Abstract
Objectives:
To present the visual sequelae of methanol poisoning and to emphasize the characteristics of methanol exposure in the Kingdom of Saudi Arabia (KSA).
Methods:
A retrospective case series was carried out on 50 sequential patients with methanol poisoning seen at the King Khaled Eye Specialist Hospital and King Saud University Hospitals in Riyadh, KSA between 2008 and 2014. All patients were examined by a neuro-ophthalmologist at least one month after methanol intoxication.
Results:
All 50 patients were young or middle-aged males. All admitted to drinking unbranded alcohol within 2-3 days before profound or relatively profound, painless, bilateral visual loss. Mean visual acuity in this group was hand motions (logMAR 2.82; range 0.1 - 5.0) with some eye to eye variability within individuals. Worse visual acuity was correlated with advancing age (Pearson correlation: oculus dextrus [right eye] - 0.37, p=0.008; oculus sinister [left eye] - 0.36, p=0.011). All patients had optic atrophy bilaterally, and all tested patients had visual field defects. Tremors with or without rigidity were present in 12 patients, and 11 of 30 patients who had neuroimaging performed had evidence of putaminal necrosis.
Conclusion:
Methanol intoxication causes visual loss within 12-48 hours due to relatively severe, painless, bilateral optic nerve damage that may be somewhat variable between eyes, and is generally worse with advancing age. The coincidence of bilateral optic nerve damage and bilateral putaminal necrosis in a young or middle-aged male is very suspicious for methanol-induced damage.
Methanol is a clear and colorless alcohol that tastes and smells the same as ethanol, but causes much less behavioral intoxication.1 It is commonly used in industry as a component of products such as antifreeze, windshield-wiper fluid, and model airplane fuel.2 In the Kingdom of Saudi Arabia (KSA), it can be found as a solvent in some brands of perfume and cologne. Ingestion of methanol may be accidental, or due to a suicide attempt. But the most common situation occurs as isolated episodes, or epidemics of methanol poisoning and its toxic optic neuropathy, which are seen due to the contamination of handmade liquor, smuggled alcohol, and so forth with methyl alcohol.2,3 Death from methanol toxicity has been reported to range between 8-36%,4-6 and permanent loss of vision has been observed in another 20-40% of patients who survive the acute injury.2,5,7 Vision loss is painless and often occurs in both eyes within one to 3 days; vision in some patients may either improve, or decline over subsequent weeks.8 The optic disk in acute intoxication has a hyperemic appearance with edema of the peripapillary retina;9 however, the optic nerve gradually becomes pale within 30-60 days after ingestion. Large, sluggishly reactive pupils have been reported to occur frequently in acute methanol poisoning, sometimes leaving both pupils permanently dilated.5,9 Neurological signs, such as confusion and coma are common in acutely hospitalized patients, and putaminal hemorrhage, and/or necrosis occur less frequently.1,2,10 This study evaluates ophthalmologic, neurologic, and neuroimaging signs in 50 consecutive patients seen at 2 major ophthalmologic centers in KSA due to visual loss after methanol poisoning.
Methods
This is a retrospective case series of 50 consecutive patients seen at the King Khaled Eye Specialist Hospital or King Saud University hospitals, Riyadh, KSA between September 2008 and September 2014 who admitted to drinking unbranded alcohol, perfume, or cologne within one to 3 days before losing vision in both eyes. This paper has the ethical approval of the Research Department of King Khaled Eye Specialist Hospital and the Research Committee of the Ophthalmology Department of the King Saud University College of Medicine, Riyadh, KSA. Exclusion criteria included denial by patient and family of any experience that might have included methanol ingestion, or an obvious alternative diagnosis for the patient’s neuro-ophthalmologic abnormalities. All patients received a complete neuro-ophthalmologic assessment during a stable clinical phase more than one month after exposure. Medical records, ophthalmologic testing, and neuroimaging were reviewed. None of the patients were examined during the acute phase of intoxication, but some had provided medical records and fundus photos from the time of initial evaluation elsewhere. A number of patients reported being treated with bicarbonate and folate and hemodialysis, but none received ethanol, or fomepizole.5-7,11 Patients were excluded from the study if documentation was inadequate for diagnosis or evaluation. Clinical characteristics were recorded, including gender, age, visual acuity, visual fields, pupillary reaction, fundus features, other neurological signs, and cranial and orbital neuroimaging observations. Goldmann perimetry was performed based on clinical indications.
Statistical analysis was performed using Statistical Package for Social Sciences version 14 (SPSS Inc., Chicago, IL, USA). Snellen acuity was converted to the logarithm of the minimum angle of resolution (logMAR) when necessary for statistical analysis. Some patients were studied with a GE Discovery 3 Tesla MRI Unit using standard protocols.
Results
All patients reported acute, profound, painless, bilateral visual loss a month or more prior to evaluation. The demographic features of this patient population are presented in Table 1. All 50 patients were male with a mean age of 38.1 years. No patient was examined after acute methanol intoxication, but several were able to provide fundus photography from their initial evaluation. Figure 1 illustrates the acute (approximately one week after methanol exposure) fundoscopic appearance in the eyes of 2 patients (Figures 1A & 1B) that show pallid, mild optic disk edema, and retinal edema. These images can be compared with the fundoscopic appearance of 2 different patients, taken approximately 6 weeks after methanol exposure (Figures 1C & 1D) with flat, very pale optic disks, and no residual retinal edema. Many patients (41 out of 50) had fixed, dilated pupils bilaterally when examined. The mean Snellen visual acuity (VA) in this patient group was hand motions (logMAR 2.82) but varied between 20/25 (logMAR 0.1), and no light perception (logMAR 5.0). Visual acuity in the 2 eyes of each patient was strongly correlated (Pearson correlation=0.67, p=0.001), although a few patients exhibited surprising asymmetry (in one patient, 20/25 in one eye and light perception in the other). Worse VA was correlated with advancing patient age (Pearson correlation VA oculus dextrus (OD [right eye]) versus age 0.37, p=0.008; VA oculus sinister [OS] [left eye] versus age 0.36, p=0.011). Goldman visual fields were performed in 19 patients and were abnormal in all, most with central visual field loss, but some with nerve fiber bundle, or paracentral defects. All patients had optic atrophy. Most patients had otherwise, normal neurologic examinations, but 13 patients had either tremors, or tremors with rigidity. Magnetic resonance imaging (MRI) was performed in 30 patients, almost all of whom had somewhat small optic nerves bilaterally on imaging (Figure 2A). Eleven patients had necrosis of the putamen, and occasionally the claustrum bilaterally (Figures 2B & 2C) that could be asymmetric (Figure 2D), or mild (Figure 2E). Two patients had additional foci of demyelination in the subcortical and deep hemispheric white matter (Figure 2F). Not all patients with abnormal neuroimaging had an abnormal neurologic examination, but observation of putaminal necrosis correlated strongly with the presence of tremor and/or rigidity (Fisher’s exact test, p=0.015). Putaminal necrosis did not correlate with VA (Mann-Whitney test: OD; p=0.825, OS; p=0.434), or with patient age (independent sample t-test, p=0.925). Of particular interest was the presence of the 11778 primary Leber Hereditary Optic Neuropathy (LHON) mutation in one patient.
Table 1.
Demographic characteristics of patients with methanol poisoning included in a study in Saudi Arabia.
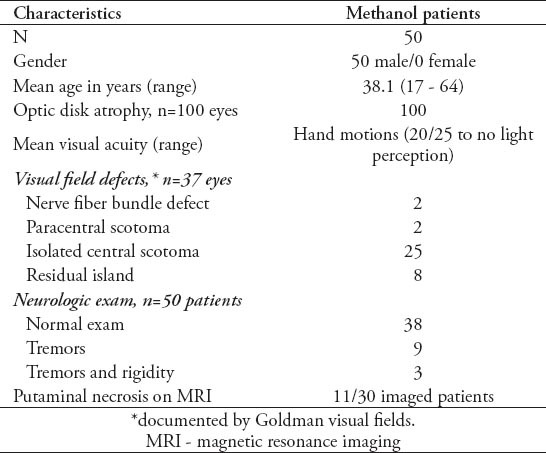
Figure 1.
Acute and chronic fundoscopic changes. Fundus images of 4 patients with the right optic disk displayed on the left side and the left optic disk displayed on the right side. Images showing the optic disks of 2 patients taken within one week of methanol exposure showing modest pallid edema of the optic disks extending onto the peripapillary retina (Figures 1A & 1B). Images depicting the optic disks of 2 different patients taken approximately 6 weeks after methanol exposure, and showing flat, moderately pale optic disks bilaterally with no residual optic disk or retinal edema (Figures 1C & 1D).
Figure 2.
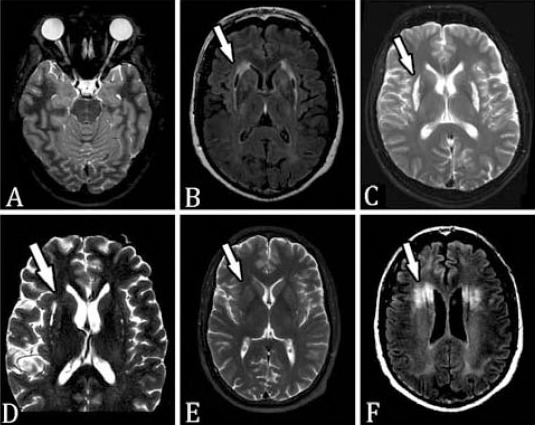
Neuroimaging of methanol poisoning: A) axial fat suppressed T2W image showing attenuated caliber optic nerves bilaterally with abnormal signal intensity more obvious on the right side; B) Flair and C) T2W images of 2 different patients illustrating typical appearance of necrosis of the putamin and claustrum bilaterally (arrows); D) T2W image showing asymmetric injury to the basal ganglia, right worse than left in this patient (arrow). E) T2W image showing bilateral mild necrosis of the putamin (arrow); and F) flair image showing additional foci of demyelination in the subcortical and deep white matter sparing the thin rim of white matter adjacent to the cortex (present in 2 patients) (arrow).
Discussion
This study evaluated 50 consecutive patients seen at 2 major ophthalmologic centers in KSA due to visual loss due to methanol poisoning. All were male, young, or middle aged, and sought ophthalmologic evaluation because of visual loss at least one month after methanol intoxication that was often severe. Mean VA was hand motions, and all 100 eyes had optic atrophy. In addition, almost one quarter of these 50 patients had neurologic signs (tremors and rigidity), and more than one third of those receiving neuroimaging had putaminal necrosis.
The minimum lethal dose of methanol is generally considered to be 30 ml of 40% methanol, but as little as 10 ml of methanol may cause blindness.4,9 Variability in lethal and blinding methanol doses between individuals is probably due to individual variation in susceptibility, concomitant ingestion of ethanol, unreliability of patient history regarding the amount ingested, and possibly other variations in individual susceptibility. For example, Prabhakaran et al12 reported 2 young women who presented with methanol poisoning. They had similar plasma methanol concentrations at approximately the same time after ingestion, but one patient developed brain death, and the other recovered without sequelae.12 The pathway of methanol metabolism2 and some of the mechanisms of injury to the optic nerve3,13 and brain14,15 are now relatively well understood. Methanol itself is relatively non-toxic and can be excreted unaltered through the lungs and kidneys. However, the major route of excretion is metabolism in the liver by alcohol dehydrogenase to formaldehyde, and then by formaldehyde dehydrogenase to formic acid.1,2,16 Formaldehyde is relative toxic, but its levels after methanol ingestion typically remain relatively low because metabolism to formic acid occurs rapidly.1 Formic acid is much more toxic because it easily invades cells, inhibits cytochrome c oxidase and aerobic metabolism, and induces acidosis, and a crisis of energy production. The metabolism of formic acid to CO2 and H2O occurs slowly by pathways requiring folate, which is generally present in relatively low levels in the human liver. The selective optic nerve toxicity of methanol is striking and quite likely occurs because formic acid prevents mitochondrial oxidative phosphorylation by inhibiting cytochrome oxidase activity. Methanol studies by Hayreh et al and others17-19 on animal models confirmed the action of methanol toxicity through inhibition of cytochrome oxidase in the laminar and retrolaminar regions of the optic nerve. Their histopathologic studies documented disruption of axonal flow, mitochondrial edema and clustering, fragmentation of neurotubules and neurofilaments, and axonal blistering. They also described alterations in glial cells, including astrocytic edema, and edema of the oligodendroglial cytoplasm.
The laboratory model created by Sadun20 following an epidemic of toxic optic neuropathy in Cuba treated rats with high doses of formic acid. Optic disc edema was observed with prelaminar axonal vacuolization and mitochondrial changes. These findings may indicate that the prelaminar region of the optic nerve head is particularly vulnerable to a decrease in available energy, which is consistent with studies of the human optic nerve that show a line of high cytochrome oxidase activity anterior to the cribriform plate.21 Other authors have described edema of oligodendrocytes in the retrolaminar region with progressive demyelination of the optic nerve.13 Although the optic nerve appears to be the main target of methanol poisoning, studies in both humans and animals have demonstrated retinal toxicity as well.3,22 Peripapillary retinal edema is commonly observed after acute methanol intoxication.9,22 Murray et al,23 using an experimental rat model of exposure to methanol, documented early alterations of the electroretinogram (ERG) followed by mitochondrial edema and disruption in the photoreceptor inner segment, retinal pigment epithelium, and optic nerve on subsequent electron microscopy.
A number of neuropathologic14 and neuroimaging24,25 studies have described the typical central nervous system (CNS) pathology of methanol poisoning. The most common CNS injury is bilateral hemorrhagic or non-hemorrhagic necrosis of the putamen, occurring in roughly half of all patients. Other lesions commonly noted include diffuse white matter hypodensity and bilateral occipital necrosis. In addition, some patients develop necrosis of the subcortical grey and white matter, cerebellar cortical lesions, bilateral intracerebral hemorrhage, bilateral tegmental necrosis, and diffuse cerebral edema. The particular vulnerability of the putamen is not fully understood but may be related to higher concentrations of formic acid there, contributory blood flow patterns in the basal ganglia, increased metabolic sensitivity of striatal neurons to methanol metabolites, or increased vulnerability to apoptosis. Other disorders associated with similar putaminal lesions include CO2 inhalation, hypoxic-ischemic injury, acute cyanide intoxication, certain mitochondrial DNA mutation syndromes,26,27 Wilson’s disease, Kearns Sayre syndrome, and Leigh disease, most of which create a potentially toxic setting for mitochondria.
Optic nerve, retina, and basal ganglia are the tissues most at risk from methanol intoxication. The association of optic nerve damage and basal ganglia necrosis with mitochondrial disease has been recognized previously in the association of LHON with basal ganglia disease (LHON and dystonia), although in this setting chronic basal ganglia disease usually precedes visual loss.27 It is not clear why distal optic nerve and putamen share this vulnerability, although both likely have relatively high metabolic rates. In addition, optic nerve axons and putaminal neurons may share metabolic or apoptotic sensitivity to methanol metabolites, or formic acid and formaldehyde may accumulate in these structures in higher concentrations than elsewhere, or decreased blood flow may occur in short posterior ciliary arteries and the basal veins of Rosenthal due to hypotension. Under any circumstance, the acute involvement of both optic nerves and basal ganglia in a young or middle-aged man, often with acidosis, encephalopathy, and abdominal discomfort is very suspicious for methanol intoxication. Similar visual and neurologic injury to our patients has been reported previously in large outbreaks of methanol poisoning.5,7,9 Usually, there is a latent period of 12-36 hours between the time of ingestion and the onset of visual symptoms, probably because of slow metabolism of methanol and gradual accumulation of formic acid.3 Pupils are sometimes large and sluggish bilaterally.9 Visual loss is painless, bilateral, and commonly severe; variable between patients (probably for a variety of reasons including volume ingested and age); sometimes surprisingly asymmetric; and may either increase or decrease over the initial weeks after injury.5,7,9 Possibly as many as one half of surviving patients exhibit some component of visual loss.6
Accurate diagnosis and rapid treatment of methanol ingestion are necessary to prevent death, and to minimize the ocular and neurological sequelae. Restoration of normal pH is the first priority in treatment because this inhibits formic acid entry into cells and improves both survival and recovery of vision.7 Sodium bicarbonate, hemodialysis, and/or peritoneal dialysis1,28 may be necessary to treat the metabolic acidosis.2 Classically, systemic treatment involves oral or intravenous ethanol, a competitive inhibitor of liver alcohol dehydrogenase (LADH), the first step in methanol metabolism, aiming for blood concentration of approximately 22 mmol/L to avoid more CNS depression.20 More recently, the ideal treatment includes correction of the systemic acidosis,6 folate supplementation, inhibition of methanol metabolism using fomepizole,11,28,29 an aldehyde dehydrogenase inhibitor and dialysis, often in combination.8,11,29,30 Folic acid analogs have been employed in the management of acute methanol intoxication.20 Early treatment with oral or intravenous steroids, and vitamin B1 have been shown in several studies to improve final visual outcome, even if given as long as 4-10 days after ingestion.31
Large epidemics of death and visual loss after methanol ingestion have been reported a number of times,4-6 but the presentation of these patients in KSA was somewhat different than reported elsewhere. In general, the number of individuals involved in a particular episode of methanol exposure in KSA was less than 10, with ingestion occurring in a remote setting. On occasion, individuals drank unbranded alcohol, but most often methanol was obtained by mistake when individuals thought that ethanol, rather than methanol, was the solvent in purchased cologne or perfume. Individuals would typically seek medical attention within the next 12-48 hours because of abdominal pain, encephalopathy, and/or blurred vision, most often as a single patient and not as part of a larger group of affected individuals. Patients were often hesitant to admit to drinking alcohol, particularly in the presence of family members. This situation is complicated for emergency room personnel in KSA because a patient suffering from methanol intoxication can appear as an isolated affected individual hours after ingestion, sometimes critically ill with an inaccurate or incomplete history, and relatively non-specific signs and symptoms. Diagnosis in this setting depends on a high level of suspicion,32 eliciting the typical history and symptoms, recognition of a substantial metabolic acidosis, and diagnosis of progressive visual loss. Unfortunately, most of the patients reported here were not correctly diagnosed, and received no treatment appropriate for methanol intoxication other than symptomatic treatment of acidosis. None received fomepizole, ethanol, or dialysis; a few were treated with intravenous methylprednisolone on the assumption that visual loss and optic disk edema were due to optic nerve inflammation, possibly with some effect.30
The ophthalmologic examination provides extremely useful information in this setting. Visual loss can be partial or complete, and can develop from hours to several days after methanol ingestion. The degree of pupillary light reflex impairment may reflect the severity of the systemic toxicity.9 Other pertinent acute ophthalmologic observations included hyperemic or pallid optic disk edema that is often fairly mild and retinal edema extending along the arcades (Figures 1A & 1B).3 Patients also sometimes have cystoid macular edema, pseudo-cherry red spot, retinal hemorrhages, and engorgement of retinal veins. Optic atrophy with or without deep excavation of the disk frequently develops weeks after severe intoxication (Figures 1C & 1D).4,9
Confirming the cause of visual loss during an ophthalmologic evaluation taking place a month or more after the inciting event was occasionally problematic. Patients sometimes needed to be questioned several times regarding events preceding visual loss or interviewed when family members were not present. Records from an initial hospitalization sometimes clarified the clinical circumstances, or at times patients would report that a friend or relative accompanying them at a party had died unexpectedly. Methanol injury is a particularly likely diagnosis in a young or middle-aged man with no major pre-morbid medical risk factors who has bilateral, relatively severe visual loss due to optic nerve disease, particularly if associated with acidosis and bilateral putaminal necrosis.
There are several limitations to this study. No patient was evaluated after acute methanol intoxication, although some patients were able to provide medical records and/or fundus photographs from a previous evaluation within the first week after exposure. Available information did not generally permit comparison between visual performance at the time of acute presentation, and the evaluation reported here that generally took place more than one month later. Ophthalmologic data were not available from patients who died before receiving medical attention, or while being cared for in an acute care facility. Similarly, no ophthalmologic data was available from patients who chose not to obtain an ophthalmologic evaluation because of visual loss. Finaly, this report may reflect a selection bias for more severe visual loss, since patients who recovered completely, or almost completely may not have sought ophthalmologic follow-up. Nevertheless, the visual acuities reported here are in general agreement with previous reports.5-9
In conclusion, The discovery of simple diagnostic techniques and new therapies for methanol poisoning should be included in future studies to avoid dramatic loss of vision in patients.
Footnotes
Related Articles.
Hajar S, Al Hazmi A, Wasli M, Mousa A, Rabiu M. Prevalence and causes of blindness and diabetic retinopathy in Southern Saudi Arabia. Saudi Med J 2015; 36: 449-455.
Khandekar RB, Al-Towerki AA, Al-Katan H, Al-Mesfer SS, Abboud EB, Al-Hussain HM, et al. Ocular malignant tumors. Review of the Tumor Registry at a tertiary eye hospital in central Saudi Arabia. Saudi Med J 2014; 35: 377-384.
Nizamuddin SH, Bawazeer AM. Causes of uveitis in a tertiary center in Western Saudi Arabia. Saudi Med J 2013; 34: 379-387.
Gedik S, Gonul S, Koktekir BE, Bakbak B. In vivo confocal microscopy of corneal endothelium in patients with retinitis pigmentosa. Saudi Med J 2012; 33: 1330-1133.
References
- 1.Barceloux DG, Bond GR, Krenzelok EP, Cooper H, Vale JA. American Academy of Clinical Toxicology Ad Hoc Committee on the Treatment Guidelines for Methanol Poisoning. American Academy of Clinical Toxicology Practice Guidelines on the Treatment of Methanol Poisoning. J Toxicol Clin Toxicol. 2002;40:415–446. doi: 10.1081/clt-120006745. [DOI] [PubMed] [Google Scholar]
- 2.Kraut JA, Kurtz I. Toxic alcohol ingestions: clinical features, diagnosis, and management. Clin J Am Soc Nephrol. 2008;3:208–225. doi: 10.2215/CJN.03220807. [DOI] [PubMed] [Google Scholar]
- 3.Ingemansson SO. Clinical observations on ten cases of methanol poisoning with particular reference to ocular manifestations. Acta Ophthalmol (Copenh) 1984;62:15–24. doi: 10.1111/j.1755-3768.1984.tb06753.x. [DOI] [PubMed] [Google Scholar]
- 4.Bennett IL, Jr, Cary FH, Mitchell GL, Jr, Cooper MN. Acute methyl alcohol poisoning: a review based on experiences in an outbreak of 323 cases. Medicine (Baltimore) 1953;32:431–463. doi: 10.1097/00005792-195312000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hovda KE, Hunderi OH, Tafjord AB, Dunlop O, Rudberg N, Jacobsen D. Methanol outbreak in Norway 2002-2004: epidemiology, clinical features and prognostic signs. J Intern Med. 2005;258:181–190. doi: 10.1111/j.1365-2796.2005.01521.x. [DOI] [PubMed] [Google Scholar]
- 6.Paasma R, Hovda KE, Jacobsen D. Methanol poisoning and long term sequelae - a six years follow-up after a large methanol outbreak. BMC Clin Pharmacol. 2009;9:5. doi: 10.1186/1472-6904-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paasma R, Hovda KE, Tikkerberi A, Jacobsen D. Methanol mass poisoning in Estonia: outbreak in 154 patients. Clin Toxicol (Phila) 2007;45:152–157. doi: 10.1080/15563650600956329. [DOI] [PubMed] [Google Scholar]
- 8.Sanaei-Zadeh H, Zamani N, Shadnia S. Outcomes of visual disturbances after methanol poisoning. Clin Toxicol (Phila) 2011;49:102–107. doi: 10.3109/15563650.2011.556642. [DOI] [PubMed] [Google Scholar]
- 9.Benton CD, Jr, Calhoun FP., Jr The ocular effects of methyl alcohol poisoning: report of a catastrophe involving three hundred and twenty persons. Trans Am Acad Ophthalmol Otolaryngol. 1952;56:875–885. [PubMed] [Google Scholar]
- 10.Jain N, Himanshu D, Verma SP, Parihar A. Methanol poisoning: characteristic MRI findings. Ann Saudi Med. 2013;33:68–69. doi: 10.5144/0256-4947.2012.26.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovda KE, Froyshov S, Gudmundsdottir H, Rudberg N, Jacobsen D. Fomepizole may change indication for hemodialysis in methanol poisoning: prospective study in seven cases. Clin Nephrol. 2005;64:190–197. doi: 10.5414/cnp64190. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran V, Ettler H, Mills A. Methanol poisoning: two cases with similar plasma methanol concentrations but different outcomes. CMAJ. 1993;148:981–984. [PMC free article] [PubMed] [Google Scholar]
- 13.Sharpe JA, Hostovsky M, Bilbao JM, Rewcastle NB. Methanol optic neuropathy: a histopathological study. Neurology. 1982;32:1093–1100. doi: 10.1212/wnl.32.10.1093. [DOI] [PubMed] [Google Scholar]
- 14.Karayel F, Turan AA, Sav A, Pakis I, Akyildiz EU, Ersoy G. Methanol intoxication: pathological changes of central nervous system (17 cases) Am J Forensic Med Pathol. 2010;31:34–36. doi: 10.1097/PAF.0b013e3181c160d9. [DOI] [PubMed] [Google Scholar]
- 15.Reddy NJ, Sudini M, Lewis LD. Delayed neurological sequelae from ethylene glycol, diethylene glycol and methanol poisonings. Clin Toxicol (Phila) 2010;48:967–973. doi: 10.3109/15563650.2010.532803. [DOI] [PubMed] [Google Scholar]
- 16.Lee SL, Shih HT, Chi YC, Li YP, Yin SJ. Oxidation of methanol, ethylene glycol, and isopropanol with human alcohol dehydrogenases and the inhibition by ethanol and 4-methylpyrazole. Chem Biol Interact. 2011;191:26–31. doi: 10.1016/j.cbi.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Amat G, Tephly TR, McMartin KE, Makar AB, Hayreh MS, Hayreh SS, et al. Methyl alcohol poisoning. II. Development of a model for ocular toxicity in methyl alcohol poisoning using the rhesus monkey. Arch Ophthalmol. 1977;95:1847–1850. doi: 10.1001/archopht.1977.04450100149021. [DOI] [PubMed] [Google Scholar]
- 18.Hayreh MS, Hayreh SS, Baumbach GL, Cancilla P, Martin-Amat G, Tephly TR, et al. Methyl alcohol poisoning III. Ocular toxicity. Arch Ophthalmol. 1977;95:1851–1858. doi: 10.1001/archopht.1977.04450100153022. [DOI] [PubMed] [Google Scholar]
- 19.Baumbach GL, Cancilla PA, Martin-Amat G, Tephly TR, McMartin KE, Makar AB, et al. Methyl alcohol poisoning. IV. Alterations of the morphological findings of the retina and optic nerve. Arch Ophthalmol. 1977;95:1859–1865. doi: 10.1001/archopht.1977.04450100161023. [DOI] [PubMed] [Google Scholar]
- 20.Sadun A. Acquired mitochondrial impairment as a cause of optic nerve disease. Trans Am Ophthalmol Soc. 1998;96:881–923. [PMC free article] [PubMed] [Google Scholar]
- 21.Barron MJ, Griffiths P, Turnbull DM, Bates D, Nichols P. The distributions of mitochondria and sodium channels reflect the specific energy requirements and conduction properties of the human optic nerve head. Br J Ophthalmol. 2004;88:286–290. doi: 10.1136/bjo.2003.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKellar MJ, Hidajat RR, Elder MJ. Acute ocular methanol toxicity: clinical and electrophysiological features. Aust N Z J Ophthalmol. 1997;25:225–230. doi: 10.1111/j.1442-9071.1997.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 23.Eells JT, Henry MM, Summerfelt P, Wong-Riley MT, Buchmann EV, Kane M, et al. Therapeutic photobiomodulation for methanol-induced retinal toxicity. Proc Natl Acad Sci U S A. 2003;100:3439–3444. doi: 10.1073/pnas.0534746100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanaei-Zadeh H. Typical bilateral putaminal lesions of methanol intoxication. J Emerg Med. 2012;42:178–179. doi: 10.1016/j.jemermed.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Taheri MS, Moghaddam HH, Moharamzad Y, Dadgari S, Nahvi V. The value of brain CT findings in acute methanol toxicity. Eur J Radiol. 2010;73:211–214. doi: 10.1016/j.ejrad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Leng Y, Liu Y, Fang X, Li Y, Yu L, Yuan Y, et al. The mitochondrial DNA 10197 G>A mutation causes MELAS/Leigh overlap syndrome presenting with acute auditory agnosia. Mitochondrial DNA. 2015;26:208–212. doi: 10.3109/19401736.2014.905860. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Amero KK, Bosley TM, Bohlega S, McLean D. Complex I respiratory defect in LHON plus dystonia with no mitochondrial DNA mutation. Br J Ophthalmol. 2005;89:1380–1381. doi: 10.1136/bjo.2005.072819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hovda KE, Andersson KS, Urdal P, Jacobsen D. Methanol and formate kinetics during treatment with fomepizole. Clin Toxicol (Phila) 2005;43:221–227. [PubMed] [Google Scholar]
- 29.Sanaei-Zadeh H, Zamani N, Shahmohammadi F. Can fomepizole be substituted by abacavir in the treatment of methanol poisoning? J Med Toxicol. 2011;7:179–180. doi: 10.1007/s13181-011-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanaei-Zadeh H. Is high-dose intravenous steroid effective on preserving vision in acute methanol poisoning? Optom Vis Sci. 2012;89:244. doi: 10.1097/OPX.0b013e3182495363. [DOI] [PubMed] [Google Scholar]
- 31.Stelmach MZ, O’Day J. Partly reversible visual failure with methanol toxicity. Aust N Z J Ophthalmol. 1992;20:57–64. doi: 10.1111/j.1442-9071.1992.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 32.Al Aseri Z, Altamimi S. Keeping a high index of suspicion: lessons learned in the management of methanol ingestion. BMJ Case Rep. 2009;2009 doi: 10.1136/bcr.09.2008.1013. pii:bcr09.2008.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]


