Abstract
AIM: To investigate a pathophysiological role of cathepsin W (CatW), a putative thiol-dependent cysteine protease, which is specifically expressed in cytotoxic lymphocytes, in different types of chronic inflammation of the gastric mucosa.
METHODS: Gastric and duodenal biopsies of patients with Helicobacter pylori (H pylori)-associated active gastritis (Hp, n = 19), chemically induced reactive gastritis (CG, n = 17), autoimmune atrophic gastritis (AIG, n = 20), lymphocytic corpus gastritis (LG, n = 29), celiac disease (CD, n = 10), and corresponding controls (n = 24) were analyzed by immunohistochemistry for the expression of CatW and CD45. Furthermore, immunohistochemical double staining with anti-CD3 and anti-cathepsin was performed for the samples of AIG.
RESULTS: Median values of CatW-expressing cells among CD45-positive immune cells were between 2% and 6% for normal gastric mucosa, CG, and LG, whereas the corresponding value was significantly increased for AIG (24.7%, P<0.001) and significantly decreased for HP (0.7%, P<0.05). Double staining with anti-CD3 and anti-CatW antibodies revealed that >90% of CatW-expressing cells in gastric mucosa of AIG were T cells. Duodenal mucosa had significantly more CatW/CD45-positive cells than normal gastric mucosa (median: 17.8% vs 2%, P<0.01). The corresponding proportion of CatW/CD45-positive cells was decreased in CD compared to duodenal mucosa (median: 2.1% vs 17.8%, P<0.05).
CONCLUSION: The opposite findings regarding the presence of CatW-positive cells in AIG (increase) and CD (decrease) reflects the different cellular composition of immune cells involved in the pathogenesis of these diseases.
Keywords: Cathepsin W, Inflammation, Gastritis, Immuno-histochemistry, NK cells
INTRODUCTION
Gastritis, by definition, is a histopathological entity characterized by the chronic and active inflammation of the gastric mucosa. The classification of gastritis, according to the updated Sydney system[1] is organized along traditional lines with active and chronic gastritis and includes specific forms of chronic gastritis defined by topography, morphology, and etiology and is graded with the help of a semiquantitative visual scale. The discovery of Helicobacter pylori (H pylori) has dramatically altered the etiological concepts of several gastric diseases. H pylori is the major cause of gastritis, gastric ulcer and has been classified as a definite human carcinogen playing a key role in the pathogenesis of gastric cancer and MALT lymphoma[2,3]. The host response to H pylori and its bacterial products is composed of T-, B-lymphocytes and plasma cells defined as chronicity of the gastritis. The infiltration of the lamina propria and gastric epithelium by polymorph nuclear leukocytes (mostly neutrophil granulocytes) reflects the activity of the inflammation.
NSAID-associated or bile-induced chemical reactive gastritis, causing 20-30% of gastritis, as well as autoimmune and lymphocytic gastritis (LG) are distinct entities. The presence of atrophy in the gastric corpus with diffuse atrophy of parietal and chief cells are the main characteristics of autoimmune atrophic gastritis (AIG) together with a hyperplasia of ECL cells. This disease is associated with serum anti-parietal and anti-intrinsic factor (IF) antibodies that cause IF deficiency, which can lead eventually to pernicious anemia in some patients. It has been shown that AIG is associated with infiltration of CD4+ T lymphocytes into the gastric mucosa, where they contribute to tissue destruction and gastric atrophy[4].
Chemically induced reactive gastritis (CG), caused by the use of nonsteroidal anti-inflammatory agents (NSAIDs) or bile reflux, is characterized by regenerative processes that lead to foveolar hyperplasia, mucosal edema, hemorrhage, capillary ectasia, proliferation of ascending smooth muscle fibers, and apical fibrosis. Usually, these lesions exhibit a slight superficial infiltration of lymphocytes and plasma cells. NSAIDs might additionally cause epithelial damage such as erosions and ulcers[1].
LG is a rare form of gastritis. The essential diagnostic feature is the presence of increased intraepithelial lymphocytes (mostly T cells), at least 25 lymphocytes per 100 epithelial cells. In the lamina propria, there are often additional neutrophil granulocytes among lymphocytes and plasma cells at the surface epithelium and within foveolar epithelium[5-7]. LG is believed to represent a special form of Helicobacter-mediated gastritis, mostly found in the corpus and fundus, although H pylori is only rarely found morphologically[6,7]. Three different types of LG can be distinguished depending on the endoscopic appearance: LG without typical endoscopic findings, the varioliform gastritis with thickened rugal folds, and Ménétriers disease with prominent rugal folds.
In human beings, the group of thiol-dependent cathepsins comprises 11 different cysteine proteases that are mostly localized in the lysosomal compartment[8]. During the last decade, it has become evident that most of these enzymes in addition to their role in lysosomal protein degradation[9] can mediate or regulate specific processes in various tissues and cell types. These functions include ‘limited proteolysis’ leading to the activation of granzymes[10], the maturation of hormones[11,12] and the regulation of antigen presentation[13]. As today, there is limited knowledge concerning the role of thiol-dependent cathepsins in gastric physiology and related disorders. Notably, cathepsins B and L were found to be associated with the carcinogenesis and metastasis of gastric and esophageal cancer[14-16]. Furthermore, cathepsins X and K were found to be specifically expressed in gastric epithelium[17], whereas cathepsin W (CatW) was found to be specifically expressed in lymphocyte-like cells within the gastric and intestinal tissue[18]. This finding was in line with the restricted expression pattern of CatW, which is expressed in NK cells and in cytotoxic T cells[19-21]. Although, its enzymatic activity and major function has not been characterized, there are some data supporting a regulatory role of this putative protease in the cytotoxic process for the NK-92 cell model[22]. However, cells from CatW-deficient mice were not affected in their cell-mediated cytotoxicity in vitro[23]. The specific association between CatW and cytotoxic cells and the potential involvement of these cells in chronic inflammation, prompted us to investigate the expression of CatW in different types of gastritis.
MATERIALS AND METHODS
Materials
A total of 119 formalin-fixed, paraffin-embedded biopsy specimens from patients suffering from different gastrointestinal disorders were retrieved from the archives of the institutes of Pathology Bayreuth and Magdeburg. The selection was based on clinical and histological evidence of the corresponding diagnosis including H pylori-associated chronic active gastritis (n = 19), CG (n = 17), AIG (n = 20), lymphocytic corpus gastritis without endoscopic findings (n = 29) as well as samples of duodenal mucosa of 10 patients with celiac disease (CD). Furthermore, healthy controls of both gastric (n = 14) and duodenal (n = 10) mucosa were examined immunohistochemically. Histological diagnosis was made by hematoxylin-eosin and Warthin-Starry stainings.
Methods
Detection of cathepsin W and CD45 by immunohist-ochemistry After the removal of paraffin by xylol, two sets of serial sections of all tissue specimens were subsequently dehydrated using increasing concentrations of ethanol. In order to demask the antigens, the samples were boiled thrice in 0.01 mol/L sodium citrate buffer (pH 6.0) for 10 min in a microwave (600 W), and incubated for 30 min at room temperature in 1×staining buffer containing 5% RPMI, 5% FCS, and 0.05% sodium azide in aqua dest (pH 7.4-7.6). The subsequent staining procedure on each one serial slide was performed using the monoclonal anti-CatW antibody CW-401B1 (1:1 000 in 1×staining buffer, 1 h at room temperature) and the Vectastain ABC-AP Kit (Vector, Burlingame) following the manufacturer’s instructions. In addition, a matching set of serial sections was stained with anti-CD45 (LCA, DakoCytomation, Glostrup, Denmark). The used antibody is a mixture of two mAbs (2B11 and PD7/26) representing all isoforms (2B11) and the isoform CD45R0 (PD7/26). Immunostaining was performed as described elsewher[24]. All samples were counterstained with hematoxylin, dehydrated and mounted using DEPEXÔ (Serva, Heidelberg, Germany).
Detection of cathepsin W and CD3 by double immu-nohistochemistry in autoimmune atrophic gastritis To distinguish between CatW-positive-stained natural killer cells and cytotoxic T lymphocytes, a third series of sections of the above used tissue specimens with AIG was pretreated and stained with the anti-CatW antibody and detected with Vectastain ABC-AP Kit as described above. After washing the slides thoroughly in PBS, subsequent staining with the anti-CD3-antibody (1:100; anti-human CD3, A 0452; DakoCytomation, Hamburg, Germany) was performed; marking the CD3-complex of T cells.
For revealing positive immunohistochemical reaction, the iVIEWÔ DAB Detection Kit (Ventana, Germany) was used as chromogen substrate, and then specimens were counterstained with hematoxylin and mounted with DEPEXÔ.
For all immunohistochemical analyses, two persons counted the numbers of stained cells in three independent high-power fields. Mann-Whitney U test was used for group comparison. For all statistical analyses, P<0.05 was considered to indicate statistical significance.
RESULTS
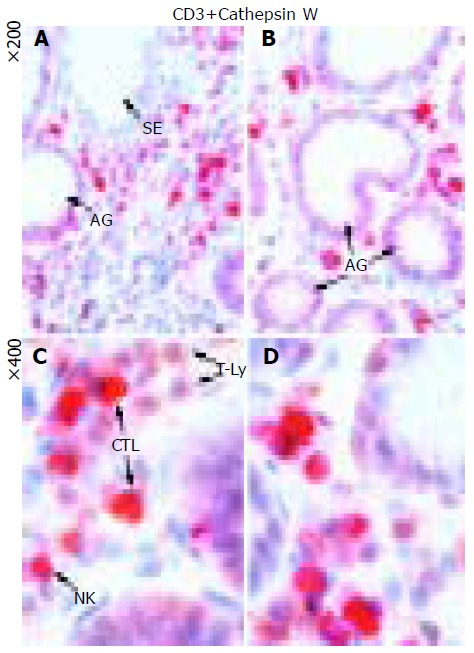
Generally, a consistent staining pattern exhibiting CatW-positive cells with a lymphocytic phenotype was obtained in all samples. The tissue specimens of the different chronic gastrointestinal disorders contained various amounts of CatW-expressing cells. In normal gastric mucosa, CatW-expressing cells were rarely detected (0.5±1 cells/field), whereas the different forms of gastritis revealed higher absolute numbers of 4.1-41 cells per observation field (Figure 2). Other immune cells including polymorph nuclear neutrophils, macrophages, and plasma cells as well as epithelial and stroma cells did not express CatW. AIG showed extensive mucosal atrophy, replacement of the oxyntic glands by intestinal metaplastic epithelium and mononuclear infiltrate within the lamina propria with approximately 25% of CD45+ cells expressing CatW (Figure 1C). CD with subtotal villous atrophy and dense inflammatory infiltrate revealed a fraction of about 5% CatW+/CD45+ cells (Figure 1D). In chronic H pylori gastritis, the gastric mucosa contained high absolute numbers of CatW+ cells among the dense infiltrate consisting of CD45+ lymphocytes, macrophages, plasma cells as well as neutrophils (Figure 1E).
Figure 2.
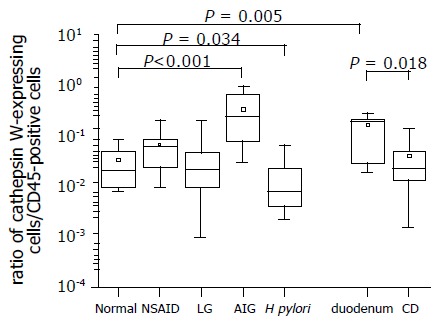
Presence of CatW-expressing cells among infiltrating immune cells in samples of patients with gastritis. The distribution of CatW- and LCA (leukocyte common antigen, CD45)-expressing cells was separately analyzed by immunohistochemistry in serial sections of tissue specimens. The number of CD45-positive cells was considered as 100% presented as a ratio of 1 at the Y-axis. The ratio of CatW+/LCA+ cells illustrates the proportion of CatW-expressing cells among leukocytes present in the gastric/duodenal mucosa. Data are shown as box plot for each group (antral mucosa: normal; chemically induced gastritis: NSAID; lymphocytic gastritis: LG; autoimmune gastritis: AIG; H pylori-induced gastritis: H pylori; normal duodenum: duodenum; celiac disease: CD). Boxes represent the 25th, 50th, and 75th percentile values (horizontal lines of the box) and means (squares). Mean values of absolute numbers of CatW-expressing cells per observation field were 0.5, 4.1, 4.2, 10.7, and 41 for normal antral mucosa, H pylori gastritis, NSAID-associated gastritis, LG and AIG, respectively. Tissue specimens from normal duodenum revealed 16 CatW-expressing cells per field, while samples from patient with CD had 6.3 CatW-positive cells in average per field.
Figure 1.
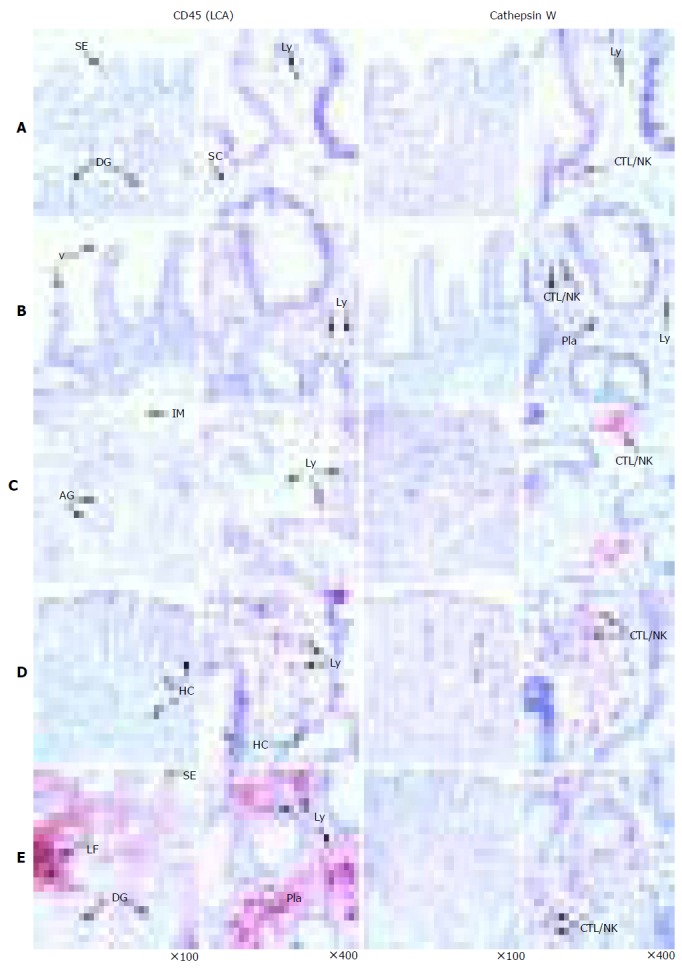
Immunohistochemical detection of CatW in gastrointestinal tissue specimens. The cellular distribution of CatW expression is presented by red staining, whereas the nuclei are counterstained with hematoxylin (blue). The distribution of CD45 (LCA) is shown as brownish staining. The negative controls (using isotype controls) did not reveal any signals (not shown). The tissue samples were as follows: normal gastric mucosa (A), duodenum (B), AIG (C), CD (D) and H pylori gastritis (E). AG: Atrophic glands, CTL: cytotoxic T-lymphocyte, DG: deep gastric glands, HC: hyperplastic crypts, IM: intestinal metaplasia, LF: lymph follicle, Ly: lymphocyte, Pla: plasma cell, NK: natural killer cell, SC: stroma cells, SE: surface epithelium, T-Ly: T lymphocyte, and V: duodenal villi.
Notably, the relative proportion of CatW-expressing cells among the infiltrating leukocytes, determined by CD45, differed remarkably for the investigated types of chronic inflammation (Figure 2). The highest proportion of CatW-expressing CD45+ cells was found in AIG reaching 24.7% (ranging from 8% to 70%). Normal gastric mucosa, which comprised samples without gastritis, revealed a fraction of 2% (ranging from 1% to 5%). The difference between both groups was found to be significant (P<0.001). Surprisingly, for CD as another autoimmune-triggered intestinal disease investigated in this study, a significant reduction of the relative number of CatW-expressing cells was observed compared to healthy duodenal mucosa (2.1% vs 17.8%, P<0.05, Figure 2). Normal duodenal mucosa contained significantly more CatW-positive cells than normal gastric mucosa (P<0.01). Furthermore, the proportion of CatW+/CD45+ cells was analyzed in the specimens with H pylori-associated chronic active gastritis. Here, a significant decreased proportion of 0.7% (ranging from 0.5% to 2%, P<0.05) compared to normal gastric mucosa was detected (Figure 2).
For the other two chronic gastric disorders (CG and LG) analyzed in this study, the proportions of CatW+/CD45+ cells were not changed and similar to normal gastric mucosa (Figure 2). CatW was identified in about 6% and 2.2% of the CD45+ cells in CG and LG, respectively.
As exemplarily shown in Figure 3, the double staining permitted a clear classification of the lymphocyte-like infiltrate of the lamina propria in AIG. The great majority (>90%) of CatW-expressing cells represented CD3+ T lymphocytes, whereas CatW+ NK cells (CD3-) were rarely detected. Whereas CatW-/CD3+ positive T cells were abundantly seen in the samples of AIG, a high proportion of CatW-expressing cytotoxic T cells (CTL) was detected, too. CatW+ NK cells (NK) were only rarely spotted in the lamina propria.
Figure 3.
Detection of CatW and CD3+ T cells in tissue specimens of AIG. The immunohistochemical double staining allowed a differentiation of both CatW-expressing cell types as well as a separation of CTLs from the other T cells (T-Ly) among the infiltrating cells of the lamina propria: Whereas CatW–/CD3+ positive T cells could be clearly distinguished by the brownish staining and were abundantly seen in the samples of AIG, an additional cytoplasmic red staining detected the CatW-expressing CTL. The exclusively red staining marked the CatW+ NK cells, which were only rarely spotted in the lamina propria.
DISCUSSION
This study revealed two new and important findings. First, it provides evidence that the elevated number of CatW-expressing cells in AIG is a rather specific event for this disease and does not just represent a general phenomenon of mucosal inflammation. Second, data provide evidence that the cellular distribution of CatW is different in peripheral blood and mucosal compartment[25,26].
The elevated number of CatW-positive cells in AIG supports a pathophysiological role of these cells in this type of gastritis as discussed previously[18]. The specific expression pattern of CatW[19-21] and its potential involvement in cytotoxic processes[22] imply an involvement of CatW in Th1-mediated inflammation like AIG. According to the current model of AIG, first, self-antigen-reactive Th1 cells, which express IFN-g, IL-2 and are capable for transendothelial migration, are activated by self-antigens of gastric mucosa and participate in tissue destruction and resulting inflammation. Second, Th2 cells, being sessile in the draining lymph nodes and activated by the self-antigens released from tissue destruction, support autoantibody production and might develop into memory cells[27]. Although, IFN-g, which is mainly released by NK cells is one of the predominant cytokines increased in AIG[28-30], there are data implying that NK cells are dispensable for the onset of AIG. In mice models with neonatal thymectomy and subsequent depletion of NK cells, the disease incidence was not changed suggesting that other cells such as CD8+ T cells are the primary source of IFN-g[31]. Furthermore, it has been shown by several groups that in the experimental mouse model, CD4-positive T cells are mainly responsible for the development of gastric lesions[32-34]. Interestingly, the predominant role of CD4-positive T cells has not been fully confirmed. D’Elios and co-workers showed an association between AIG and Th1-differentiated T cells that possessed cytotoxic activity[35]. Other studies investigating patient-derived samples identified an upregulation of CD16+ and CD8+ cells suggesting a potential role of these cytotoxic cells in human AIG[36,37]. Based on these reports, an interaction of the CD4+ cells with autoreactive CD8+ effector cells might lead to the development of autoimmune gastritis that involves also regulatory T cells[38,39]. Taking into consideration the data from the human disease that showed a predominant involvement of T cells in AIG[35,36], the higher proportion of CD8+ T cell in mucosal compartments[26] and the specific expression pattern of CatW in cytotoxic T and NK cells[21], we assume that in AIG mostly CD8+ T cells contribute to the high proportion of CatW+/CD45+ cells. By performing double staining immunohistochemistry, we were able to confirm this hypothesis by showing a very low proportion of CatW+ NK cells, whereas the number of CatW-expressing cytotoxic T lymphocytes was increased significantly. This observation supports a specific role of CTLs in the pathophysiological processes of AIG. For comparison, tissue samples of CD, another prototype of T-cell mediated autoimmune disease, were also investigated. Surprisingly, a decrease of the CatW+/CD45+ cells was determined in CD. This phenomenon is presumably caused by the massive increase of CatW-negative CD4+ T cells, which make up the majority of infiltrating immune cells in this disease[39,40]. The observation, that the absolute number of CatW-expressing cells is significantly higher in normal intestinal samples compared to gastric samples, is in line with the distribution of immune cells in these compartments.
It is well known that H pylori leads to an infiltration of neutrophils and a predominant Th1-dominated immune response[41]. The analysis of lymphocytic subsets in chronic gastritis revealed that most of the infiltrating lymphocytes were found to be CD4+ T cells[42], whereas neutrophils and cytotoxic T cells seem to play an important role with respect to the activity of H pylori gastritis[43,44]. Furthermore, a significant increase of IL-2 receptor positive cells, including NK cells, was reported in chronic gastritis[44]. Taking into consideration the involvement of different subsets of immune cells in H pylori-mediated gastritis, the relative decrease of CatW-expressing cells can be attributed to the massive infiltration of CD45+ leukocytes (CD4+ T cells, B cells, and granulocytes). Although, there is no doubt that these cells in general participate in the immunological response by the secretion of cytokines and mediating cytotoxic effects, the lower numbers of CatW-expressing cells compared to AIG implies that they do not play a predominant role in the pathogenesis of H pylori gastritis[17].
No differences in the CatW+/CD45+ proportions for the CG and LG were detected compared to healthy controls. These findings are in line with the pathogenesis of both types of gastritis. NSAIDs, as the most common cause of CG, lead primarily to an inhibition of cyclooxygenases that subsequently leads to lower levels of prostaglandins and thromboxane and less lymphocytic infiltration compared to H pylori gastritis and AIG[45,46]. Therefore, CG showed normal numbers or only a minor increase of inflammatory cells, mostly being lymphocytes and plasma cells, in the mucosa[1]. Since the typical histological picture includes only a slight amount of inflammatory cells, the minimal presence of CatW-expressing cells, comparable to healthy gastric mucosa, is in concordance with these studies.
As the name implies, LG is characterized by the presence of large numbers of mature lymphocytes infiltrating the surface and foveolar epithelium[7]. Based on the guidelines, 25-40 intraepithelial lymphocytes per 100 epithelial cells are necessary for this diagnosis. Intraepithelial lymphocytes comprise mostly suppressor T cells showing cytotoxic ability through production of TIA1 and granzyme B[7,47]. The unchanged CatW+/CD45+ proportion in LG obviously corresponds to the normal fraction of CatW-expressing cells among the physiological infiltrate of inflammatory cells in gastric mucosa. It is notable that LG is thought to be related to H pylori infection, as shown by their improvement after eradication[6], indicating that LG may represent an atypical host immune response to this infection, even if Helicobacter is only rarely found morphologically[5,6].
The numeric differences of CatW-expressing cells and the differences of the relative portion of CatW-expressing cells among infiltrating CD45+ leukocytes in the investigated types of chronic gastroduodenal inflammation imply a distinct involvement of cytotoxic cells expressing CatW in the pathogenesis among these diseases. Whereas CatW-expressing CTLs seem to contribute to the pathogenesis of AIG and to a lesser extent in H pylori-associated gastritis, the low absolute and relative numbers of CatW-positive cells in the inflammatory infiltrate in CG and LG suggest that CatW-expressing cells do not play a major role in these diseases.
The identification of CTL as the predominant CatW-expressing cell type in gastric mucosa, as shown for AIG, supports the idea that the CatW-expression pattern might differ between certain subpopulations of cytotoxic cells. This hypothesis is further supported by the recent finding that cytotoxic cells of gastric mucosa can express an alternatively spliced isoform that has not been detected in peripheral NK cells so far[48].
ACKNOWLEDGMENTS
The authors thank Ursula Stolz, Claudia Miethke, and Carola Kügler for their skilful technical assistance while performing immunohistochemical procedures.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
Supported by the “Deutsche Forschungsgemeinschaft”, Germany (We2170/3-1) and the NBL-3 program of the “Bundesministerium für Forschung und Te chnik” (NBL3/01ZZ0407/PFG1)
References
- 1.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogelman JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 3.Asaka M, Takeda H, Sugiyama T, Kato M. What role does Helicobacter pylori play in gastric cancer? Gastroenterology. 1997;113:S56–S60. doi: 10.1016/s0016-5085(97)80013-3. [DOI] [PubMed] [Google Scholar]
- 4.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 5.Dixon MF, Wyatt JI, Burke DA, Rathbone BJ. Lymphocytic gastritis--relationship to Campylobacter pylori infection. J Pathol. 1988;154:125–132. doi: 10.1002/path.1711540204. [DOI] [PubMed] [Google Scholar]
- 6.Müller H, Volkholz H, Stolte M. Healing of lymphocytic gastritis by eradication of Helicobacter pylori. Digestion. 2001;63:14–19. doi: 10.1159/000051867. [DOI] [PubMed] [Google Scholar]
- 7.Haot J, Hamichi L, Wallez L, Mainguet P. Lymphocytic gastritis: a newly described entity: a retrospective endoscopic and histological study. Gut. 1988;29:1258–1264. doi: 10.1136/gut.29.9.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turk V, Turk B, Turk D. Lysosomal cysteine proteases: facts and opportunities. EMBO J. 2001;20:4629–4633. doi: 10.1093/emboj/20.17.4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirschke H, Barrett AJ, Rawlings ND. Proteinases 1: lysosomal cysteine proteinases. Protein Profile. 1995;2:1581–1643. [PubMed] [Google Scholar]
- 10.Pham CT, Ley TJ. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, et al. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedrichs B, Tepel C, Reinheckel T, Deussing J, von Figura K, Herzog V, Peters C, Saftig P, Brix K. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111:1733–1745. doi: 10.1172/JCI15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 14.Hughes SJ, Glover TW, Zhu XX, Kuick R, Thoraval D, Orringer MB, Beer DG, Hanash S. A novel amplicon at 8p22-23 results in overexpression of cathepsin B in esophageal adenocarcinoma. Proc Natl Acad Sci USA. 1998;95:12410–12415. doi: 10.1073/pnas.95.21.12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lauritzen C, Pedersen J, Madsen MT, Justesen J, Martensen PM, Dahl SW. Active recombinant rat dipeptidyl aminopeptidase I (cathepsin C) produced using the baculovirus expression system. Protein Expr Purif. 1998;14:434–442. doi: 10.1006/prep.1998.0976. [DOI] [PubMed] [Google Scholar]
- 16.Dohchin A, Suzuki JI, Seki H, Masutani M, Shiroto H, Kawakami Y. Immunostained cathepsins B and L correlate with depth of invasion and different metastatic pathways in early stage gastric carcinoma. Cancer. 2000;89:482–487. [PubMed] [Google Scholar]
- 17.Bühling F, Peitz U, Krüger S, Küster D, Vieth M, Gebert I, Roessner A, Weber E, Malfertheiner P, Wex T. Cathepsins K, L, B, X and W are differentially expressed in normal and chronically inflamed gastric mucosa. Biol Chem. 2004;385:439–445. doi: 10.1515/BC.2004.051. [DOI] [PubMed] [Google Scholar]
- 18.Buhling F, Kellner U, Guenther D, Kahl S, Brömme D, Weber E, Malfertheiner P, Wex T. Characterization of novel anti-cathepsin W antibodies and cellular distribution of cathepsin W in the gastrointestinal tract. Biol Chem. 2002;383:1285–1289. doi: 10.1515/BC.2002.144. [DOI] [PubMed] [Google Scholar]
- 19.Linnevers C, Smeekens SP, Brömme D. Human cathepsin W, a putative cysteine protease predominantly expressed in CD8+ T-lymphocytes. FEBS Lett. 1997;405:253–259. doi: 10.1016/s0014-5793(97)00118-x. [DOI] [PubMed] [Google Scholar]
- 20.Brown J, Matutes E, Singleton A, Price C, Molgaard H, Buttle D, Enver T. Lymphopain, a cytotoxic T and natural killer cell-associated cysteine proteinase. Leukemia. 1998;12:1771–1781. doi: 10.1038/sj.leu.2401164. [DOI] [PubMed] [Google Scholar]
- 21.Wex T, Bühling F, Wex H, Günther D, Malfertheiner P, Weber E, Brömme D. Human cathepsin W, a cysteine protease predominantly expressed in NK cells, is mainly localized in the endoplasmic reticulum. J Immunol. 2001;167:2172–2178. doi: 10.4049/jimmunol.167.4.2172. [DOI] [PubMed] [Google Scholar]
- 22.Wex T, Wex H, Hartig R, Wilhelmsen S, Malfertheiner P. Functional involvement of cathepsin W in the cytotoxic activity of NK-92 cells. FEBS Lett. 2003;552:115–119. doi: 10.1016/s0014-5793(03)00895-0. [DOI] [PubMed] [Google Scholar]
- 23.Ondr JK, Pham CT. Characterization of murine cathepsin W and its role in cell-mediated cytotoxicity. J Biol Chem. 2004;279:27525–27533. doi: 10.1074/jbc.M400304200. [DOI] [PubMed] [Google Scholar]
- 24.Borscheri N, Roessner A, Röcken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. Am J Surg Pathol. 2001;25:1297–1303. doi: 10.1097/00000478-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hayday A, Theodoridis E, Ramsburg E, Shires J. Intraepithelial lymphocytes: exploring the Third Way in immunology. Nat Immunol. 2001;2:997–1003. doi: 10.1038/ni1101-997. [DOI] [PubMed] [Google Scholar]
- 26.Neutra MR, Mantis NJ, Kraehenbuhl JP. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–1009. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 27.Katakai T, Mori KJ, Masuda T, Shimizu A. Differential localization of Th1 and Th2 cells in autoimmune gastritis. Int Immunol. 1998;10:1325–1334. doi: 10.1093/intimm/10.9.1325. [DOI] [PubMed] [Google Scholar]
- 28.Barrett SP, Gleeson PA, de Silva H, Toh BH, van Driel IR. Interferon-gamma is required during the initiation of an organ-specific autoimmune disease. Eur J Immunol. 1996;26:1652–1655. doi: 10.1002/eji.1830260737. [DOI] [PubMed] [Google Scholar]
- 29.Chen H, Paul WE. Cultured NK1.1+ CD4+ T cells produce large amounts of IL-4 and IFN-gamma upon activation by anti-CD3 or CD1. J Immunol. 1997;159:2240–2249. [PubMed] [Google Scholar]
- 30.Romagnani S. Induction of TH1 and TH2 responses: a key role for the 'natural' immune response? Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 31.La Gruta NL, Van Driel IR, Toh BH, Gleeson PA. The role of natural killer cells in the induction of autoimmune gastritis. Autoimmunity. 2001;34:147–154. doi: 10.3109/08916930109001962. [DOI] [PubMed] [Google Scholar]
- 32.De Silva HD, Van Driel IR, La Gruta N, Toh BH, Gleeson PA. CD4+ T cells, but not CD8+ T cells, are required for the development of experimental autoimmune gastritis. Immunology. 1998;93:405–408. doi: 10.1046/j.1365-2567.1998.00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinelli TM, van Driel IR, Alderuccio F, Gleeson PA, Toh BH. Analysis of mononuclear cell infiltrate and cytokine production in murine autoimmune gastritis. Gastroenterology. 1996;110:1791–1802. doi: 10.1053/gast.1996.v110.pm8964405. [DOI] [PubMed] [Google Scholar]
- 34.Alderuccio F, Toh BH, Gleeson PA, van Driel IR. A novel method for isolating mononuclear cells from the stomachs of mice with experimental autoimmune gastritis. Autoimmunity. 1995;21:215–221. doi: 10.3109/08916939509008018. [DOI] [PubMed] [Google Scholar]
- 35.D'Elios MM, Bergman MP, Azzurri A, Amedei A, Benagiano M, De Pont JJ, Cianchi F, Vandenbroucke-Grauls CM, Romagnani S, Appelmelk BJ, et al. H(+),K(+)-atpase (proton pump) is the target autoantigen of Th1-type cytotoxic T cells in autoimmune gastritis. Gastroenterology. 2001;120:377–386. doi: 10.1053/gast.2001.21187. [DOI] [PubMed] [Google Scholar]
- 36.Vargas JA, Alvarez-Mon M, Manzano L, Albillos A, Fernandez-Corugedo A, Albarrán F, Durántez A. Functional defect of T cells in autoimmune gastritis. Gut. 1995;36:171–175. doi: 10.1136/gut.36.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitomi H, Tanabe S, Igarashi M, Katsumata T, Arai N, Kikuchi S, Kiyohashi A, Okayasu I. Autoimmune enteropathy with severe atrophic gastritis and colitis in an adult: proposal of a generalized autoimmune disorder of the alimentary tract. Scand J Gastroenterol. 1998;33:716–720. doi: 10.1080/00365529850171657. [DOI] [PubMed] [Google Scholar]
- 38.Shevach EM, McHugh RS, Piccirillo CA, Thornton AM. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 39.Shan L, Molberg Ø, Parrot I, Hausch F, Filiz F, Gray GM, Sollid LM, Khosla C. Structural basis for gluten intolerance in celiac sprue. Science. 2002;297:2275–2279. doi: 10.1126/science.1074129. [DOI] [PubMed] [Google Scholar]
- 40.Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Raia V, Auricchio S, Picard J, Osman M, Quaratino S, Londei M. Association between innate response to gliadin and activation of pathogenic T cells in coeliac disease. Lancet. 2003;362:30–37. doi: 10.1016/S0140-6736(03)13803-2. [DOI] [PubMed] [Google Scholar]
- 41.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 42.Raptopoulou-Gigi M, Polyzonis M, Orphanou-Koumerkeridou H, Agorastos J, Vacolas J, Gigis P. Immunohistological phenotyping of the stomach infiltrating lymphocytes in chronic gastritis. Allergol Immunopathol (Madr) 1990;18:87–89. [PubMed] [Google Scholar]
- 43.Fiocca R, Villani L, Luinetti O, Gianatti A, Perego M, Alvisi C, Turpini F, Solcia E. Helicobacter colonization and histopathological profile of chronic gastritis in patients with or without dyspepsia, mucosal erosion and peptic ulcer: a morphological approach to the study of ulcerogenesis in man. Virchows Arch A Pathol Anat Histopathol. 1992;420:489–498. doi: 10.1007/BF01600253. [DOI] [PubMed] [Google Scholar]
- 44.Ohtani N, Ohtani H, Nakayama T, Naganuma H, Sato E, Imai T, Nagura H, Yoshie O. Infiltration of CD8+ T cells containing RANTES/CCL5+ cytoplasmic granules in actively inflammatory lesions of human chronic gastritis. Lab Invest. 2004;84:368–375. doi: 10.1038/labinvest.3700039. [DOI] [PubMed] [Google Scholar]
- 45.Wallace JL, Zamuner SR, McKnight W, Dicay M, Mencarelli A, del Soldato P, Fiorucci S. Aspirin, but not NO-releasing aspirin (NCX-4016), interacts with selective COX-2 inhibitors to aggravate gastric damage and inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G76–G81. doi: 10.1152/ajpgi.00295.2003. [DOI] [PubMed] [Google Scholar]
- 46.Tomisato W, Tsutsumi S, Hoshino T, Hwang HJ, Mio M, Tsuchiya T, Mizushima T. Role of direct cytotoxic effects of NSAIDs in the induction of gastric lesions. Biochem Pharmacol. 2004;67:575–585. doi: 10.1016/j.bcp.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Oberhuber G, Bodingbauer M, Mosberger I, Stolte M, Vogelsang H. High proportion of granzyme B-positive (activated) intraepithelial and lamina propria lymphocytes in lymphocytic gastritis. Am J Surg Pathol. 1998;22:450–458. doi: 10.1097/00000478-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Meinhardt C, Peitz U, Treiber G, Wilhelmsen S, Malfertheiner P, Wex T. Identification of a novel isoform predominantly expressed in gastric tissue and a triple-base pair polymorphism of the cathepsin W gene. Biochem Biophys Res Commun. 2004;321:975–980. doi: 10.1016/j.bbrc.2004.07.056. [DOI] [PubMed] [Google Scholar]


