 2013;86:222–30. doi: 10.1016/j.bcp.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIBA J, SYSON K, BARONIAN G, ZANELLA-CLEON I, KALSCHEUER R, KREMER L, BORNEMANN S, MOLLE V. Mycobacterium tuberculosis maltosyltransferase GlgE, a genetically validated antituberculosis target, is negatively regulated by Ser/Thr phosphorylation. J Biol Chem. 2013;288:16546–56. doi: 10.1074/jbc.M112.398503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMASSU A, DAFFÉ M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem. J. 1994;297:351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMASSU A, LANÉELLE M-A, DAFFÉ M. Revised structure of a trehalose-containing immunoreactive glycolipid of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 1991;78:171–176. doi: 10.1016/0378-1097(91)90153-2. [DOI] [PubMed] [Google Scholar]
- LEMASSU A, LEVY-FREBAULT VV, LANEELLE MA, DAFFE M. Lack of correlation between colony morphology and lipooligosaccharide content in the Mycobacterium tuberculosis complex. J Gen Microbiol. 1992;138:1535–41. doi: 10.1099/00221287-138-7-1535. [DOI] [PubMed] [Google Scholar]
- LENGELER KB, TIELKER D, ERNST JF. Protein-O-mannosyltransferases in virulence and development. Cell. Mol. Life Sci. 2007 doi: 10.1007/s00018-007-7409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI S, KANG J, YU W, ZHOU Y, ZHANG W, XIN Y, MA Y. Identification of M. tuberculosis Rv3441c and M. smegmatis MSMEG_1556 and essentiality of M. smegmatis MSMEG_1556. PLoS One. 2012;7:e42769. doi: 10.1371/journal.pone.0042769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI W, XIN Y, MCNEIL MR, MA Y. rmlB and rmlC genes are essential for growth of mycobacteria. Biochem Biophys Res Commun. 2006;342:170–178. doi: 10.1016/j.bbrc.2006.01.130. [DOI] [PubMed] [Google Scholar]
- LINARES C, BERNABEU A, LUQUIN M, VALERO-GUILLEN PL. Cord factors from atypical mycobacteria (Mycobacterium alvei, Mycobacterium brumae) stimulate the secretion of some pro-inflammatory cytokines of relevance in tuberculosis. Microbiology. 2012;158:2878–85. doi: 10.1099/mic.0.060681-0. [DOI] [PubMed] [Google Scholar]
- LIU CF, TONINI L, MALAGA W, BEAU M, STELLA A, BOUYSSIE D, JACKSON MC, NIGOU J, PUZO G, GUILHOT C, BURLET-SCHILTZ O, RIVIERE M. Bacterial protein-O-mannosylating enzyme is crucial for virulence of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 2013;110:6560–5. doi: 10.1073/pnas.1219704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOUGHEED KE, OSBORNE SA, SAXTY B, WHALLEY D, CHAPMAN T, BOULOC N, CHUGH J, NOTT TJ, PATEL D, SPIVEY VL, KETTLEBOROUGH CA, BRYANS JS, TAYLOR DL, SMERDON SJ, BUXTON RS. Effective inhibitors of the essential kinase PknB and their potential as anti-mycobacterial agents. Tuberculosis (Edinb) 2011;91:277–86. doi: 10.1016/j.tube.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUDWICZAK P, GILLERON M, BORDAT Y, MARTIN C, GICQUEL B, PUZO G. Mycobacterium tuberculosis phoP mutant: lipoarabinomannan molecular structure. Microbiology. 2002;148:3029–3037. doi: 10.1099/00221287-148-10-3029. [DOI] [PubMed] [Google Scholar]
- LY D, KASMAR AG, CHENG TY, DE JONG A, HUANG S, ROY S, BHATT A, VAN SUMMEREN RP, ALTMAN JD, JACOBS WR, JR., ADAMS EJ, MINNAARD AJ, PORCELLI SA, MOODY DB. CD1c tetramers detect ex vivo T cell responses to processed phosphomycoketide antigens. J Exp Med. 2013;210:729–41. doi: 10.1084/jem.20120624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNETT J, STOKES RW. Selection of transposon mutants of Mycobacterium tuberculosis with increased macrophage infectivity identifies fadD23 to be involved in sulfolipid production and association with macrophages. Microbiology. 2007;153:3133–40. doi: 10.1099/mic.0.2007/007864-0. [DOI] [PubMed] [Google Scholar]
- MA Y, MILLS JA, BELISLE JT, VISSA V, HOWELL M, BOWLIN K, SCHERMAN MS, MCNEIL MR. Determination of the pathway for rhamnose biosynthesis in mycobacteria: cloning, sequencing and expression of the Mycobacterium tuberculosis gene encoding alpha-D-glucose-1-phosphate thymidylyltransferase. Microbiology. 1997;143:937–945. doi: 10.1099/00221287-143-3-937. [DOI] [PubMed] [Google Scholar]
- MA Y, PAN F, MCNEIL MR. Formation of dTDP-rhamnose is essential for growth of mycobacteria. J. Bacteriol. 2002;184:3392–3395. doi: 10.1128/JB.184.12.3392-3395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MA Y, STERN RJ, SCHERMAN MS, VISSA VD, YAN W, JONES VC, ZHANG F, FRANZBLAU SG, LEWIS WH, MCNEIL MR. Drug targeting Mycobacterium tuberculosis cell wall synthesis: Genetics of dTDP-Rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrob. Agents Chemother. 2001;45:1407–1416. doi: 10.1128/AAC.45.5.1407-1416.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGNET S, HARTKOORN RC, SZEKELY R, PATO J, TRICCAS JA, SCHNEIDER P, SZANTAI-KIS C, ORFI L, CHAMBON M, BANFI D, BUENO M, TURCATTI G, KERI G, COLE ST. Leads for antitubercular compounds from kinase inhibitor library screens. Tuberculosis (Edinb) 2010;90:354–60. doi: 10.1016/j.tube.2010.09.001. [DOI] [PubMed] [Google Scholar]
- MAHAPATRA S, BASU J, BRENNAN PJ, CRICK DC. Structure, biosynthesis and genetics of the mycolic acid-arabinogalactan-peptidoglycan complex. In: COLE ST, EISENACH KD, MCMURRAY DN, JACOBS WR JR., editors. Tuberculosis and the Tubercle Bacillus. ASM Press; Washington, D.C.: 2005. [Google Scholar]
- MAKAROV V, MANINA G, MIKUSOVA K, MOLLMANN U, RYABOVA O, SAINT-JOANIS B, DHAR N, PASCA MR, BURONI S, LUCARELLI AP, MILANO A, DE ROSSI E, BELANOVA M, BOBOVSKA A, DIANISKOVA P, KORDULAKOVA J, SALA C, FULLAM E, SCHNEIDER P, MCKINNEY JD, BRODIN P, CHRISTOPHE T, WADDELL S, BUTCHER P, ALBRETHSEN J, ROSENKRANDS I, BROSCH R, NANDI V, BHARATH S, GAONKAR S, SHANDIL RK, BALASUBRAMANIAN V, BALGANESH T, TYAGI S, GROSSET J, RICCARDI G, COLE ST. Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan synthesis. Science. 2009;324:801–804. doi: 10.1126/science.1171583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALAGA W, CONSTANT P, EUPHRASIE D, CATALDI A, DAFFÉ M, REYRAT J-M, GUILHOT C. Deciphering the genetic bases of the structural diversity of phenolic glycolipids in strains of the Mycobacterium tuberculosis complex. J. Biol. Chem. 2008;283:15177–15184. doi: 10.1074/jbc.M710275200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARLAND Z, BEDDOE T, ZAKER-TABRIZI L, LUCET IS, BRAMMANANTH R, WHISSTOCK JC, WILCE MC, COPPEL RL, CRELLIN PK, ROSSJOHN J. Hijacking of a substrate-binding protein scaffold for use in mycobacterial cell wall biosynthesis. J Mol Biol. 2006;359:983–997. doi: 10.1016/j.jmb.2006.04.012. [DOI] [PubMed] [Google Scholar]
- MAROLDA CL, TATAR LD, ALAIMO C, AEBI M, VALVANO MA. Interplay of the Wzx translocase and the corresponding polymerase and chain length regulator proteins in the translocation and periplasmic assembly of lipopolysaccharide O antigen. J. Bacteriol. 2006;188:5124–5135. doi: 10.1128/JB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARRAKCHI H, BARDOU F, LANEELLE M-A, DAFFÉ M. A comprehensive overview of mycolic acid structure and biosynthesis. In: DAFFÉ M, REYRAT J-M, editors. The mycobacterial cell envelope. ASM Press; Washington, DC: 2008. [Google Scholar]
- MARUTA K, HATTORI K, NAKADA T, KUBOTA M, SUGIMOTO T, KURIMOTO M. Cloning and sequencing of trehalose biosynthesis genes from Rhizobium sp. M-11. Biosci Biotechnol Biochem. 1996;60:717–20. doi: 10.1271/bbb.60.717. [DOI] [PubMed] [Google Scholar]
- MARUTA K, HATTORI K, NAKADA T, KUBOTA M, SUGIMOTO T, KURIMOTO M. Cloning and sequencing of trehalose biosynthesis genes from Arthrobacter sp. Q36. Biochim Biophys Acta. 1996;1289:10–3. doi: 10.1016/0304-4165(95)00139-5. [DOI] [PubMed] [Google Scholar]
- MARUTA K, MITSUZUMI H, NAKADA T, KUBOTA M, CHAEN H, FUKUDA S, SUGIMOTO T, KURIMOTO M. Cloning and sequencing of a cluster of genes encoding novel enzymes of trehalose biosynthesis from thermophilic archaebacterium Sulfolobus acidocaldarius. Biochim Biophys Acta. 1996;1291:177–81. doi: 10.1016/s0304-4165(96)00082-7. [DOI] [PubMed] [Google Scholar]
- MATSUHASHI M. Biosynthesis in the bacterial cell wall. Tanpakushitsu Kakusan Koso. 1966;11:875–886. [PubMed] [Google Scholar]
- MATSUNAGA I, BHATT A, YOUNG DC, CHENG T-Y, EYLES SJ, BESRA GS, BRIKEN V, PORCELLI SA, COSTELLO CE, JACOBS WR, JR, MOODY DB. Mycobacterium tuberculosis pks12 produces a novel polyketide presented by CD1c to T cells. J Exp Med. 2004;200:1559–1569. doi: 10.1084/jem.20041429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATSUNAGA I, SUGITA M. Mycoketide: a CD1c-presented antigen with important implications in mycobacterial infection. Clin Dev Immunol. 2012;2012:981821. doi: 10.1155/2012/981821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCNEIL M, DAFFE M, BRENNAN PJ. Evidence for the nature of the link between the arabinogalactan and peptidoglycan of mycobacterial cell walls. J Biol Chem. 1990;265:18200–6. [PubMed] [Google Scholar]
- MCNEIL MR, DAFFÉ M, BRENNAN PJ. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J. Biol. Chem. 1991;266:13217–13223. [PubMed] [Google Scholar]
- MENDES V, MARANHA A, LAMOSA P, DA COSTA MS, EMPADINHAS N. Biochemical characterization of the maltokinase from Mycobacterium bovis BCG. BMC Biochem. 2010;11:21. doi: 10.1186/1471-2091-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIAH F, KOLIWER-BRANDL H, REJZEK M, FIELD RA, KALSCHEUER R, BORNEMANN S. Flux through trehalose synthase flows from trehalose to the alpha anomer of maltose in mycobacteria. Chem Biol. 2013;20:487–93. doi: 10.1016/j.chembiol.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHELL SL, WHELAN AO, WHEELER PR, PANICO M, EASTON RL, ETIENNE T, HASLAM SM, DELL A, MORRIS HR, REASON AJ, HERRMANN J-L, YOUNG DB, HEWINSON RG. The MBP83 antigen from Mycobacterium bovis contains O-linked mannose and (1->3)-mannobiose moieties. J. Biol. Chem. 2003;278:16423–16432. doi: 10.1074/jbc.M207959200. [DOI] [PubMed] [Google Scholar]
- MIKUSOVA K, HUANG H, YAGI T, HOLSTERS M, VEREECKE D, D'HAEZE W, SCHERMAN MS, BRENNAN PJ, MCNEIL MR, CRICK DC. Decaprenylphosphoryl arabinofuranose, the donor of the D-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-step epimerization of decaprenylphosphoryl ribose. J. Bacteriol. 2005;187:8020–8025. doi: 10.1128/JB.187.23.8020-8025.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIKUSOVA K, MIKUS M, BESRA GS, HANCOCK I, BRENNAN PJ. Biosynthesis of the linkage region of the mycobacterial cell wall. J. Biol. Chem. 1996;271:7820–7828. doi: 10.1074/jbc.271.13.7820. [DOI] [PubMed] [Google Scholar]
- MIKUSOVA K, YAGI T, STERN R, MCNEIL MR, BESRA GS, CRICK DC, BRENNAN PJ. Biosynthesis of the galactan component of the mycobacterial cell wall. J. Biol. Chem. 2000;275:33890–33897. doi: 10.1074/jbc.M006875200. [DOI] [PubMed] [Google Scholar]
- MILLS JA, MOTICHKA K, JUCKER M, WU HP, UHLIK BC, STERN RJ, SCHERMAN MS, VISSA VD, PAN F, KUNDU M, MA YF, MCNEIL MR. Inactivation of the mycobacterial rhamnosyltransferase, which is needed for the formation of the arabinogalactan-peptidoglycan linker, leads to irreversible loss of viability. J. Biol. Chem. 2004;279:43540–43546. doi: 10.1074/jbc.M407782200. [DOI] [PubMed] [Google Scholar]
- MINNIKIN DE. Lipids: Complex lipids, their chemistry, biosynthesis and roles. In: RATLEDGE C, STANFORD J, editors. The Biology of Mycobacteria. Academic Press; London: 1982. [Google Scholar]
- MINNIKIN DE, DOBSON G, SESARDIC D, RIDELL M. Mycolipenates and mycolipanolates of trehalose from Mycobacterium tuberculosis. J. Gen. Microbiol. 1985;131:1369–1374. doi: 10.1099/00221287-131-6-1369. [DOI] [PubMed] [Google Scholar]
- MIR M, ASONG J, LI X, CARDOT J, BOONS GJ, HUSSON RN. The extracytoplasmic domain of the Mycobacterium tuberculosis Ser/Thr kinase PknB binds specific muropeptides and is required for PknB localization. PLoS Pathog. 2011;7:e1002182. doi: 10.1371/journal.ppat.1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA AK, ALDERWICK LJ, RITTMANN D, TATITURI RV, NIGOU J, GILLERON M, EGGELING L, BESRA GS. Identification of an alpha(1->6) mannopyranosyltransferase (MptA), involved in Corynebacterium glutamicum lipomannan biosynthesis, and identification of its orthologue in Mycobacterium tuberculosis. Mol Microbiol. 2007;65:1503–1517. doi: 10.1111/j.1365-2958.2007.05884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA AK, ALDERWICK LJ, RITTMANN D, WANG C, BHATT A, JACOBS WR, JR., TAKAYAMA K, EGGELING L, BESRA GS. Identification of a novel alpha(1->6) mannopyranosyltransferase MptB from Corynebacterium glutamicum by deletion of a conserved gene, NCgl1505, affords a lipomannan- and lipoarabinomannan-deficient mutant. Mol. Microbiol. 2008b;68:1595–1613. doi: 10.1111/j.1365-2958.2008.06265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA AK, DRIESSEN NN, APPELMELK BJ, BESRA GS. Lipoarabinomannan and related glycoconjugates: structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev. 2011;35:1126–57. doi: 10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISHRA AK, KLEIN C, GURCHA SS, ALDERWICK LJ, BABU P, HITCHEN PG, MORRIS HR, DELL A, BESRA GS, EGGELING L. Structural characterization and functional properties of a novel lipomannan variant isolated from a Corynebacterium glutamicum pimB' mutant. Antonie Van Leeuwenhoek. 2008a;94:277–87. doi: 10.1007/s10482-008-9243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKE Y, TOYONAGA K, MORI D, KAKUTA S, HOSHINO Y, OYAMADA A, YAMADA H, ONO K, SUYAMA M, IWAKURA Y, YOSHIKAI Y, YAMASAKI S. C-type lectin MCL is an FcRgamma-coupled receptor that mediates the adjuvanticity of mycobacterial cord factor. Immunity. 2013;38:1050–62. doi: 10.1016/j.immuni.2013.03.010. [DOI] [PubMed] [Google Scholar]
- MOHAMMADI T, VAN DAM V, SIJBRANDI R, VERNET T, ZAPUN A, BOUHSS A, DIEPEVEEN-DE BRUIN M, NGUYEN-DISTECHE M, DE KRUIJFF B, BREUKINK E. Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. Embo J. 2011;30:1425–32. doi: 10.1038/emboj.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLE V, KREMER L. Division and cell envelope regulation by Ser/Thr phosphorylation: Mycobacterium shows the way. Mol Microbiol. 2010;75:1064–77. doi: 10.1111/j.1365-2958.2009.07041.x. [DOI] [PubMed] [Google Scholar]
- MOLLE V, KREMER L, GIRARD-BLANC C, BESRA GS, COZZONE AJ, PROST JF. An FHA phosphoprotein recognition domain mediates protein EmbR phosphorylation by PknH, a Ser/Thr protein kinase from Mycobacterium tuberculosis. Biochemistry. 2003;42:15300–9. doi: 10.1021/bi035150b. [DOI] [PubMed] [Google Scholar]
- MOODY DB, ULRICHS T, MUHLECKER W, YOUNG DC, GURCHA SS, GRANT E, ROSAT JP, BRENNER MB, COSTELLO CE, BESRA GS, PORCELLI SA. CD1c-mediated T-cell recognition of isoprenoid glycolipids in Mycobacterium tuberculosis infection. Nature. 2000;404:884–8. doi: 10.1038/35009119. [DOI] [PubMed] [Google Scholar]
- MORITA YS, FUKUDA T, SENA CB, YAMARYO-BOTTE Y, MCCONVILLE MJ, KINOSHITA T. Inositol lipid metabolism in mycobacteria: biosynthesis and regulatory mechanisms. Biochim Biophys Acta. 2011;1810:630–41. doi: 10.1016/j.bbagen.2011.03.017. [DOI] [PubMed] [Google Scholar]
- MORITA YS, SENA CCB, WALLER RF, KUROKAWA K, SERNEE MF, NAKATANI F, HAITES RE, BILLMAN-JACOBE H, MCCONVILLE MJ, MAEDA Y, KINOSHITA T. PimE is a polyprenol-phosphate-mannose-dependent mannosyltransferase that transfers the fifth mannose of phosphatidylinositol mannoside in mycobacteria. J. Biol. Chem. 2006;281:25143–25155. doi: 10.1074/jbc.M604214200. [DOI] [PubMed] [Google Scholar]
- MORITA YS, VELASQUEZ R, TAIG E, WALLER RF, PATTERSON JH, TULL D, WILLIAMS SJ, BILLMAN-JACOBE H, MCCONVILLE MJ. Compartmentalization of lipid biosynthesis in mycobacteria. J. Biol. Chem. 2005;280:21645–21652. doi: 10.1074/jbc.M414181200. [DOI] [PubMed] [Google Scholar]
- MOUGOUS JD, PETZOLD CJ, SERARATNE RH, LEE DH, AKEY DL, LIN FL, MUNCHEL SE, PRATT MR, RILEY LW, LEARY JA, BERGER JM, BERTOZZI CR. Identification, function and structure of the mycobacterial sulfotransferase that initiates sulfolipid-1 biosynthesis. Nat Struct Mol Biol. 2004;11:721–729. doi: 10.1038/nsmb802. [DOI] [PubMed] [Google Scholar]
- MUNOZ M, LANÉELLE M-A, LUQUIN M, TORRELLES J, JULIAN E, AUSINA V, DAFFÉ M. Occurence of an antigenic triacyl trehalose in clinical isolates and reference strains of Mycobacterium tuberculosis. FEMS Microbiol. Lett. 1997;157:251–259. doi: 10.1111/j.1574-6968.1997.tb12781.x. [DOI] [PubMed] [Google Scholar]
- MURAKAMI S. Multidrug efflux transporter, AcrB--the pumping mechanism. Curr Opin Struct Biol. 2008;18:459–65. doi: 10.1016/j.sbi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- MURPHY HN, STEWART GR, MISCHENKO VV, APT AS, HARRIS R, MCALISTER MSB, DRISCOLL PC, YOUNG DB, ROBERTSON BD. The OtsAB pathway is essential for trehalose biosynthesis in Mycobacterium tuberculosis. J. Biol. Chem. 2005;280:14524–14529. doi: 10.1074/jbc.M414232200. [DOI] [PubMed] [Google Scholar]
- NAKADA T, MARUTA K, TSUSAKI K, KUBOTA M, CHAEN H, SUGIMOTO T, KURIMOTO M, TSUJISAKA Y. Purification and properties of a novel enzyme, maltooligosyl trehalose synthase, from Arthrobacter sp. Q6. Biosci Biotechnol Biochem. 1995;59:2210–4. doi: 10.1271/bbb.59.2210. [DOI] [PubMed] [Google Scholar]
- NAMBIAR JK, PINTO R, AGUILO JI, TAKATSU K, MARTIN C, BRITTON WJ, TRICCAS JA. Protective immunity afforded by attenuated, PhoP-deficient Mycobacterium tuberculosis is associated with sustained generation of CD4+ T-cell memory. Eur J Immunol. 2012;42:385–92. doi: 10.1002/eji.201141903. [DOI] [PubMed] [Google Scholar]
- NERES J, POJER F, MOLTENI E, CHIARELLI LR, DHAR N, BOY-ROTTGER S, BURONI S, FULLAM E, DEGIACOMI G, LUCARELLI AP, READ RJ, ZANONI G, EDMONDSON DE, DE ROSSI E, PASCA MR, MCKINNEY JD, DYSON PJ, RICCARDI G, MATTEVI A, COLE ST, BINDA C. Structural basis for benzothiazinone-mediated killing of Mycobacterium tuberculosis. Sci Transl Med. 2012;4:150ra121. doi: 10.1126/scitranslmed.3004395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON GL, BUCHMEIER N, FAHEY RC. Biosynthesis and functions of mycothiol, the unique protective thiol of Actinobacteria. Microbiol. Mol. Biol. Rev. 2008;72:471–494. doi: 10.1128/MMBR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEYROLLES O, GUILHOT C. Recent advances in deciphering the contribution of Mycobacterium tuberculosis lipids to pathogenesis. Tuberculosis (Edinb) 2011;91:187–95. doi: 10.1016/j.tube.2011.01.002. [DOI] [PubMed] [Google Scholar]
- NIGOU J, GILLERON M, CAHUZAC B, BOUNERY JD, HEROLD M, THURNHER M, PUZO G. The phosphatidyl-myo-inositol anchor of the lipoarabinomannans from Mycobacterium bovis bacillus Calmette-Guérin. Heterogeneity, structure, and role in the regulation of cytokine secretion. J. Biol. Chem. 1997;272:23094–23103. doi: 10.1074/jbc.272.37.23094. [DOI] [PubMed] [Google Scholar]
- NIGOU J, VASSELON T, RAY A, CONSTANT P, GILLERON M, BESRA GS, SUTCLIFFE I, TIRABY G, PUZO G. Mannan chain length controls lipoglycans signaling via and binding to TLR2. J. Immunol. 2008;180:6696–6702. doi: 10.4049/jimmunol.180.10.6696. [DOI] [PubMed] [Google Scholar]
- NIKONENKO BV, REDDY VM, PROTOPOPOVA M, BOGATCHEVA E, EINCK L, NACY CA. Activity of SQ641, a capuramycin analog, in a murine model of tuberculosis. Antimicrob Agents Chemother. 2009;53:3138–9. doi: 10.1128/AAC.00366-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIMOTO T, NAKANO M, NAKADA T, CHAEN H, FUKUDA S, SUGIMOTO T, KURIMOTO M, TSUJISAKA Y. Purification and properties of a novel enzyme, trehalose synthase, from Pimelobacter sp. R48. Biosci Biotechnol Biochem. 1996;60:640–4. doi: 10.1271/bbb.60.640. [DOI] [PubMed] [Google Scholar]
- NOLL H, BLOCH H, ASSELINEAU J, LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956;20:299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- NORTH EJ, JACKSON M, LEE RE. New Approaches to Target the Mycolic Acid Biosynthesis Pathway for the Development of Tuberculosis Therapeutics. Curr Pharm Des. 2013 doi: 10.2174/1381612819666131118203641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ONAJOLE OK, PIERONI M, TIPPARAJU SK, LUN S, STEC J, CHEN G, GUNOSEWOYO H, GUO H, AMMERMAN NC, BISHAI WR, KOZIKOWSKI AP. Preliminary structure-activity relationships and biological evaluation of novel antitubercular indolecarboxamide derivatives against drug-susceptible and drug-resistant Mycobacterium tuberculosis strains. J Med Chem. 2013;56:4093–103. doi: 10.1021/jm4003878. [DOI] [PubMed] [Google Scholar]
- ORTALO-MAGNÉ A, DUPONT MA, LEMASSU A, ANDERSEN AB, GOUNON P, DAFFÉ M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiol. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- ORTALO-MAGNÉ A, LEMASSU A, LANÉELLE MA, BARDOU F, SILVE G, GOUNON P, MARCHAL G, DAFFÉ M. Identification of the surface-exposed lipids on the cell envelopes of Mycobacterium tuberculosis and other mycobacterial species. J. Bacteriol. 1996;178:456–461. doi: 10.1128/jb.178.2.456-461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PABST MJ, GROSS JM, BROZNA JP, GOREN MB. Inhibition of macrophage priming by sulfatide from Mycobacterium tuberculosis. J. Immunol. 1988;140:634–640. [PubMed] [Google Scholar]
- PALMA-NICOLAS JP, HERNANDEZ-PANDO R, SEGURA E, IBARRA-SANCHEZ MJ, ESTRADA-GARCIA I, ZENTELLA-DEHESA A, LOPEZ-MARIN LM. Mycobacterial di-O-acyl trehalose inhibits Th-1 cytokine gene expression in murine cells by down-modulation of MAPK signaling. Immunobiology. 2010;215:143–52. doi: 10.1016/j.imbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- PAN F, JACKSON M, MA Y, MCNEIL M. Cell wall core galactofuran synthesis is essential for growth of mycobacteria. J. Bacteriol. 2001;183:3991–3998. doi: 10.1128/JB.183.13.3991-3998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN Y-T, CARROLL JD, ASANO N, PASTUSZAK I, EDAVANA VK, ELBEIN AD. Trehalose synthase converts glycogen to trehalose. FEBS Journal. 2008;275:3408–3420. doi: 10.1111/j.1742-4658.2008.06491.x. [DOI] [PubMed] [Google Scholar]
- PAN YT, KOROTH EDAVANA V, JOURDIAN WJ, EDMONDSON R, CARROLL JD, PASTUSZAK I, ELBEIN AD. Trehalose synthase of Mycobacterium smegmatis: purification, cloning, expression, and properties of the enzyme. Eur J Biochem. 2004;271:4259–69. doi: 10.1111/j.1432-1033.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- PARISH T, LIU J, NIKAIDO H, STOKER NG. A Mycobacterium smegmatis mutant with a defective inositol monophosphate phosphatase gene homolog has altered cell envelope permeability. J. Bacteriol. 1997;179:7827–7833. doi: 10.1128/jb.179.24.7827-7833.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASSEMAR C, ARBUES A, MALAGA W, MERCIER I, MOREAU F, LEPOURRY L, NEYROLLES O, GUILHOT C, ASTARIE-DEQUEKER C. Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host-pathogen interactions. Cell Microbiol. 2013 doi: 10.1111/cmi.12214. [DOI] [PubMed] [Google Scholar]
- PASTORET S, FRAIPONT C, DEN BLAAUWEN T, WOLF B, AARSMAN ME, PIETTE A, THOMAS A, BRASSEUR R, NGUYEN-DISTECHE M. Functional analysis of the cell division protein FtsW of Escherichia coli. J Bacteriol. 2004;186:8370–9. doi: 10.1128/JB.186.24.8370-8379.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTERSON JH, WALLER RF, JEEVARAJAH D, BILLMAN-JACOBE H, MCCONVILLE MJ. Mannose metabolism is required for mycobacterial growth. Biochem J. 2003;372:77–86. doi: 10.1042/BJ20021700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULSEN IT, BROWN MH, SKURRAY RA. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVELKA MS, JR., MAHAPATRA S, CRICK DC. Genetics of peptidoglycan biosynthesis. Microbiol Spectrum. 2014;2(4) doi: 10.1128/microbiolspec.MGM2-0034-2013. MGM2-0034-2013. doi:10.1128/microbiolspec.MGM2-0034-2013. [DOI] [PubMed] [Google Scholar]
- PENG W, ZOU L, BHAMIDI S, MCNEIL MR, LOWARY TL. The galactosamine residue in mycobacterial arabinogalactan is alpha-linked. J Org Chem. 2012;77:9826–32. doi: 10.1021/jo301393s. [DOI] [PubMed] [Google Scholar]
- PENUMARTI N, KHULLER GK. Subcellular distribution of mannophosphoinositides in Mycobacterium smegmatis during growth. Experientia. 1983;39:882–884. doi: 10.1007/BF01990417. [DOI] [PubMed] [Google Scholar]
- PITARQUE S, HERRMANN JL, DUTEYRAT JL, JACKSON M, STEWART GR, LECOINTE F, PAYRE B, SCHWARTZ O, YOUNG DB, MARCHAL G, LAGRANGE PH, PUZO G, GICQUEL B, NIGOU J, NEYROLLES O. Deciphering the molecular bases of Mycobacterium tuberculosis binding to the lectin DC-SIGN reveals an underestimated complexity. Biochem J. 2005;392:615–24. doi: 10.1042/BJ20050709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITARQUE S, LARROUY-MAUMUS G, PAYRÉ B, JACKSON M, PUZO G, NIGOU J. The immunomodulatory lipoglycans, lipoarabinomannan and lipomannan, are exposed at the mycobacterial cell surface. Tuberculosis. 2008;88:560–565. doi: 10.1016/j.tube.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POCE G, BATES RH, ALFONSO S, COCOZZA M, PORRETTA GC, BALLELL L, RULLAS J, ORTEGA F, DE LOGU A, AGUS E, LA ROSA V, PASCA MR, DE ROSSI E, WAE B, FRANZBLAU SG, MANETTI F, BOTTA M, BIAVA M. Improved BM212 MmpL3 inhibitor analogue shows efficacy in acute murine model of tuberculosis infection. PLoS One. 2013;8:e56980. doi: 10.1371/journal.pone.0056980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORCELLI SA, MODLIN RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol. 1999;17:297–329. doi: 10.1146/annurev.immunol.17.1.297. [DOI] [PubMed] [Google Scholar]
- PRIGOZHIN DM, MAVRICI D, HUIZAR JP, VANSELL HJ, ALBER T. Structural and biochemical analyses of Mycobacterium tuberculosis N-acetylmuramyl-L-alanine amidase Rv3717 point to a role in peptidoglycan fragment recycling. J Biol Chem. 2013;288:31549–55. doi: 10.1074/jbc.M113.510792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUECH V, GUILHOT C, PEREZ E, TROPIS M, ARMITIGE LY, GICQUEL B, DAFFE M. Evidence for a partial redundancy of the fibronectin-binding proteins for the transfer of mycoloyl residues onto the cell wall arabinogalactan termini of Mycobacterium tuberculosis. Mol Microbiol. 2002;44:1109–22. doi: 10.1046/j.1365-2958.2002.02953.x. [DOI] [PubMed] [Google Scholar]
- RAETZ CRH, REYNOLDS CM, TRENT MS, BISHOP RE. Lipid A modification systems in Gram-negative bacteria. Annu. Rev. Biochem. 2007;76:295–329. doi: 10.1146/annurev.biochem.76.010307.145803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAETZ CRH, WHITFIELD C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002;71:635–700. doi: 10.1146/annurev.biochem.71.110601.135414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAGAS A, ROUSSEL L, PUZO G, RIVIERE M. The Mycobacterium tuberculosis cell-surface glycoprotein Apa as a potential adhesin to colonize target cells via the innate immune system pulmonary C-type lectin surfactant protein A. J. Biol. Chem. 2007;282:5133–5142. doi: 10.1074/jbc.M610183200. [DOI] [PubMed] [Google Scholar]
- RAINCZUK AK, YAMARYO-BOTTE Y, BRAMMANANTH R, STINEAR TP, SEEMANN T, COPPEL RL, MCCONVILLE MJ, CRELLIN PK. The lipoprotein LpqW is essential for the mannosylation of periplasmic glycolipids in Corynebacteria. J Biol Chem. 2012;287:42726–38. doi: 10.1074/jbc.M112.373415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANA AK, SINGH A, GURCHA SS, COX LR, BHATT A, BESRA GS. Ppm1-encoded polyprenyl monophosphomannose synthase activity is essential for lipoglycan synthesis and survival in mycobacteria. PLoS One. 2012;7:e48211. doi: 10.1371/journal.pone.0048211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO SP, LAKSHMINARAYANA SB, KONDREDDI RR, HERVE M, CAMACHO LR, BIFANI P, KALAPALA SK, JIRICEK J, MA NL, TAN BH, NG SH, NANJUNDAPPA M, RAVINDRAN S, SEAH PG, THAYALAN P, LIM SH, LEE BH, GOH A, BARNES WS, CHEN Z, GAGARING K, CHATTERJEE AK, PETHE K, KUHEN K, WALKER J, FENG G, BABU S, ZHANG L, BLASCO F, BEER D, WEAVER M, DARTOIS V, GLYNNE R, DICK T, SMITH PW, DIAGANA TT, MANJUNATHA UH. Indolcarboxamide is a preclinical candidate for treating multidrug-resistant tuberculosis. Sci Transl Med. 2013;5:214ra168. doi: 10.1126/scitranslmed.3007355. [DOI] [PubMed] [Google Scholar]
- RAO V, FUJIWARA N, PORCELLI SA, GLICKMAN MS. Mycobacterium tuberculosis controls host innate immune activation through cyclopropane modification of a glycolipid effector molecule. J. Exp. Med. 2005;201:535–543. doi: 10.1084/jem.20041668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND JB, MAHAPATRA S, CRICK DC, PAVELKA MS., JR. Identification of the namH gene, encoding the hydroxylase responsible for the N-glycolylation of the mycobacterial peptidoglycan. J Biol Chem. 2005;280:326–33. doi: 10.1074/jbc.M411006200. [DOI] [PubMed] [Google Scholar]
- REAL G, FAY A, ELDAR A, PINTO SM, HENRIQUES AO, DWORKIN J. Determinants for the subcellular localization and function of a nonessential SEDS protein. J Bacteriol. 2008;190:363–76. doi: 10.1128/JB.01482-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY VM, EINCK L, NACY CA. In vitro antimycobacterial activities of capuramycin analogues. Antimicrob Agents Chemother. 2008;52:719–21. doi: 10.1128/AAC.01469-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REED MB, DOMENECH P, MANCA C, SU H, BARCZAK AK, KREISWIRTH BN, KAPLAN G, BARRY CE., III A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature. 2004;431:84–87. doi: 10.1038/nature02837. [DOI] [PubMed] [Google Scholar]
- REMUINAN MJ, PEREZ-HERRAN E, RULLAS J, ALEMPARTE C, MARTINEZ-HOYOS M, DOW DJ, AFARI J, MEHTA N, ESQUIVIAS J, JIMENEZ E, ORTEGA-MURO F, FRAILE-GABALDON MT, SPIVEY VL, LOMAN NJ, PALLEN MJ, CONSTANTINIDOU C, MINICK DJ, CACHO M, REBOLLO-LOPEZ MJ, GONZALEZ C, SOUSA V, ANGULO-BARTUREN I, MENDOZA-LOSANA A, BARROS D, BESRA GS, BALLELL L, CAMMACK N. Tetrahydropyrazolo[1,5-a]Pyrimidine-3-Carboxamide and N-Benzyl-6',7'-Dihydrospiro[Piperidine-4,4'-Thieno[3,2-c]Pyran] Analogues with Bactericidal Efficacy against Mycobacterium tuberculosis Targeting MmpL3. PLoS One. 2013;8:e60933. doi: 10.1371/journal.pone.0060933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN H, DOVER LG, ISLAM ST, ALEXANDER DC, CHEN JM, BESRA GS, LIU J. Identification of the lipooligosaccharide biosynthetic gene cluster from Mycobacterium marinum. Mol. Microbiol. 2007;63:1345–1359. doi: 10.1111/j.1365-2958.2007.05603.x. [DOI] [PubMed] [Google Scholar]
- RICHMOND JM, LEE J, GREEN DS, KORNFELD H, CRUIKSHANK WW. Mannose-capped lipoarabinomannan from Mycobacterium tuberculosis preferentially inhibits sphingosine-1-phosphate-induced migration of Th1 cells. J Immunol. 2012;189:5886–95. doi: 10.4049/jimmunol.1103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICK PD, BARR K, SANKARAN K, KAJIMURA J, RUSH JS, WAECHTER CJ. Evidence that the wzxE gene of Escherichia coli K-12 encodes a protein involved in the transbilayer movement of a trisaccharide-lipid intermediate in the assembly of enterobacterial common antigen. J. Biol. Chem. 2003;278:16534–16542. doi: 10.1074/jbc.M301750200. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ JE, RAMIREZ AS, SALAS LP, HELGUERA-REPETTO C, GONZALEZ-Y-MERCHAND J, SOTO CY, HERNANDEZ-PANDO R. Transcription of genes involved in sulfolipid and polyacyltrehalose biosynthesis of Mycobacterium tuberculosis in experimental latent tuberculosis infection. PLoS One. 2013;8:e58378. doi: 10.1371/journal.pone.0058378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMAIN F, HORN C, PESCHER P, NAMANE A, RIVIERE M, PUZO G, BARZU O, MARCHAL G. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacterium tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 1999;67:5567–5572. doi: 10.1128/iai.67.11.5567-5572.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMBOUTS Y, ALIBAUD L, CARRERE-KREMER S, MAES E, TOKARSKI C, ELASS E, KREMER L, GUERARDEL Y. Fatty acyl chains of Mycobacterium marinum lipooligosaccharides: structure, localization and acylation by PapA4 (MMAR_2343) protein. J Biol Chem. 2011;286:33678–88. doi: 10.1074/jbc.M111.273920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMBOUTS Y, BURGUIERE A, MAES E, CODDEVILLE B, ELASS E, GUERARDEL Y, KREMER L. Mycobacterium marinum lipooligosaccharides are unique caryophyllose-containing cell wall glycolipids that inhibit tumor necrosis factor-alpha secretion in macrophages. J Biol Chem. 2009;284:20975–88. doi: 10.1074/jbc.M109.011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE NL, COMPLETO GC, LIN S-J, MCNEIL MR, PALCIC MM, LOWARY TL. Expression, purification and characterization of a galactofuranosyltransferase involved in Mycobacterium tuberculosis arabongalactan biosynthesis. J Am Chem Soc. 2006;128:6721–6729. doi: 10.1021/ja058254d. [DOI] [PubMed] [Google Scholar]
- ROUSSEAU C, NEYROLLES O, BORDAT Y, GIROUX S, SIRAKOVA TD, PREVOST M-C, KOLATTUKUDY PE, GICQUEL B, JACKSON M. Deficiency in mycolipenate- and mycosanoate-derived acyltrehaloses enhances early interactions of Mycobacterium tuberculosis with host cells. Cell. Microbiol. 2003b;5:405–415. doi: 10.1046/j.1462-5822.2003.00289.x. [DOI] [PubMed] [Google Scholar]
- ROUSSEAU C, TURNER OC, RUSH E, BORDAT Y, SIRAKOVA TD, KOLATTUKUDY PE, RITTER S, ORME IM, GICQUEL B, JACKSON M. Sulfolipid deficiency does not affect the virulence of Mycobacterium tuberculosis H37Rv in mice and guinea pigs. Infect. Immun. 2003a;71:4684–4690. doi: 10.1128/IAI.71.8.4684-4690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY R, USHA V, KERMANI A, SCOTT DJ, HYDE EI, BESRA GS, ALDERWICK LJ, FUTTERER K. Synthesis of alpha-glucan in mycobacteria involves a hetero-octameric complex of trehalose synthase TreS and Maltokinase Pep2. ACS Chem Biol. 2013;8:2245–55. doi: 10.1021/cb400508k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ N. Bioinformatics identification of MurJ (MviN) as the peptidoglycan lipid II flippase in Escherichia coli. Proc. Natl. Acad. Sci. USA. 2008;105:15553–15557. doi: 10.1073/pnas.0808352105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL DG. Mycobacterium tuberculosis and the intimate discourse of a chronic infection. Immunol Rev. 2011;240:252–68. doi: 10.1111/j.1600-065X.2010.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYNDAK M, WANG S, SMITH I. PhoP, a key player in Mycobacterium tuberculosis virulence. Trends Microbiol. 2008;16:528–34. doi: 10.1016/j.tim.2008.08.006. [DOI] [PubMed] [Google Scholar]
- SAADAT S, BALLOU CE. Pyruvylated glycolipids from Mycobacterium smegmatis. Structures of two oligosaccharide components. J Biol Chem. 1983;258:1813–8. [PubMed] [Google Scholar]
- SAAVEDRA R, SEGURA E, LEYVA R, ESPARZA LA, LOPEZ-MARIN LM. Mycobacterial di-O-acyl-trehalose inhibits mitogen- and antigen-induced proliferation of murine T cells in vitro. Clin. Diagn. Lab. Immunol. 2001;8:1081–1088. doi: 10.1128/CDLI.8.6.1081-1088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAAVEDRA R, SEGURA E, TENORIO EP, LOPEZ-MARIN LM. Mycobacterial trehalose-containing glycolipid with immunomodulatory activity on human CD4+ and CD8+ T-cells. Microbes Infect. 2006;8:533–40. doi: 10.1016/j.micinf.2005.08.005. [DOI] [PubMed] [Google Scholar]
- SACKSTEDER KA, PROTOPOPOVA M, BARRY CE, 3RD, ANDRIES K, NACY CA. Discovery and development of SQ109: a new antitubercular drug with a novel mechanism of action. Future Microbiol. 2012;7:823–37. doi: 10.2217/fmb.12.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKAGUCHI I, IKEDA N, NAKAYAMA M, KATO Y, YANO I, KANEDA K. Trehalose 6,6'-dimycolate (Cord factor) enhances neovascularization through vascular endothelial growth factor production by neutrophils and macrophages. Infect Immun. 2000;68:2043–52. doi: 10.1128/iai.68.4.2043-2052.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMBOU T, DINADAYALA P, STADTHAGEN G, BARILONE N, BORDAT Y, CONSTANT P, LEVILLAIN F, NEYROLLES O, GICQUEL B, LEMASSU A, DAFFÉ M, JACKSON M. Capsular glucan and intracellular glycogen of Mycobacterium tuberculosis: biosynthesis and impact on the persistence in mice. Mol Microbiol. 2008;70:762–74. doi: 10.1111/j.1365-2958.2008.06445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANI M, HOUBEN ENG, GEURTSEN J, PIERSON J, DE PUNDER K, VAN ZON M, WEVER B, PIERSMA SR, JIMENEZ CR, DAFFE M, APPELMELK BJ, BITTER W, VAN DER WEL N, PETERS PJ. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog. 2010;6:e1000794. doi: 10.1371/journal.ppat.1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANKI AK, BOUCAU J, RONNING DR, SUCHECK SJ. Antigen 85C-mediated acyl-transfer between synthetic acyl donors and fragments of the arabinan. Glycoconj J. 2009;26:589–96. doi: 10.1007/s10719-008-9211-z. [DOI] [PubMed] [Google Scholar]
- SANYAL S, FRANK CG, MENON AK. Distinct flippases translocate glycerophospholipids and oligosaccharide diphosphate dolichols across the endoplasmic reticulum. Biochemistry. 2008;47:7937–7946. doi: 10.1021/bi800723n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARTAIN MJ, BELISLE JT. N-Terminal clustering of the O-glycosylation sites in the Mycobacterium tuberculosis lipoprotein SodC. Glycobiology. 2009;19:38–51. doi: 10.1093/glycob/cwn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASSETTI CM, RUBIN EJ. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA. 2003;100:12989–12994. doi: 10.1073/pnas.2134250100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHERMAN MS, KALBE-BOURNONVILLE L, BUSH D, XIN Y, DENG L, MCNEIL MR. Polyprenylphosphate-pentoses in mycobacteria are synthesized from 5-phosphoribose pyrophosphate. J. Biol. Chem. 1996;271:29652–29658. doi: 10.1074/jbc.271.47.29652. [DOI] [PubMed] [Google Scholar]
- SCHERMAN MS, WESTON A, DUNCAN K, WHITTINGTON A, UPTON R, DENG L, COMBER R, FRIEDRICH JD, MCNEIL MR. Biosynthetic origin of mycobacterial cell wall arabinosyl residues. J. Bacteriol. 1995;177:7125–7130. doi: 10.1128/jb.177.24.7125-7130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOENEN H, BODENDORFER B, HITCHENS K, MANZANERO S, WERNINGHAUS K, NIMMERJAHN F, AGGER EM, STENGER S, ANDERSEN P, RULAND J, BROWN GD, WELLS C, LANG R. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J Immunol. 2010;184:2756–60. doi: 10.4049/jimmunol.0904013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEBACH JR, CASADEVALL A, SCHNEERSON R, DAI Z, WANG X, ROBBINS JB, GLATMAN-FREEDMAN A. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun. 2001;69:5671–8. doi: 10.1128/IAI.69.9.5671-5678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWEBACH JR, GLATMAN-FREEDMAN A, GUNTHER-CUMMINS L, DAI Z, ROBBINS JB, SCHNEERSON R, CASADEVALL A. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect. Immun. 2002;70:2566–2575. doi: 10.1128/IAI.70.5.2566-2575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEELIGER JC, HOLSCLAW CM, SCHELLE MW, BOTYANSZKI Z, GILMORE SA, TULLY SE, NIEDERWEIS M, CRAVATT BF, LEARY JA, BERTOZZI CR. Elucidation and chemical modulation of sulfolipid-1 biosynthesis in Mycobacterium tuberculosis. J Biol Chem. 2012;287:7990–8000. doi: 10.1074/jbc.M111.315473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEIDEL M, ALDERWICK LJ, BIRCH HL, SAHM H, EGGELING L, BESRA GS. Identification of a novel arabinofuranosyltransferase AftB involved in a terminal step of cell wall arabinan biosynthesis in Corynebacterianeae, such as Corynebacterium glutamicum and Mycobacterium tuberculosis. J. Biol. Chem. 2007;282:14729–14740. doi: 10.1074/jbc.M700271200. [DOI] [PubMed] [Google Scholar]
- SENA CBC, FUKUDA T, MIYANAGI K, MATSUMOTO S, KOBAYASHI K, MURAKAMI Y, MAEDA Y, KINOSHITA T, MORITA YS. Controlled expression of branch-forming mannosyltransferase is critical for mycobacterial lipoarabinomannan biosynthesis. J. Biol. Chem. 2010;285:13326–13336. doi: 10.1074/jbc.M109.077297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHABAANA AK, KULANGARA K, SEMAC I, PAREL Y, ILANGUMARAN S, DHARMALINGAM K, CHIZZOLINI C, HOESSLI DC. Mycobacterial lipoarabinomannans modulate cytokine production in human T helper cells by interfering with raft/microdomain signalling. Cell Mol Life Sci. 2005;62:179–87. doi: 10.1007/s00018-004-4404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHARMA K, GUPTA M, KRUPA A, SRINIVASAN N, SINGH Y. EmbR, a regulatory protein with ATPase activity, is a substrate of multiple serine/threonine kinases and phosphatase in Mycobacterium tuberculosis. FEBS J. 2006b;273:2711–21. doi: 10.1111/j.1742-4658.2006.05289.x. [DOI] [PubMed] [Google Scholar]
- SHARMA K, GUPTA M, PATHAK M, GUPTA N, KOUL A, SARANGI S, BAWEJA R, SINGH Y. Transcriptional control of the mycobacterial embCAB operon by PknH through a regulatory protein, EmbR, in vivo. J Bacteriol. 2006a;188:2936–44. doi: 10.1128/JB.188.8.2936-2944.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHI L, BERG S, LEE A, SPENCER JS, ZHANG J, VISSA V, MCNEIL MR, KHOO K-H, CHATTERJEE C. The carboxy terminus of EmbC from Mycobacterium smegmatis mediates chain length extension of the arabinan in lipoarabinomannan. J. Biol. Chem. 2006;281:19512–19526. doi: 10.1074/jbc.M513846200. [DOI] [PubMed] [Google Scholar]
- SHI L, ZHOU R, LIU Z, LOWARY TL, SEEBERGER PH, STOCKER BL, CRICK DC, KHOO K-H, CHATTERJEE C. Transfer of the first arabinofuranose residue to galactan is essential for Mycobacterium smegmatis viability. J. Bacteriol. 2008;190:5248–5255. doi: 10.1128/JB.00028-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGER B, SCHUBERT K, DONOVAN C, BRAMKAMP M. The lipid II flippase RodA determines morphology and growth in Corynebacterium glutamicum. Mol Microbiol. 2013;90:966–82. doi: 10.1111/mmi.12411. [DOI] [PubMed] [Google Scholar]
- SILVA CL, EKIZLERIAN SM, FAZIOLI RA. Role of cord factor in the modulation of infection caused by mycobacteria. Am J Pathol. 1985;118:238–47. [PMC free article] [PubMed] [Google Scholar]
- SIMONNEY N, MOLINA JM, MOLIMARD M, OKSENHENDLER E, LAGRANGE PH. Comparison of A60 and three glycolipid antigens in an ELISA test for tuberculosis. Clinical Microbiology and Infection. 1996;2:214–222. doi: 10.1016/s1198-743x(14)65145-4. [DOI] [PubMed] [Google Scholar]
- SIMONNEY N, MOLINA JM, MOLIMARD M, OKSENHENDLER E, PERRONNE C, LAGRANGE PH. Analysis of the immunological humoral response to Mycobacterium tuberculosis glycolipid antigens (DAT, PGLTb1) for diagnosis of tuberculosis in HIV-seropositive and -seronegative patients. Eur J Clin Microbiol Infect Dis. 1995;14:883–91. doi: 10.1007/BF01691495. [DOI] [PubMed] [Google Scholar]
- SINGH A, CROSSMAN DK, MAI D, GUIDRY L, VOSKUIL MI, RENFROW MB, STEYN AJ. Mycobacterium tuberculosis WhiB3 maintains redox homeostasis by regulating virulence lipid anabolism to modulate macrophage response. PLoS Pathog. 2009;5:e1000545. doi: 10.1371/journal.ppat.1000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINGLA D, ANURAG M, DASH D, RAGHAVA GP. A web server for predicting inhibitors against bacterial target GlmU protein. BMC Pharmacol. 2011;11:5. doi: 10.1186/1471-2210-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIRAKOVA TD, THIRUMALA AK, DUBEY VS, SPRECHER H, KOLATTUKUDY PE. The Mycobacterium tuberculosis pks2 gene encodes the synthase for the hepta- and octamethyl branched fatty acids required for sulfolipid synthesis. J. Biol. Chem. 2001;276:16833–16839. doi: 10.1074/jbc.M011468200. [DOI] [PubMed] [Google Scholar]
- ŠKOVIEROVÁ H, LARROUY-MAUMUS G, PHAM H, BELANOVÁ M, BARILONE N, DASGUPTA A, MIKUŠOVÁ K, GICQUEL B, GILLERON M, BRENNAN PJ, PUZO G, NIGOU J, JACKSON M. Biosynthetic origin of the galactosamine substituent of arabinogalactan in Mycobacterium tuberculosis. J. Biol. Chem. 2010;285:41348–41355. doi: 10.1074/jbc.M110.188110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ŠKOVIEROVÁ H, LARROUY-MAUMUS G, ZHANG J, KAUR D, BARILONE N, KORDULAKOVA J, GILLERON M, GUADAGNINI S, BELANOVA M, PREVOST M-C, GICQUEL B, PUZO G, CHATTERJEE D, BRENNAN PJ, NIGOU J, JACKSON M. AftD, a novel essential arabinofuranosyltransferase from mycobacteria. Glycobiology. 2009;19:1235–1247. doi: 10.1093/glycob/cwp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYDEN RA, JACKSON M, ZUCKER J, RAMIREZ MV, DAWSON CC, CREW R, SAMPSON NS, THOMAS ST, JAMSHIDI N, SISK P, CASPI R, CRICK DC, MCNEIL MR, PAVELKA MS, NIEDERWEIS M, SIROY A, DONA V, MCFADDEN J, BOSHOFF H, LEW JM. Updating and curating metabolic pathways of TB. Tuberculosis (Edinb) 2013;93:47–59. doi: 10.1016/j.tube.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH GT, SWEREDOSKI MJ, HESS S. O-linked glycosylation sites profiling in Mycobacterium tuberculosis culture filtrate proteins. J Proteomics. 2013 doi: 10.1016/j.jprot.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG F, GUAN Z, RAETZ CRH. Biosynthesis of undecaprenyl phosphate-galactosamine and undecaprenyl phosphate-glucose in Francisella novicida. Biochemistry. 2009;48:1173–1182. doi: 10.1021/bi802212t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERANDEO P, DEHO G, POLISSI A. The lipopolysaccharide transport system of Gram-negative bacteria. Biochim. Biophys. Acta. 2009;1791:594–602. doi: 10.1016/j.bbalip.2009.01.011. [DOI] [PubMed] [Google Scholar]
- STADTHAGEN G, SAMBOU T, GUERIN M, BARILONE N, BOUDOU F, KORDULAKOVA J, CHARLES P, ALZARI PM, LEMASSU A, DAFFÉ M, PUZO G, GICQUEL B, RIVIERE M, JACKSON M. Genetic basis for the biosynthesis of methylglucose lipopolysaccharides in Mycobacterium tuberculosis. J Biol Chem. 2007;282:27270–6. doi: 10.1074/jbc.M702676200. [DOI] [PubMed] [Google Scholar]
- STANLEY SA, GRANT SS, KAWATE T, IWASE N, SHIMIZU M, WIVAGG C, SILVIS M, KAZYANSKAYA E, AQUADRO J, GOLAS A, FITZGERALD M, DAI H, ZHANG L, HUNG DT. Identification of novel inhibitors of M. tuberculosis growth using whole cell based high-throughput screening. ACS Chem Biol. 2012;7:1377–84. doi: 10.1021/cb300151m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN RJ, LEE TY, LEE TJ, YAN W, SCHERMAN MS, VISSA VD, KIM SK, WANNER BL, MCNEIL MR. Conversion of dTDP-4-keto-6-deoxyglucose to free dTDP-4-keto-rhamnose by the rmlC gene products of Escherichia coli and Mycobacterium tuberculosis. Microbiology. 1999;145:663–671. doi: 10.1099/13500872-145-3-663. [DOI] [PubMed] [Google Scholar]
- STOKES RW, NORRIS-JONES R, BROOKS DE, BEVERIDGE TJ, DOXSEE D, THORSON LM. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect. Immun. 2004;72:5676–5686. doi: 10.1128/IAI.72.10.5676-5686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOOP EJ, MISHRA AK, DRIESSEN NN, VAN STEMPVOORT G, BOUCHIER P, VERBOOM T, VAN LEEUWEN LM, SPARRIUS M, RAADSEN SA, VAN ZON M, VAN DER WEL NN, BESRA GS, GEURTSEN J, BITTER W, APPELMELK BJ, VAN DER SAR AM. Mannan core branching of lipo(arabino)mannan is required for mycobacterial virulence in the context of innate immunity. Cell Microbiol. 2013;15:2093–108. doi: 10.1111/cmi.12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SULZENBACHER G, CANAAN S, BORDAT Y, NEYROLLES O, STADTHAGEN G, ROIG-ZAMBONI V, RAUZIER J, MAURIN D, LAVAL F, DAFFE M, CAMBILLAU C, GICQUEL B, BOURNE Y, JACKSON M. LppX is a lipoprotein required for the translocation of phthiocerol dimycocerosates to the surface of Mycobacterium tuberculosis. EMBO J. 2006;25:1436–44. doi: 10.1038/sj.emboj.7601048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUPPLY P, MARCEAU M, MANGENOT S, ROCHE D, ROUANET C, KHANNA V, MAJLESSI L, CRISCUOLO A, TAP J, PAWLIK A, FIETTE L, ORGEUR M, FABRE M, PARMENTIER C, FRIGUI W, SIMEONE R, BORITSCH EC, DEBRIE AS, WILLERY E, WALKER D, QUAIL MA, MA L, BOUCHIER C, SALVIGNOL G, SAYES F, CASCIOFERRO A, SEEMANN T, BARBE V, LOCHT C, GUTIERREZ MC, LECLERC C, BENTLEY SD, STINEAR TP, BRISSE S, MEDIGUE C, PARKHILL J, CRUVEILLER S, BROSCH R. Genomic analysis of smooth tubercle bacilli provides insights into ancestry and pathoadaptation of Mycobacterium tuberculosis. Nat Genet. 2013;45:172–9. doi: 10.1038/ng.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAHLAN K, WILSON R, KASTRINSKY DB, ARORA K, NAIR V, FISCHER E, BARNES SW, WALKER JR, ALLAND D, BARRY CE, 3RD, BOSHOFF HI. SQ109 targets MmpL3, a membrane transporter of trehalose monomycolate involved in mycolic acid donation to the cell wall core of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56:1797–809. doi: 10.1128/AAC.05708-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAYAMA K, WANG C, BESRA GS. Pathway to synthesis and processing of mycolic acids in Mycobacterium tuberculosis. Clin. Microbiol. Rev. 2005;18:81–101. doi: 10.1128/CMR.18.1.81-101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEITELBAUM R, GLATMAN-FREEDMAN A, CHEN B, ROBBINS JB, UNANUE E, CASADEVALL A, BLOOM BR. A monoclonal antibody recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRELLES JB, AZAD AK, SCHLESINGER LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J. Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- TORRELLES JB, KHOO KH, SIELING PA, MODLIN RL, ZHANG N, MARQUES AM, TREUMANN A, RITHNER CD, BRENNAN PJ, CHATTERJEE D. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem. 2004;279:41227–39. doi: 10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- TORRELLES JB, SCHLESINGER LS. Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRELLES JB, SIELING PA, ARCOS J, KNAUP R, BARTLING C, RAJARAM MV, STENGER S, MODLIN RL, SCHLESINGER LS. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011;286:35438–46. doi: 10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRELLES JB, SIELING PA, ZHANG N, KEEN MA, MCNEIL MR, BELISLE JT, MODLIN RL, BRENNAN PJ, CHATTERJEE D. Isolation of a distinct Mycobacterium tuberculosis mannose-capped lipoarabinomannan isoform responsible for recognition by CD1b-restricted T cells. Glycobiology. 2012;22:1118–27. doi: 10.1093/glycob/cws078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAN AT, WEN D, WEST NP, BAKER EN, BRITTON WJ, PAYNE RJ. Inhibition studies on Mycobacterium tuberculosis N-acetylglucosamine-1-phosphate uridyltransferase (GlmU) Org Biomol Chem. 2013;11:8113–26. doi: 10.1039/c3ob41896k. [DOI] [PubMed] [Google Scholar]
- TREFZER C, RENGIFO-GONZALEZ M, HINNER MJ, SCHNEIDER P, MAKAROV V, COLE ST, JOHNSSON K. Benzothiazinones: prodrugs that covalently modify the decaprenylphosphoryl-beta-D-ribose 2'-epimerase DprE1 of Mycobacterium tuberculosis. J Am Chem Soc. 2010;132:13663–5. doi: 10.1021/ja106357w. [DOI] [PubMed] [Google Scholar]
- TREFZER C, SKOVIEROVA H, BURONI S, BOBOVSKA A, NENCI S, MOLTENI E, POJER F, PASCA MR, MAKAROV V, COLE ST, RICCARDI G, MIKUSOVA K, JOHNSSON K. Benzothiazinones are suicide inhibitors of mycobacterial decaprenylphosphoryl-beta-D-ribofuranose 2'-oxidase DprE1. J Am Chem Soc. 2012;134:912–5. doi: 10.1021/ja211042r. [DOI] [PubMed] [Google Scholar]
- TREUMANN A, XIDONG F, MCDONNELL L, DERRICK PJ, ASHCROFT AE, CHATTERJEE D, HOMANS SW. 5-methylthiopentose: a new substituent on liporabinomannan in Mycobacterium tuberculosis. J. Mol. Biol. 2002;316:89–100. doi: 10.1006/jmbi.2001.5317. [DOI] [PubMed] [Google Scholar]
- TSENG TT, GRATWICK KS, KOLLMAN J, PARK D, NIES DH, GOFFEAU A, SAIER MH., JR. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol. 1999;1:107–25. [PubMed] [Google Scholar]
- TURNBULL WB, SHIMIZU KH, CHATTERJEE D, HOMANS SW, TREUMANN A. Identification of the 5-methylthiopentose substituent in Mycobacterium tuberculosis lipoarabinomannan. Angew. Chem. Int. Ed. 2004;43:3918–3922. doi: 10.1002/anie.200454119. [DOI] [PubMed] [Google Scholar]
- TURNBULL WB, STALFORD SA. Methylthioxylose--a jewel in the mycobacterial crown? Org Biomol Chem. 2012;10:5698–706. doi: 10.1039/c2ob25630d. [DOI] [PubMed] [Google Scholar]
- UGALDE JE, PARODI AJ, UGALDE RA. De novo synthesis of bacterial glycogen: Agrobacterium tumefaciens glycogen synthase is involved in glucan initiation and elongation. Proc. Natl. Acad. Sci. USA. 2003;100:10659–10663. doi: 10.1073/pnas.1534787100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UH E, JACKSON ER, SAN JOSE G, MADDOX M, LEE RE, LEE RE, BOSHOFF HI, DOWD CS. Antibacterial and antitubercular activity of fosmidomycin, FR900098, and their lipophilic analogs. Bioorg Med Chem Lett. 2011;21:6973–6. doi: 10.1016/j.bmcl.2011.09.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DER WOUDE AD, SARKAR D, BHATT A, SPARRIUS M, RAADSEN SA, BOON L, GEURTSEN J, VAN DER SAR AM, LUIRINK J, HOUBEN EN, BESRA GS, BITTER W. Unexpected link between lipooligosaccharide biosynthesis and surface protein release in Mycobacterium marinum. J Biol Chem. 2012;287:20417–29. doi: 10.1074/jbc.M111.336461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDERVEN BC, HARDER JD, CRICK DC, BELISLE JT. Export-mediated assembly of mycobacterial glycoproteins parallels eukaryotic pathways. Science. 2005;309:941–943. doi: 10.1126/science.1114347. [DOI] [PubMed] [Google Scholar]
- WALLIS RS, KIM P, COLE S, HANNA D, ANDRADE BB, MAEURER M, SCHITO M, ZUMLA A. Tuberculosis biomarkers discovery: developments, needs, and challenges. Lancet Infect Dis. 2013;13:362–72. doi: 10.1016/S1473-3099(13)70034-3. [DOI] [PubMed] [Google Scholar]
- WALTERS SB, DUBNAU E, KOLESNIKOVA I, LAVAL F, DAFFÉ M, SMITH I. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 2006;60:312–330. doi: 10.1111/j.1365-2958.2006.05102.x. [DOI] [PubMed] [Google Scholar]
- WANG F, SAMBANDAN D, HALDER R, WANG J, BATT SM, WEINRICK B, AHMAD I, YANG P, ZHANG Y, KIM J, HASSANI M, HUSZAR S, TREFZER C, MA Z, KANEKO T, MDLULI KE, FRANZBLAU S, CHATTERJEE AK, JOHNSON K, MIKUSOVA K, BESRA GS, FUTTERER K, JACOBS WR, JR., SCHULTZ PG. Identification of a small molecule with activity against drug-resistant and persistent tuberculosis. Proc Natl Acad Sci U S A. 2013;110:E2510–7. doi: 10.1073/pnas.1309171110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG R, KLEGERMAN ME, MARSDEN I, SINNOTT M, GROVES MJ. An anti-neoplastic glycan isolated from Mycobacterium bovis (BCG vaccine) Biochem. J. 1995;311:867–872. doi: 10.1042/bj3110867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG X, RIBEIRO AA, GUAN Z, RAETZ CRH. Identification of undecaprenyl phosphate-b-D-galactosamine in Francisella novicida and its function in lipid A modification. Biochemistry. 2009;48:1162–1172. doi: 10.1021/bi802211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARRIER T, TROPIS M, WERNGREN J, DIEHL A, GENGENBACHER M, SCHLEGEL B, SCHADE M, OSCHKINAT H, DAFFE M, HOFFNER S, EDDINE AN, KAUFMANN SH. Antigen 85C inhibition restricts Mycobacterium tuberculosis growth through disruption of cord factor biosynthesis. Antimicrob Agents Chemother. 2012;56:1735–43. doi: 10.1128/AAC.05742-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WELIN A, WINBERG ME, ABDALLA H, SARNDAHL E, RASMUSSON B, STENDAHL O, LERM M. Incorporation of Mycobacterium tuberculosis lipoarabinomannan into macrophage membrane rafts is a prerequisite for the phagosomal maturation block. Infect Immun. 2008;76:2882–7. doi: 10.1128/IAI.01549-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESTON AR, STERN RJ, LEE RE, NASSAU PM, MONSEY D, MARTIN SL, SCHERMAN MS, BESRA GS, DUNCAN K, MCNEIL MR. Biosynthetic origin of mycobacterial cell wall galactofuranosyl residues. Tuber. Lung Dis. 1997;78:123–131. doi: 10.1016/s0962-8479(98)80005-1. [DOI] [PubMed] [Google Scholar]
- WHEATLEY RW, ZHENG RB, RICHARDS MR, LOWARY TL, NG KK. Tetrameric structure of the GlfT2 galactofuranosyltransferase reveals a scaffold for the assembly of mycobacterial Arabinogalactan. J Biol Chem. 2012;287:28132–43. doi: 10.1074/jbc.M112.347484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITFIELD C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 2006;75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- WILKINSON KA, NEWTON SM, STEWART GR, MARTINEAU AR, PATEL J, SULLIVAN SM, HERRMANN JL, NEYROLLES O, YOUNG DB, WILKINSON RJ. Genetic determination of the effect of post-translational modification on the innate immune response to the 19 kDa lipoprotein of Mycobacterium tuberculosis. BMC Microbiol. 2009;9:93. doi: 10.1186/1471-2180-9-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLUCKA BA, MCNEIL MR, DE HOFFMANN E, CHOJNACKI T, BRENNAN PJ. Recognition of the lipid intermediate for arbinogalactan/arabinomannan biosynthesis and its relation to the mode of action of ethambutol on mycobacteria. J. Biol. Chem. 1994;269:23328–23335. [PubMed] [Google Scholar]
- WOODRUFF PJ, CARLSON BL, SIRIDECHADILOK B, PRATT MR, SENARATNE RH, MOUGOUS JD, RILEY LW, WILLIAMS SJ, BERTOZZI CR. Trehalose is required for growth of Mycobacterium smegmatis. J. Biol. Chem. 2004;279:28835–28843. doi: 10.1074/jbc.M313103200. [DOI] [PubMed] [Google Scholar]
- YAGI T, MAHAPATRA S, MIKUSOVA K, CRICK DC, BRENNAN PJ. Polymerization of mycobacterial arabinogalactan and ligation to peptidoglycan. J. Biol. Chem. 2003;278:26497–26504. doi: 10.1074/jbc.M302216200. [DOI] [PubMed] [Google Scholar]
- YAN A, GUAN Z, RAETZ CRH. An undecaprenyl phosphate-aminoarabinose flippase required for polymyxin resistance in Escherichia coli. J. Biol. Chem. 2007;282:36077–36089. doi: 10.1074/jbc.M706172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG L, SINHA T, CARLSON TK, KEISER TL, TORRELLES JB, SCHLESINGER LS. Changes in the major cell envelope components of Mycobacterium tuberculosis during in vitro growth. Glycobiology. 2013;23:926–34. doi: 10.1093/glycob/cwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG J, AMIN AG, HOLEMANN A, SEEBERGER PH, CHATTERJEE D. Development of a plate-based scintillation proximity assay for the mycobacterial AftB enzyme involved in cell wall arabinan biosynthesis. Bioorg Med Chem. 2010;18:7121–31. doi: 10.1016/j.bmc.2010.07.040. [DOI] [PubMed] [Google Scholar]
- ZHANG J, ANGALA SK, PRAMANIK PK, LI K, CRICK DC, LIAV A, JOZWIAK A, SWIEZEWSKA E, JACKSON M, CHATTERJEE D. Reconstitution of Functional Mycobacterial Arabinosyltransferase AftC Proteoliposome and Assessment of Decaprenylphosphorylarabinose Analogues as Arabinofuranosyl Donors. ACS Chem Biol. 2011;6:819–28. doi: 10.1021/cb200091m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG J, KHOO K-H, WU S-W, CHATTERJEE C. Characterization of a distinct arabinofuranosyltransferase in Mycobacterium smegmatis. J. Am. Chem. Soc. 2007;129:9650–9662. doi: 10.1021/ja070330k. [DOI] [PubMed] [Google Scholar]
- ZHANG L, ENGLISH D, ANDERSEN BR. Activation of human neutrophils by Mycobacterium tuberculosis-derived sulfolipid I. J. Immunol. 1991;146:2730–2736. [PubMed] [Google Scholar]
- ZHANG L, GOREN MB, HOLZER TJ, ANDERSEN BR. Effect of Mycobacterium tuberculosis-derived sulfolipid I on human phagocytic cells. Infect. Immun. 1988;56:2876–2883. doi: 10.1128/iai.56.11.2876-2883.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG N, TORRELLES JB, MCNEIL MR, ESCUYER VE, KHOO K-H, BRENNAN PJ, CHATTERJEE D. The Emb proteins of mycobacteria direct arabinosylation of lipoarabinomannan and arabinogalactan via an N-terminal recognition region and a C-terminal synthetic region. Mol. Microbiol. 2003;50:69–76. doi: 10.1046/j.1365-2958.2003.03681.x. [DOI] [PubMed] [Google Scholar]
- ZHANG W, JONES VC, SCHERMAN MS, MAHAPATRA S, CRICK D, BHAMIDI S, XIN Y, MCNEIL MR, MA Y. Expression, essentiality, and a microtiter plate assay for mycobacterial GlmU, the bifunctional glucosamine-1-phosphate acetyltransferase and N-acetylglucosamine-1-phosphate uridyltransferase. Int J Biochem Cell Biol. 2008;40:2560–71. doi: 10.1016/j.biocel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG YJ, IOERGER TR, HUTTENHOWER C, LONG JE, SASSETTI CM, SACCHETTINI JC, RUBIN EJ. Global assessment of genomic regions required for growth in Mycobacterium tuberculosis. PLoS Pathog. 2012;8:e1002946. doi: 10.1371/journal.ppat.1002946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHENG J, WEI C, ZHAO L, LIU L, LENG W, LI W, JIN Q. Combining blue native polyacrylamide gel electrophoresis with liquid chromatography tandem mass spectrometry as an effective strategy for analyzing potential membrane protein complexes of Mycobacterium bovis bacillus Calmette-Guerin. BMC Genomics. 2011;12:40. doi: 10.1186/1471-2164-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZLOTTA AR, VAN VOOREN J-P, DENIS O, DROWART A, DAFFÉ M, LEFEVRE P, SCAHNDENE L, DE COCK M, DE BRUYN J, VANDENBUSSCHE P, JURION F, PALFLIET K, SIMON J, SCHULMAN CC, CONTENT J, HUYGEN K. What are the immunologically active components of bacille Calmette-Guérin in therapy of superficial bladder cancer? Int. J. Cancer. 2000;87:844–852. doi: 10.1002/1097-0215(20000915)87:6<844::aid-ijc14>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- ZUBER B, CHAMI M, HOUSSIN C, DUBOCHET J, GRIFFITHS G, DAFFE M. Direct visualization of the outer membrane of mycobacteria and corynebacteria in their native state. J. Bact. 2008;190:5672–5680. doi: 10.1128/JB.01919-07. [DOI] [PMC free article] [PubMed] [Google Scholar]