Abstract
The patient was a 57-year-old woman presenting with jaundice as the chief complaint. She began vomiting on July 10, 2003. Jaundice was noted and admitted to our hospital for thorough testing. Tests on admission indicated severe hepatitis, based on: aspartate aminotransferase (AST), 1 076 IU/L; alanine aminotransferase (ALT), 1 400 IU/L; total bilirubin (TB), 20.9 mg/dL; and prothrombin time rate (PT%), 46.9%. Acute hepatitis A (HA) was diagnosed based on negative hepatitis B surface antigen and hepatitis C virus RNA and positive immunoglobulin (Ig) M HA antibody, but elevation of anti-nuclear antigen (Ã-320) and IgG (3 112 mg/dL) led to suspicion of autoimmune hepatitis (AIH). Plasma exchange was performed for 3 d from July 17, and steroid pulse therapy was performed for 3 d starting on July 18, followed by oral steroid therapy. Liver biopsy was performed on August 5, and the results confirmed acute hepatitis and mild chronic inflammation. Levels of AST and ALT normalized, so dose of oral steroid was markedly reduced. Steroid therapy was terminated after 4 mo, as the patient had glaucoma. Starting 3 mo after cessation of steroid therapy, levels of AST and ALT began to increase again. Another liver biopsy was performed and AIH was diagnosed based on serum data and biopsy specimen. Oral steroid therapy was reinitiated. Levels of AST and ALT again normalized. The present case was thus considered to represent AIH triggered by acute HA.
Keywords: Acute hepatitis A, Autoimmune hepatitis, International criteria for AIH, HLA
INTRODUCTION
Autoimmune hepatitis (AIH) is a chronic progressive hepatitis characterized clinically by positive anti-nuclear antibody (ANA) and hypergammaglobulinemia, and histologically by portal inflammatory cell infiltration (plasmacyte dominant) and piecemeal necrosis. However, the exact mechanisms of onset are unknown. Based on past reports, AIH appears to be induced by antibody-dependent cell-mediated cytotoxicity, which involves both antibody-mediated and cellular immunity against specific liver antigens on hepatocyte membranes[1]. Several studies have documented the involvement of genetic factors, including human lymphocyte antigen (HLA) types such as DR3 and DR4[2]. Furthermore, cases of AIH have been reported in which viruses causing acute hepatitis such as hepatitis A (HA) virus[3-7] HBV[8] and Epstein-Barr virus[9] have acted as a trigger. We report herein the case of a patient with AIH that appears to have been triggered by acute HA.
CASE REPORT
The patient was a 57-year-old woman with jaundice as the chief complaint.
History of present illness
The patient had been seeing a local doctor for hypertension, with no sign of hepatic pathology. On July 2, 2003, she developed hives and was given fexofenadine hydrochloride. Around this time, she began to feel sick. While she had eaten raw foods within the previous month, she had not eaten raw oysters. Starting on July 10, she began vomiting and visited the local doctor on July 14. Jaundice was noted, and the patient was referred and admitted to our hospital for thorough testing.
Physical findings on admission
The patient was 163 cm tall and weighed 100 kg. She was thus very obese, and although yellowing of the bulbar conjunctiva was noted, no other abnormalities were identified.
Test findings on admission
Aspartate aminotransferase (AST), 1 076 IU/L; alanine aminotransferase (ALT), 1 400 IU/L; total bilirubin (TB), 20.9 mg/dL; prothrombin time rate (PT%), 46.9%; hepatitis B surface antigen (HBsAg) negative; hepatitis C virus (HCV) RNA negative; and immunoglobulin (Ig) M HA antibody positive (titer, 1.9 cut of index). Acute HA was therefore diagnosed. Furthermore, as levels of ANA (×320) and IgG (3 112 mg/dL) were elevated, AIH was suspected. Drug lymphocyte stimulation test (DLST) yielded negative results for fexofenadine hydrochloride. The patient scored 1 point according to the diagnostic criteria for acute drug-induced hepatic injury established by the International Consensus Meeting (ICM)[10], excluding the possibility of drug-induced hepatic injury. Computed tomography showed no hepatic atrophy, but thickening of the gallbladder wall was apparent, suggesting acute hepatitis (Figure 1). HLA assessment conducted after admission revealed: A24(9); B52(5); B61(40); DR15(2); and DR9 (Table 1).
Figure 1.
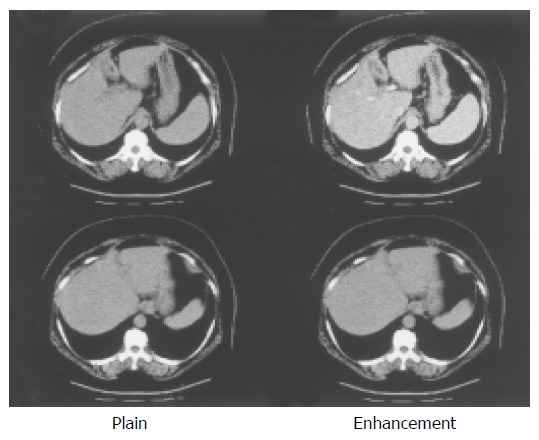
Liver parenchyma is not atrophic. Thickening of the gall bladder wall is also seen. This finding is compatible with acute hepatitis.
Table 1.
Labo data on admission
| WBC | 6900/uL | BS | 129 mg/dL |
| Neutro | 60.90% | HbA1c | 6.40% |
| Eosin | 3.60% | CRP | 1.59 mg/dL |
| Baso | 0.50% | Na | 137 mEq/L |
| Mono | 9.10% | K | 3.6 mEq/L |
| Lympho | 25.90% | Cl | 99 mEq/L |
| RBC | 502×104/uL | ||
| Hb | 15.2 g/dL | ||
| Ht | 44.70% | ||
| Plt | 16.5×104/uL | ANA | 320 times |
| AMA | - | ||
| TP | 8.5 g/dL | IgG | 3 112 mg/dL |
| Alb | 3.4 g/dL | IgM | 3 112 mg/dL |
| BUN | 11 mg/dL | IgA | 631 mg/dL |
| Cr | 0.9 mg/dL | ||
| T-Bil | 20.9 mg/dL | IgM HA | + |
| D-Bil | 16.9 mg/dL | HBsAg | - |
| AST | 1 076 IU/L | HCV Ab | - |
| ALT | 1 400 IU/L | CMV IgM | - |
| PT% | 46.90% | EBV IgM | - |
| LDH | 498 U/L | - | |
| G-GTP | 129 IU/L | - | |
| ChE | 242 U/L | DLST |
Clinical course following admission
Although AST and ALT levels started to improve, TB and PT% deteriorated to 27.5 mg/dL and 40.8%, respectively. To avoid onset of fulminant hepatitis, plasma exchange was performed for 3 d starting on July 17. From July 18, steroid pulse therapy was performed for 3 d, followed by oral steroid therapy. Levels of AST, ALT, TB, and PT% improved. Results of liver biopsy on August 5 confirmed acute hepatitis and mild chronic inflammation (mild fibrous enlargement of portal area was shown in two of eight portal areas and marked inflammatory cell infiltration of liver parenchyma was identified). The patient scored seven points according to international diagnostic criteria for AIH[11], which was thus excluded at this time. Acute HA was therefore diagnosed. At first, 50 mg/d of oral steroid (prednisolone) was administered, with dose reduced by 10 mg/d every week. Levels of AST, ALT, and TB gradually improved, and the patient was discharged while receiving 20 mg/d of steroid. After discharge, dose of oral steroid was reduced by 5 mg/d every week. As the patient also had glaucoma, oral steroid therapy was terminated after 4 mo. Levels of AST and ALT remained low, but began to increase around 3 mo after cessation of therapy. Since IgM HA antibody was negative at this time, AIH was suspected to be based on serum data such as ANA and IgG levels, and liver biopsy was performed on April 13 (Figure 2). AIH was diagnosed, and 600 mg/d of ursodeoxycholic acid was administered. This therapy proved ineffective, and oral steroid therapy (40 mg/d) was reinstated. AST and ALT levels normalized, and dose of oral steroid was gradually reduced. The patient is currently undergoing treatment in an outpatient basis with oral steroid therapy 1 mg/d, and no further elevation of AST or ALT levels has been seen.
Figure 2.
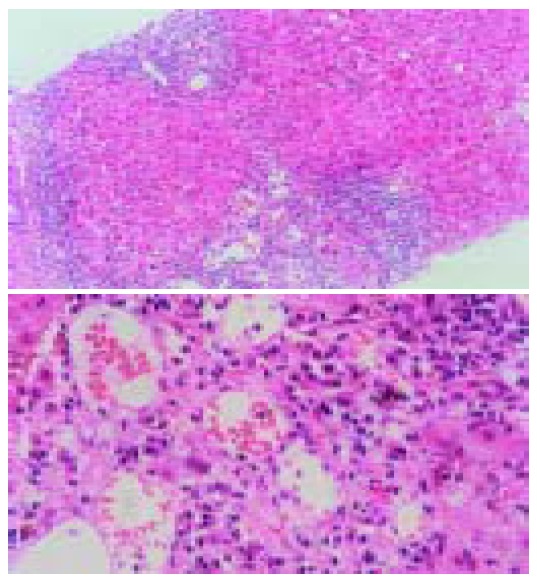
This liver biopsy specimen show the histopathologic appearance of chronic hepatitis. Interface hepatitis is present with bridging fibrosis, which fibrosis connects portal and cetral arreas. However, dominant plasmacyte infiltrates and rossete formation are not present.
DISCUSSION
On admission, although drug-induced hepatic injury was suspected in the present patient, acute HA was diagnosed due to positive IgM HA antibody. However, since ANA was ×320 and IgG was 3 110 mg/dL, AIH could not be excluded as a possibility, and we could not be certain that the acute hepatic injury was caused by HA alone. DLST for the prescribed drug was negative, and the patient scored one point according to the diagnostic criteria for acute drug-induced hepatic injury established by the ICM[9]. Drug-induced hepatic injury thus seemed unlikely.
In the present patient, AIH was suspected at the onset of acute hepatitis, and assessment was performed in accordance with the international diagnostic criteria for AIH[11]. However, as the patient scored seven points, AIH was not initially diagnosed. Oral steroid therapy was terminated after 4 mo because transaminase levels remained normal and the patient also had glaucoma. However, transaminase levels again increased 3 mo after terminating oral steroid therapy. Since AIH had been suspected based on serum data such as ANA and IgG, liver biopsy was performed again. At this time, the patient scored 21 points according to international diagnostic criteria for AIH[11], and AIH was thus diagnosed.
While viral infection has been known to trigger AIH, few cases of AIH following acute HA have been described[3-7]. In most of these cases, AIH did not occur at the same time as acute hepatitis, with AIH often occurring several months after the onset of acute HA. According to international diagnostic criteria, the present patient did not display AIH at the time of acute hepatitis. However, since levels of ANA and IgG were high, excessive immune reactions were present. We suspect that these excessive immune reactions may lead to onset of AIH. No studies have investigated AIH during the early stages of acute hepatitis, and excessive immune reactions during acute HA have not been documented. One report documented a case of AIH that occurred after several months of abnormal liver function following acute HA[5]. While immune reactions are not often assessed in acute HA, as was the case with the present patient, excessive immune reactions may occur during acute HA.
Vento and colleagues[3] reported a patient with a deficiency of specific suppressor T cells for asialoglycoprotein receptor who developed type I AIH following subclinical HA, suggesting the possibility that immunological abnormalities including antigen presentations were involved in the onset of AIH following acute HA. HLA typings of patients with AIH triggered by acute HA were compared, but no specific tendencies were identified (Table 2). Since the sample of patients was small, larger subject populations need to be studied in future.
Table 2.
Sample of patients
| Author | Age (yr) | Sex | Term | HLA |
| Male/Female | (WK) | |||
| Vento S et al | 18 | M | 16 | A2, B15, B40, DR7, DR4 |
| Vento S et al | 13 | F | 15 | A1, A19, B8, B44, DR7, DR3 |
| Rahaman SM et al | 55 | F | 10 | NT |
| Huppertz HI et al | 7 | M | 10 | A1, A2, Bw52, Bw62, Cw3, Cw6, DR7, DRw8, DQw2 |
| Hilzenrat N et al | 55 | F | 7 | A26, B38, DRB1*0401, DRB44*01, DQB1*02 |
| Bertran EM et al | 27 | M | 10 | NT |
| Our case | 57 | F | 28 | A24, B52, B61, DR15, DR9 |
Herein, we presented the case of a patient with AIH that appears to have been triggered by acute HA. Given the existence of such patients and the need for careful follow-up of patients with acute HA, the clinical features of AIH patients after acute hepatitis need to be investigated for early diagnosis of AIH.
Footnotes
Science Editor Guo SY Language Editor Elsevier HK
References
- 1.Eggink HF, Houthoff HJ, Huitema S, Gips CH, Poppema S. Cellular and humoral immune reactions in chronic active liver disease. I. Lymphocyte subsets in liver biopsies of patients with untreated idiopathic autoimmune hepatitis, chronic active hepatitis B and primary biliary cirrhosis. Clin Exp Immunol. 1982;50:17–24. [PMC free article] [PubMed] [Google Scholar]
- 2.Czaja AJ, Carpenter H, Santrach PJ, Moore SB. DR human leukocyte antigens and disease severity in chronic hepatitis C. J Hepatol. 1996;24:666–673. doi: 10.1016/s0168-8278(96)80261-3. [DOI] [PubMed] [Google Scholar]
- 3.Vento S, Garofano T, Di Perri G, Dolci L, Concia E, Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991;337:1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- 4.Huppertz HI, Treichel U, Gassel AM, Jeschke R, Meyer zum Büschenfelde KH. Autoimmune hepatitis following hepatitis A virus infection. J Hepatol. 1995;23:204–208. doi: 10.1016/0168-8278(95)80336-x. [DOI] [PubMed] [Google Scholar]
- 5.Rahaman SM, Chira P, Koff RS. Idiopathic autoimmune chronic hepatitis triggered by hepatitis A. Am J Gastroenterol. 1994;89:106–108. [PubMed] [Google Scholar]
- 6.Hilzenrat N, Zilberman D, Klein T, Zur B, Sikuler E. Autoimmune hepatitis in a genetically susceptible patient: is it triggered by acute viral hepatitis A? Dig Dis Sci. 1999;44:1950–1952. doi: 10.1023/a:1026645629103. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz Bertrán E, Rosa Salazar V, Hostalet Robles F, Correa Estañ JA, Belda Abad G, Muñoz Ramírez E. [Autoimmune hepatitis caused by acute hepatitis due to hepatitis A virus] Gastroenterol Hepatol. 2002;25:501–504. [PubMed] [Google Scholar]
- 8.Laskus T, Slusarczyk J. Autoimmune chronic active hepatitis developing after acute type B hepatitis. Dig Dis Sci. 1989;34:1294–1297. doi: 10.1007/BF01537282. [DOI] [PubMed] [Google Scholar]
- 9.Vento S, Guella L, Mirandola F, Cainelli F, Di Perri G, Solbiati M, Ferraro T, Concia E. Epstein-Barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346:608–609. doi: 10.1016/s0140-6736(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 10.Danan G, Benichou C. Causality assessment of adverse reactions to drugs. I. A novel method based on the conclusions of international consensus meeting: application to drug-induced liver injuries. J Clin Epidemiol. 1993;46:1323–1330. doi: 10.1016/0895-4356(93)90101-6. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, Cooksley WG, Czaja AJ, Desmet VJ, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. Hepatol. 1999;31:929–93. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]


