Abstract
Objectives:
To evaluate the role of anti-Mullerian hormone (AMH) and inhibin B in the evaluation of the effectiveness of short- (3 months) and long-term (6 months or more) metformin therapy in Iraqi women with polycystic ovarian syndrome (PCOS).
Methods:
This cross-sectional study was carried out at the Biochemistry Department, College of Medicine, University of Baghdad, Baghdad, Iraq from June 2010 to May 2011. It included 38 volunteers of women patients with PCOS, aged 18-38 years, who were classified into: Group I (GI, n=20); Group II included women in GI that were followed up after they were treated with metformin hydrochloride tablet 500 mg 3 times daily for 3 months; and GIII included 18 women that were already on metformin hydrochloride treatment 500 mg tablet 3 times daily for 6 months to 3 years. Investigations included serum measurement of insulin, AMH, inhibin B, androgen hormones using enzyme-linked immunosorbent assay, and mini Vidus techniques.
Results:
The mean ± standard error of the mean value of serum AMH levels was significantly decreased in post metformin treatment women (3 months; GII) compared with those before treatment (GI), and those women on prolonged treatment (GIII) (p<0.01 for both). However, there was no significant difference in serum AMH between GI and GIII. With respect to serum inhibin B, both women of GI and GII had significant decrease compared with GIII, with no significant changes between GI and GII.
Conclusion:
This study showed the efficacy of serum AMH measurement as a prognostic biochemical marker in the follow up of metformin treatment of PCOS women.
Polycystic ovary syndrome (PCOS) is one of the most common causes of female infertility, affecting approximately 8% of women of reproductive age,1 while the incidence of PCOS in Iraq was reported to be 41%.2 In 2006, the Androgen Excess Society evidence-based definition considers PCOS as a mainly hyperandrogenic disorder, and therefore sustains a diagnosis of PCOS only in the presence of clinical and/or biochemical hyperandrogenism, which should be accompanied by either oligo-ovulation and/or polycystic ovarian morphology.3 Anti-Mullerian hormone (AMH), also known as Mullerian-inhibiting substance, is a member of the transforming growth factor-β (TGFβ) superfamily.4,5 The AMH is a disulfide-linked glycoprotein with a molecular weight of 140 KDa.6 In females, the AMH is mainly secreted by the granulose cells of the ovarian early developing follicles. The expression of AMH is localized in granulose cells of the primary, pre-antral, and small antral follicles, suggesting an important role of AMH in human folliculogenesis. Since AMH is secreted exclusively in the gonads, its serum concentrations in women are thought to reflect the size of the ovarian follicle pool. Serum AMH levels are 2-3 folds increased in PCOS women,7,8 which is in line with the increased number of AMH-producing pre-antral and small antral follicles.9 Increased serum AMH in women with hyperandrogenism and/or oligo-anovulation could indicate to clinicians the presence of PCOS when reliable ultrasound is not available.10 Insulin resistance (IR) and secondary hyperinsulinemia affects approximately 65-70% of women with PCOS.11 Many of these women are also obese, which further exacerbates their IR. Hyperinsulinemia may also directly cause premature follicular atresia and antral follicle arrest.12 Polycystic ovary syndrome is an endocrine-metabolic disorder, closely tied to IR, and a compensatory hyperinsulinemia. The latter may have preferentially impaired oocyte developmental competence in PCOS patients with obesity. Insulin may induce local androgen production, reducing circulating sex hormone binding globulin (SHBG) levels, thereby increasing the bioavailability of testosterone, which results in oocyte of lower quality, and post-maturity of follicles.13,14 Metformin (1,1-dimethylbiguanide hydrochloride, originally sold as Glucophage,™ Bristol-Myers Squibb Company, Princeton, NJ, USA) is an oral antidiabetic drug in the biguanide class, and is approved by the US Food and Drug Administration for the treatment of type 2 diabetes mellitus.15 Primary clinical action is to inhibit hepatic glucose production, although it also decreases intestinal glucose uptake, and increases insulin sensitivity in peripheral tissues. Metformin has antilipolytic effects, lowering circulating free fatty acid concentrations, which ultimately aids in reducing gluconeogenesis.16 The use of metformin in PCOS is associated with increased menstrual cyclicity, improved ovulation, and a reduction in circulating androgen levels. Metabolic benefits are enhanced in the presence of weight loss, and weight loss itself may be enhanced in the presence of metformin.17,18 Metformin is available in 500, 850, and 1000 mg tablets with a target dose of 1500-2550 mg per day. Many studies in PCOS have used a dose of 850 mg twice a day for 6 months.19
Methods
This cross-sectional study was carried out at the Department of Biochemistry, College of Medicine, University of Baghdad, and at Kamal Al-Samaraee Hospital, Baghdad, Iraq from June 2010 to May 2011. It included 38 women with PCOS, age range 18-38 years, and mean age 28.09±1.29 years. These PCOS women were allocated into 3 groups: Group I (GI) consisted of 20 women diagnosed to have PCOS who were studied on 2-4 cycle day; Group II (GII) which included the 20 women in group I, and who were followed up after they were treated with metformin hydrochloride tablet 500 mg (Glucophage™ tablet, Bristol-Myers Squibb Company, New York, USA) 3 times daily for 3 months and were studied on a 2-4 cycle day; and Group III (GIII) consisted of 18 women diagnosed to have PCOS and who were already on metformin hydrochloride treatment 500 mg tablet 3 times daily for 6 months to 3 years, and this group was also studied on day 2-4 of their menstrual cycle. Individual questions to each woman patient included; age, married or not, number of children, previous history of high blood pressure or diabetes mellitus, and gynecological diseases, regular or irregular menstrual cycle, amenorrhea or oligomenorrhea, and acne or hirsutism. Pregnant women and diabetic PCOS women have been excluded from this study. Formal consent was obtained from each women patient. Ethical approval was obtained from the Scientific Committee of the Biochemistry Department, College of Medicine, University of Baghdad, Baghdad, Iraq. Diagnosis of PCOS was based on the Rotterdam Consensus Group Criteria for Definition of PCOS.20 The diagnosis of PCOS was based on the presence of at least 2 of the following 3 criteria: 1. oligo-ovulation and/or anovulation; 2. clinical and/or biochemical signs of hyperandrogenism; and 3. polycystic ovaries on ultrasound defined as the presence of 12, or more follicles in either ovary measuring 2-9 mm in diameter, and/or increased ovarian volume greater than 10 ml. Other causes for hyperandrogenism that mimic PCOS such as, congenital adrenal hyperplasia, Cushing syndrome, or androgen secreting tumors were excluded from this study. The ultrasound study was performed at Kamal Al-Samaraee Hospital, Ultrasound Department under the supervision of a specialist gynecologist. Ovarian morphology was objectively assessed by pelvic ultrasound. Most ultrasound examinations were performed transvaginally to optimize image quality. All ultrasound investigations were achieved in real-time on the same machine (AG 50149 with a transvaginal 3.5 MHz probe, Siemens, Germany) and interpreted under the highest possible magnification. Five milliliters of peripheral venous blood was aspirated from each women of GI, GII, and GIII after 10-12 hours overnight fast on day 2-4 of their menstrual cycle. Blood samples were collected before anthropometric measurements and after transvaginal ultrasound. If a dominant follicle (>16 mm) was present by vaginal ultrasound, the woman’s blood sample was not obtained, and she was therefore excluded from this study. The sample blood was transferred into a plain test tube and allowed to clot for 20-30 minutes, followed by centrifugation at 2500 rpm for 10-15 minutes. The separated serum was stored at -20 ºC until the day of assay. Investigations included serum measurements of AMH,21 and inhibin B.22 Serum follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol 17-β (E2), prolactin, free testosterone, androstenedione, dehydroepiandrosterone sulfate (DHEAS), and insulin were measured using enzyme linked immunosorbent assay (ELISA) and mini Vidus techniques according to the reported methods.23 The homeostasis model assessment of insulin resistance (HOMA-IR) calculation uses fasting plasma glucose and insulin concentrations to estimate insulin resistance (HOMA-IR) using a mathematical model (HOMA-IR = [glucose in mmol/l insulin in µIU/ml]/22.5) was calculated.23 All material kits for the measured parameters were provided from Human GmbH.65205 Wiesbaden, Germany. The ELISA study was performed using Biotech instruments (Winooski, Vermont, USA).
The Statistical Package for Social Sciences version 15 (SPSS Inc, Chicago, IL, USA), and Minitab analysis programs were used for all statistical studies. Analysis of variance (ANOVA) and Student’s t-tests were used to test for statistical significance. Linear regression was utilized to test for correlation between different studied parameters, and the significance of the R-value was assessed by related t-test. P<0.05 was considered statistically significant.
Results
Table 1 shows that the mean±standard error of mean (SEM) values of serum AMH levels was 4.49±0.54 ng/ml in GI, 3.03±0.53 ng/ml in GII, and 4.88±0.91 ng/ml in GIII with a significant decrease in GII compared with GI (p=0.01), and GIII (p=0.01). However, the PCOS women in GIII did not show a significant difference from those women in GI. Table 1 also shows that the mean±SEM values of serum inhibin B levels was 88.60±17.87 pg/ml in GI, 84.60±9.01 pg/ml in GII, and 160.83±22.17 pg/ml in GIII. There was no significant difference in serum inhibin B between the women of GII and GI, while it was significantly decreased in both GI and GII when compared with GIII (for both, p=0.01). Table 2 shows the mean ±SEM values of age and body mass index (BMI) of GI, GII, and GIII. There was no significant difference in age among the studied groups. The mean value of BMI of GII was significantly decreased in comparison with that of GIII (p=0.01), but with a insignificant decrease compared to GI (30.85±0.95 Kg/m2). The women of GIII were the more obese one. Table 2 also shows that the mean values of the ovarian follicles number and ovarian volume of women of GII were significantly decreased when compared with those of GI (p=0.01, p=0.05), and GIII (for both, p=0.01). Table 3 shows the mean±SEM values of the measured serum fasting glucose, insulin, and HOMA-IR for the studied PCOS groups. The mean of serum glucose levels was significantly decreased in GII and GIII when compared with GI (for both, p=0.001). However, the mean value of insulin resistance-HOMA-IR was significantly decreased in GII compared with GI (p=0.039), while that of GIII did not differ significantly from that of GI. Table 4 shows the mean±SEM values of the measured androgen hormones. The mean values of serum free testosterone and DHEA-S levels did not differ significantly among the 3 groups. However, the mean value of serum androstenedione levels was significantly decreased in women of GI and GII when compared with those of GIII (for both; p=0.01). The mean±SEM values of serum prolactin, FSH, LH, estradiol 17-β and LH/FSH ratio did not differ significantly among GI, GII, and GIII (data not shown). The study also revealed significant positive correlation between serum levels of AMH and the values of ovarian volume (r=0.476, p=0.05) along with a significant positive correlation between serum levels of LH and the number of ovarian follicles (r=0.626, p=0.01) in women of GI. In GIII, there was a significant positive correlation between serum levels of AMH and the LH/FSH ratio (r=0.568, p=0.05), and between the values of BMI with those of ovarian volume (r=0.57, p=0.05).
Table 1.
Mean ± standard error of mean values of anti-Mullerian hormone (AMH) and inhibin B in different groups of women with polycystic ovary syndrome.
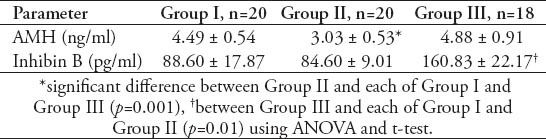
Table 2.
Mean ± standard error of mean values of age, body mass index (BMI), number of ovarian follicles and ovarian volume in different groups of women with polycystic ovary syndrome.
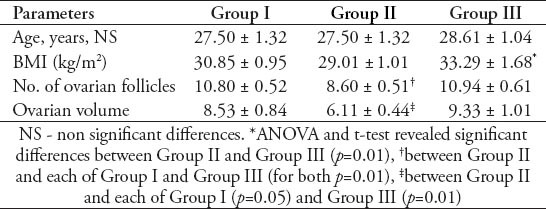
Table 3.
Mean ± standard error of mean values of fasting serum glucose, insulin, and HOMA-IR in different groups of women with polycystic ovary syndrome.
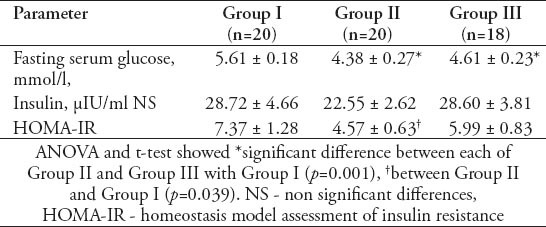
Table 4.
Mean ± standard error of mean values of free testosterone, androstenedione, and DHEAS in different groups of women with polycystic ovary syndrome.
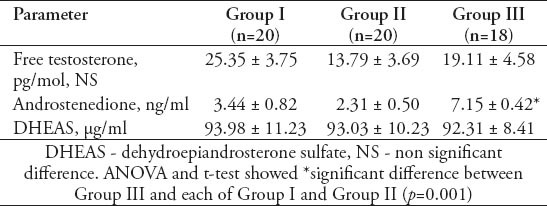
Discussion
The AMH is secreted by the granulosa cells of small antral and pre-antral follicles in the ovary. It diminishes aromatase induction by FSH in antral follicles and inhibits recruitment of primordial follicles.24 Several studies have reported higher levels of AMH in women with PCOS than in controls.25,26 An increased production of AMH induces a decrease in the sensitivity of follicles to FSH at receptor level, which is necessary for their growth. It leads to an increase of the number of antral follicles on the detriment of their size: the number of small antral follicles 2-5 mm in size increases, restraining, thus the selection of the dominant follicle. Such a situation is clinically characterized by anovulation cycles, manifesting themselves as oligo- or amenorrhea.6 The present study found that the significant and positive effect of ovarian volume on AMH levels in GI, and the importance of obesity as one of the factors tightly bound with AMH regulation is in agreement with Chen et al.27
The results of the present study are consistent with previous studies, in which the treatment with insulin sensitizer metformin resulted in the reduction of AMH levels.16,28 The AMH levels are significantly elevated in women with PCOS, and they may serve as a marker for evaluation of treatment efficacy with metformin. Furthermore, obese PCOS patients are more likely to respond to metformin therapy with maximal doses (2550 mg/day) as compared with the ones with low body mass index.29 However, other studies did not find significant changed in serum AMH after metformin treatment in PCOS,30 which may be attributed to a low dose (twice 850 mg/day for 6 months). Also, it has been found that despite the improvement of metabolic parameters and the reduction of androgen levels, AMH levels did not change after metformin treatment, and the dose and possibly the time of use, of metformin are factors associated with the reduction of AMH levels.31 The reason for the reduction in AMH concentrations after metformin remains controversial. In a prospective study,32 metformin acutely improved IR indexes and restored ovarian morphology. Metformin seems to suppress the hepatic gluconeogenesis. Also, metformin improves the peripheral resistance to insulin, increase the consumption of glucose in skeletal muscles, and decrease the intestinal glucose absorption. Metformin enhances insulin action at cell levels by enhancing the caption of glucose in adipose and muscular cells, and by increasing the ligation to the insulin receptors. Also, the beneficial role of metformin is due to: increase in the hepatic production of SHBG, thus lowering the circulating free testosterone, decrease the adrenal androgen production, decrease androgen production in the ovary, normalize LH and slightly increase FSH levels, and decrease the insulin concentrations. Moreover, metformin has been demonstrated to induce regular menstrual cycles, increase ovulation, ameliorate hirsutism, and produce a slight weight loss.16 It has been shown that 6 months of androgen suppression by metformin treatment failed to influence circulating AMH levels.33 Also, it has been found that metformin treatment of PCOS patients results in significant reduction in circulating AMH. Suppression of AMH occurs only after protracted treatment (after 4 months). The protracted delay suppression of AMH, in contrast to rapid suppression of androgen, may be secondary to the development of a cohort of follicles that underwent initial recruitment in an environment of reduced insulin stimulation.34
The present study revealed significant positive correlation between LH and AMH levels, and between LH/FSH ratio and AMH levels in PCOS women under metformin treatment for 6 months-3 years (GIII), which may reflect the role of serum concentration of AMH in the regulation of menstrual cycle and ovulation. It has been hypothesized that inhibin B secretion may be reduced in obese women as a result of functional impairment of the granulosa cells because of IR.35 Hyperinsulinemia, which is more common in obese women, may reduce inhibin B production either by a direct effect on the granulosa cells, or through impairment of insulin-like growth factor-I (IGF-I) action on these cells.28 There was a significant increase in mean inhibin B levels in GIII PCOS women compared with GI and GII. These results reflected that improvement of insulin action of GII PCOS as demonstrated by significant decreased of HOMA-IR of this group compared with that in GI (pre-treatment) may interpret the low levels of inhibin B in GII PCOS women. Moreover, GIII PCOS women did not show significant correction of their HOMA-IR, which may be linked to a significant increase of inhibin B compared to that in GI.
The present study noted a significant decrease in the BMI of GII women compared with those of GIII women. However, the PCOS of women in GII and GIII still have had a significant increase in BMI. The sustained elevated BMI in both groups (GII and GIII) after short and long metformin treatment of the present study may highlight the important role of lifestyle habits in improving obesity, and subsequent efficacy of metformin treatment. In agreement with Palomba et al,36 the present study confirmed a significant decrease in the number of ovarian follicles in PCOS women treated with metformin for 3 months compared with themselves before treatment, and to PCOS women treated from 6 months-3 years. The mean ovarian volume in GII PCOS women was significantly decreased compared with GI and GIII (Table 2). In patients with PCOS, there is a barrier that keeps follicles from becoming the dominant follicle. In addition to the very low levels of FSH, high levels of AMH decrease the sensitivity of follicles to FSH. Thus, follicles cannot develop into a dominant follicle, which leads to an accumulation of small antral follicles 2-9 mm in diameter. The AMH also inhibit the activity of the aromatase enzyme, suggesting that AMH contributes to the severity of PCOS.37,38
The present study found that there was significant positive correlation between BMI and ovarian volume in GIII PCOS women, which may explain the increased ovarian volume of those women since they were considered obese, and demonstrate the significant association of lifestyle habit in improvement of PCOS treatment with metformin. It has been shown that metformin enters the cell and directly stimulates the tyrosine kinase activity of the intracellular portion of the b-subunit of the insulin receptor. During the therapy with metformin, the reduction in hyperinsulinemia observed may be due to improvements in hepatic extraction and insulin sensitivity. It has been also found that metformin reduce basal levels of FFA, which has a role in reducing glucose disposal in the skeletal muscle and impairment of insulin sensitivity.14,16
The limitation of the present study is the relatively small number of PCOS women, and the inability to collect and store follicular fluid for measurement of AMH and other hormones, and compare their values with those of blood serum.
In conclusion, serum AMH is a useful prognostic biochemical marker for metformin treatment in PCOS women. Metformin has beneficial effects on follicle growth in women with PCOS. Duration of metformin treatment for more than 3 months is not advised, as there was no improvement in biochemical and clinical features of PCOS women during these periods of treatment. Future studies must include evaluation of the association of serum AMH with leptin and kisspeptin in PCOS.
References
- 1.Carmina E, Azziz R. Diagnosis, phenotype, and prevalence of polycystic ovary syndrome. Fertil Steril. 2006;86(Supp 1):S7–S8. doi: 10.1016/j.fertnstert.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Aflatoonian A, Seyedhassani SM, Tabibnejad N. The epidemiological and etiological aspects of infertility in Yazd province of Iran. Iranian Journal of Reproductive Medicine. 2009;7:117–122. [Google Scholar]
- 3.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Position statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 4.Itman C, Mendis S, Barakat B, Loveland LK. All in the family: TGF-β family action in testis development. Reproduction. 2006;132:233–236. doi: 10.1530/rep.1.01075. [DOI] [PubMed] [Google Scholar]
- 5.Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: a new marker for ovarian function. Reproduction. 2006;131:1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 6.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2009;16:113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 7.Bako AU, Morad S, Atiomo WA. Polycystic ovary syndrome: an overview. Reviews in Gynaecological Practice. 2005;5:115–122. [Google Scholar]
- 8.Pellatt L, Hanna L, Brincat M, Galea R, Brain H, Whitehead S, Mason H. Granulosa cell production of anti-Mullerian hormone is increased in polycystic ovaries. J Clin Endocrinol Metab. 2007;92:240–245. doi: 10.1210/jc.2006-1582. [DOI] [PubMed] [Google Scholar]
- 9.Pigny P, Jonard S, Robert Y, Dewailly D. Serum anti-Mullerian hormone as a surrogate for antral follicle count for definition of the polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:941–945. doi: 10.1210/jc.2005-2076. [DOI] [PubMed] [Google Scholar]
- 10.Wiweko B, Maidarti M, Priangga MD, Shafira N, Fernando D, Sumapraja K, et al. Anti-mullerian hormone as a diagnostic and prognostic tool for PCOS patients. J Assist Reprod Genet. 2014;31:1311–1316. doi: 10.1007/s10815-014-0300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DE, Park SY, Park SY, Lee SR, Chung HW, Jeong K. Clinical and biochemical profiles according to Homeostasis Model Assessment-insulin Resistance (HOMA-IR) in Korean women with polycystic ovary syndrome. J Menopausal Med. 2014;20:104–110. doi: 10.6118/jmm.2014.20.3.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boomsma CM, Fauser BC, Macklon NS. Pregnancy complications in women with polycystic ovary syndrome. Semin Reprod Med. 2008;26:72–84. doi: 10.1055/s-2007-992927. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Yu G, Yang D, Li S, Lu S, Wu X, et al. Prevalence and predictors of metabolic abnormalities in Chinese women with PCOS: a cross-sectional study. BMC Endocr Disord. 2014;14:76. doi: 10.1186/1472-6823-14-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NP. Metformin use in women with polycystic ovary syndrome. Ann Transl Med. 2014;2:56–63. doi: 10.3978/j.issn.2305-5839.2014.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestler JE. Metformin in the treatment of infertility in PCOS: an alternative perspective. Fertil Steril. 2008;90:14–16. doi: 10.1016/j.fertnstert.2008.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neagu M, Cristescu C. Anti-Műllerian hormone -- a prognostic marker for metformin therapy efficiency in the treatment of women with infertility and polycystic ovary syndrome. J Med Life. 2012;5:462–464. [PMC free article] [PubMed] [Google Scholar]
- 17.Nestler JE. Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med. 2008;358:47–54. doi: 10.1056/NEJMct0707092. [DOI] [PubMed] [Google Scholar]
- 18.Tan S, Hahn S, Benson S, Dietz T, Lahner H, Moeller LC, et al. Metformin improves polycystic ovary syndrome symptoms irrespective of pre-treatment insulin resistance. Eur J Endocrinol. 2007;157:669–676. doi: 10.1530/EJE-07-0294. [DOI] [PubMed] [Google Scholar]
- 19.Badawy A, Elnashar A. Treatment options for polycystic ovary syndrome. Int J Womens Health. 2011;3:25–35. doi: 10.2147/IJWH.S11304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91:456–488. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 21.Kricka LJ. Interferences in immunoassay-still a threat. Clin Chem. 2000;46:1037–1038. [PubMed] [Google Scholar]
- 22.Groome NP, Evans LW. Does measurement of inhibin have a clinical role?Ann Clin Biochem. 2000;37:419–431. doi: 10.1177/000456320003700401. [DOI] [PubMed] [Google Scholar]
- 23.Burits CA, Ashwood ER, Bruns DE, editors. Tietz Fundamentals of Clinical Chemistry. 6th ed. St. Louis (MO): Saunders Elsevier; 2008. pp. 373–401. (735-801). [Google Scholar]
- 24.Carlsson IB, Scott JE, Visser JA, Ritvos O, Themnen AP, Hovatta O. Anti-Mullerian hormone inhibits initiation of growth of human primordial ovarian follicles in vitro. Hum Reprod. 2006;21:2223–2227. doi: 10.1093/humrep/del165. [DOI] [PubMed] [Google Scholar]
- 25.La Marca A, Volpe A. Anti-Mullerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool?Clin Endocrinol (Oxf) 2006;64:603–610. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 26.Catteau-Jonard S, Jamin SP, Leclerc A, Gonzalès J, Dewailly D, di Clemente N. Anti-Mullerian hormone, its receptor, FSH receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93:4456–4461. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 27.Chen MJ, Yang WS, Chen CL, Wu MY, Yang YS, Ho HN. The relationship between anti-Mullerian hormone, androgen and insulin resistance on the number of antral follicles in women with the polycystic ovary syndrome. Hum Reprod. 2008;23:952–957. doi: 10.1093/humrep/den015. [DOI] [PubMed] [Google Scholar]
- 28.Fleming R, Harborne L, MacLaughlin DT, Ling D, Norman J, Sattar N, et al. Metformin reduces serum mullerian-inhibiting substance levels in women with polycystic ovary syndrome after protracted treatment. Fertil Steril. 2005;83:130–136. doi: 10.1016/j.fertnstert.2004.05.098. [DOI] [PubMed] [Google Scholar]
- 29.Tomova A, Deepinder F, Robeva R, Kirilov G, Mechandjiev Z, Kumanov P. Anti-Müllerian hormone in women with polycystic ovary syndrome before and after therapy with metformin. Horm Metab Res. 2011;43:723–727. doi: 10.1055/s-0031-1286307. [DOI] [PubMed] [Google Scholar]
- 30.Grigoryan O, Absatarova J, Andreeva E, Melnichenko G, Dedov I. Effect of metformin on the level of anti-Mullerian hormone in therapy of polycystic ovary syndrome in obese women. Minerva Ginecol. 2014;66:85–89. [PubMed] [Google Scholar]
- 31.Nascimento AD, Silva Lara LA, Japur de Sá Rosa-e-Silva AC, Ferriani RA, Reis RM. Effects of metformin on serum insulin and anti-Mullerian hormone levels and on hyperandrogenism in patients with polycystic ovary syndrome. Gynecol Endocrinol. 2013;29:246–249. doi: 10.3109/09513590.2012.736563. [DOI] [PubMed] [Google Scholar]
- 32.Bayrak A, Terbell H, Urwitz-Lane R, Mor E, Stanczyk FZ, Paulson RJ. Acute effects of metformin therapy include improvement of insulin resistance and ovarian morphology. Fertil Steril. 2007;87:870–875. doi: 10.1016/j.fertnstert.2006.08.096. [DOI] [PubMed] [Google Scholar]
- 33.Carlsen SM, Vanky E, Fleming R. Anti-Müllerian hormone concentrations in androgen-suppressed women with polycystic ovary syndrome. Hum Reprod. 2009;24:1732–1738. doi: 10.1093/humrep/dep074. [DOI] [PubMed] [Google Scholar]
- 34.Richard SL, Ricardo A. Androgen excess disorders. Danforth’s Obstetrics and Gynecology. 8th ed. Philadelphia (PA): Lippincott Williams &Wilkins; 2003. pp. 663–672. [Google Scholar]
- 35.Welt CK, Taylor AE, Martin KA, Hall JE. Serum inhibin B in polycystic ovary syndrome: regulation by insulin and luteinizing hormone. J Clin Endocrinol Metab. 2002;87:5559–5565. doi: 10.1210/jc.2002-020546. [DOI] [PubMed] [Google Scholar]
- 36.Palomba S, Falbo A, Russo T, Orio F, Tolino A, Zullo F. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod. 2010;25:1005–1013. doi: 10.1093/humrep/dep466. [DOI] [PubMed] [Google Scholar]
- 37.Nardo LG, Yates AP, Roberts SA. The relationships between AMH, androgens, insulin resistance and basal ovarian follicular status in non-obese subfertile women with and without polycystic ovary syndrome. Hum Reprod. 2009;24:2917–2923. doi: 10.1093/humrep/dep225. [DOI] [PubMed] [Google Scholar]
- 38.Begawy AF, El-Mazny AN, Abou-Salem NA, El-Taweel NE. Anti-mullerian hormone in polycystic ovary syndrome and normo-ovulatory women: correlation with clinical, hormonal and ultrasonographic parameters. Middle East Fertility Society Journal. 2010;15:253–258. [Google Scholar]


