Abstract
AIM: Clinical and experimental data suggest that gut-derived endotoxins are an important pathogenic factors for progression of chronic liver disease. Recently, a C-T (-159) polymorphism in the promoter region of the CD14 gene was detected and found to confer increased CD14 expression and to be associated with advanced alcoholic liver damage. Here, we investigated this polymorphism in patients with less advanced alcoholic liver disease (ALD) and chronic hepatitis C virus (HCV) infection.
METHODS: CD14 genotyping was performed by PCR-RFLP analysis in (a) 121 HCV patients, (b) 62 patients with alcohol-associated cirrhosis (Alc-Ci), (c) 118 individuals with heavy alcohol abuse without evidence of advanced liver damage (Alc-w/o Ci), and (d) 247 healthy controls. Furthermore, serum levels of soluble CD14 (sCD14) and transaminases were determined.
RESULTS: The TT genotype was significantly more frequent in Alc-Ci compared to Alc-w/o Ci or controls (40.3% vs 23.7% or 24.0%, respectively). In Alc-w/o Ci, serum levels of transaminases did not differ significantly between patients with different CD14 genotypes. In HCV patients, TT-homozygotes had significantly higher sCD14 levels and sCD14 serum levels were significantly higher in patients with advanced fibrosis or cirrhosis. However, no association was found between CD14 genotypes and histological staging or grading.
CONCLUSION: Considering serum transaminases as surrogate markers for alcoholic liver damage, the CD14 polymorphism seems to exhibit different effects during the course of ALD. Differences in genotype distribution between cirrhotic HCV patients and alcoholics and the known functional impact of this polymorphism on CD14 expression levels further indicate differences in the pathophysiological role of CD14 and CD14-mediated lipopolysaccharides signal transduction with regard to the stage as well as the type of the underlying liver disease.
Keywords: CD14 gene, Alcoholic liver disease, Chronic hepatitis C infection
INTRODUCTION
Individuals affected by chronic liver disease show a broad spectrum of responses to the same etiologic agent. As one cause for this finding and increasing evidence indicates that genetic factors influence the natural history of chronic liver disease[1].
In case of alcoholic liver disease (ALD), early studies have focused on polymorphic forms of enzymes involved in alcohol metabolism[2-4]. Recent studies have shown that genetically determined variations in the inflammatory response contribute to different susceptibility for the development of various liver diseases, notably viral hepatitis[5,6] and ALD[7-10]. These findings together with accumulating clinical and experimental data indicate that the extent of the inflammatory response to the etiological agent is crucial in the pathogenesis of chronic hepatitis C virus (HCV) as well as alcoholic and other liver disease.
Several lines of evidence indicate that bacterial products, primarily endotoxins (lipopolysaccharides; LPS) induce and perpetuate hepatic inflammation. Endotoxin levels are correlated with the severity of ALD in experimental models[11] as well as in patients with chronic liver disease[12] and reducing gut endotoxin alleviates hepatic injury[13]. Endogenous LPS are constitutively produced within the gut by the death of Gram-negative bacteria and are absorbed into intestinal capillaries. In low concentrations, LPS can also be demonstrated in the portal venous blood in healthy humans[14].
Increased LPS levels have been shown in patients with ALD as well as patients with chronic hepatitis C infection[12,15-17]. Several factors promote endotoxemia in this setting, including increased translocation of endotoxins and reduced hepatic clearance[11,18].
Circulating endotoxin is bound mainly to the LPS binding protein. This complex has a high affinity for the CD14 receptor[19]. CD14 is expressed on mature monocytes and macrophages, such as Kupffer cells[20,21]. Furthermore, hepatocytes as well as activated hepatic stellate cells (HSC) get stimulated by LPS via membrane-bound CD14[20,23].
A soluble CD14 (sCD14) is constitutively present in the circulation, and is apparently derived both from secretion of CD14 and from enzymatically cleaved glycosylphosphatidylinositol-anchored tissue CD14[24,25]. sCD14 is believed to play a key role as an intermediate in the neutralization of LPS under physiological conditions. sCD14 accelerates the transfer between LPS micelles and lipoproteins by acting as a carrier. sCD14 also enhances the release of monocyte-bound LPS, transferring LPS into plasma and into lipoproteins[26]. This results in a decreased cellular response to LPS, such as induction of TNF and interleukin-6 synthesis[27].
Recently, a polymorphism of the CD14 gene (C-T transition at bp -159 from the major transcription start site) was described. This polymorphism is located within the Sp1 transcription factor binding site, known to affect CD14 expression. The T allele promotes CD14 gene transcription as shown in in vitro on monocytes[28,29].
Functional relevance of the polymorphism has been suggested in several studies finding an association with myocardial infarction[29,30] ulcerative colitis[31] asthma[32] and chronic spondyloarthropathy[33]. Notably, all these diseases have an inflammatory component.
Recently, Jarvelainen et al[34] investigated the frequency of this CD14 polymorphism in autopsy series of Finnish patients with ALD. Interestingly, the T allele was associated with advanced ALD, especially with cirrhosis, but not with steatosis or less advanced stages of fibrosis. A subsequent analysis of a relatively small cohort of 30 patients with primary biliary cirrhosis revealed no significant association of the CD14 polymorphism with the severity of the disease and found no differences in the genotype distribution as compared to healthy controls[35]. Poullis et al[36] analyzed the effect of the CD14 polymorphism on liver function tests and found some evidence indicating that the TT genotype, associated with higher serum levels of sCD14, may offer protection from the development of fatty liver disease. This hypothesis is supported by recent studies indicating an association between sCD14 levels and insulin sensitivity[37,38].
As in ALD in HCV patients, elevated serum levels of endotoxin as well as sCD14 have been reported[12,16,39] but the C-T (-159) promotor polymorphism of the CD14 gene had not been investigated in this group of patients so far.
Here we analyzed the association of this CD14 polymorphism with the histological stage of fibrosis and the grade of inflammation in hepatic tissue of HCV patients, and their corresponding sCD14 serum levels, respectively.
Furthermore, the aim of this study was to verify the association of the CD14 polymorphism with the degree of alcoholic liver damage, previously found in the Finnish study[35], in our cohort of patients with ALD from southern Germany.
MATERIALS AND METHODS
Patients and controls
The following groups of patients were studied retrospectively: (a) 121 patients (80 males and 41 females; mean age: 37.211.5 years) with chronic hepatitis C (positive for HCV-RNA and anti-HCV) consecutively admitted to the medical department of the University of Regensburg. All patients were negative for hepatitis B surface antigen or antibodies to HIV and none of them had evidence of other types of liver disease. Risk factors for acquisition of hepatitis C infection were previous intravenous drug abuse in 25.6%, receipt of blood or blood products before the introduction of donor screening in 15.7%, and other factors or unknown in 58.7%. Liver biopsies were obtained from all patients before initiation of antiviral therapy (naive patients) by percutaneous Menghini-needle biopsy. In these patients fibrosis and inflammation were graded and staged numerically according to a score proposed by Desmet et al[40] by a single pathologist.
The majority of patients received treatment with interferon-a+ribavirin. For 91 patients, data of qualitative HCV-PCR analysis 3 mo after initiation of antiviral therapy were available.
(b) sixty-two patients with alcoholic cirrhosis consecutively admitted to the medical department of the University of Regensburg (49 males and 13 females; mean age: 53.18.9 years). Patients were considered to have alcohol-related cirrhosis, if alcohol intake had been in excess of 100 g/d for more than 10 years and if testing for viral, metabolic, and immune etiologies was negative. Liver cirrhosis was diagnosed based on typical clinical, laboratory, ultrasound, and gastroscopical findings.
(c) one hundred and eighteen heavy drinkers without evidence of liver damage (90 males and 28 females; mean age: 42.59.1 years). These unrelated individuals of German descent were recruited from an in-patient abstention program at the Department of Psychiatry of the University of Regensburg. Each subject met the criteria of alcohol dependence according to ICD-10[41]. At the day of admission, several serum parameters were analyzed including transaminases (alanine aminotransferase [ALT], AST, and g-glutamyl transferase [g-GT]), and anti-HCV- and HBV-antibodies. Previous and current drinking habits, as well as family history of non-ALDs, and potential previous complications of advanced liver damages as ascites or esophageal varices were examined. Furthermore, within 14 d after admission, a clinical and ultrasound examination of the abdomen was performed.
Only alcohol-dependent subjects with an alcohol consumption of at least 80 g/d for more than 5 years were included. Furthermore, clinical or sonomorphological indications for advanced liver damage and serological evidence of HBV or HCV infection were exclusion criteria.
(d)two hundred and forty seven healthy subjects (119 females and 128 males), genotyped already in a previous study[42], served as controls.
Patients and controls were Caucasians and their geographical origin was southern Germany. Informed consent was obtained from all patients and the study was approved by the local ethics committee.
DNA isolation and CD14 genotyping
Genomic DNA specimens were prepared from 200μL blood using the QIAamp blood kit following the manufacturer's instructions (Qiagen, Hilden, Germany). The C-T polymorphisms at position -159 of the CD14 gene was analyzed by performing PCR and subsequent restriction fragment length polymorphism analysis. PCR was performed under standard conditions (35 cycles, annealing temperature: 55 °C) in a total reaction volume of 50 μL containing 2 μLof diluted genomic DNA, using the following pair of primers: forward: 5-CCG AGA TGT TCC CAG CAC AG-3 and reverse: 5-CTG CTT TGC TTG TGC CTC TT-3. PCR products were digested by HaeIII, and the resulting fragments were separated by electrophoresis in a 2% agarose gel and visualized by ethidium bromide staining. With the -159 T polymorphic base, the recognition sequence 5-GG/CC-3 is modified to 5-GG/TC-3, which is not cut by HaeIII.
Serum sCD14 levels
sCD14 concentrations were analyzed in the serum of 97 patients with chronic hepatitis C infection using a sandwich ELISA following the instructor抯 manual (Biosource Europe, Nivelles, Belgium).
Statistical analysis
Results are expressed as mean±D (range), median, or percent. Genotype frequencies are reported with their group percentages. Comparisons between clinical subgroups were made using the Student's unpaired t-test. A two-sided c2 test was used for comparison of qualitative variables. A P value <0.05 was considered statistically significant.
All computations were performed by using the SPSS-10 for Windows statistical computer package (SSPS, Inc., Chicago, IL, USA).
RESULTS
CD14 genotype frequency in patients with chronic alcohol abuse and controls
Initially, we wanted to confirm the finding of the previous Finnish study by Jarvelainen et al[34] indicating higher prevalence of the C-159T promotor polymorphism of the CD14 endotoxin receptor in patients with advanced ALD.
CD14 genotyping was performed in patients with chronic alcohol abuse and liver cirrhosis (n = 62), or without clinical or sonomorphological signs of liver cirrhosis (n = 118), respectively. Furthermore, 247 healthy controls were genotyped. Results are summarized in Table 1.
Table 1.
Frequency of (–159) CD14 genotypes (%) in patients with chronic alcohol abuse and cirrhosis, compared to alcoholics without clinical or sonomorphological signs of cirrhosis and controls
|
CD14 genotype frequency |
|||
| CC | CT | TT | |
| Alcoholics with cirrhosis (n=62) | 5 (8.1%) | 32 (51.6%) | 25 (40.3%) |
| Alcoholics w/o cirrhosis (n=118) | 32 (27.1%) | 58 (49.2%) | 28 (23.7%) |
| Controls (n=247) | 64 (25.6%) | 128 (51.8%) | 55 (22.3%) |
Distribution of the individual genotypes in the control group (CC: 64/247 [25.6%], CT 128/247 [51.8%], and TT: 55/247 [22.3%]) was similar as in cohorts of other healthy individuals in previous reports[36,43,44].
Frequency of the C-159T polymorphism did not differ significantly between alcoholics without cirrhosis and controls. However, in accordance to the study by Jarvelainen et al[34] the TT genotype was significantly more frequent in alcoholics with cirrhosis (40.3%), compared to alcoholics without cirrhosis (23.7%; P = 0.020) and controls (22.3%; P = 0.006), respectively.
Comparison of ALT and AST levels between TT-homozygotes and alcoholics with genotype CC or CT revealed no significant differences (42.061.0 U/L vs 33.633.8 U/L, and 34.927.9 U/L vs 28.228 U/L, respectively).
CD14 genotype frequency in patients with chronic hepatitis C infection
Next we wanted to analyze the frequency of the C-159T CD14 polymorphism in patients with a different etiology of chronic liver disease. Genotyping of 121 patients with chronic hepatitis C infection revealed no significant differences compared to healthy controls (CC: 26/121 [21.5%], CT: 66/121 [54.5%], and TT: 29/121 [24.0%]).
Comparing TT-homozygote HCV patients and HCV patients with genotype CC or CT we found no differences in demographic features such as age (38.012.3 years vs 37.111.4 years) and distribution of sexes (58.6% vs 68.5% males), and in the frequencies of potential routes of HCV infection (24.1% vs 26.1% i.v. drug users; 17.2% vs 15.2% recipients of blood transfusions), serum HCV-RNA levels (2.0106 copies/mL vs 1.6106 copies/mL) or frequencies of different HCV-genotypes (58.7% vs 66.3% type 1; 34.5% vs 25.0% type 2, Table 2). The number of initial virological responders to interferon therapy (no HCV-RNA detectable in the serum, 3 mo after initiation of therapy) did not differ between the genotypes (14/21 [66.7%] vs46/70 [65.7%]).
Table 2.
Clinical and biological characteristics of 121 patients with chronic hepatitis C infection according to CD14 –159 genotypes (TT vs CC and CT)
| Clinical and biological characteristics | All patients |
CD14 –159 genotype |
|
| TT | CC or CT | ||
| Age (yr; mean±SD) | 37.2±11.5 | 38.0±12.3 | 37.1±11.4 |
| Male gender (%) | 66.1 | 58.6 | 68.5 |
| HCV transmission route (%) | |||
| - i.v. drug use | 25.6 | 24.1 | 26.1 |
| - Post-transfusion | 15.7 | 17.2 | 15.2 |
| - Other factors or unknown | 58.7 | 58.6 | 58.7 |
| HCV genotypes (%) | |||
| - Type 1 | 64.5 | 58.7 | 66.3 |
| - Type 3 | 27.3 | 34.5 | 25 |
| - Others than type 1 or 3 | 8.3 | 6.9 | 8.7 |
| Viral load | |||
| (106 copies/mL; median) | 1.7 | 2 | 1.6 |
| Initial virological response to anti-HCV treatment1 (%) | 65.9 | 66.7 | 65.7 |
HCV-RNA negative, 3 mo after the start of antiviral therapy; data available from 91/121 patients.
Moreover and in contrast to patients with chronic alcohol abuse, frequency of TT-homozygotes did not differ between HCV patients with different severity of liver damage (Figure 1). TT-homozygotes were similarly frequent in patients with no or only mild, periportal fibrosis (staging 1 or 2) and HCV patients with more advanced septal fibrosis or cirrhosis (staging 3 or 4), respectively (20/85 [23.5%] vs 9/36 [25.5%], respectively).
Figure 1.
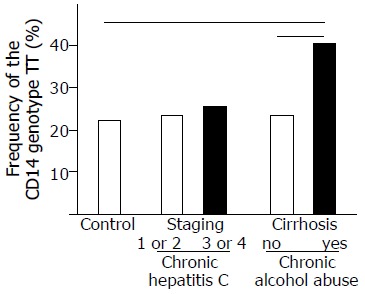
Frequency of the TT genotype at position -159 of the CD14 gene in patients with chronic hepatitis C infection, chronic alcohol abuse, and controls. The TT genotype was significantly more frequent in alcoholics with cirrhosis (25/62; [40.3%]), compared to alcoholics without clinical or sonomorphological signs of cirrhosis (28/118; [23.7%]; P = 0.020) and controls (55/247 [22.3%]; P = 0.006). In contrast, in HCV patients with no or only mild fibrosis (stage 1 or 2) and patients with prominent fibrosis or cirrhosis (stage 3 or 4) the frequency of the TT genotype was not significantly different (20/85 [23.5%]vs 9/36 [25.5%]).
The frequency of the CD14 genotypes was similar in HCV patients with mild or only minimal hepatic inflammation (grading 1 or 2) and patients with more severe hepatic inflammation or necrosis (grading >2): 14/63 [22.2%] vs 15/58 [25.9%], respectively. As in patients with chronic alcohol abuse, the comparison of ALT and AST levels between TT-homozygotes and hepatitis C patients with genotype CC or CT revealed no significant differences (80.465.3 U/L vs 80.278.1 U/L, and 33.920.7 U/L vs 36.128.7 U/L, respectively).
Soluble CD14 serum levels in patients with chronic hepatitis C infection
It has been reported that sCD14 levels were elevated in patients with chronic liver disease[39]. Furthermore, studies investigating sCD14 levels in children recruited from a general population sample or in patients with ulcerative colitis found an association between sCD14 serum levels and CD14 -159 genotypes[28,31].
Therefore, we investigated sCD14 levels in the serum of 87 patients with chronic hepatitis C infection. Mean serum CD14 levels (3.8±0.4 μg¸/mL) were comparable to previous studies analyzing serum of patients with chronic liver disease[39].
HCV patients with no or only mild, periportal fibrosis, had significantly lower sCD14 serum levels than HCV patients with more advanced septal fibrosis or cirrhosis, respectively (3.5±0.4 μg/mL vs 3.8±0.9 μg/mL; P = 0.004).
Moreover, TT-homozygote HCV patients (n = 20) had significantly higher sCD14 serum levels than HCV patients with -159 CD14 genotype CT or TT (n = 77), respectively (3.5±0.4 μg/mL vs 3.7±0.7 μg/mL; P = 0.012, Figure 2).
Figure 2.
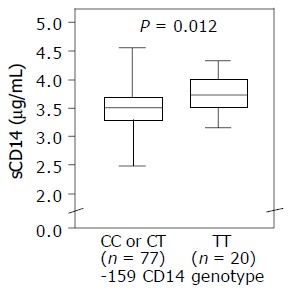
Serum levels of sCD14 comparing TT-homozygote HCV patients and patients with genotypes CC or CT, respectively. Box plots illustrate median values and interquartile distance.
DISCUSSION
There is a remarkable variation in the course of chronic liver disease in different patients. Recent studies suggest that the natural history of chronic liver disease is influenced by a number of gene polymorphism[1]. These genetic factors could explain the broad spectrum of response of the same etiologic agent seen in these individuals.
Recently, the C-T (-159) polymorphism in the promotor region of the CD14 gene was identified as a risk factor for the development of alcoholic liver cirrhosis in the Finnish population based on autopsy series[34]. The Finnish population has a limited number of founders and is characterized by national and regional isolation[45,46].
The first aim of our study was to re-evaluate the results of this previous study in a cohort of patients with chronic alcohol abuse from southern Germany.
In line with the previous Finnish study[35] the -159 CD14 genotype TT was significantly more frequent in patients with alcoholic liver cirrhosis compared to healthy controls or alcohol-dependent subjects without evidence of advanced liver damage.
Interestingly, serum aminotransferase levels did not vary significantly between patients with different genotypes in the group of alcoholics without evidence of advanced liver damage. Considering serum levels of aminotransferases as surrogate markers for hepatocellular damage, our data are in line with those of Jarvelainen et al[34] who also could not find an association between the -159 genotypes and histological steatosis or less advanced stages of fibrosis, respectively. Taken together, these findings indicate that the functional CD14 polymorphism and the associated CD14 expression, respectively, may exhibit different effects during the course of ALD.
On one hand, the CD14 polymorphism has been shown to affect expression levels of membrane-bound CD14[28,44]. Higher expression levels of membrane-bound CD14 predispose hepatic cells for higher cytokine expression in response to endotoxins and cause increased inflammatory activity[23,47].
On the other hand, the CD14 polymorphism plays a significant role in regulating serum levels of sCD14 as TT-homozygotes have raised levels of sCD14[28,31]. Increased sCD14 can decrease the inflammatory response of monocytes to endotoxin by enhanced clearance[26]. Furthermore, a strong correlation between sCD14 and the endotoxin neutralizing capacity of plasma has been shown[48].
Increased expression of sCD14 in TT-homozygotes may lead to enhanced clearance of portal endotoxin and subsequently low-grade hepatic stimulation. In line with this hypothesis, Poullis et al[36] found the TT polymorphism to be associated with significantly reduced serum levels of ALT and g-GT. Similarly, Fernandez-Real et al., reported an inverse correlation between serum levels of sCD14 and ALT in individuals without known liver disease during routine-checkup visits[38].
These data indicate that the CD14 polymorphism seems to affect two mechanisms with opposite effects on hepatic inflammation by regulating the expression of membrane bound as well as sCD14. During the course of ALD the relative pathophysiological impact of each of these two mechanisms may vary.
Interestingly, there is an association between CD14 expression and the degree of fibrosis[49] and CD14 is de novoexpressed on HSC during their activation process[23]. These cells are present in acute as well as chronically diseased livers, and there is a positive correlation between the degree of fibrosis and the accumulation of activated HSC[50,51]. Activated HSC synthesize and secrete excessive ECM molecules. In addition, it is recognized that they also play an important role in hepatic inflammation[52] and LPS-mediated NF-kB activation in HSC increases their resistance to apoptosis[23]. Therefore, with progressing liver damage and fibrosis, the number as well as type of CD14-expressing cells varies leading to differences in the LPS effects and CD14-mediated signal transduction.
In summary, the balance between potentially anti-inflammatory effects of sCD14 and proinflammatory effects of mCD14 may shift toward mCD14 during progression of ALD.
This imbalance may be further enhanced by higher endotoxin levels in patients with severe liver damage[11,16,17]. High LPS levels in more advanced liver damage may saturate the scavenger effects of sCD14. Furthermore, higher LPS levels promote hepatic inflammation leading to increased SP1-binding activity[53]. Since the -159 CD14 promotor polymorphism potentially affects binding of the transcription factor SP-1, high LPS levels in more advanced liver disease might promote CD14 expression particularly in carriers of the T-allele.
In summary, this hypothesis could explain that TT genotype promotes fibrosis in late stages of ALD, while in earlier stages pro- and anti-inflammatory mechanisms may be in balance regardless of the genotype.
The second aim of this study was to investigate the role of the -159 CD14 promotor polymorphism in HCV patients.
TT-homozygote HCV patients had significantly higher sCD14 serum levels than HCV patients with -159 CD14 genotype CT or TT, respectively. Moreover, HCV patients with advanced liver fibrosis or cirrhosis had significantly higher sCD14 levels than patients with less advanced stages of liver fibrosis. Similarly, a correlation between sCD14 levels and hepatic fibrosis has been shown previously in patients with ALD[39]. However, in contrast to patients with ALD, liver cirrhosis was not associated with the TT-homozygous state in patients with chronic hepatitis C infection. These findings indicate that the functional CD14 polymorphism and the expression of CD14 may exhibit different effects during the course of ALD and chronic HCV infection.
One explanation for this finding could be different expression patterns of membrane bound and sCD14, respectively, depending on the underlying cause of chronic liver disease. Furthermore, pathophysiological mechanism of viral and ALD may be differently affected by endotoxins, binding of sCD14 or CD14-mediated signal transduction.
It is known that sCD14 reveals inhibitory action on lymphocyte function[54,55]. Hepatic inflammation in HCV patients is characterized by lymphocyte infiltration while in alcoholic hepatitis there is a predominant infiltration with neutrophils. Furthermore, endotoxemia has been shown to exaggerate hepatic inflammation in murine viral infections[56,57] and to be associated with the response to antiviral therapy in HCV patients[58].
The origin of endotoxemia in HCV patients seems to be multifactorial, likely depending on impaired phagocytic functions and reduced T-cell-mediated antibacterial activity[57]. Furthermore, one cannot exclude the passage of LPS from the gut flora to the blood stream, condition owing to altered intestinal permeability, similar as shown in models of ALD[11,18]. In patients with alcoholic cirrhosis, the mean endotoxin concentration was significantly higher than in patients with non-alcoholic cirrhosis and higher endotoxin concentrations were more frequently observed in patients with alcoholic cirrhosis[12]. Therefore, it may be speculated that different LPS levels in patients with ALD and patients with chronic HCV infection may indirectly account for varying pathophysiological effects of CD14-mediated signal transduction.
There are several studies indicating that alcohol potentiates the proinflammatory effects of endotoxins in hepatic diseases. Peripheral blood mononuclear cells or purified monocytes from patients with alcoholic liver cirrhosis, stimulated in vitro with LPS, displayed a marked increase of proinflammatory cytokines compared with healthy controls[15]. Furthermore, alcohol was shown to increase the expression of CD14 on Kupffer cells and subsequently increased their responsiveness to endotoxins[59,60]. Interestingly, elegant studies by Jarvelainen et al[61] in an animal model indicate that the synergistic effect of endotoxins and alcohol may vary depending on the time of exposure.
Finally, as for most genetic association studies, it has to be considered that the single nucleotide polymorphism investigated is in linkage disequilibrium to a different disease-associated genetic variation. Theoretically, this potential genetic variation may affect particularly the pathophysiology of alcoholics but not viral liver disease. However, the functional relevance of the CD14/-159 polymorphism demonstrated in several studies and the important role of LPS and CD14 signaling in the pathophysiology of chronic liver disease clearly argue against this hypothesis.
In summary, comparison of the frequency of the -159 CD14 genotypes revealed significant differences between the two etiologies of liver diseases investigated in this study. In ALD, the impact of the -159 CD14 polymorphism may vary during the course of the disease, potentially via varying effects of soluble and membrane-bound forms of CD14. It will be interesting to elucidate the importance of this CD14 polymorphism in patients with other underlying liver diseases as non alcoholic fatty or autoimmune liver disease. These studies could potentially lead to therapeutic or prevention strategies selectively for those subgroups of patients that are particularly prone for the pathophysiological effects of gut-derived endotoxins.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
Supported by grants from the Else Kroner Fresenius-Stiftung to Hellerbrand C and the Deutsche Forschungsgemeinschaft (Schn 620/3-1) to Schnabl B
Co-first-authors: C Meiler and M Muhlbauer
References
- 1.Bataller R, North KE, Brenner DA. Genetic polymorphisms and the progression of liver fibrosis: a critical appraisal. Hepatology. 2003;37:493–503. doi: 10.1053/jhep.2003.50127. [DOI] [PubMed] [Google Scholar]
- 2.Yamauchi M, Maezawa Y, Mizuhara Y, Ohata M, Hirakawa J, Nakajima H, Toda G. Polymorphisms in alcohol metabolizing enzyme genes and alcoholic cirrhosis in Japanese patients: a multivariate analysis. Hepatology. 1995;22:1136–1142. doi: 10.1016/0270-9139(95)90621-5. [DOI] [PubMed] [Google Scholar]
- 3.Day CP, Bashir R, James OF, Bassendine MF, Crabb DW, Thomasson HR, Li TK, Edenberg HJ. Investigation of the role of polymorphisms at the alcohol and aldehyde dehydrogenase loci in genetic predisposition to alcohol-related end-organ damage. Hepatology. 1991;14:798–801. doi: 10.1002/hep.1840140509. [DOI] [PubMed] [Google Scholar]
- 4.Carr LG, Hartleroad JY, Liang Y, Mendenhall C, Moritz T, Thomasson H. Polymorphism at the P450IIE1 locus is not associated with alcoholic liver disease in Caucasian men. Alcohol Clin Exp Res. 1995;19:182–184. doi: 10.1111/j.1530-0277.1995.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 5.Mühlbauer M, Bosserhoff AK, Hartmann A, Thasler WE, Weiss TS, Herfarth H, Lock G, Schölmerich J, Hellerbrand C. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 6.Yee LJ, Tang J, Herrera J, Kaslow RA, van Leeuwen DJ. Tumor necrosis factor gene polymorphisms in patients with cirrhosis from chronic hepatitis C virus infection. Genes Immun. 2000;1:386–390. doi: 10.1038/sj.gene.6363696. [DOI] [PubMed] [Google Scholar]
- 7.Takamatsu M, Yamauchi M, Maezawa Y, Ohata M, Saitoh S, Toda G. Correlation of a polymorphism in the interleukin-1 receptor antagonist gene with hepatic fibrosis in Japanese alcoholics. Alcohol Clin Exp Res. 1998;22:141S–144S. doi: 10.1111/acer.1998.22.s3_part1.141s. [DOI] [PubMed] [Google Scholar]
- 8.Grove J, Daly AK, Bassendine MF, Gilvarry E, Day CP. Interleukin 10 promoter region polymorphisms and susceptibility to advanced alcoholic liver disease. Gut. 2000;46:540–545. doi: 10.1136/gut.46.4.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grove J, Daly AK, Bassendine MF, Day CP. Association of a tumor necrosis factor promoter polymorphism with susceptibility to alcoholic steatohepatitis. Hepatology. 1997;26:143–146. doi: 10.1002/hep.510260119. [DOI] [PubMed] [Google Scholar]
- 10.Takamatsu M, Yamauchi M, Maezawa Y, Saito S, Maeyama S, Uchikoshi T. Genetic polymorphisms of interleukin-1beta in association with the development of alcoholic liver disease in Japanese patients. Am J Gastroenterol. 2000;95:1305–1311. doi: 10.1111/j.1572-0241.2000.02030.x. [DOI] [PubMed] [Google Scholar]
- 11.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 12.Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- 13.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 14.Jacob AI, Goldberg PK, Bloom N, Degenshein GA, Kozinn PJ. Endotoxin and bacteria in portal blood. Gastroenterology. 1977;72:1268–1270. [PubMed] [Google Scholar]
- 15.Caradonna L, Mastronardi ML, Magrone T, Cozzolongo R, Cuppone R, Manghisi OG, Caccavo D, Pellegrino NM, Amoroso A, Jirillo E, et al. Biological and clinical significance of endotoxemia in the course of hepatitis C virus infection. Curr Pharm Des. 2002;8:995–1005. doi: 10.2174/1381612024606983. [DOI] [PubMed] [Google Scholar]
- 16.Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165–172. doi: 10.1016/0168-8278(95)80424-2. [DOI] [PubMed] [Google Scholar]
- 17.Chan CC, Hwang SJ, Lee FY, Wang SS, Chang FY, Li CP, Chu CJ, Lu RH, Lee SD. Prognostic value of plasma endotoxin levels in patients with cirrhosis. Scand J Gastroenterol. 1997;32:942–946. doi: 10.3109/00365529709011206. [DOI] [PubMed] [Google Scholar]
- 18.Rivera CA, Bradford BU, Seabra V, Thurman RG. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am J Physiol. 1998;275:G1252–G1258. doi: 10.1152/ajpgi.1998.275.6.G1252. [DOI] [PubMed] [Google Scholar]
- 19.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 20.Matsuura K, Ishida T, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Upregulation of mouse CD14 expression in Kupffer cells by lipopolysaccharide. J Exp Med. 1994;179:1671–1676. doi: 10.1084/jem.179.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracy TF, Fox ES. CD14-lipopolysaccharide receptor activity in hepatic macrophages after cholestatic liver injury. Surgery. 1995;118:371–377. doi: 10.1016/s0039-6060(05)80347-2. [DOI] [PubMed] [Google Scholar]
- 22.Nanbo A, Nishimura H, Muta T, Nagasawa S. Lipopolysaccharide stimulates HepG2 human hepatoma cells in the presence of lipopolysaccharide-binding protein via CD14. Eur J Biochem. 1999;260:183–191. doi: 10.1046/j.1432-1327.1999.00141.x. [DOI] [PubMed] [Google Scholar]
- 23.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 24.Pugin J, Heumann ID, Tomasz A, Kravchenko VV, Akamatsu Y, Nishijima M, Glauser MP, Tobias PS, Ulevitch RJ. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 25.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 26.Kitchens RL, Wolfbauer G, Albers JJ, Munford RS. Plasma lipoproteins promote the release of bacterial lipopolysaccharide from the monocyte cell surface. J Biol Chem. 1999;274:34116–34122. doi: 10.1074/jbc.274.48.34116. [DOI] [PubMed] [Google Scholar]
- 27.Wurfel MM, Hailman E, Wright SD. Soluble CD14 acts as a shuttle in the neutralization of lipopolysaccharide (LPS) by LPS-binding protein and reconstituted high density lipoprotein. J Exp Med. 1995;181:1743–1754. doi: 10.1084/jem.181.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, Martinez FD. A Polymorphism* in the 5' flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 1999;20:976–983. doi: 10.1165/ajrcmb.20.5.3494. [DOI] [PubMed] [Google Scholar]
- 29.Hubacek JA, Rothe G, Pit'ha J, Skodová Z, Stanĕk V, Poledne R, Schmitz G. C(-260)--& gt; T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. doi: 10.1161/01.cir.99.25.3218. [DOI] [PubMed] [Google Scholar]
- 30.Unkelbach K, Gardemann A, Kostrzewa M, Philipp M, Tillmanns H, Haberbosch W. A new promoter polymorphism in the gene of lipopolysaccharide receptor CD14 is associated with expired myocardial infarction in patients with low atherosclerotic risk profile. Arterioscler Thromb Vasc Biol. 1999;19:932–938. doi: 10.1161/01.atv.19.4.932. [DOI] [PubMed] [Google Scholar]
- 31.Obana N, Takahashi S, Kinouchi Y, Negoro K, Takagi S, Hiwatashi N, Shimosegawa T. Ulcerative colitis is associated with a promoter polymorphism of lipopolysaccharide receptor gene, CD14. Scand J Gastroenterol. 2002;37:699–704. doi: 10.1080/00365520212504. [DOI] [PubMed] [Google Scholar]
- 32.Woo JG, Assa'ad A, Heizer AB, Bernstein JA, Hershey GK. The -159 C--& gt; T polymorphism of CD14 is associated with nonatopic asthma and food allergy. J Allergy Clin Immunol. 2003;112:438–444. doi: 10.1067/mai.2003.1634. [DOI] [PubMed] [Google Scholar]
- 33.Repo H, Anttonen K, Kilpinen SK, Palotie A, Salven P, Orpana A, Leirisalo-Repo M. CD14 and TNfa promoter polymorphisms in patients with acute arthritis. Special reference to development of chronic spondyloarthropathy. Scand J Rheumatol. 2002;31:355–361. doi: 10.1080/030097402320817086. [DOI] [PubMed] [Google Scholar]
- 34.Järveläinen HA, Orpana A, Perola M, Savolainen VT, Karhunen PJ, Lindros KO. Promoter polymorphism of the CD14 endotoxin receptor gene as a risk factor for alcoholic liver disease. Hepatology. 2001;33:1148–1153. doi: 10.1053/jhep.2001.24236. [DOI] [PubMed] [Google Scholar]
- 35.Corpechot C, Poupon R. Promoter polymorphism of the CD14 endotoxin receptor gene and primary biliary cirrhosis. Hepatology. 2002;35:242–243. doi: 10.1053/jhep.2002.30279. [DOI] [PubMed] [Google Scholar]
- 36.Poullis AP, Shetty AK, Risley PD, Collinson PO, Mendall MA. Effect of the CD14 promoter polymorphism on liver function tests and its association with alcohol and obesity. Eur J Gastroenterol Hepatol. 2003;15:1317–1322. doi: 10.1097/00042737-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-Real JM, Broch M, Richart C, Vendrell J, López-Bermejo A, Ricart W. CD14 monocyte receptor, involved in the inflammatory cascade, and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1780–1784. doi: 10.1210/jc.2002-020173. [DOI] [PubMed] [Google Scholar]
- 38.Fernández-Real JM, López-Bermejo A, Broch M, Vendrell J, Richart C, Ricart W. Circulating soluble CD14 monocyte receptor is associated with increased alanine aminotransferase. Clin Chem. 2004;50:1456–1458. doi: 10.1373/clinchem.2003.030015. [DOI] [PubMed] [Google Scholar]
- 39.Oesterreicher C, Pfeffel F, Petermann D, Müller C. Increased in vitro production and serum levels of the soluble lipopolysaccharide receptor sCD14 in liver disease. J Hepatol. 1995;23:396–402. doi: 10.1016/0168-8278(95)80197-9. [DOI] [PubMed] [Google Scholar]
- 40.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 41.Janca A, Ustün TB, Early TS, Sartorius N. The ICD-10 symptom checklist: a companion to the ICD-10 classification of mental and behavioural disorders. Soc Psychiatry Psychiatr Epidemiol. 1993;28:239–242. doi: 10.1007/BF00788743. [DOI] [PubMed] [Google Scholar]
- 42.Hubacek JA, Stüber F, Fröhlich D, Book M, Wetegrove S, Rothe G, Schmitz G. The common functional C(-159)T polymorphism within the promoter region of the lipopolysaccharide receptor CD14 is not associated with sepsis development or mortality. Genes Immun. 2000;1:405–407. doi: 10.1038/sj.gene.6363691. [DOI] [PubMed] [Google Scholar]
- 43.Lichy C, Meiser H, Grond-Ginsbach C, Buggle F, Dörfer C, Grau A. Lipopolysaccharide receptor CD14 polymorphism and risk of stroke in a South-German population. J Neurol. 2002;249:821–823. doi: 10.1007/s00415-002-0726-0. [DOI] [PubMed] [Google Scholar]
- 44.Hubacek J, Pitha J, Skodova Z, Poledne R. Is the CD14 receptor gene a marker for smoking dependence? Med Sci Monit. 2002;8:BR172–BR174. [PubMed] [Google Scholar]
- 45.Nevanlinna HR. The Finnish population structure. A genetic and genealogical study. Hereditas. 1972;71:195–236. doi: 10.1111/j.1601-5223.1972.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 46.de la Chapelle A. Disease gene mapping in isolated human populations: the example of Finland. J Med Genet. 1993;30:857–865. doi: 10.1136/jmg.30.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 48.Hiki N, Berger D, Mimura Y, Frick J, Dentener MA, Buurman WA, Seidelmann M, Kaminishi M, Beger HG. Release of endotoxin-binding proteins during major elective surgery: role of soluble CD14 in phagocytic activation. World J Surg. 2000;24:499–506. doi: 10.1007/s002689910080. [DOI] [PubMed] [Google Scholar]
- 49.Su GL, Rahemtulla A, Thomas P, Klein RD, Wang SC, Nanji AA. CD14 and lipopolysaccharide binding protein expression in a rat model of alcoholic liver disease. Am J Pathol. 1998;152:841–849. [PMC free article] [PubMed] [Google Scholar]
- 50.Ballardini G, Degli Esposti S, Bianchi FB, de Giorgi LB, Faccani A, Biolchini L, Busachi CA, Pisi E. Correlation between Ito cells and fibrogenesis in an experimental model of hepatic fibrosis. A sequential stereological study. Liver. 1983;3:58–63. doi: 10.1111/j.1600-0676.1983.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 51.Knittel T, Kobold D, Piscaglia F, Saile B, Neubauer K, Mehde M, Timpl R, Ramadori G. Localization of liver myofibroblasts and hepatic stellate cells in normal and diseased rat livers: distinct roles of (myo-)fibroblast subpopulations in hepatic tissue repair. Histochem Cell Biol. 1999;112:387–401. doi: 10.1007/s004180050421. [DOI] [PubMed] [Google Scholar]
- 52.Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120–1130. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]
- 53.Sakuta T, Matsushita K, Yamaguchi N, Oyama T, Motani R, Koga T, Nagaoka S, Abeyama K, Maruyama I, Takada H, et al. Enhanced production of vascular endothelial growth factor by human monocytic cells stimulated with endotoxin through transcription factor SP-1. J Med Microbiol. 2001;50:233–237. doi: 10.1099/0022-1317-50-3-233. [DOI] [PubMed] [Google Scholar]
- 54.Rey Nores JE, Bensussan A, Vita N, Stelter F, Arias MA, Jones M, Lefort S, Borysiewicz LK, Ferrara P, Labéta MO. Soluble CD14 acts as a negative regulator of human T cell activation and function. Eur J Immunol. 1999;29:265–276. doi: 10.1002/(SICI)1521-4141(199901)29:01<265::AID-IMMU265>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 55.Arias MA, Rey Nores JE, Vita N, Stelter F, Borysiewicz LK, Ferrara P, Labéta MO. Cutting edge: human B cell function is regulated by interaction with soluble CD14: opposite effects on IgG1 and IgE production. J Immunol. 2000;164:3480–3486. doi: 10.4049/jimmunol.164.7.3480. [DOI] [PubMed] [Google Scholar]
- 56.Gut JP, Schmitt S, Bingen A, Anton M, Kirn A. Probable role of endogenous endotoxins in hepatocytolysis during murine hepatitis caused by frog virus 3. J Infect Dis. 1984;149:621–629. doi: 10.1093/infdis/149.4.621. [DOI] [PubMed] [Google Scholar]
- 57.Jirillo E, Caccavo D, Magrone T, Piccigallo E, Amati L, Lembo A, Kalis C, Gumenscheimer M. The role of the liver in the response to LPS: experimental and clinical findings. J Endotoxin Res. 2002;8:319–327. doi: 10.1179/096805102125000641. [DOI] [PubMed] [Google Scholar]
- 58.Amati L, Cozzolongo R, Manghisi OG, Cuppone R, Pellegrino NM, Caccavo D, Jirillo E. The immune responsiveness in hepatitis C virus infected patients: effects of interferon-alfa/ribavirin combined treatment on the lymphocyte response with special reference to B cells. Curr Pharm Des. 2004;10:2093–2100. doi: 10.2174/1381612043384231. [DOI] [PubMed] [Google Scholar]
- 59.Devière J, Content J, Denys C, Vandenbussche P, Schandene L, Wybran J, Dupont E. Excessive in vitro bacterial lipopolysaccharide-induced production of monokines in cirrhosis. Hepatology. 1990;11:628–634. doi: 10.1002/hep.1840110416. [DOI] [PubMed] [Google Scholar]
- 60.Järveläinen HA, Oinonen T, Lindros KO. Alcohol-induced expression of the CD14 endotoxin receptor protein in rat Kupffer cells. Alcohol Clin Exp Res. 1997;21:1547–1551. [PubMed] [Google Scholar]
- 61.Kono H, Wheeler MD, Rusyn I, Lin M, Seabra V, Rivera CA, Bradford BU, Forman DT, Thurman RG. Gender differences in early alcohol-induced liver injury: role of CD14, NF-kappaB, and TNF-alpha. Am J Physiol Gastrointest Liver Physiol. 2000;278:G652–G661. doi: 10.1152/ajpgi.2000.278.4.G652. [DOI] [PubMed] [Google Scholar]
- 62.Järveläinen HA, Fang C, Ingelman-Sundberg M, Lindros KO. Effect of chronic coadministration of endotoxin and ethanol on rat liver pathology and proinflammatory and anti-inflammatory cytokines. Hepatology. 1999;29:1503–1510. doi: 10.1002/hep.510290508. [DOI] [PubMed] [Google Scholar]


